Abstract
Induced pluripotent stem (iPS) cells, which are morphologically and functionally similar with embryonic stem (ES) cells, have been successfully generated from somatic cells through defined reprogramming transcription factors. Forkhead class O3a (FoxO3a) has been recently reported to play an important role in the homeostasis and maintenance of certain types of stem cells; however, the role of FoxO3a in the reprogramming process and differentiation of iPS cells remains unclear. In this study, we investigate the function of FoxO3a during the reprogramming process and characterize the properties of iPS cells from FoxO3a-wild type and -null mouse embryonic fibroblasts (MEFs). Our results show that the FoxO3a-null iPS cells are similar to the wild-type iPS cells in the levels of ES cell markers, alkaline phosphatase activity, and formation of teratoma in vivo. The reprogramming process is delayed in the FoxO3a-null MEFs compared to the wild-type MEFs; whereas the overexpression of FoxO3a partially recovers the impaired reprogramming efficiency in the null group. More importantly, FoxO3a deficiency impairs the neuronal lineage differentiation potential of iPS cells in vitro. These results suggest that FoxO3a affects the reprogramming kinetics and the neuronal lineage differentiation potential of the resulting iPS cells. Therefore, this study demonstrates a novel function of FoxO3a in cell reprogramming, which will help the development of alternative strategies for generating iPS cells.
Introduction
Embryonic stem (ES) cells are promising sources for cell transplantation in regenerative medicine and tissue replacement due to their plasticity and pluripotency. However, their clinical application raises ethical concerns and includes the risk of donor-host rejection [1]. More recently, it has been reported that fibroblasts can be reprogrammed to induced pluripotent stem (iPS) cells by ectopic expression of transcription factors Oct3/4, Sox2, either Klf4 and c-Myc or Nanog and LIN28 [2–4]. These iPS cells are morphologically and functionally similar, but not identical, to ES cells [3]. Current reprogramming technology allows the induction of patient- and disease-specific iPS cells, which could be applied in cell replacement without concern for immune rejection [5]. In addition, iPS cells have been directly and efficiently differentiated into many specified cell types in vitro, such as motor neurons [6], dopaminergic neurons [7], cardiac cells [8], and hepatocytes [9]. The transplanted dopaminergic neurons derived from iPS cells could survive and function in an animal model of Parkinson's disease [10]. However, the tumorigenicity due to the genetic manipulation during the reprogramming process of iPS cells prohibits its clinical application. Even after long-term differentiation, a small number of iPS cells may still remain undifferentiated and could be expanded [11]. So it is vital to fully understand the full differentiation of iPS cells in vitro and in vivo.
The family of forkhead class O (FoxO) proteins, consisting of FoxO1, FoxO3a, FoxO4, and FoxO6, are critical regulators in various physiological processes, including cell cycle arrest, apoptosis, and antioxidative stress, and are mediated through a distinct forkhead DNA-binding domain [12,13]. FoxO factors have been shown to be regulated by Akt-dependent phosphorylation with the stimulation of insulin or growth factors, which induces their nuclear export [14]. Many additional stress stimuli, such as DNA damage [15], nutrient deprivation [16], cytokines [17], and hypoxia [18] could also regulate the function of the FoxO family. In summary, FoxO transcription factors integrate extracellular stimuli into intracellular signals through various mechanisms.
It is reported that FoxO transcription factors play a unique role in life-span regulation downstream of the insulin receptor in caenorhabditis elegans [19], and genetic variation within FoxO3a was strongly associated with human longevity [20–22]. FoxO3a has recently been found to regulate the self-renewal and homeostasis of hematopoietic [23] and neural [24,25] stem cells (NSCs), primarily by providing resistance to oxidative stress. In addition, NSCs isolated from FoxO3a-null mice showed an impaired capability to generate different neural lineages [24] and FoxO3a-null mice exhibit a myeloproliferative syndrome, with an increased white blood cell count [26]. FoxO3a was also involved in CXCL12-mediated human neural progenitor cell proliferation through cell cycle control [27]. Although the functions of FoxO3a have been elaborately studied in somatic stem cells, it still remains unknown that whether FoxO3a regulates the reprogramming process and differentiation of iPS cells. Due to the similarity of iPS cells with ES cells, the emergence of iPS cell technology also indicates the possible role of FoxO3a in ES cells.
In this study, FoxO3a-wild type and -null mouse embryonic fibroblasts (MEFs) are used as the parental cells to generate iPS cells and the reprogramming kinetics is analyzed during this process. Further, the resulting FoxO3a-wild type and -null iPS cells are examined in their self-renewal and pluripotency in vitro and in vivo. Our results demonstrate that FoxO3a-null MEFs can also be reprogrammed to iPS cells with high expression of ES cell markers, strong alkaline phosphatase (AP) activity, and the resulting iPS cells can form teratomas with three germ layer structures in vivo. However, the reprogramming process is delayed when FoxO3a is deficient, which could be partially rescued by the overexpression of FoxO3a in the null MEFs. Moreover, the resulting FoxO3a-null iPS cells are defective in their differentiation into neuronal lineages in vitro. These cumulative findings illustrate a novel function of FoxO3a in cell reprogramming, which will help the development of alternative strategies for generating iPS cells.
Materials and Methods
Media for cell culture
MEFs were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen), supplemented with 10% fetal bovine serum (FBS; Invitrogen), 0.1 mM MEM nonessential amino acid (ATCC), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma-Aldrich). Plat-E cells (Cell Biolab) were maintained in DMEM, supplemented with 10% FBS, 1 μg/mL puromycin (Sigma-Aldrich), 10 μg/mL blasticidin (Sigma-Aldrich), 100 U/mL penicillin, and 100 μg/mL streptomycin. ESGRO Complete PLUS Clonal Grade Medium (Millipore) was used for iPS cell maintenance. NSCs were expanded in NeuroCult® NSC basal medium with proliferation supplement (STEMCELL Technologies), 10 ng/mL recombinant human basic fibroblast growth factor (Sigma-Aldrich), and 20 ng/mL recombinant human epidermal growth factor (R&D Systems). The medium for monoculture of iPS cell differentiation was DMEM/F12 (Invitrogen) with N-2 supplement (Invitrogen) and B-27 supplement (Invitrogen). The medium for neuron differentiation from NSCs was neural basal medium (Invitrogen) plus B-27 supplement.
Animals
FoxO3a-wild type and -null FVB/NJ and severe combined immunodeficiency (SCID) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed under pathogen-free conditions at University of Nebraska Medical Center (UNMC) with ad libitum access to food and water. All animal experiments and procedures were approved by the UNMC Institutional Animal Care and Use Committee in compliance with ethical guidelines set forth by the National Institutes of Health.
Isolation and culture of MEFs
Embryos were isolated from 14-day (E14) pregnant mice. The head and visceral tissues were removed and the remaining bodies were washed in sterile Dulbecco's phosphate buffered saline (Invitrogen) and treated with 0.25% trypsin/1 mM ethylenediaminetetraacetic acid solution (Sigma-Aldrich). After trypsinization, an equal amount of MEF medium was added, and the cell suspension was filtered through a 40-μm cell strainer to remove tissue debris. MEFs were used within three passages to avoid replicative senescence.
Retroviral packaging and infection
Plat-E cells were used as the virus packaging cells and pMXs-based retroviral vectors were introduced into these cells with Lipofectamine™ LTX (Invitrogen). Virus-containing supernates derived from these Plat-E cultures were filtered through a 0.45-μm filter supplemented with 10 μg/mL polybrene (Millipore). After retroviral infection, the medium was switched to MEF medium for 24 h, and then to the serum-free ESGRO medium (Millipore) [28].
Teratoma formation assay
Teratoma formation [2] was assessed as previously described with modifications. Briefly, suspensions of undifferentiated iPS cells were subcutaneously injected into the dorsal flank of SCID mice. Six weeks after injection, tumors were weighed, fixed in 4% paraformaldehyde (PFA), and embedded in paraffin. Sections were stained with hematoxylin and eosin for histological analysis. Immunohistochemistry staining specific for Tuj (Abcam), alpha smooth muscle actin (α-SMA) (Abcam), and alpha-fetoprotein (AFP) (Dako) was carried out on teratoma sections to identify ectoderm, mesoderm, and endoderm structures, respectively.
Neural differentiation of iPS cells
To progressively differentiate iPS cells to NSCs and neurons [29], embryonic bodies were formed in bacteriological grade dishes with suspension culture for 4 days, and then plated onto the adhesive tissue culture surface. Selection of Nestin-positive cells was initiated by switching to serum-free insulin, transferrin, selenium chloride, and fibronectin (ITSFn) medium. After 6–10 days, cell expansion was performed by switching to NSC medium. According to the established protocol for monolayer culture [30], undifferentiated iPS cells were plated onto 0.1% gelatin-coated coverslips, and then subjected to western blotting analysis and immunostaining with specific antibodies, including Oct3/4 (Santa Cruz), Nestin (DSHB), Sox2 (Cell Signaling Technologies), Tuj, and glial fibrillary acidic protein (GFAP) (Cell Signaling Technologies).
Western blot
Western blot was performed as previously described [31]. The protein mixtures were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to an Immuno-Blot polyvinylindene fluoride membrane (Bio-Rad). After blocked in tris-buffered saline/Tween (0.1%) with 5% fat-free milk for 1 h, the membrane was then incubated with primary antibodies specific for FoxO3a, Oct3/4, Tuj, GFAP, Nestin, and β-actin overnight at 4°C, followed by horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technologies). The signal was visualized by enhanced chemiluminescent solution (Pierce) and captured with CL-X PosureTM Film (Pierce).
AP staining
After the recommended protocol from AP detection kit (Millipore), FoxO3a-wild type and -null iPS cells were fixed with 4% PFA for 1–2 min, and then washed with 1× Rinse Buffer. After incubated with the staining solution for 15 min, cells were washed with 1× Rinse Buffer and the number of AP+ colonies was counted under light microscope.
Statistical analyses
Data were expressed as means±standard deviation and statistically evaluated by analysis of variance followed by the Tukey's test for paired observations. Significance was considered as a p-value of<0.05. All assays were performed at least twice, with triplicate samples in each experiment.
Results
The deficiency of FoxO3a delayed the reprogramming process from MEFs to iPS cells
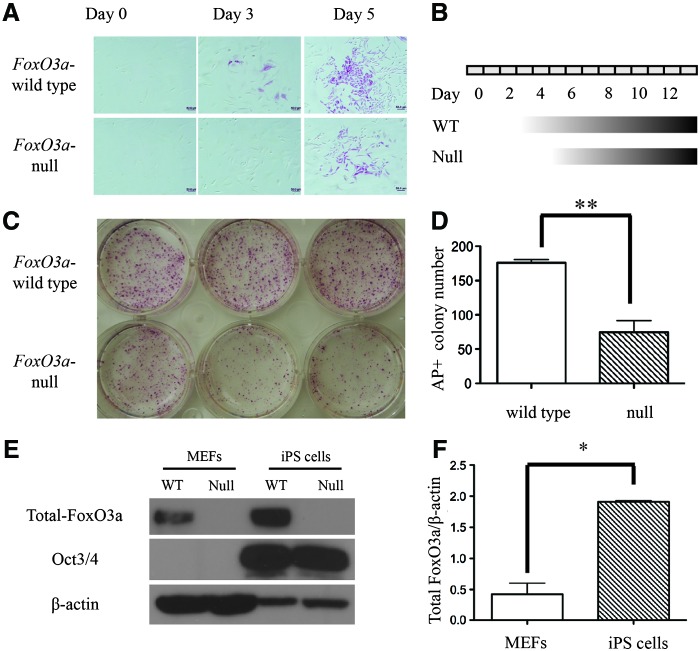
After the protocol as previously described [28], we isolated and cultured MEFs from FoxO3a-wild type and -null mice, and infected cells with retroviruses encoding Yamanaka factors, including Oct3/4, Sox2, Klf4, and c-Myc. We found that the reprogramming kinetics of FoxO3a-null MEFs was slower compared with wild-type MEFs and the AP was activated after the introduction of Yamanaka factors at day 3 in FoxO3a-wild type MEFs, which is consistent with previous observations [32,33]; however, AP activity was only detected at day 5 in FoxO3a-null MEFs (Fig. 1A, B). In addition, the total yield of AP+ colonies was significantly higher in the FoxO3a-wild type MEFs compared with the FoxO3a-null MEFs (Fig. 1C, D), indicating that the deficiency of FoxO3a delayed the reprogramming kinetics. Moreover, we also observed that the expression level of FoxO3a was highly upregulated during the reprogramming process from MEFs to iPS cells, and the pluripotency marker Oct3/4 was only detected in the iPS cells (Fig. 1E, F). These data suggest that FoxO3a may be involved in the generation of iPS cells from MEFs. As expected, FoxO3a was totally absent at the protein level in the FoxO3a-null MEFs (Fig. 1E).
FIG. 1.
Reprogramming kinetics of Forkhead class O3a (FoxO3a)-wild type and -null mouse embryonic fibroblasts (MEFs). (A) At the day 0, 3, and 5 after infection with Yamanaka factors (Oct3/4, Sox2, Klf4, and c-Myc), FoxO3a-wild type and -null MEFs were fixed with 4% paraformaldehyde in phosphate buffered saline, and then subjected to alkaline phosphatase (AP) staining. (B) Schematic representation of the kinetics of FoxO3a-wild type and -null induced pluripotent stem (iPS) cell generation from MEFs. (C) The reprogramming six-well plate was stained with AP at day 12 after infection with viruses of Yamanaka factors. (D) The total number of AP+ colonies in FoxO3-wild type and -null MEFs. (E) Western blot assay of extracts from FoxO3a-wild and -null MEFs and iPS cells using FoxO3a (75D8) Rabbit mAb and Oct3/4 mAb with β-actin as a loading control. (F) The expression of FoxO3a in MEFs and iPS cells was normalized with β-actin (*p<0.05; **p<0.01). Scale bar=50 μm. Color images available online at www.liebertpub.com/scd
Overexpression of FoxO3a improved the reprogramming efficiency
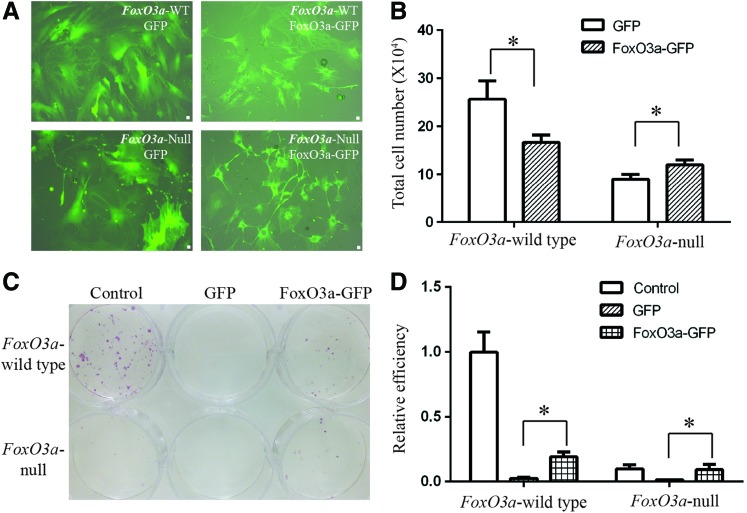
To further confirm the vital function of FoxO3a during reprogramming process, we constructed the pMXs-base retrovirus encoding FoxO3a-green fluorescent protein (GFP) fusion protein, and then transduced in FoxO3a-wild type and -null MEFs. As indicated by GFP expression, we observed that the infection efficiency was high in MEFs (Fig. 2A). When the Yamanaka factors were introduced into FoxO3a-wild type and -null MEFs together with GFP or FoxO3a-GFP, we found that there was no significant difference of reprogramming efficiency between GFP group and FoxO3a-GFP group either in FoxO3a-wild type or -null MEFs (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd), probably due to the counteraction of Yamanaka factors to FoxO3a. Thus, we firstly infected FoxO3a-wild type and -null MEFs with GFP or FoxO3a-GFP. After 5–7 days, these cells were counted and replanted in a new six-well plate with the same cell numbers for iPS generation. Due to the antiproliferation effect of FoxO3a [12], the total cell number of FoxO3a-GFP group was much lower than the GFP group in FoxO3a-wild type MEFs; however, we observed the protection of FoxO3a in the null MEFs (Fig. 2B). Although the virus infection significantly impaired the final yield of AP+ colonies compared to the control group, the overexpression of FoxO3a-GFP dramatically increased the reprogramming efficiency in both FoxO3a-wild type and -null MEFs (Fig. 2C, D).
FIG. 2.
Recovery of reprogramming efficiency with overexpression of FoxO3a-GFP in the wild-type and null MEFs. (A) The merged pictures of light and GFP in the FoxO3a-wild type and -null MEFs with overexpression of either GFP or FoxO3a-GFP. (B) The total cell numbers of FoxO3a-wild type and -null MEFs after 5–7 days infection of either GFP or FoxO3a-GFP retroviruses. (C) Overview of AP staining in the control group (without GFP or FoxO3a-GFP infection), GFP group, and FoxO3a-GFP group with the overexpression of Yamanaka factors for 15–20 days. (D) The number of AP+ colonies was normalized to the control group of FoxO3a-wild type MEFs. *p<0.05. Scale bar=50 μm. Color images available online at www.liebertpub.com/scd
To determine the level of reactive oxidative stress (ROS), we performed the dihydroethidium (DHE) staining, the indicative of ROS and found that the deficit of FoxO3a increased levels of ROS in MEFs (Supplementary Fig. S2A, B). FoxO3a protects cells against oxidative stress by directly regulating the expression of enzyme manganese superoxide dismutase (MnSOD) [34]. Western blotting analysis also demonstrated the ROS-related changes, including the decrease of MnSOD expression, one of the major cellular antioxidant defense system, and the increase of 4-hydroxynonenal (4-HNE) level, which is produced by the lipid peroxidation chain reaction during oxidant stress (Supplementary Fig. S2C). Generally, MEFs progressively decrease proliferation due to cellular senescence and the higher levels of senescence markers-p16 and p21 were detected in the FoxO3a-null MEFs compared to the wild-type MEFs (Supplementary Fig. S2D). These results suggest that all the changes induced by FoxO3a deficiency contribute to the delay of reprogramming process in MEFs.
Similarity of FoxO3a-null iPS cells with the wild-type iPS cells
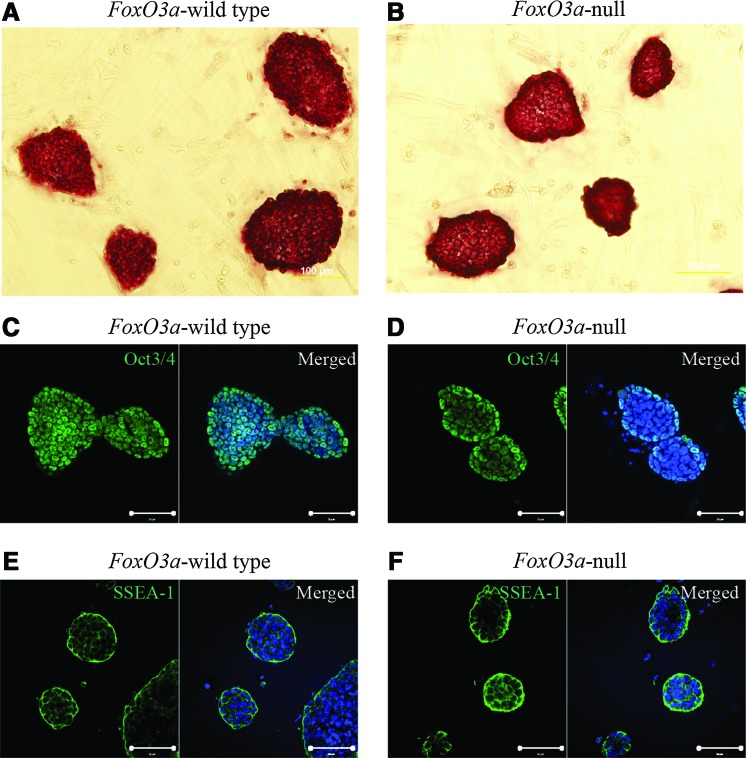
Although FoxO3a-null MEFs could form AP+ colonies with ES cell morphology through ectopic expression of Yamanaka factors, it remains questionable as to whether these ES cell-like colonies were true iPS cells with the capability of self-renewal, proliferation, and differentiation. Both FoxO3a-wild type and -null colonies were isolated and continuously propagated in vitro with serum-free ESGRO complete medium and subjected to AP activity analysis, immunofluorescence staining, and teratoma formation assay. The results demonstrated that both FoxO3a-wild type and -null iPS cells had strong AP activity (Fig. 3A, B), and there was no difference in the immunofluorescence staining with regards to the expression level of the ES cell markers Oct3/4 and SSEA-1 between these two kinds of iPS cells (Fig. 3C–F). In addition, the real-time polymerase chain reaction (RT-PCR) results showed that the fibroblast-specific genes, including Col1a1, Col3a1, Twist1, Twist2, Snai1, and Dkk3, were silenced to the similar levels of ES cells (Supplementary Fig. S3A), while the ES cell-specific genes, including Nanog, Zfp42, Utf1, Dppa5a, and Eras, were significantly upregulated (Supplementary Fig. S3B). Using specific primers to detect exogenous and endogenous genes expression used for reprogramming, we observed that ectopic Oct3/4 and Sox2 were silenced and the endogenous genes were activated to the similar levels with ES cells (Supplementary Fig. S3C). The expression of Nanog was further confirmed by immunofluorescence staining with specific antibody (Supplementary Fig. S3D, E).
FIG. 3.
Characterization of FoxO3a-wild type and -null iPS cells. (A) AP staining (20×magnification) in FoxO3a-wild type iPS cell. (B) AP staining (20×magnification) in FoxO3a-null iPS cells. Immunofluorescence staining with antibodies specific for Oct3/4 (C) and SSEA-1 (E) in FoxO3a-wild type iPS cells and nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Immunofluorescence staining with antibodies specific for Oct3/4 (D) and SSEA-1 (F) in FoxO3a-null iPS cells and nuclear staining with DAPI (blue). Scale bar for A and B=100 μm, scale bar for C, D, E, and F=50 μm. Color images available online at www.liebertpub.com/scd
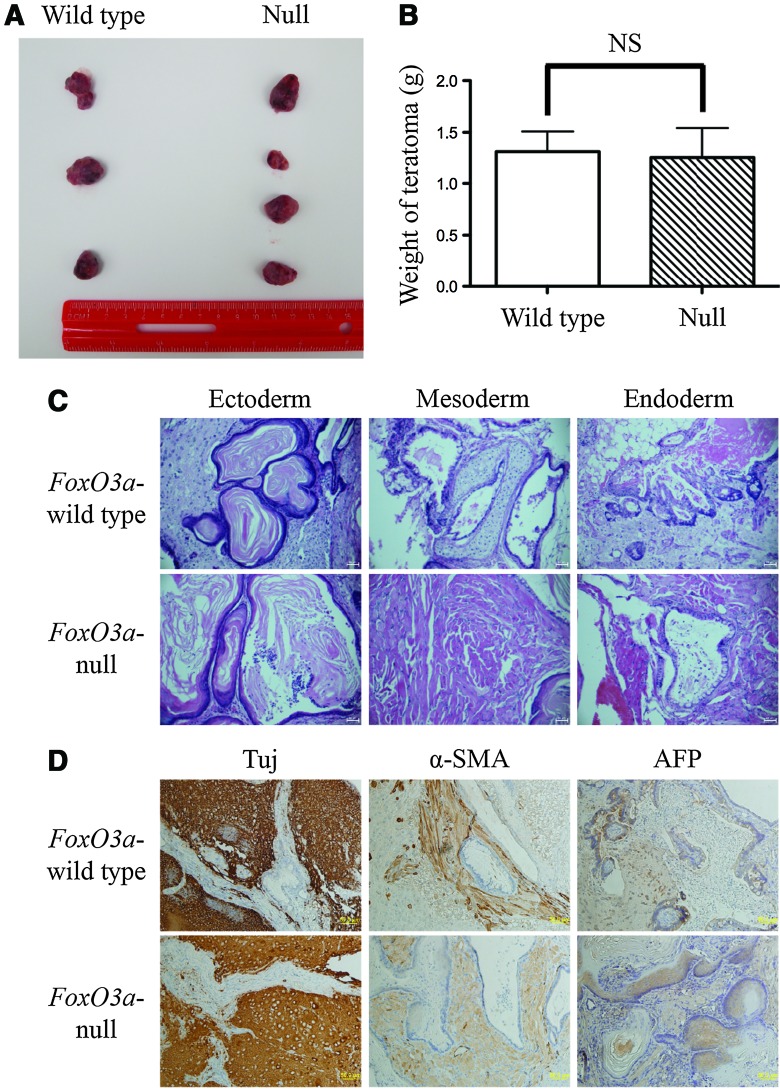
To further confirm the pluripotency property of the resulting FoxO3a-wild type and -null iPS cells in vivo, these cells were subcutaneously injected into the flank of SCID mice. We observed that the teratomas formed 5 weeks post injection in both FoxO3a-wild type and -null iPS cell groups (Fig. 4A), whereas there was no difference in the weight of tumors between the two groups (Fig. 4B). In addition, histological examination showed that both teratoma tissues contained three germ layer structures, specifically epidermis (ectoderm), muscle, and cartilage (mesoderm), and intestinal epithelium (endoderm) (Fig. 4C). Further immunohistochemistry analysis demonstrated that both paraffin sections of teratomas contained cells positive for three lineage-specific markers: Tuj (neuron-specific, ectoderm), α-SMA (muscle-specific, mesoderm), and AFP (fetal liver-specific, endoderm) (Fig. 4D).
FIG. 4.
Teratoma formation assay of FoxO3a-wild type and -null iPS cells. (A) FoxO3a-wild type and -null iPS cells were subcutaneously injected into the dorsal flank of severe combined immunodeficiency mice and teratomas formed after 5 weeks. (B) Weight of teratomas derived from FoxO3a-wild type and -null iPS cells. (C) Hematoxylin and eosin stain staining of paraffin sections of teratoma derived from FoxO3a-wild type (upper panels) and -null iPS cells (lower panels). (D) Immunohistochemistry staining for Tuj, α-SMA, and AFP was carried out on paraffin sections of teratomas derived from FoxO3a-wild type (upper panels) and -null (lower panels) iPS cells. Scale bar=50 μm. α-SMA, alpha smooth muscle actin. Color images available online at www.liebertpub.com/scd
FoxO3a deficiency impairs the neuronal lineage differentiation in vitro
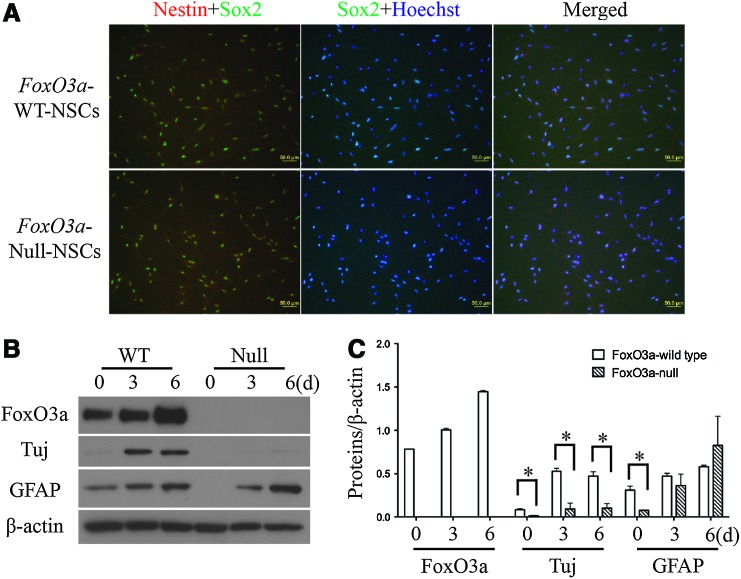
It was reported that NSCs isolated from FoxO3a-null mice showed an impaired capability to generate different neural lineages [24], and considering the difficulty of comparing the ratio of endoderm, mesoderm, and ectoderm in teratoma, we desired a feasible method to assess the potential for neuronal lineage differentiation between FoxO3a-wild type and -null iPS cells. The FoxO3a-null iPS cells were differentiated into neuronal lineages in vitro either with or without embryonic formation. We first differentiated FoxO3a-wild type and -null iPS cells into NSCs with serum-free ITSFn medium to select Nestin-positive cells from embryonic bodies [29]. Our results demonstrated that both FoxO3a-wild type and -null iPS cells could normally differentiate into NSCs in vitro (Fig. 5A). However, the NSCs derived from FoxO3a-wild type iPS cells showed higher potential for neuron differentiation, compared to NSCs derived from FoxO3a-null iPS cells (Fig. 5B, C), and the expression level of FoxO3a was observed to be increased during the differentiation process (Fig. 5B, C). Immunofluorescence staining results showed that the knockout of FoxO3a decreased the proportions of both neurons (Tuj+) and astrocytes (GFAP+) during differentiation (Supplementary Fig. S4A, B), suggesting that FoxO3a not only contributes to the proper reprogramming, but is also involved in the regulation of neuronal lineage differentiation.
FIG. 5.
Progressive differentiation of iPS cells into neuronal lineages. (A) Identification of neural stem cells (NSCs) derived from iPS cells with immunofluorescence staining of Nestin (red), Sox2 (green), and nuclear staining with Hoechst (blue). The upper panels were the NSCs derived from FoxO3a-wild type iPS cells and the lower panels were from FoxO3a-null iPS cells. (B) Western blot analysis of FoxO3a, Tuj, and GFAP during the differentiation process of NSCs derived from iPS cells with β-actin as a loading control. (C) The expression of FoxO3a, Tuj, and GFAP in FoxO3a-wild type and -null NSCs with normalization to β-actin (*p<0.05). Scale bar=50 μm. Color images available online at www.liebertpub.com/scd
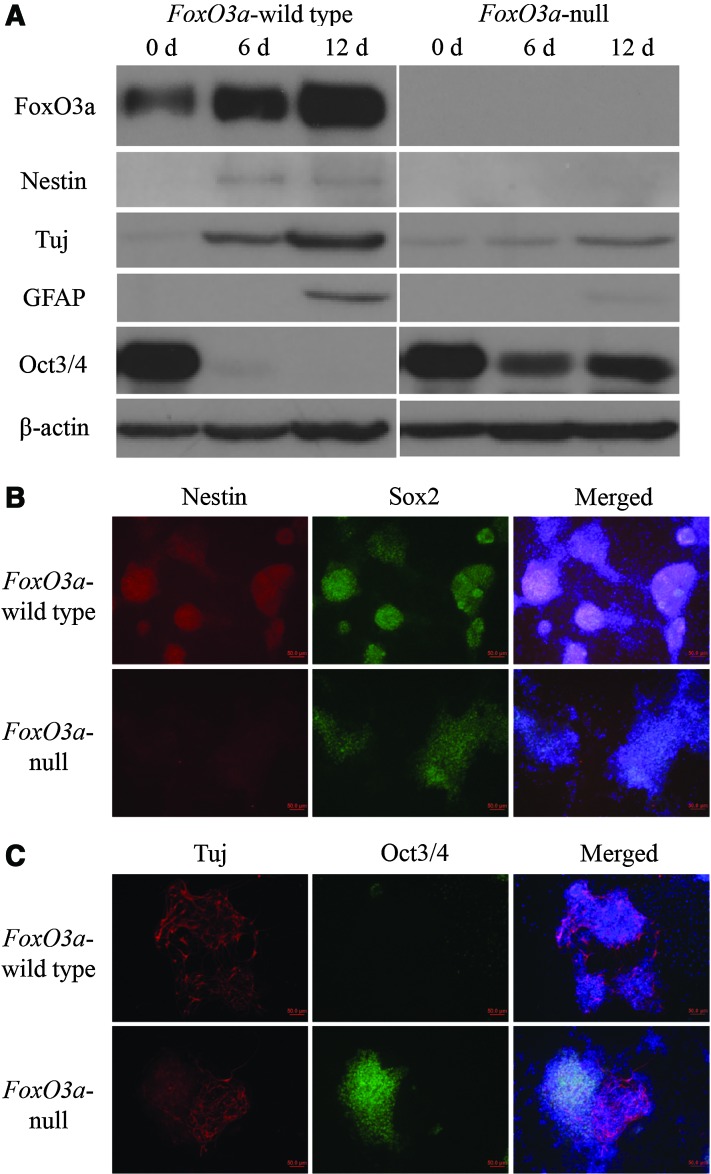
Using an adherent monolayer culture system, ES cells could be directly driven into a neuronal state without embryonic body formation [30]; however, it is still unknown if FoxO3a-wild type and -null iPS cells could be directly converted into neuroectodermal lineages, while omitting the formation of embryonic body, so these two types of iPS cells were dissociated to single cells and plated in monoculture differentiation medium onto 0.1% gelatin-coated six-well plates. Our results showed that most FoxO3a-wild type iPS cells differentiated into neural lineages after 6 days, and Oct3/4, the pluripotency marker of ES cells, was not detectable with either western blot or immunofluorescence staining (Fig. 6A, C), whereas the NSC marker Nestin, the neuron marker Tuj, and the astrocyte marker GFAP all progressively increased, suggesting that FoxO3a-wild type iPS cells successfully differentiated into neuronal lineages in monoculture condition (Fig. 6A–C). However, there were still high levels of Oct3/4 in FoxO3a-null iPS cells even after 6 and 12 days differentiation (Fig. 6A–C), indicating that some of FoxO3a-null iPS cells remained undifferentiated. In addition, the expression levels of Nestin, Tuj, and GFAP in FoxO3a-null iPS cells were lower than that in FoxO3a-wild type iPS cells (Fig. 6A–C). Together, these results indicate that the knockout of FoxO3a induces iPS cells refractory to fully differentiate into neuronal lineages in vitro.
FIG. 6.
Conversion of iPS cells into neuronal lineages in adherent monoculture. (A) FoxO3a-wild type and -null iPS cells were plated onto 0.1% gelatin-coated tissue culture plastic in Dulbecco's modified Eagle's medium/F12 with N2 and B27. At 0, 6, and 12 days, protein samples were collected for FoxO3a, Nestin (neural stem cell marker), Tuj (neuronal lineage marker), GFAP (astrocyte marker), and Oct3/4 (pluripotency marker). (B) At 6 days, differentiated cells were fixed with 4% paraformaldehyde and stained with Nestin (red), Sox2 (green), and DAPI (blue). Upper panels were from FoxO3a-wild type iPS cells and lower panels were from FoxO3a-null iPS cells. (C) Tuj (red), Oct3/4 (green), and DAPI (blue) were examined through immunofluorescence staining at 6 days. Upper panels were from FoxO3a-wild type iPS cells and lower panels were from FoxO3a-null iPS cells. Scale bar=50 μm. Color images available online at www.liebertpub.com/scd
Discussion
In our previous study, mouse astrocytes have been successfully reprogrammed into iPS cells and possess more potential for neuronal differentiation than iPS cells from MEFs [35,36]. Here we investigated the reprogramming process and neuronal differentiation of iPS cells derived from FoxO3a-wild type and -null MEFs, and our results demonstrate that the iPS cell reprogramming kinetics is delayed due to FoxO3a deficiency and that the reprogramming process upregulates the expression of FoxO3a, suggesting a critical function of FoxO3a in the process of iPS cell generation. Overexpression of FoxO3a before Yamanaka factors promotes the final yield of iPS cell colonies in both FoxO3a-wild type and -null MEFs, confirming its vital function during reprogramming process. In addition, FoxO3a-null iPS cells are refractory to fully differentiate in vitro, specifically into neuronal lineages. However, FoxO3a-null iPS cells are similar to wild-type iPS cells with regards to their expression levels of ES cell markers, AP activity, and capability of teratoma formation. These results suggest that FoxO3a mainly functions in the reprogramming process and neuronal differentiation of iPS cells.
The functions of FoxO3a are extremely complicate and sometime contradictory. It not only functions as a trigger for apoptosis and inhibits the cell proliferation [37], but also protects cells against from oxidative stress through directly regulating the expression of antioxidants, such as MnSOD and Catalase [38]. Thus, the overexpression of FoxO3a with virus could only protect MEFs in the null group but not in the wild-type group (Fig. 2B); however, the improvement of reprogramming efficiency are observed in both FoxO3a-wild type and -null MEF groups (Fig. 2C, D), which may be due to the ROS generated by reprogramming factors [39,40]. We have observed that the deficiency of FoxO3a downregulates MnSOD expression, which subsequently increases the ROS level in MEFs and produces more 4-HNE (Supplementary Fig. S2). This resulted high ROS condition unfavors the generation of iPS cells and FoxO3a mainly functions its antioxidative effect during the reprogramming process.
One significant characteristic of MEF cells is their cellular senescence, which is defined as an irreversible state of G1 cell cycle arrest after a serial of passages [41]. Various stresses, such as virus infection and oxidative stress, speed up the cellular senescence and progressively induce the CDK inhibitor-p16 and p21 in aging cells [42]. We also observed the upregulation of p16 and p21 in FoxO3a-null MEFs, compared to the wild-type MEFs. Thus, we only use the FoxO3a-wild type and -null MEFs derived from littermates with the same passage to avoid the artificial effect of passages to reprogramming efficiency and the FoxO3a-heterozygous males are mated with the FoxO3a-heterozygous females to isolate MEFs.
Recently it has been reported that FoxO1, another member of FoxO family, is an essential regulator of pluripotency in both human and mouse ES cells through directly regulating the gene expression of Oct3/4 and Sox2 and the knockdown of FoxO3a with shRNA results in the loss of pluripotency in mouse ES cells without modulating the expression of Oct3/4 and Sox2 [43]. However, our data show that the FoxO3a-null iPS cells are strongly AP positive and express high levels of ES cell markers, such as Oct3/4, SSEA1 (Fig. 3), and Nanog (Supplementary Fig. S3). Moreover, the transgenes of Yamanaka factors are silenced in FoxO3a-wild type and -null iPS cells, while the endogenous ES-specific network is activated (Supplementary Fig. S3); which indicates the complete reprogramming of these iPS cells. The reason may be due to the selection process during the generation of iPS cells from FoxO3a-null MEFs. The criteria for choosing iPS cell colonies are based on both the morphology and AP activity. The colonies chosen are already AP+ and show the ES cell morphology; thus, excluding the non-ES cell-like colonies. Interestingly, the effects of FoxO3a knockout on the NSCs are only significant in adult mice, but not on the NSCs from embryonic or postnatal mice; thus, it may take a longer time for the defect of self-renewal in the FoxO3a-null iPS cells to become apparent.
As the teratoma formation assay is currently regarded as the gold standard test for the pluripotency of ES and iPS cells, the capacity of full differentiation of FoxO3a-wild type and -null iPS cells were examined using teratoma formation in vivo and followed by differentiation into neuronal lineages in vitro. Our results demonstrate that both FoxO3a-wild type and -null iPS cells could form cell lineages of all three germ layers. Thus, the deficiency of FoxO3a in iPS cells does not influence the pluripotency property of generating different germ layer structures in vivo.
Although the pluripotency of FoxO3a-null iPS cells is similar to the wild-type iPS cell in vivo, there is still a dramatic difference in their neuronal differentiation properties. The results show that both FoxO3a-wild type and -null iPS cells could differentiate into NSCs with ITSFn selection medium (Fig. 5A); however, the NSCs derived from FoxO3a-null iPS cells showed lower potential for both neuron and astrocyte differentiation than the NSCs derived from FoxO3a-wild type iPS cells (Fig. 5B, C and Supplementary Fig. S4), which suggests that FoxO3a is essential for the proper differentiation of iPS cells in vitro. In addition, another method of direct differentiation of iPS cells into neurons has indicated that a part of FoxO3a-null iPS cells still remain in an undifferentiated state with high levels of Oct3/4 (Fig. 6).
Many other studies have also demonstrated that FoxO3a plays a vital role in the proper differentiation of hematopoietic and NSCs. FoxO3a-null mice exhibit a myeloproliferative syndrome and NSCs isolated from FoxO3a-null mice have an impaired ability to generate different neural lineages [23,24,26]. In addition, the expression of FoxO3a is upregulated after differentiation into neuronal lineages with both monoculture and progressive differentiation, suggesting the critical function of FoxO3a in the proper neuronal differentiation of iPS cells. Recently, induced NSCs (iNSCs) have been directly generated from mouse and human fibroblasts omitting the pluripotent stage [44,45] and FoxO3a may be also involved in the reprogramming process and proper differentiation of these iNSCs.
FoxO3a is widely expressed in the original cell-MEFs, the reprogrammed-iPS cells, and also in the neuronal lineages, including NSCs, neurons, and astrocytes; it seems that the expression of FoxO3a progressively increases. As the reprogramming process and pluripotency stage are two different procedures, FoxO3a may regulate a different subset of genes with its FoxO binding motif. As reported in the NSCs, FoxO3a is required for the expression of hypoxia-dependent genes, such as Ddit4. FoxO3a may also be able to directly bind the regulatory regions of Ddit4, a proven target of hypoxia-induced factor 1 in the iPS cells.
In summary, our findings suggest new functions of FoxO3a in the pluripotency and differentiation of iPS and ES cells. Understanding the complete and proper differentiation of iPS cells in vitro and in vivo could contribute to the ongoing efforts to develop new cell therapies in regenerative diseases.
Supplementary Material
Acknowledgments
We kindly thank Mrs. Kristin M. Leland and Mr. Randall J. Ambroz for the critical reading of the article and Drs. Yunlong Huang, and Hui Peng for the technical support of this work. Julie Ditter, Lenal Bottoms, Johna Belling, and Robin Taylor provided outstanding administrative and secretarial support. This work was supported in part by research grants by the National Institutes of Health: R01 NS 41858-01, R01 NS 061642-01, R01 NS 61642-2S1, R21 MH 083525-01, P01 NS043985, and P20 RR15635-01 (J.Z.), National Natural Science Foundation of China (NSFC) no. 81028007 (J.Z.) and National Natural Science Foundation of China (NSFC) no. 81271419 (C.T.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Nishikawa S. Goldstein RA. Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9:725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Yu J. Vodyanik MA. Smuga-Otto K. Antosiewicz-Bourget J. Frane JL. Tian S. Nie J. Jonsdottir GA. Ruotti V, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 5.Robinton DA. Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karumbayaram S. Novitch BG. Patterson M. Umbach JA. Richter L. Lindgren A. Conway AE. Clark AT. Goldman SA, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–811. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper O. Hargus G. Deleidi M. Blak A. Osborn T. Marlow E. Lee K. Levy A. Perez-Torres E. Yow A. Isacson O. Differentiation of human ES and Parkinson's disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci. 2010;45:258–266. doi: 10.1016/j.mcn.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter L. Carr C. Yang CT. Stuckey DJ. Clarke K. Watt SM. Efficient differentiation of human induced pluripotent stem cells generates cardiac cells that provide protection following myocardial infarction in the rat. Stem Cells Dev. 2012;21:977–986. doi: 10.1089/scd.2011.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q. Yang Y. Zhang J. Wang GY. Liu W. Qiu DB. Hei ZQ. Ying QL. Chen GH. Efficient derivation of functional hepatocytes from mouse induced pluripotent stem cells by a combination of cytokines and sodium butyrate. Chin Med J (Engl) 2011;124:3786–3793. [PubMed] [Google Scholar]
- 10.Hargus G. Cooper O. Deleidi M. Levy A. Lee K. Marlow E. Yow A. Soldner F. Hockemeyer D, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci U S A. 2010;107:15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu W. Wang SJ. Zhou GD. Liu W. Cao Y. Zhang WJ. Residual undifferentiated cells during differentiation of induced pluripotent stem cells in vitro and in vivo. Stem Cells Dev. 2012;21:521–529. doi: 10.1089/scd.2011.0131. [DOI] [PubMed] [Google Scholar]
- 12.Monsalve M. Olmos Y. The complex biology of FOXO. Curr Drug Targets. 2011;12:1322–1350. doi: 10.2174/138945011796150307. [DOI] [PubMed] [Google Scholar]
- 13.Cui M. Huang Y. Zhao Y. Zheng J. New insights for FOXO and cell-fate decision in HIV infection and HIV associated neurocognitive disorder. Adv Exp Med Biol. 2009;665:143–159. doi: 10.1007/978-1-4419-1599-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salih DA. Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H. Regan KM. Lou Z. Chen J. Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- 16.Greer EL. Dowlatshahi D. Banko MR. Villen J. Hoang K. Blanchard D. Gygi SP. Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomis RR. Alarcon C. Nadal C. Van Poznak C. Massague J. C/EBPbeta at the core of the TGFbeta cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell. 2006;10:203–214. doi: 10.1016/j.ccr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Bakker WJ. Harris IS. Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cells. 2007;28:941–953. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 19.Lin K. Dorman JB. Rodan A. Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 20.Willcox BJ. Donlon TA. He Q. Chen R. Grove JS. Yano K. Masaki KH. Willcox DC. Rodriguez B. Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flachsbart F. Caliebe A. Kleindorp R. Blanche H. von Eller-Eberstein H. Nikolaus S. Schreiber S. Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soerensen M. Dato S. Christensen K. McGue M. Stevnsner T. Bohr VA. Christiansen L. Replication of an association of variation in the FOXO3A gene with human longevity using both case-control and longitudinal data. Aging Cell. 2010;9:1010–1017. doi: 10.1111/j.1474-9726.2010.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyamoto K. Araki KY. Naka K. Arai F. Takubo K. Yamazaki S. Matsuoka S. Miyamoto T. Ito K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Renault VM. Rafalski VA. Morgan AA. Salih DA. Brett JO. Webb AE. Villeda SA. Thekkat PU. Guillerey C, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paik JH. Ding Z. Narurkar R. Ramkissoon S. Muller F. Kamoun WS. Chae SS. Zheng H. Ying H, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yalcin S. Marinkovic D. Mungamuri SK. Zhang X. Tong W. Sellers R. Ghaffari S. ROS-mediated amplification of AKT/mTOR signalling pathway leads to myeloproliferative syndrome in Foxo3(−/−) mice. EMBO J. 2010;29:4118–4131. doi: 10.1038/emboj.2010.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y. Peng H. Cui M. Whitney NP. Huang Y. Zheng JC. CXCL12 increases human neural progenitor cell proliferation through Akt-1/FOXO3a signaling pathway. J Neurochem. 2009;109:1157–1167. doi: 10.1111/j.1471-4159.2009.06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao L. Feng W. Sun Y. Bai H. Liu J. Currie C. Kim J. Gama R. Wang Z, et al. Generation of iPS cells using defined factors linked via the self-cleaving 2A sequences in a single open reading frame. Cell Res. 2009;19:296–306. doi: 10.1038/cr.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SH. Lumelsky N. Studer L. Auerbach JM. McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 30.Ying QL. Stavridis M. Griffiths D. Li M. Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 31.Cui M. Huang Y. Tian C. Zhao Y. Zheng J. FOXO3a inhibits TNF-alpha- and IL-1beta-induced astrocyte proliferation: implication for reactive astrogliosis. Glia. 2011;59:641–654. doi: 10.1002/glia.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wernig M. Meissner A. Foreman R. Brambrink T. Ku M. Hochedlinger K. Bernstein BE. Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 33.Brambrink T. Foreman R. Welstead GG. Lengner CJ. Wernig M. Suh H. Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgering BM. Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- 35.Tian C. Wang Y. Sun L. Ma K. Zheng JC. Reprogrammed mouse astrocytes retain a “memory” of tissue origin and possess more tendencies for neuronal differentiation than reprogrammed mouse embryonic fibroblasts. Protein Cell. 2011;2:128–140. doi: 10.1007/s13238-011-1012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghorpade A. Reprogrammed astrocytes with old “memories” blossom into region-specific neurons. Protein Cell. 2011;2:87–89. doi: 10.1007/s13238-011-1025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunet A. Bonni A. Zigmond MJ. Lin MZ. Juo P. Hu LS. Anderson MJ. Arden KC. Blenis J. Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 38.Ferber EC. Peck B. Delpuech O. Bell GP. East P. Schulze A. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 2012;19:968–979. doi: 10.1038/cdd.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida Y. Takahashi K. Okita K. Ichisaka T. Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Utikal J. Polo JM. Stadtfeld M. Maherali N. Kulalert W. Walsh RM. Khalil A. Rheinwald JG. Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dirac AM. Bernards R. Reversal of senescence in mouse fibroblasts through lentiviral suppression of p53. J Biol Chem. 2003;278:11731–11734. doi: 10.1074/jbc.C300023200. [DOI] [PubMed] [Google Scholar]
- 42.Chen JH. Ozanne SE. Hales CN. Methods of cellular senescence induction using oxidative stress. Methods Mol Biol. 2007;371:179–189. doi: 10.1007/978-1-59745-361-5_14. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X. Yalcin S. Lee DF. Yeh TY. Lee SM. Su J. Mungamuri SK. Rimmele P. Kennedy M, et al. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat Cell Biol. 2011;13:1092–1099. doi: 10.1038/ncb2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thier M. Worsdorfer P. Lakes YB. Gorris R. Herms S. Opitz T. Seiferling D. Quandel T. Hoffmann P, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Tian C. Ambroz RJ. Sun L. Wang Y. Ma K. Chen Q. Zhu B. Zheng JC. Direct conversion of dermal fibroblasts into neural progenitor cells by a novel cocktail of defined factors. Curr Mol Med. 2012;12:126–137. doi: 10.2174/156652412798889018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.