Abstract
Background
Follicular variant of papillary thyroid carcinoma (FVPTC) shares features of papillary (PTC) and follicular (FTC) thyroid carcinomas on a clinical, morphological, and genetic level. MicroRNA (miRNA) deregulation was extensively studied in PTCs and FTCs. However, very limited information is available for FVPTC. The aim of this study was to assess miRNA expression in FVPTC with the most comprehensive miRNA array panel and to correlate it with the clinicopathological data.
Methods
Forty-four papillary thyroid carcinomas (17 FVPTC, 27 classic PTC) and eight normal thyroid tissue samples were analyzed for expression of 748 miRNAs using Human Microarray Assays on the ABI 7900 platform (Life Technologies, Carlsbad, CA). In addition, an independent set of 61 tumor and normal samples was studied for expression of novel miRNA markers detected in this study.
Results
Overall, the miRNA expression profile demonstrated similar trends between FVPTC and classic PTC. Fourteen miRNAs were deregulated in FVPTC with a fold change of more than five (up/down), including miRNAs known to be upregulated in PTC (miR-146b-3p, -146-5p, -221, -222 and miR-222-5p) and novel miRNAs (miR-375, -551b, 181-2-3p, 99b-3p). However, the levels of miRNA expression were different between these tumor types and some miRNAs were uniquely dysregulated in FVPTC allowing separation of these tumors on the unsupervised hierarchical clustering analysis. Upregulation of novel miR-375 was confirmed in a large independent set of follicular cell derived neoplasms and benign nodules and demonstrated specific upregulation for PTC. Two miRNAs (miR-181a-2-3p, miR-99b-3p) were associated with an adverse outcome in FVPTC patients by a Kaplan–Meier (p<0.05) and multivariate Cox regression analysis (p<0.05).
Conclusions
Despite high similarity in miRNA expression between FVPTC and classic PTC, several miRNAs were uniquely expressed in each tumor type, supporting their histopathologic differences. Highly upregulated miRNA identified in this study (miR-375) can serve as a novel marker of papillary thyroid carcinoma, and miR-181a-2-3p and miR-99b-3p can predict relapse-free survival in patients with FVPTC thus potentially providing important diagnostic and predictive value.
Introduction
Papillary thyroid carcinoma (PTC) is the most common type of thyroid malignancy (1). It comprises several variants that are classified on the basis of their morphologic features. Follicular variant of papillary carcinoma (FVPTC) is the most common variant, and is diagnosed in about 15–30% of PTC (1,2). Historically, all thyroid tumors with follicular growth were diagnosed as follicular thyroid carcinomas (FTC). The first histologic description of FVPTC occurred in 1960, but nevertheless, it was not until 1977 when this entity was further characterized in a landmark paper by Chem and Rosai and found its way into daily clinical practice (2,3). Since then, the scientific debate about this remarkable entity is ongoing. While these tumors have characteristic nuclear features of PTC, they demonstrate a follicular growth pattern and complete lack of well-formed papillae (2). Moreover, the metastatic pattern of these tumors often resembles classic PTC with cervical lymph-node metastasis, but some cases may demonstrate distant hematologic metastases, a finding more common in FTC (4).
At the molecular level, FVPTC also appears as an entity located between PTC and FTC. It frequently harbors RAS mutations and the PAX8/PPARγ rearrangement can also be detected; both events are common in follicular tumors and are rare in classic PTC (5). On the other hand, it may have BRAF gene mutations—but, in contrast to the usual BRAF V600E found in classic PTC, FVPTC has a distinct BRAF K601E mutation. BRAF K601E is very rare in FTC and was only described in one case in previous publications (6,7). In addition, the overall gene expression profile in FVPTC is distinct from classic PTC, underlining these differences (8,9). In summary, FVPTC shares features of classic PTC and FTC on the morphological, genetic, and prognostic level.
MicroRNAs (miRNAs) are small endogenous, noncoding RNAs that regulate gene expression in practically every major cellular function, and as a consequence, deregulated miRNAs have been linked to a variety of different cancers throughout the human body, including thyroid carcinomas (10,11). Thyroid tumors encompass different miRNA profiles depending on the histological subtype (12,13). Some miRNAs like miR-221 or -222 have been reported in both classic PTC and FTC, while others like miR-146b and miR-31 have been reported in PTC only (14).
The goal of this study was to examine miRNA expression in a large series of FVPTC using the most comprehensive miRNA profile available and correlate it with the clinicopathological data.
Materials and Methods
Tissue samples and patient characteristics
For miRNA expression profiling, 52 neoplastic and non-neoplastic formalin-fixed paraffin embedded (FFPE) thyroid tissue samples were analyzed including 17 FVPTC, 27 classic PTC, and 8 normal thyroid tissues. The tissues were received from the University Hospital Zürich, Switzerland, and surrounding pathology institutes.
Two groups of patients were included in this study: (a) patients with an adverse clinical outcome (ACO) defined as recurrent tumor relapse or patient death, and (b) a control group of patients that had a normal (favorable) outcome without tumor relapse or death. For the ACO group, the records were searched at the Department of Nuclear Medicine, Canton Zürich, for thyroid carcinoma cases that had been operated between 1990 and 2006 and had one of the following characteristics: tumor relapse after first radioiodine therapy, distant metastases, or tumor-associated death. All patients from this group were matched by age, stage, and sex to the group of patients with a favorable outcome. Patient characteristics are summarized in Table 1. The study was approved by the Cantonal Research Ethics Board (STV 28-2006). All tumors were classified according to widely accepted diagnostic histologic criteria by two board certified pathologists, blinded to the clinicopathological outcome (M.D. and A.P.) (1). Validation of novel miRNAs was performed on 61 snap-frozen thyroid tissues (22 FVPTC, 24 PTC, 8 FTC, 2 hyperplastic nodules, and 5 normal tissues) from surgically removed thyroid samples collected at the Department of Pathology, University of Pittsburgh Medical Center following Institutional Review Board (IRB) approval.
Table 1.
Patient Characteristics
| Classic PTC | FVPTC | Total | |
|---|---|---|---|
| Sex (n) | |||
| Female | 18 | 10 | 28 |
| Male | 9 | 7 | 16 |
| Age (years) | |||
| Years±SE Mean | 47.8±3 | 50.9±4.3 | 49±3 |
| pT | |||
| pT1 | 4 | 1 | 5 |
| pT2 | 3 | 0 | 3 |
| pT3 | 20 | 15 | 35 |
| pT4 | 0 | 1 | 1 |
| Status OS (n) | |||
| Censored | 24 | 13 | 37 |
| Death | 3 | 4 | 7 |
| t follow-up OS (months) | 74.6±47 | 80.8±47.4 | 77±46.7 |
| Status TSS (n) | |||
| Censored | 25 | 16 | 41 |
| Death | 2 | 1 | 3 |
| t follow-up TSS (months) | 74.6±47 | 80.8±47.4 | 77±46.7 |
| Status relapse (n) | |||
| Censored | 8 | 8 | 16 |
| Relapse | 19 | 9 | 28 |
| t follow-up RFS (months) | 25.7±29.6 | 55.9±54 | 37.4±42.8 |
| Total (n) | 27 | 17 | 44 |
PTC, papillary thyroid carcinoma; FVPTC, follicular variant of papillary thyroid carcinoma; OS, overall survival; TSS, tumor-specific survival; RFS, relapse-free survival.
RNA isolation
RNA was isolated from FFPE tissue samples using the RecoverAll kit (Ambion, Life Technologies) according to the manufacturer's instructions. Each FFPE tissue specimen was stained with hematoxylin and eosin (H&E) to ensure that characteristic features of the thyroid tumor were present. Areas with high density and purity (>80%) of tumor cells were marked for microdissection of adjacent sections to minimize contamination from surrounding healthy thyroid tissue or infiltrating cells. Overlapping areas in up to six adjacent slides were manually microdissected from 15 μm unstained histological sections under the guidance of a H&E-stained slide using an Olympus SZ61 stereomicroscope (Olympus, Hamburg, Germany). RNA quality and quantity was assessed with a spectrophotometer (NanoDrop 1000; Thermo Scientific, Wilmington, DE). Total RNA was extracted from snap-frozen surgical specimens using Trizol reagent (Invitrogen, Life Technologies) as previously described (15).
miRNA expression analysis
Quantitation of mature miRNA expression levels in thyroid tumors and normal thyroid tissue was performed by RT-PCR using TaqMan® Human Microarray Arrays v3.0 (Applied Biosystems, Life Technologies), which was designed to detect 754 human miRNAs. The array was performed on an ABI 7900 platform (Applied Biosystems, Life Technologies). First, 150 ng of total RNA was reverse transcribed using a high-capacity cDNA archive kit (Applied Biosystems, Life Technologies) and then followed by preamplification on an ABI 7500 Real-Time PCR System (Applied Biosystems, Life Technologies). The following PCR was run on an ABI 7900 Real-Time PCR System (Applied Biosystems, Life Technologies). Out of three different endogenous controls (RNU44, RNU48, U6 snRNA), the two most stable ones were used for normalization of RNA input: RNU44 and U6 snRNA. Nonhuman miRNA ath-miR159a was used as a negative control.
MiRNA expression levels were calculated by relative quantitation using DataAssist v3.0 software (Applied Biosystems, Life Technologies) and the fold-expression changes were determined by the 2-ΔΔCt method (16). Outliers among replicates were excluded and p-values were adjusted using Benjamini–Hochberg false discovery rate. The data are presented as the fold change of miRNA expression in tumors relative to normal thyroid tissues after normalization to endogenous controls. Maximum allowed Ct values were 38. Expression of individual miRNAs was analyzed using the TaqMan® individual miRNA assays (Applied Biosystems, Life Technologies) according to the manufacturer's instructions. A RNA input of 10 ng for high-capacity cDNA archive kit (Applied Biosystems, Life Technologies) was reverse transcribed followed by amplification on an ABI 7500 Real-Time PCR System (Applied Biosystems, Life Technologies).
Patient follow-up
Complete follow-up data were collected using chart reports and the cancer registry of the Canton Zürich and recorded as overall survival (OS), tumor-specific survival (TSS), and relapse-free survival (RFS). RFS was defined as the time between a patient's thyroidectomy and tumor relapse after the first radioiodine therapy.
Statistical analysis
Descriptive statistics, Mann–Whitney U-test, Kaplan–Meier analysis, and Cox regression were calculated with SPSS V21 (IBM, Armonk, NY). Fold changes were calculated with Dataassyst V3.1 (Applied Biosystems, Life Technologies), as was the unsupervised hierarchical clustering.
Results
Seventeen FVPTC, 27 classic PTC, and 8 normal thyroid tissues were analyzed for expression of 754 miRNAs using the most updated miRNA array panel, which covers the complete Sanger Database v.14. Overall, some miRNAs were similarly expressed in FVPTC and classic PTC. However, the levels of miRNA expression were different between these tumor types, and some miRNAs were uniquely dysregulated in FVPTC and classic PTC. The unsupervised hierarchical clustering analysis showed clear separation of the two entities from each other, demonstrating individual clusters for FVPTC, PTC, and normal thyroid tissue (Fig. 1), supporting their molecular and histopathologic differences.
FIG. 1.
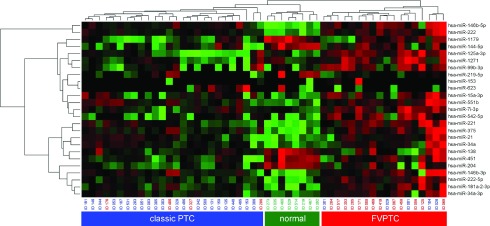
Unsupervised hierarchical clustering (distance measure: Euklidian Distance; clustering method: complete linkage) of all reported miRNAs. Follicular variant of papillary thyroid carcinoma (FVPTC), classic papillary thyroid carcinoma (PTC), and normal thyroid tissue form three distinct clusters. Color images available online at www.liebertpub.com/thy
Comparison of miRNA expression between FVPTC and classic PTC
We identified 21 miRNAs significantly (p<0.05) deregulated (more than twofold up/down) in FVPTC as compared to normal tissue, with 14 of these deregulated more than fivefold up or down (Table 2). Strongly upregulated miRNAs in FVPTC were miR-146b-3p, -146-5p, -221, -222, and miR-222-5p, -181a, which were previously reported in PTC (14,17,18). Interestingly, the level of expression for miR-146b-3p and miR-146b-5p was similar between FVPTC and classic PTC (30–33-fold for miR-146b-3p and 73–77-fold for miR-146b-5p respectively). However, expression of miR-221 and miR-222-5p that are known to be upregulated in both PTC and FTC was twice as high in FVPTC compared to classic PTC.
Table 2.
Fold Changes and p-Values of Significant Deregulated miRNAs
| miRNA | FVPTC vs. NT fold change >2 (p<0.05) | FVPTC vs. NT fold change >5 (p<0.05) | FVPTC vs. PTC fold change >5 (p<0.05) | FVPTC (RQ) | FVPTC (p-value) | Classic PTC (RQ) | Classic PTC(p-value) |
|---|---|---|---|---|---|---|---|
| hsa-let-7i-3p | × | 3.2283 | 0.0138 | 1.9262 | 0.1875 | ||
| hsa-miR-1179 | × | × | 0.0928 | 0.0269 | 0.0291 | 0.0007 | |
| hsa-miR-125a-3p | × | 12.5594 | 0.075 | 1.1127 | 0.9453 | ||
| hsa-miR-1271 | × | 2.8553 | 0.3094 | 0.4106 | 0.4034 | ||
| hsa-miR-138 | × | × | 0.1564 | 0.0091 | 0.2647 | 0.0013 | |
| hsa-miR-144-5p | × | 0.2472 | 0.0143 | 0.1392 | 0.0004 | ||
| hsa-miR-146b-3p | × | × | 29.4838 | 0.0143 | 32.8455 | 0.0138 | |
| hsa-miR-146b-5p | × | × | 77.1832 | 0 | 73.2796 | 0.0002 | |
| hsa-miR-153 | × | 2.7257 | 0.1184 | ||||
| hsa-miR-15a-3p | × | 3.8019 | 0.0138 | 3.3222 | 0.0148 | ||
| hsa-miR-181a-2-3p | × | × | 6.2228 | 0.0039 | 3.3402 | 0.0253 | |
| hsa-miR-204 | × | × | 0.1209 | 0.0053 | 0.058 | 0 | |
| hsa-miR-21 | × | 4.1654 | 0.0085 | 4.9069 | 0.0009 | ||
| hsa-miR-219-5p | × | × | 0.0372 | 0.0121 | 0.0185 | 0.0305 | |
| hsa-miR-221 | × | × | 16.1265 | 0 | 7.2353 | 0.0004 | |
| hsa-miR-222 | × | × | 16.2601 | 0.0003 | 13.5943 | 0.0008 | |
| hsa-miR-222-5p | × | × | 9.7092 | 0.0138 | 3.396 | 0.1571 | |
| hsa-miR-34a | × | 3.285 | 0.0137 | 3.05 | 0.0022 | ||
| hsa-miR-34a-3p | × | 2.5873 | 0.043 | 1.8738 | 0.0588 | ||
| hsa-miR-375 | × | × | 37.094 | 0.0003 | 20.1274 | 0.0009 | |
| hsa-miR-451 | × | 0.3051 | 0.0252 | 0.1747 | 0.0004 | ||
| hsa-miR-542-5p | × | × | 7.2879 | 0.0185 | 2.1064 | 0.4096 | |
| hsa-miR-551b | × | × | 22.5078 | 0.0138 | 15.7151 | 0.0166 | |
| hsa-miR-623 | × | 0.4794 | 0.7667 | 2.3888 | 0.6962 | ||
| hsa-miR-99b-3p | × | × | 7.0874 | 0.0509 | 1.3553 | 0.8164 |
NT, normal thyroid; ×, miRNAs with significant difference in expression between studied tissues (PTC, FVPTC, and NT).
We identified a number of downregulated miRNAs in both FVPTC and classic PTC, including miR-1179, -138, -144-5p, -199b-5p, -204, -219-5p, and miR-451 (Table 2). The levels of downregulation ranged between 3- and 54-fold. Four miRNAs were expressed significantly differently in FVPTC compared to classic PTC with a fold change more than five (miR-125a-3p, -1271, -153, and -623; Table 2).
In addition, two novel miRNAs miR-375 and miR-551b were found to be highly upregulated in FVPTC. MiR-375 showed more than a 37-fold upregulation as compared to normal thyroid tissue and miR-551b was upregulated more than 22-fold (Table 2). Analysis of their expression in classic PTC also revealed their upregulation but at a lower level (20-fold and 16-fold respectively).
Validation of miR-375 expression
MiR-375 was highly upregulated in FVPTC but at a lower level in classic PTC using the array approach. To confirm array data and to determine whether miR-375 upregulation is specific for papillary carcinoma, we studied its expression in an independent set of 61 follicular cell-derived neoplasms and normal thyroid tissue, including 22 FVPTC, 24 classic PTC, 8 FTC, 2 hyperplastic nodules and 5 normal tissues. In addition, we reanalyzed all arrayed FVPTC and classic PTC samples for miR-375 expression using individual RT-PCR reactions. MiR-375 was strongly upregulated in FVPTC and classic PTC but not in FTC, HN, or normal thyroid. A high correlation between array data and individual assays was observed (r=0.6).
Correlation of miRNA expression with outcome in FVPTC patients
The up/downregulated miRNAs identified in all FVPTC samples were correlated with the survival data. Patients with an adverse clinical outcome, that is, tumor relapse after first radioiodine therapy, distant metastases, or tumor-related death were compared to the control group. Because FVPTC are generally indolent neoplasms, overall survival (OS) is not a good clinical endpoint. Tumor-specific death (TSS) is a very rare event due to the low aggressiveness of these neoplasms, and our study did not reach statistical power for this outcome. Recurrent tumor relapse imposes a significant reduction in patient quality of life and therefore was used for outcome analysis.
Upregulation of miR-181a-2-3p and of miR-99b-3p and downregulation of miR-222 were associated with a favorable outcome in terms of tumor relapse in FVPTC (p<0.05). In the first two, Kaplan–Meier analysis reached statistical significance (p<0.05; Fig. 2). Both miRNAs were confirmed in two separate multivariate Cox regression analyses, including age, sex, and tumor stage [miR-181a-2-3p: p<0.05; Exp(B) 0.136; miR-99b-3p: Exp(B) 0.170], the only parameters that reached statistical significance.
FIG. 2.
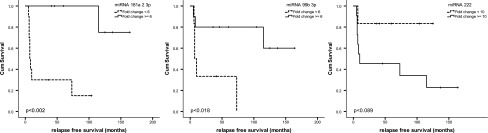
Kaplan–Meier survival plots: relapse-free survival (RFS) is reduced for all three shown miRNAs, but only miR-181a-2-3p and miR-99b reach statistical significance (log rank p<0.002 and p<0.018).
Discussion
We assessed the miRNA expression in a large series of follicular variant and classic PTCs with the most comprehensive currently available miRNA coverage. We correlated miRNA expression with clinicopathological data and patient survival, revealing potential important prognostic markers for FVPTC. Finally, we reported a novel miRNA, miR-375, which is extremely consistent and highly upregulated in FVPTC and PTC but not in follicular carcinomas, hyperplastic nodules, and normal thyroid tissue.
MiRNAs have been demonstrated to play important roles in a variety of fundamental cellular processes, including cell proliferation, differentiation, and cell death (10,14). As a result, miRNAs are directly involved in the development and progression of cancer, representing an important regulatory mechanism. In this study, we assessed the miRNAome with the most comprehensive currently available coverage of Sanger miRBase v.14 in a large series of FVPTC samples.
There are several well-characterized miRNAs known to be upregulated (miR-146b, -21, -221, -222) (14,18,19) and downregulated (miR-1, miR-138) (18,20) in PTC. In the present work, we have confirmed these findings, which underline the robustness of this analysis and also strengthen the previously published work by ourselves and others. Interestingly, the level of expression for miR-146b-3p and miR-146b-5p was similar between FVPTC and classic PTC. However, expression of miR-221 and miR-222 that are known to be upregulated in both PTC and FTC was twice as high in FVPTC compared to classic PTC. Potentially, this may be due to the fact that FVPTCs share features of both classic PTC and FTC, and miR-221 and miR-222 are important in regulation of both tumor types. In addition, we previously demonstrated a correlation between miRNA expression and the mutational status of PTC (14). We showed that both miR-221 and miR-222 were upregulated more strongly in PTC with RAS mutations, which are commonly found in FVPTC. It would be potentially interesting to determine the prevalence of RAS mutations in this cohort and correlate it with miRNA expression.
We identified several new, never before reported miRNAs being significantly upregulated in FVPTC and classic PTC. MiR-375 was upregulated more than 35-fold in FVPTC and 20-fold in classic PTC, and not found to be upregulated in normal thyroid tissue, hyperplastic nodules, and follicular carcinomas. High-level and PTC-specific upregulation may indicate a potential diagnostic utility for this miRNA. MiR-375 is known to play a role in other human malignancies, including lung and breast cancer (12,21,22). Another miRNA found to be upregulated in both tumor types was miR-551b. However, this miRNA has never been shown to play a role in any human malignancy and therefore awaits confirmation in other studies and organ systems.
PTCs are known to be a heterogenous group of tumors, sharing in general a favorable outcome. Specific mutation profiles are found to be associated with distinct histological subtypes of PTC, such as the BRAF V600E mutations with a classic PTC phenotype, whereas RAS gene mutations and the BRAF K601E substitution are more frequently found in FVPTC (23,24). The present study demonstrates that this is also true for miRNA expression. Overall, the miRNA profile demonstrated similar trends in expression between FVPTC and classic PTC. However, some miRNAs were uniquely dysregulated in FVPTC and classic PTC. For example, miR-125a-3p, -1271, and -153 showed upregulation in FVPTC but were expressed at normal level or even downregulated in classic PTC, whereas the opposite pattern of expression was found for miR-623. These miRNAs have never been reported in thyroid cancer but are known to be dysregulated in other human malignancies, including esophageal, gastric, colon, hepatocellular, pancreas, breast cancer, neuroblastomas, and leukemias (21,25–36). The unsupervised hierarchical clustering analysis showed clear separation of the two types of PTC from each other, demonstrating individual clusters for FVPTC and classic PTC, and supporting their molecular, histopathologic, and clinical differences.
FVPTC are known to have a favorable outcome. Identifying the small subset of patients who will suffer from recurrent disease is of major clinical interest. One of the important players in this scenario is the BRAF V600E mutation, which can be detected in about 40–45% of PTC (37) but at much lower frequency in FVPTC. Many studies demonstrated an association of this mutation with aggressive tumor behavior, although not all studies could confirm these findings (38). It is evident that a subset of tumors harboring the BRAF V600E mutation still has a good prognosis, underscoring the need for new molecular markers. Recently, a correlation between deregulation of miRNA expression in miR-146b, -222, and -130b and aggressiveness of PTC was reported (20,39). In this study, we were able to confirm upregulation of miR-222 and also identified novel markers of aggressiveness in FVPTC. Several miRNAs reached statistical significance when looking into RFS, including miR-181a-2-3p, -222, and -99b-3p. The Kaplan–Meier survival analysis was able to confirm a significant result for miR-181a-2-3p and miR-99b-3p. Patients had an adverse outcome if miR-222 was expressed at a higher level and miR-181a-2-3p and miR-99b-3p were expressed at a lower level and as compared to normal thyroid tissue. A multivariate analysis including age, sex, and tumor stage showed that the two latter miRNAs were the only significant parameters, predicting patient relapse. MiR-181a-2-3p has not been reported in human cancer before, but miR-99b-3p has been linked to prostate cancer (40,41). Although further studies are needed to to confirm this finding in a larger cohort of patients, these miRNAs bear the potential to serve as good molecular markers helping to stratify patients better in the future and facilitate guiding appropriate clinical management.
In conclusion, we report a comprehensive miRNA profile of FVPTC, the most common histologic type of PTC. We discovered a new miRNA (miR-375) that is upregulated in FVPTC and classic PTC, and identified several miRNAs that can predict patient relapse free survival. Further studies will have to prove their potential diagnostic and predictive clinical value.
Acknowledgments
M.D. was supported by the Fondation pour la recherche Nuovo-Soldati, the Gertrud-Hagmann-Stiftung für Malignomforschung, and the Research Support Foundation, and Y.E.N. was supported by the National Institute of Health grant R01 CA88041. We thank Prof. Steinert, Dr. Valenta, Dr. Haldemann, and Dr. Meili for providing us with clinicopathological data from their nuclear medicine departments. We also thank Dr. Flury, Cantonal Hospital Winterthur, Dr. Moll, Cantonal Hospital Münsterlingen, Prof. Diebold, Cantonal Hospital Luzern, Prof. Hochstetter, Institut Enge, Dr. Scheidegger, Viollier Basel, Dr. Baltisser, Institute Regenbogen, and Dr. Riehle, Institute Arnaboldi, for their collaboration. We also thank Alexiy Nikiforov for help with editing this manuscript and valuable comments on the submitted work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.DeLellis R. Lloyd R. Heitz P. Eng C. Pathology, Genetics of Tumours of Endocrine Organs. In: DeLellis R, editor; Lloyd R, editor; Heitz P, editor; Eng C, editor. IARC Press, Lyon [World Health Organization classification of tumours]; 2004. [Google Scholar]
- 2.Diagnostic Pathology and Molecular Genetics of the Thyroid. In: Nikiforov YEB, editor; Paul W, editor; Thompson LD, editor. First edition Lippincott Williams & Wilkins; Philadelphia: 2009. [Google Scholar]
- 3.Chem KT. Rosai J. Follicular variant of thyroid papillary carcinoma: a clinicopathologic study of six cases. Am J Surg Pathol. 1977;1:123–130. doi: 10.1097/00000478-197706000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Chetty R. Follicular patterned lesions of the thyroid gland: a practical algorithmic approach. J Clin Pathol. 2011;64:737–741. doi: 10.1136/jclinpath-2011-200121. [DOI] [PubMed] [Google Scholar]
- 5.Adeniran AJ. Zhu Z. Gandhi M. Steward DL. Fidler JP. Giordano TJ. Biddinger PW. Nikiforov YE. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–222. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 6.Nikiforov YE. Molecular analysis of thyroid tumors. Mod Pathol. 2011;24:S34–43. doi: 10.1038/modpathol.2010.167. [DOI] [PubMed] [Google Scholar]
- 7.Pennelli G. Vianello F. Barollo S. Pezzani R. Merante Boschin I. Pelizzo MR. Mantero F. Rugge M. Mian C. BRAF(K601E) mutation in a patient with a follicular thyroid carcinoma. Thyroid. 2011;21:1393–1396. doi: 10.1089/thy.2011.0120. [DOI] [PubMed] [Google Scholar]
- 8.Giordano TJ. Kuick R. Thomas DG. Misek DE. Vinco M. Sanders D. Zhu Z. Ciampi R. Roh M. Shedden K. Gauger P. Doherty G. Thompson NW. Hanash S. Koenig RJ. Nikiforov YE. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene. 2005;24:6646–6656. doi: 10.1038/sj.onc.1208822. [DOI] [PubMed] [Google Scholar]
- 9.Igci YZ. Arslan A. Akarsu E. Erkilic S. Igci M. Oztuzcu S. Cengiz B. Gogebakan B. Cakmak EA. Demiryurek AT. Differential expression of a set of genes in follicular and classic variants of papillary thyroid carcinoma. Endocr Pathol. 2011;22:86–96. doi: 10.1007/s12022-011-9157-8. [DOI] [PubMed] [Google Scholar]
- 10.Lu J. Getz G. Miska EA. Alvarez-Saavedra E. Lamb J. Peck D. Sweet-Cordero A. Ebert BL. Mak RH. Ferrando AA. Downing JR. Jacks T. Horvitz HR. Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 12.Nikiforova MN. Tseng GC. Steward D. Diorio D. Nikiforov YE. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008;93:1600–1608. doi: 10.1210/jc.2007-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dettmer M. Vogetseder A. Durso MB. Moch H. Komminoth P. Perren A. Nikiforov YE. Nikiforova MN. MicroRNA expression array identifies novel diagnostic markers for conventional and oncocytic follicular thyroid carcinomas. J Clin Endocrinol Metab. 2013;98:E1–E7. doi: 10.1210/jc.2012-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikiforova MN. Chiosea SI. Nikiforov YE. MicroRNA expression profiles in thyroid tumors. Endocr Pathol. 2009;20:85–91. doi: 10.1007/s12022-009-9069-z. [DOI] [PubMed] [Google Scholar]
- 15.Nikiforova MN. Caudill CM. Biddinger P. Nikiforov YE. Prevalence of RET/PTC rearrangements in Hashimoto's thyroiditis and papillary thyroid carcinomas. Int J Surg Pathol. 2002;10:15–22. doi: 10.1177/106689690201000104. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Pallante P. Visone R. Ferracin M. Ferraro A. Berlingieri MT. Troncone G. Chiappetta G. Liu CG. Santoro M. Negrini M. Croce CM. Fusco A. MicroRNA deregulation in human thyroid papillary carcinomas. Endocrine-related cancer. 2006;13:497–508. doi: 10.1677/erc.1.01209. [DOI] [PubMed] [Google Scholar]
- 18.He H. Jazdzewski K. Li W. Liyanarachchi S. Nagy R. Volinia S. Calin GA. Liu CG. Franssila K. Suster S. Kloos RT. Croce CM. de la Chapelle A. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA. 2005;102:19075–19080. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tetzlaff MT. Liu A. Xu X. Master SR. Baldwin DA. Tobias JW. Livolsi VA. Baloch ZW. Differential expression of miRNAs in papillary thyroid carcinoma compared to multinodular goiter using formalin fixed paraffin embedded tissues. Endocr Pathol. 2007;18:163–173. doi: 10.1007/s12022-007-0023-7. [DOI] [PubMed] [Google Scholar]
- 20.Yip L. Kelly L. Shuai Y. Armstrong MJ. Nikiforov YE. Carty SE. Nikiforova MN. MicroRNA signature distinguishes the degree of aggressiveness of papillary thyroid carcinoma. Ann Surg Oncol. 2011;18:2035–2041. doi: 10.1245/s10434-011-1733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giricz O. Reynolds PA. Ramnauth A. Liu C. Wang T. Stead L. Childs G. Rohan T. Shapiro N. Fineberg S. Kenny PA. Loudig O. Hsa-miR-375 is differentially expressed during breast lobular neoplasia and promotes loss of mammary acinar polarity. J Pathol. 2012;226:108–119. doi: 10.1002/path.2978. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa E. Osada H. Okazaki Y. Arima C. Tomida S. Tatematsu Y. Taguchi A. Shimada Y. Yanagisawa K. Yatabe Y. Toyokuni S. Sekido Y. Takahashi T. miR-375 is activated by ASH1 and inhibits YAP1 in a lineage-dependent manner in lung cancer. Cancer Res. 2011;71:6165–6173. doi: 10.1158/0008-5472.CAN-11-1020. [DOI] [PubMed] [Google Scholar]
- 23.Trovisco V. Soares P. Preto A. de Castro IV. Lima J. Castro P. Maximo V. Botelho T. Moreira S. Meireles AM. Magalhaes J. Abrosimov A. Cameselle-Teijeiro J. Sobrinho-Simoes M. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients' age but not with tumour aggressiveness. Virchows Arch. 2005;446:589–595. doi: 10.1007/s00428-005-1236-0. [DOI] [PubMed] [Google Scholar]
- 24.Adeniran AJ. Zhu ZW. Gandhi M. Steward DL. Fidler JP. Giordano TJ. Biddinger PW. Nikiforov YE. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–222. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 25.Volinia S. Galasso M. Sana ME. Wise TF. Palatini J. Huebner K. Croce CM. Breast cancer signatures for invasiveness and prognosis defined by deep sequencing of microRNA. Proc Natl Acad Sci USA. 2012;109:3024–3029. doi: 10.1073/pnas.1200010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam EK. Wang X. Shin VY. Zhang S. Morrison H. Sun J. Ng EK. Yu J. Jin H. A microRNA contribution to aberrant Ras activation in gastric cancer. Am J Trans Res. 2011;3:209–218. [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Y. Suo AL. Li ZF. Liu LY. Tian T. Ni L. Zhang WG. Nan KJ. Song TS. Huang C. MicroRNA profiling of human gastric cancer. Mol Med Report. 2009;2:963–970. doi: 10.3892/mmr_00000199. [DOI] [PubMed] [Google Scholar]
- 28.Favreau AJ. Sathyanarayana P. miR-590-5p, miR-219-5p, miR-15b and miR-628-5p are commonly regulated by IL-3, GM-CSF and G-CSF in acute myeloid leukemia. Leuk Res. 2012;36:334–341. doi: 10.1016/j.leukres.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He XX. Chang Y. Meng FY. Wang MY. Xie QH. Tang F. Li PY. Song YH. Lin JS. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene. 2012;31:3357–3369. doi: 10.1038/onc.2011.500. [DOI] [PubMed] [Google Scholar]
- 30.Lin M. Chen W. Huang J. Gao H. Ye Y. Song Z. Shen X. MicroRNA expression profiles in human colorectal cancers with liver metastases. Oncol Rep. 2011;25:739–747. doi: 10.3892/or.2010.1112. [DOI] [PubMed] [Google Scholar]
- 31.Bray I. Tivnan A. Bryan K. Foley NH. Watters KM. Tracey L. Davidoff AM. Stallings RL. MicroRNA-542-5p as a novel tumor suppressor in neuroblastoma. Cancer Lett. 2011;303:56–64. doi: 10.1016/j.canlet.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanic M. Zajac M. Gomez-Lopez G. Benitez J. Martinez-Delgado B. Integration of BRCA1-mediated miRNA and mRNA profiles reveals microRNA regulation of TRAF2 and NFkappaB pathway. Breast Cancer Res Treat. 2012;134:41–51. doi: 10.1007/s10549-011-1905-4. [DOI] [PubMed] [Google Scholar]
- 33.Feber A. Xi L. Pennathur A. Gooding WE. Bandla S. Wu M. Luketich JD. Godfrey TE. Litle VR. MicroRNA prognostic signature for nodal metastases and survival in esophageal adenocarcinoma. Ann Thorac Surg. 2011;91:1523–1530. doi: 10.1016/j.athoracsur.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalimutho M. Blanco GD. Di Cecilia S. Sileri P. Cretella M. Pallone F. Federici G. Bernardini S. Differential expression of miR-144* as a novel fecal-based diagnostic marker for colorectal cancer. Journal of Gastroenterology. 2011;46:1391–1402. doi: 10.1007/s00535-011-0456-0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X. Chen X. Lin J. Lwin T. Wright G. Moscinski LC. Dalton WS. Seto E. Wright K. Sotomayor E. Tao J. Myc represses miR-15a/miR-16-1 expression through recruitment of HDAC3 in mantle cell and other non-Hodgkin B-cell lymphomas. Oncogene. 2012;31:3002–3008. doi: 10.1038/onc.2011.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovalchuk O. Filkowski J. Meservy J. Ilnytskyy Y. Tryndyak VP. Chekhun VF. Pogribny IP. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7:2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 37.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 38.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 39.Chou CK. Chen RF. Chou FF. Chang HW. Chen YJ. Lee YF. Yang KD. Cheng JT. Huang CC. Liu RT. miR-146b is highly expressed in adult papillary thyroid carcinomas with high risk features including extrathyroidal invasion and the BRAF(V600E) mutation. Thyroid. 2010;20:489–494. doi: 10.1089/thy.2009.0027. [DOI] [PubMed] [Google Scholar]
- 40.Sun D. Lee YS. Malhotra A. Kim HK. Matecic M. Evans C. Jensen RV. Moskaluk CA. Dutta A. miR-99 family of MicroRNAs suppresses the expression of prostate-specific antigen and prostate cancer cell proliferation. Cancer Res. 2011;71:1313–1324. doi: 10.1158/0008-5472.CAN-10-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maurel M. Jalvy S. Ladeiro Y. Combe C. Vachet L. Sagliocco F. Bioulac-Sage P. Pitard V. Jacquemin-Sablon H. Zucman-Rossi J. Laloo B. Grosset CF. A functional screening identifies five micrornas controlling glypican-3: role of mir-1271 down-regulation in hepatocellular carcinoma. Hepatology. 2013;57:195–204. doi: 10.1002/hep.25994. [DOI] [PubMed] [Google Scholar]


