Abstract
Accumulating evidence shows that post-ischemic inflammation originated by Toll-like receptors (TLR) plays critical roles in ischemic stroke. However, the functions of other innate immune receptors are poorly understood in cerebral ischemia. Macrophage-inducible C-type lectin, Mincle, is one of the innate immune receptor C-type lectin-like receptor (CLR) to response against dying cells. In the present study, we showed that Mincle, its ligand SAP130, and its downstream phospho-Syk/Syk were upregulated after ischemia, and that Mincle is expressed in immune and non-immune cells in the ischemic brains of mice and human. We treated mice with piceatannol, a Syk inhibitor, and consequently the infarct volume and swelling were suppressed by piceatannol. The levels of phospho-Syk, MMP9 and ICAM-1 were downregulated, and the level of Claudin5 was uplegurated in piceatannol-treated groups. These data indicate that innate immune system, such as Mincle and Syk plays a pivotal role in the pathogenesis after the ischemia and reperfusion.
Stroke is a leading cause of human death1. Thrombolysis, the method currently available to rescue patients of severe acute ischemic stroke, involves recanalizing the supply of oxygen and glucose in order to suppress neuronal cell death2. However, because of the characteristic side effect of lysis, this strategy contains the risk of exaggerating Blood Brain Barrer (BBB) breakdown to reach a state of hemorrhagic transformation (HT)3. This ongoing dilemma for the utilization of thrombolysis is kept in balance by the narrow time window that allows its use only for 4.5 h after ischemia4. Under the circumstances explained above, new therapeutic strategies that could protect the neurovascular unit are intensely desired5.
Inflammation occurs in the endovascular area and parenchyma of the ischemic brain, which are organized by various types of cells, such as neurons, endothelial cells, and immune cells6. During the inflammatory process, a complex network of cytokines is induced, upregulated adhesion molecules induce recruitment and invasion of leukocytes, and oxidative stress and activation of various proteases enhance BBB disruption and subsequent leakage of blood6. The inflammatory reactions that follow ischemia and reperfusion are essential for the progression of ischemic injury. Inhibition of these reactions was successful in animal models7,8; however, the question of what the launching point of inflammation after ischemia is still remains.
Recent studies have found the long-sought-after answer that following ischemic stroke, innate immune receptors, especially Toll-like receptor 2 (TLR2) and TLR4, sense dying cells and function as the initiators of inflammation. This is because innate immune receptors have the property of being able to recognize common structures to respond to a wide range of ligands, which include not only non-self but also self ligands, instead of the specific recognition that the adaptive immune system utilizes9,10,11,12.
Despite many studies focusing on innate immune system, the roles of innate immune receptors, other than TLRs, after ischemic stroke remain unclear. The C-type lectin-like receptor (CLR) is a member of the innate immune receptors, which also comprise TLRs, Nod-like receptors (NLRs), and RIG-I-like receptors (RLRs)13,14,15,16. Interestingly, even though the immunomechanisms of CLRs are still not yet sufficiently understood, several CLRs are known to mediate intracellular signals via immunoreceptor tyrosine-based activation motifs (ITAMs) that are utilized by adaptive immune receptors17. Recent studies show that macrophage-inducible C-type lectin (Mincle), a member of the CLR family and a known inducible receptor for fungal pathogens, also responds to SAP130. SAP130, a subunit of histone deacetylase, diffuses out of dying cells, starting signals by associating Mincle with ITAMs followed by recruitment of a protein tyrosine kinase, Syk18,19.
Therefore, we hypothesized that Mincle, its ligand SAP130, and the downstream kinase Syk participate in the pathogenesis of ischemic stroke by initiating inflammation. To examine this hypothesis, we investigated the localization of Mincle in ischemic brains of mice and humans, changes in the expression levels of Mincle, SAP130, p-Syk, and Syk in ischemic mice, and the effect of piceatannol, a Syk inhibitor, for the treatment of acute ischemic stroke.
Results
Localization of Mincle expression in the ischemic brain of mice and human stroke patients
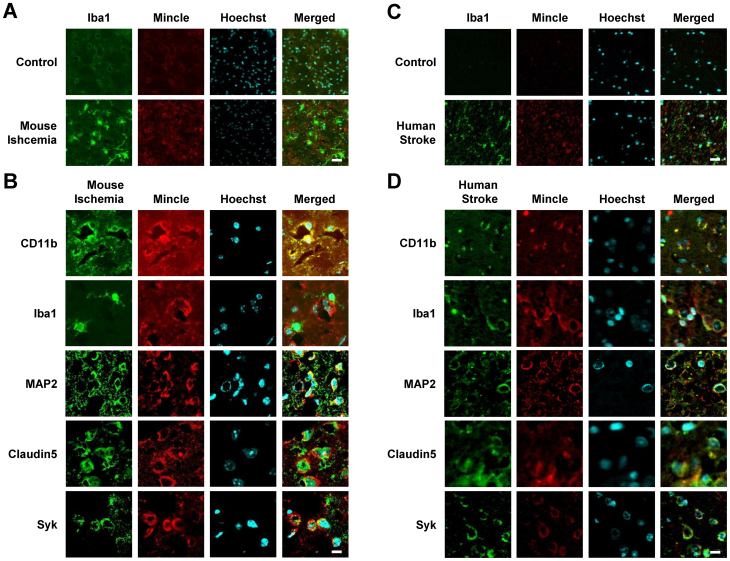
To identify what type of cells Mincle is expressed in, double immunofluorescence was performed in ischemic brain at 24 h after ischemia. CD11b and Iba1 were used as markers of immune cells, MAP2 was used as a marker of neuronal cells, and Claudin 5 was used as a marker of endothelial cells. In mice, Mincle and Iba1 were upregulated at 22 h after ischemia and reperfusion (Fig 1A). Mincle was co-localized with CD11b at 22 h after ischemia and reperfusion, which means that Mincle is expressed in immune cells (Fig 1B). However, Mincle was not co-localized with Iba1 at 22 h after ischemia and reperfusion (Fig 1B). Mincle co-localized with MAP2 at 22 h after ischemia and reperfusion, which means that Mincle is expressed in neuronal cells (Fig 1B). Mincle co-localized with Claudin5 at 22 h after ischemia and reperfusion, which means that Mincle is expressed in endothelial cells (Fig 1B). Mincle also co-localized with Syk at 22 h after ischemia and reperfusion (Fig 1B). Mincle and Iba1 were upregulated in human stroke patients (Fig 1C). Mincle co-localized with CD11b, MAP2, Claudin5, and Syk in human stroke patients as well as ischemic mice (Fig 1D). In addition, Mincle was co-localized with Iba1 (Fig 1D). These data show that Mincle is expressed in immune, neuronal, and endothelial cells after ischemic stroke in both mice and humans.
Figure 1. Localization of Mincle in brain tissue after ischemic stroke in mice and humans by double-immunostaining of Mincle and marker proteins.
(A) The expression levels of Mincle and Iba1 were upregulated after ischemia. Scale bar = 20 μm. (B) Mincle co-localized with CD11b, but not Iba1, all of which are markers of immune cells. Mincle co-localized with neuronal cell marker MAP2. Mincle co-localized with the endothelial cell marker Claudin5. Mincle co-localized with Syk, which is the downstream signal of Mincle. Scale bar = 10 μm. (C) The expression levels of Mincle and Iba1 were upregulated in human stroke patients. Scale bar = 20 μm. (D) Mincle co-localized with CD11b, MAP2, and Claudin 5 in human stroke patients. Unlike in mice, Mincle also co-localized with Iba1. Scale bar = 10 μm.
Time-course expression profiles of Mincle, SAP130, p-Syk, and Syk after transient cerebral ischemia
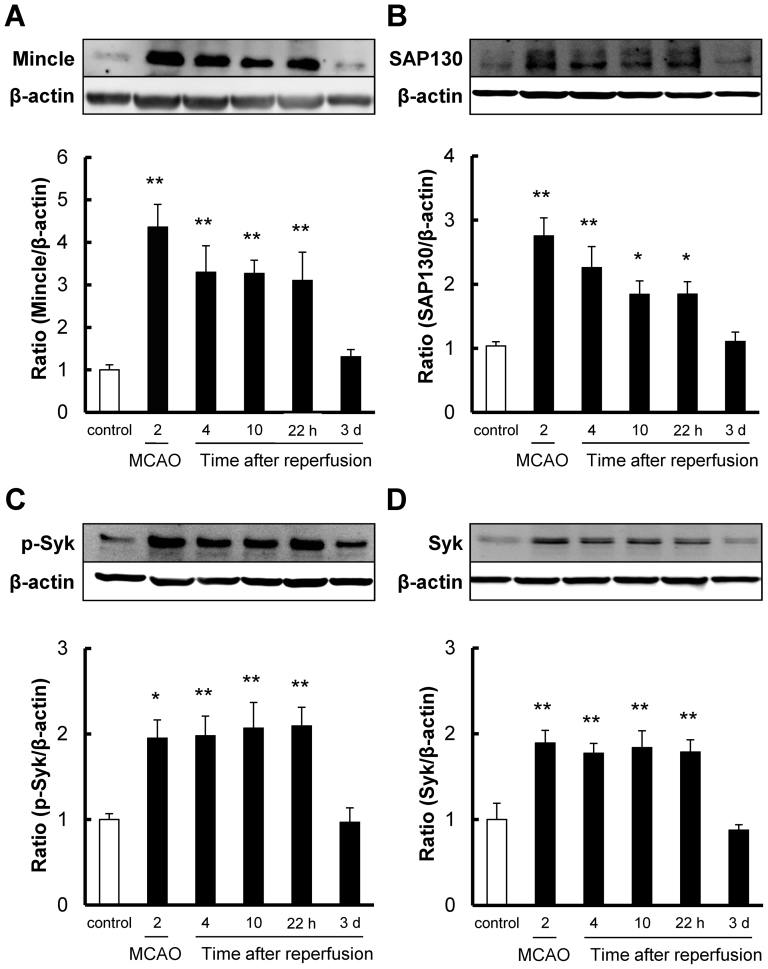
We tested changes in the protein levels of Mincle, SAP130, p-Syk, and Syk in brain tissue by western blot at 2 h after ischemia and at 4 h, 10 h, 22 h, and 3 days after reperfusion. All 4 proteins were significantly increased at 2 h after ischemia and at 4 h, 10 h, and 22 h after reperfusion, but not at 3 days post-reperfusion (Fig 2A to D). The trends of Mincle and SAP130 were similar, with expression levels peaking at 2 h after ischemia and gradually reducing up to 22 h after reperfusion (Fig 2A to B). On the other hand, p-Syk and Syk were increased at 2 h after ischemia and were sustained at the same level to 22 h after reperfusion (Fig 2C to D).
Figure 2. The time-course of changes in protein levels of Mincle, SAP130, p-Syk, and Syk after reperfusion following 2 h of focal ischemia in mice.
Quantitative analysis with western blot shows that the expression levels of (A) Mincle, (B) SAP130, (C) p-Syk, and (D) Syk were significantly increased at 2 h after ischemia and at 4 h, 10 h, and 22 h after reperfusion, but not at 3 days. Cropped blots are used in the figure. These gels were run under same experimental condition. * P < 0.05, ** P < 0.01 vs. control, n = 8.
Pharmacological inhibition of Syk activation with piceatannol reduced cerebral ischemic injury
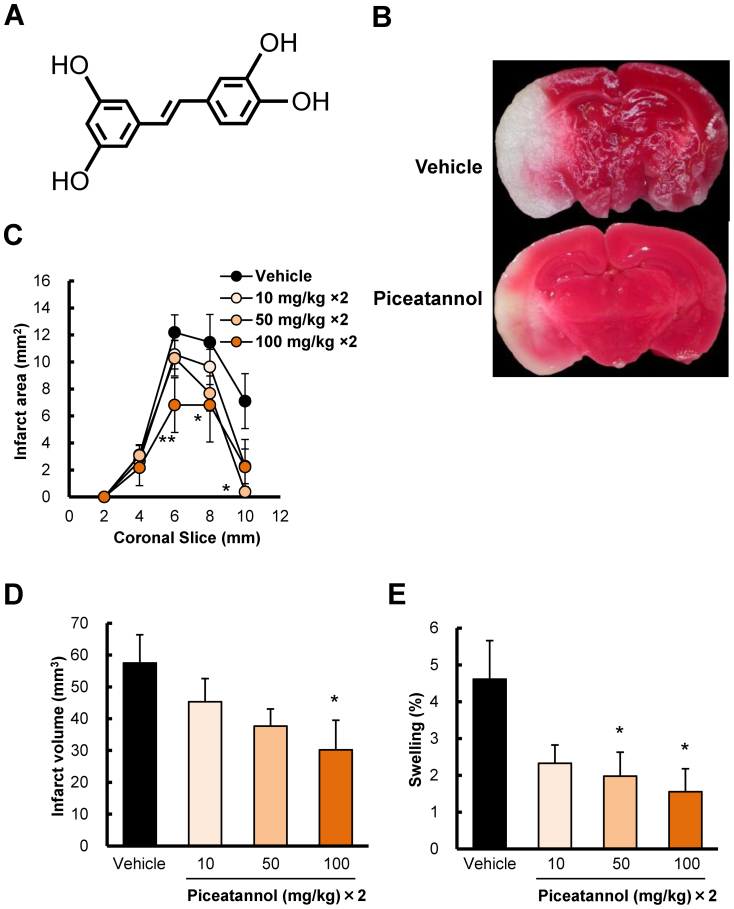
To investigate the effects of inhibition of Syk activation, a Syk inhibitor piceatannol at 10, 50, and 100 mg/kg was administered intraperitoneally (i.p.) twice: at 30 min before ischemia and just after reperfusion. Based on analysis with TTC (2,3,5-triphenyltetrazolium chloride) staining, piceatannol reduced the infarct area, infarct volume, and swelling in a dose-dependent manner, with significant differences observed at 50 and 100 mg/kg in infarct area (Fig 3B), at 100 mg/kg in infarct volume (Fig 3C), at 50 and 100 mg/kg in swelling (Fig 3D). At 100 mg/kg, i.p., piceatannol reduced the infarct volume, by approximately 50%, at 24 h after cerebral ischemia (Fig 3C).
Figure 3. The protective effects of piceatannol, a Syk inhibitor, on infarction and swelling at 22 h after reperfusion following 2 h ischemia.
Brains were removed, and the forebrains were sliced into 5 coronal 2-mm sections. Sections are identified according to the distance from the frontal limit of the forebrain. (A) A representative photograph of TTC staining of 8-mm front coronal brain sections (2 mm thick) at 22 h after reperfusion following 2 h ischemia. The upper panel is vehicle control, and the lower panel is piceatannol treatment (at 100 mg/kg × 2, i.p.). Pre- and post-treatments with piceatannol (B) at 50 and 100 mg/kg significantly reduced the infarct area and that with piceatannol (C) at 100 mg/kg significantly reduced the infarct volume. (D) Piceatannol at 50 and 100 mg/kg significantly reduced swelling. * P < 0.05, ** P < 0.01 vs. vehicle, n = 8–10.
The mechanisms of the protective effects of piceatannol in cerebral ischemia
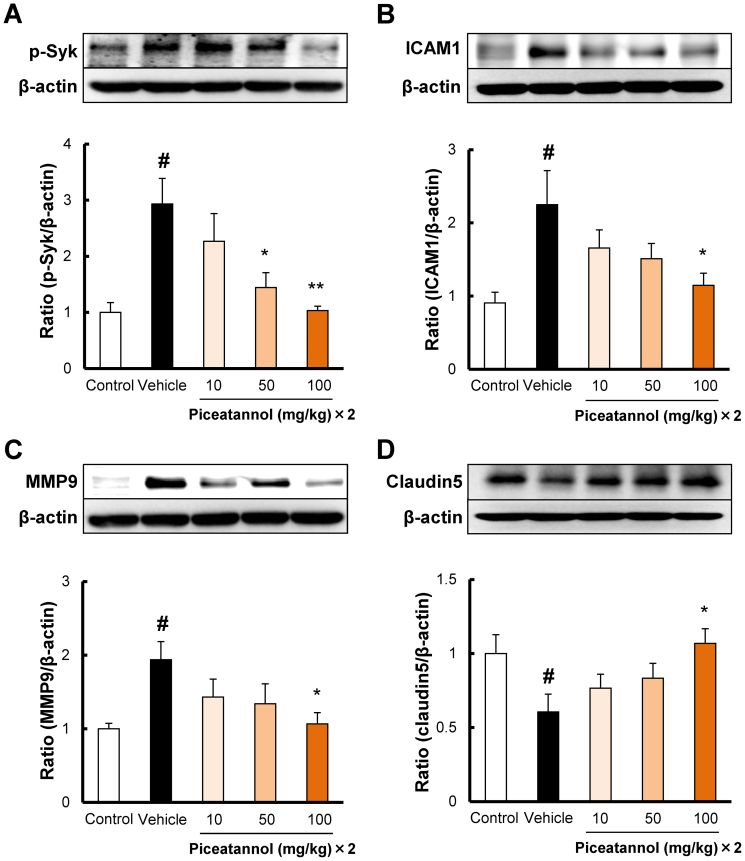
To determine the mechanisms of the protective effects of piceatannol, we examined the changes in the levels of 4 proteins involved in the pathogenesis of cerebral ischemia by western blot. Piceatannol is a known inhibitor of Syk activation, and therefore, we tested whether piceatannol can suppress the expression level of phospho-Syk. In the vehicle group, the phospho-Syk level was significantly upregulated at 24 h after ischemia (Fig 4A). Piceatannol at 50 and 100 mg/kg i.p. significantly reduced the level of phospho-Syk in ischemic brain (Fig 4A). It has been reported that ICAM1 promotes leukocyte binding to endothelial cells during ischemia and reperfusion and aggravates ischemic damage20. ICAM1 level increased at 24 h after ischemia in the vehicle group (Fig 4B). Piceatannol at 100 mg/kg i.p. reduced the level of ICAM1 after ischemia (Fig 4B). MMP9 plays a detrimental role in the progression of ischemia21. In the vehicle group, MMP9 level increased at 24 h after ischemia (Fig 4C). Piceatannol at 100 mg/kg i.p. decreased the level of MMP9 after ischemia (Fig 4C). Recent studies demonstrated that tight junction proteins play a crucial role in BBB dysfunction, leading to leakage of water (brain edema) or blood (hemorrhagic transformation)22. The level of Claudin5, an important member of the family of tight-junction proteins, decreased at 24 h after ischemia (Fig 4D). Piceatannol at 100 mg/kg, i.p., increased the level of Claudin5 after ischemia (Fig 4D).
Figure 4. The mechanisms of the protective effects of piceatannol after cerebral ischemia.
Quantitative analysis with western blot shows that the expression level of (A) p-Syk was increased at 22 h after reperfusion, and administration of piceatannol intraperitoneally at 50 and 100 mg/kg, i.p. significantly reduced p-Syk. (B) ICAM1 was increased after 22 h after reperfusion, and administration of piceatannol intraperitoneally at 100 mg/kg, i.p. significantly reduced ICAM1. (C) MMP9 was increased 22 h after reperfusion, and administration of piceatannol intraperitoneally at 100 mg/kg, i.p. significantly reduced MMP9. (D) Claudin5 expression was decreased after 22 h after reperfusion, and administration of piceatannol intraperitoneally at 100 mg/kg, i.p. significantly ameliorated Claudin5 expression. Cropped blots are used in the figure. These gels were run under same experimental condition. # P < 0.05 vs. control, * P < 0.05, ** P < 0.01 vs. vehicle, n = 6.
Discussion
Mincle, a member of the CLR family that is able to sense several exogenous ligands such as fungus, yeast, and mycobacteria, was first identified as a direct transcriptional target of NF-IL6 in macrophages18,23. In addition, it was reported that Mincle recognizes an endogenous ligand, nuclear protein SAP130, as it exits dying cells, which suggests that Mincle may be involved in the pathogenesis of cerebral ischemia, because the pathology also causes the release of self-components19.
In the present study, we found that Mincle was expressed in immune, neuronal, and endothelial cells after cerebral ischemia (see Fig. 1 and Supplementary Fig. S1 online). This is the first observation that an innate immune receptor, although why Mincle was not co-localized with Iba1 in mice is unkown, is expressed in endothelial cells in the brain after ischemia (see Fig. 1 and Supplementary Fig. S1 online). We also found that the protein expression levels of Mincle, its ligand SAP130, and the downstream signal Syk were upregulated or activated after cerebral ischemia and reperfusion. Chronological analysis showed that the trend of expression level in Mincle, which was quickly and strongly induced at 2 h after ischemia and gradually decreased, is very similar with that in SAP130. This means that SAP130 may be the primary ligand of Mincle, although other endogenous ligands have not yet been identified.
On the other hand, the downstream kinase Syk was upregulated and activated almost equally from 2 h to 24 h after ischemia. One possible explanation for this difference is that Syk also reportedly associates with other receptors in addition to Mincle. Syk is also known to be the downstream signal for other CLRs such as Dectin1 and Dectin224,25, other innate receptors such as TLR4 (which is gradually increased from the onset of cerebral ischemia to 24 h)26, and the complement system, which is a part of the innate immune system27. Mincle knock-out mice showed better outcome after stroke, and the absence of Mincle protected neurons against apoptosis caused by in vitro glucose deprivation28. Although immune mechanisms in the brain may have several differences from other organs29, these findings indicate that Syk plays a critical role in cerebral ischemia by driving signals from innate immune receptors (Fig 5). Piceatannol, a Syk inhibitor that is derived from passion fruit seeds, rescued cerebral ischemic damage, as measured by infarct volume and cerebral edema. This observation is in concordance with a report that oral administration of a Syk inhibitor, R788, for 6 days exerts protective effects against intestinal ischemia induced tissue injury30, and with an our recent study that Piceatannol reduces retinal damage after ischemia31.
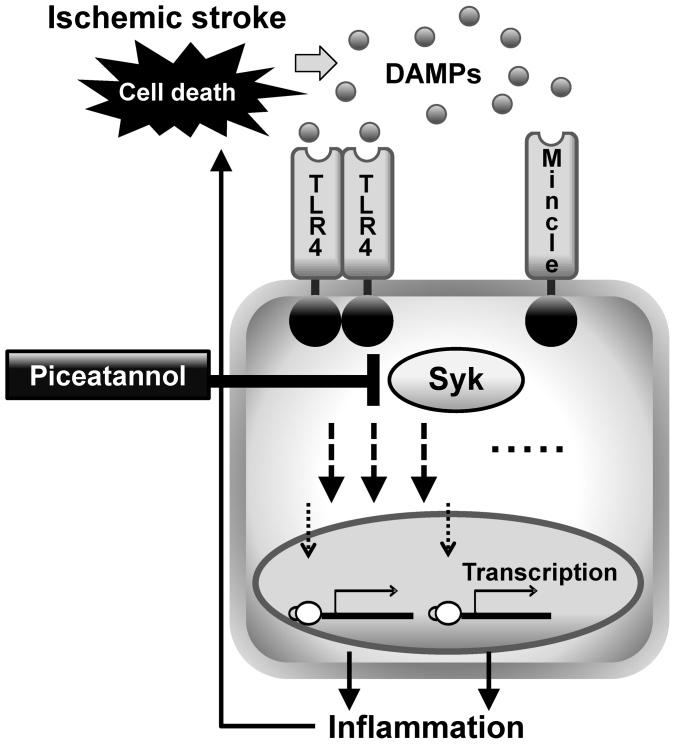
Figure 5. Putative mechanism for Mincle and Syk in ischemic stroke.
Damage-associated molecular patterns (DAMPs) turn on innate immunity via receptors, such as TLR4 and Mincle. The downstream kinase Syk drives inflammatory cascades and cytokines, and the Syk inhibitor piceatannol reduces tissue injury induced by ischemia and reperfusion.
Recent reports suggest that infiltrating leukocytes, rather than resident microglia, are the primary contributors to the injury after cerebral ischemia10. Intercellular adhesion molecules, such as ICAM1, promote aggregation of leukocytes in injured brain20. MMP9 exacerbates disruption of the BBB, which is composed of tight-junction proteins, and damages neurons21. As mechanisms to explain the protective effects of piceatannol, administration of piceatannol was found to reduce the expression levels of p-Syk, ICAM1, and MMP9, and to increase that of Claudin5, a tight-junction protein. Taken together, our results suggest that piceatannol may protect the neurovascular unit against ischemic stroke-induced injury22,32.
Studies of the innate immune system have elucidated the principles of many diseases, especially autoimmune and inflammatory diseases. In rheumatoid arthritis, a representative autoimmune disease, intracellular kinases, such as Syk and JAK, are a primary focus as innovative targets to reduce inflammation. Inhibition of these kinases can control both innate and adaptive immune responses, and clinical trials of these inhibitors are ongoing33. The pathology of ischemic stroke also starts with the innate immune response and is connected to adaptive immunity similarly to autoimmune diseases. However, if the pathological process of cerebral ischemia progresses too fast, so-called “Stroke” occurs. In this context, these kinase inhibitors, which can suppress several signals of innate immune responses, may also be innovative candidates for the treatment of ischemic stroke. However, further studies including the early and chronic stages of stroke are required to comprehensively understand the innate and adaptive immune response and the involvement of these kinases in cerebral ischemia.
In conclusion, the expressions of Mincle, the ligand SAP130, and the downstream signal p-Syk/Syk increased after cerebral ischemia and reperfusion. The Syk inhibitor piceatannol remarkably reduced cerebral tissue damage. These findings indicate that Mincle and Syk, which are critical components of the innate immune response, are involved in the mechanism of injury after ischemia and reperfusion.
Methods
Animals
The experimental designs and all procedures were in accordance with the guidelines of the World Medical Association's Declaration of Helsinki and the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and the study was approved by the Experimental Committee of Gifu Pharmaceutical University. Mice were randomized and operators were blinded. All efforts were made to minimize both suffering and the number of animals used. Experiments were performed using male ddY mice aged 4–5weeks (Japan SLC Ltd., Shizuoka, Japan). The mice (weighing 22 to 28 g) were housed at 24 ± 2°C under a 12-h light/dark cycle (lights on from 07:00–19:00 h).
Focal cerebral ischemia and drug treatment
The filament middle cerebral artery occlusion (MCAO) model used, as described previously34. Mice were anesthetized with 2.0 to 3.0% isoflurane (Merck Hoei Ltd., Osaka, Japan) and maintained with 1.0 to 1.5% isoflurane (both in 70% N2O/30% O2) by means of an animal general anesthesia machine (Soft Lander; Sin-ei Industry Co. Ltd., Saitama, Japan). Body temperature was maintained at 37.0–37.5°C with the aid of a heating pad and heating lamp. After a midline skin incision, the left external carotid artery was exposed, and its branches were occluded. An 8–0 nylon monofilament (Ethicon, Somerville, NJ, USA) coated with a mixture of silicone resin (Xantopren; Bayer Dental, Osaka, Japan) was inserted into the left internal carotid artery through the external carotid artery stump so as to occlude the origin of the middle cerebral artery. Then, the left common carotid artery was occluded. After 2 h of occlusion, the animal was reanesthetized briefly and reperfusion initiated via withdrawal of the monofilament.
After the surgery, the mice were kept in the preoperative condition (room temperature; 24 ± 2°C) until sampling. Doses of 10, 50, and 100 mg/kg of Piceatannol dissolved in 0.5% carboxymethylcellulose was injected intraperitoneally (i.p.) twice; 30 min before occlusion and just after reperfusion.
Assessment of cerebral infarction
To analyze infarct volume, mice were euthanized using sodium pentobarbital (Nissan Kagaku, Tokyo, Japan) at 24 h after MCAO, and forebrains were coronally sectioned into five slices (2 mm thick). These were placed in 2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich Co., St. Louis, MO, USA) at 37°C for 30 min, and then fixed in 10% buffered formalin. Digital images of the caudal aspect of each slice were obtained using a digital camera (Coolpix 4500, Nikon, Tokyo, Japan). Infarct, ipsilateral hemisphere, and contralateral hemisphere areas were measured using image processing software (Image-J ver. 1.43 h; National Institutes of Health, Bethesda, MD, USA), and infarct volume was calculated as previously reported34.
Western blotting
Mice were deeply anesthetized and decapitated at 24 h after ischemia. Brains were quickly removed, and the brains were cut into 3 mm coronal sections and separated into ipsilateral side and contralateral side. Tissues were homogenized in 10 ml/g tissue ice-cold lysis buffer (50 mM Tris-HCl, pH 8.0, containing 150 mM NaCl, 50 mM EDTA, 1% Triton X-100, and protease/phosphatase inhibitor mixture). A sample obtained from the sham-control or ischemic hemisphere was loaded, and the separated proteins were subsequently transferred. For immunoblotting, the following primary antibodies were used: anti-Mincle antibody (1:1000; MBL, Nagoya, Japan), anti-SAP130 antibody (1:2000; Abcam, Cambridge, MA, USA), anti-phospho-Syk antibody (1:1000, Cell Signaling, Danvers, MA,USA), anti-Syk antibody (1:1000, Cell Signaling), anti-ICAM1 antibody (1:1000, Abcam), anti-MMP9 antibody (1:1000; Millipore, Billerica, MA, USA), and anti-Claudin5 antibody (1:1000, Invitrogen, Eugene, OR). The secondary antibody was anti-rat IgG (1:5000, Thermo Scientific, Rockford, IL, USA), anti-goat IgG (1:5000, Thermo Scientific), and anti-rabbit IgG (1:5000; Thermo Scientific). The immunoreactive bands were visualized using ImmunoStar LD (Wako Pure Chemical Industries). The band intensity was measured using a Lumino imaging analyzer (LAS-4000: Fuji film, Tokyo, Japan). The differences of expression levels were analyzed using MultiGauge software (Fujifilm) by measuring the intensity of the bands.
Human tissue specimens
Human tissue specimens were obtained from patients who had undergone surgery for reasons of clinical necessity at the Department of Neurosurgery, Gifu University Hospital. There were no additional interventions in the patients enrolled in this study. The use of surgical specimens for immunohistochemistry was approved by the institutional review board of Gifu University (#25–46), and representative signed informed written consent. The stroke patient was a woman of 60's who suffered large hemispheric infarction due to cardiogenic embolism and underwent internal and external decompression at 24 h after symptom onset because of brain herniation. The tissue specimen was temporal cortex of the infarct brain removed by decompressive surgery. The control patient was a woman of 20's who presented intractable epilepsy caused by cavernous malformation in the temporal lobe and had no other medical history, and the neurological status was completely normal with the exception of seizures. The patient underwent the anterior temporal lobectomy including the removal of vascular malformation. The tissue specimen was the histologically normal cortex of the removed temporal lobe.
Double-immunostaining
For double-immunostaining, frozen tissues from mice and paraffin-embedded human specimens were used. Mice were injected with sodium pentobarbital (Nembutal; 50 mg/kg, i.p.), then perfused through the left ventricle with 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Brains were removed after 15 min perfusion fixation at 4°C, then immersed in the same fixative solution overnight at 4°C. They were then immersed in 25% sucrose in 0.1 M PB for 24 h, and finally frozen in liquid nitrogen. Coronal sections (14 μm thick) between from 0.4 and 1.0 mm anterior to bregma were cut on a cryostat at −20°C, and stored at −80°C until use. The human specimens were deparaffinized and rehydrated before the immunohistochemical procedures. The sections were blocked with 10% goat serum in PBS and incubated overnight at 4°C with the following primary antibodies: anti-Mincle antibody (1:100, MBL), anti-CD11b antibody (1:100, Millipore), anti-Iba1 antibody (1:200, Wako, Osaka, Japan), anti-MAP2 antibody (1;100, Millipore), and anti-Claudin5 antibody (1:100, Invitrogen) anti-Syk antibody (1:100, Cell signaling), anti-VE-cadherin antibody (1:200, Abcam), anti-CD31 antibody (1:200, Abcam). Then, they were incubated for 3 h with Alexa Fluor 546 F (ab')2 fragment of goat anti-rat IgG and Alexa Fluor 488 F (ab')2 fragment of goat anti-rabbit IgG. The sections were observed under a confocal microscope (FV10i, Olympus, Tokyo, Japan).
Statistical analysis
All data are presented as means ± standard error (SE). Student's t-test was used for comparisons of two experimental groups, and Dunnett's test was used for multiple group comparisons. Stat View software version 5.0 (SAS Institute Inc., Cary, NC, USA) was used, and P<0.05 was considered statistically significant.
Author Contributions
Y.S., K.M., K.T., M.N., M.S. and H.H. designed the experiments. Y.S. and Y.N. performed the experiments. T.T. and S.Y. contributed the clinical samples. Y.S. performed the analysis and wrote the paper. All authors contributed to the editing of paper and to scientific discussion.
Supplementary Material
Supplementary Information
References
- Lloyd-Jones D. et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 119, 480–486 (2009). [DOI] [PubMed] [Google Scholar]
- Zivin J. A., Fisher M., DeGirolami U., Hemenway C. C. & Stashak J. A. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science. 230, 1289–1292 (1985). [DOI] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 333, 1581–1587 (1955). [DOI] [PubMed] [Google Scholar]
- Hacke W. et al. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N Engl J Med. 359, 1317–1329 (2008). [DOI] [PubMed] [Google Scholar]
- Berislav V. Zlokovic. Neurodegeneration and the neurovascular unit. Nat Med. 16, 1370–1371 (2010). [DOI] [PubMed] [Google Scholar]
- Costantino, Iadecola. & Josef, Anrather. The immunology of stroke: from mechanisms to translation. Nat Med. 17, 796–808 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulafia D. P. et al. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 29, 534–544 (2009). [DOI] [PubMed] [Google Scholar]
- Jung J. E., Kim G. S. & Chan P. H. Neuroprotection by interleukin-6 is mediated by signal transducer and activator of transcription 3 and antioxidative signaling in ischemic stroke. Stroke. 42, 3574–3579 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S. C. et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 104, 13798–13803 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichita T. et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med. 18, 911–917 (2012). [DOI] [PubMed] [Google Scholar]
- Poltorak A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282, 2085–2088 (1988). [DOI] [PubMed] [Google Scholar]
- Park J. S. et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 290, 917–924 (2006). [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T. B. & Gringhuis S. I. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 9, 465–479 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T. & Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 11, 373–384 (2010). [DOI] [PubMed] [Google Scholar]
- Fritz J. H., Ferrero R. L., Philpott D. J. & Girardin S. E. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 7, 1250–1257 (2006). [DOI] [PubMed] [Google Scholar]
- Satoh T. et al. “LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses”. Proc Natl Acad Sci U S A. 107, 1512–1517 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey M. B., Lanier L. L. & Nakamura M. C. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol Rev. 208, 50–65 (2005). [DOI] [PubMed] [Google Scholar]
- Wells C. A. et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J immunol. 180, 7404–7413 (2008). [DOI] [PubMed] [Google Scholar]
- Yamasaki S. et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 9, 1179–1188 (2008). [DOI] [PubMed] [Google Scholar]
- Connolly E. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 97, 209–216 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi M. et al. Role for matrix metalloproteinase 9 after cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 20, 1681–1689 (2000). [DOI] [PubMed] [Google Scholar]
- Mishiro K. et al. A broad-spectrum matrix metalloproteinase inhibitor prevents hemorrhagic complications induced by tissue plasminogen activator in mice. Neuroscience. 205, 39–48 (2012). [DOI] [PubMed] [Google Scholar]
- Matsumoto M. et al. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol. 163, 5039–5048 (1999). [PubMed] [Google Scholar]
- Robinson M. J. et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 206, 2037–2051 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison S. E. & Brown G. D. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 13, 817–822 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyakkoku K. et al. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 171, 258–267 (2010). [DOI] [PubMed] [Google Scholar]
- Shi Y. et al. Protein-tyrosine kinase Syk is required for pathogen engulfment in complement-mediated phagocytosis. Blood. 107, 4554–4562 (2006). [DOI] [PubMed] [Google Scholar]
- Manzanero S., Hsieh Y. H., Gelderblom M., Wells C. & Arumugam T. V. The immune receptor Mincle mediates ischemic stroke-induced injury. Stroke 2012 Conference 7, 19–19 (2012). [Google Scholar]
- Suzuki Y. et al. Pharmacological inhibition of TLR4-NOX4 signal protects against neuronal death in transient focal ischemia. Sci Rep. 2, 896 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamuk O. N. et al. Spleen tyrosine kinase inhibition prevents tissue damage after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 299, 391–399 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka F. et al. Toll-Like Receptor 4 Mediates Retinal Ischemia Reperfusion Injury Through Nuclear Factor-κB and Spleen Tyrosine Kinase Activation. IOVS. 54, 5807–5816 (2013). [DOI] [PubMed] [Google Scholar]
- Connolly E. S. Jr. et al. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 97, 209–216 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyttaris V. C. Kinase inhibitors: a new class of antirheumatic drugs. Drug Des Devel Ther. 6, 245–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H. et al. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci U S A. 94, 2007–2012 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information