Abstract
Neuropeptide Y (NPY) and NPY receptors are widely expressed in various organs and cell types and have been shown to have pleiotropic functions. However, their presence or role in human embryonic stem cells (hESCs) remains unknown. We now show that undifferentiated hESCs primarily express NPY and its Y1 and Y5 receptors. Inhibition of NPY signalling using either the selective NPY Y1 or Y5 receptor antagonist reduces the maintenance of self-renewal and proliferation of undifferentiated hESCs. We also provide compelling evidence that exogenous NPY supports the long-term growth of undifferentiated hESCs in the absence of feeder cell factors using only knockout serum replacement media. Further, NPY facilitates the use of chemically defined medium made up of N2/B27 supplement and basic fibroblast growth factor (bFGF) for hESC feeder-free culture. Our results indicate that both Y1 and Y5 receptors appear to be involved in the NPY-mediated activation of AKT/protein kinase B and extracellular signal-regulated kinase 1/2 (ERK1/2) in hESCs. Notably, only Y1 receptor, but not Y5 receptor, is responsible for the NPY-induced activation of cAMP-response element binding (CREB) in hESCs. These results provide the first evidence that NPY and its Y1 and Y5 receptors have potential role in maintaining hESC self-renewal and pluripotency. We demonstrate the underlying importance of NPY signalling and its usefulness in the development of a defined and xeno-free culture condition for the large-scale propagation of undifferentiated hESCs.
Keywords: human embryonic stem cells, self-renewal, NPY, NPY Y1 receptor, NPY Y5 receptor
Introduction
Human embryonic stem cells (hESCs) are characterized by their capacity for self-renewal and their potential to generate almost all cell types. Thus, they are considered to be a promising and unlimited source of cellular transplantation and tissue engineering. However, the development of a serum-free, xeno-free medium that supports the derivation, large-scale propagation and long-term culture of hESCs is still one of the major hurdles for their clinical utility. Therefore, understanding the molecular mechanisms that control hESC self-renewal, and the identification of molecules and factors in this self-renewal, is crucial. Various molecules have been studied to develop defined culture conditions for hESCs, but so far the role of neuropeptide Y (NPY) signalling through G-protein-coupled receptors (GPCRs) has not been investigated.
NPY, one of the most abundant neuropeptides in the brain, is a highly conserved 36-residue peptide that is structurally similar to peptide YY and pancreatic polypeptide [1, 2]. The proteolytic processing of proNPY, the 69-amino acid precursor protein, leads to the mature secreted form of NPY. The amino acid sequence of mature human and mouse NPY are identical, while porcine NPY (leucine at position 17) and human NPY (methionine at position 17) differ in a single amino acid; however, this difference does not seem to affect the biological activity of NPY [3].
The growth factor properties of NPY were demonstrated in various cell types including neurons [4–6], endothelial cells [7, 8], vascular smooth muscle cells [9–11] and adipocyte precursor cells [12]. The mitotic response to NPY varies according to the type of tissue and different signalling pathways that are linked to multiple NPY receptors. NPY exerts its effects by binding to six NPY receptors (designated Y1 to Y6), which belong to the GPCR superfamily [13]. Mammals have five subtypes of NPY receptors, Y1, Y2, Y4, Y5 and Y6 (a pseudogene in human beings), and NPY preferentially binds to Y1, Y2 and Y5. The NPY receptor is coupled to G-proteins (Gi/o) that mediate intracellular calcium mobilization and the inhibition of adenylate cyclase and 3′,5′-cyclic adenosine monophosphate (cAMP) production, which leads to mitogen-activated protein kinase (MAPK) activation [6, 14–16].
In this study, we demonstrate that NPY supports the undifferentiated proliferation and long-term self-renewal of two-independent hESC lines, H9 and HUES-7, under feeder-free culture condition with or without knockout serum replacement (KSR). We also demonstrate that the effects of NPY on hESCs are mediated by the NPY Y1 and Y5 receptors and involve the activation of AKT, ERK1/2 and CREB signalling.
Materials and methods
Reagents
Human NPY (Tyr-Pro-Ser-Lys-Pro-Asp-Asn-Pro-Gly-Glu-Asp-Ala-Pro-Ala-Glu-Asp-Leu-Ala-Arg-Tyr-Tyr-Ser-Ala-Leu-Arg-His-Tyr-Ile-Asn-Leu-Ile-Thr-Arg-Gln-Arg-Tyr-NH2; Sigma, St. Louis, MO, USA), two random-sequence control peptides (SC-10; N-WFGDVNQKPI-C, SC-36; N-SNSRPPRKNDVNTMADAYKFLQDLDTYYGDRAR VRF-C) (PepTron, Daejon, Korea), and selective agonists for NPY Y1 ([Leu31, Pro34]-NPY) and NPY Y5 receptor ([cPP1–7, NPY19–23, Ala31, Aib32, Gln34]– human pancreatic polypeptide [hPP]) (Tocris Bioscience, Ellisville, MO, USA) were dissolved as stock solution in phosphate-buffered saline (PBS) to a final concentration of 3 μM, stored as aliquot in the deep freezer (–70°C) and thawed once. Prior to use, they were diluted with culture medium to yield the desired final concentrations (0.005–3 μM). Peptides were added to culture dish on a daily basis unless otherwise indicated.
The selective NPY receptor antagonists BIBP3226 (Y1; Tocris Bioscience) and L152804 (Y5; Tocris Bioscience), AKT inhibitor (Calbiochem; La Jolla, CA, USA), ERK1/2 inhibitor (U0126) (Calbiochem) and PKA inhibitor (H89) (Calbiochem) were dissolved in dimethyl sulphoxide (DMSO) to a final concentration of 10 μM, and stored as aliquot in the deep-freezer (–70°C). Prior to use, they were diluted with culture medium to yield the desired concentrations (3–10 mM). Control cultures were incubated with 0.1% (v/v) DMSO alone or PBS. In our experiments, the DMSO concentration in all test media did not exceed 0.1%. NPY antagonists and kinase inhibitors were added to culture dish on a daily basis unless otherwise indicated. Peptides and antagonists used in this study are listed in Table S1.
hESC culture
Two hESC lines H9 (NIH Code, WA09; WiCell Research Institute, Madison, WI, USA) and HUES-7 (Harvard University, Cambridge, MA, USA) were routinely maintained as described previously [17]. For feeder-free culture, hESCs were grown on plates coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) in mouse embryonic fibroblast (MEF)-conditioned medium (MEF-CM) or unconditioned medium (UM) with or without human NPY. This cultured hESCs were passaged once per week following collagenase IV (1 mg/ml; Invitrogen, Carlsbad, CA, USA) or dispase (1 mg/ml; Invitrogen) treatment. The MEF-CM was prepared using γ-irradiated MEFs as previously described [18], and the MEF-CM was supplemented with 8 ng/ml bFGF. UM contains 80% DMEM/F12, 20% KSR, 1% non-essential amino acid (NEAA), 1 μM L-glutamine, 0.1 μM β-mercaptoethanol (Sigma) and 4 ng/ml bFGF. For feeder- and serum-free culture, hESCs were grown in N2/B27-based medium [19] containing DMEM/F12, 1 × N2/B27 (Invitrogen), 1% NEAA, 1 mM L-glutamine, 0.1 mM β-mercaptoethanol and 20 ng/ml bFGF with or without NPY and transforming growth factor (TGF)-β (R&D Systems, Minneapolis, MN, USA). Culture medium was changed daily. NPY, NPY antagonists and agonists and kinase inhibitors were added every day to fresh culture medium unless otherwise indicated.
Alkaline phosphatase staining and activity assay
Staining for alkaline phosphatase (ALP) was performed with a commercially available ALP kit according to the manufacturer’s instruction (Sigma). Images of ALP+ cells were recorded by using a HP Scanjet G4010. The bight field images were also obtained using an Olympus microscope (IX51, Olympus, Tokyo, Japan). ALP activity was determined using an ALP assay kit (Sigma) according to the manufacturer’s instructions. Aliquots of lysate (100 μg) were used for the assay. The ALP activity was determined by using a FLUOStar OPTIMA microtitre plate reader (BMG Labtech GmbH, Offenburg, Germany) at 405 nm wavelength.
Flow cytometry
hESCs were harvested and analysed for the expression of stage-specific embryonic antigen-4 (SSEA-4) and ALP by flow cytometry (Supporting Information ‘Materials and methods’).
Karyotype analysis
hESCs cultured in UM supplemented with 0.5 μM NPY (NPY medium) for 15 passages were processed for G-banding by GenDix (Seoul, Korea). Representative images were taken using ChIPS-Karyo (Chromosome Image Processing System, GenDix).
Embryoid body differentiation
To examine the potential of hESC differentiation, human embryoid bodies (hEBs) were prepared by culturing hESCs in hEB culture medium (DMEM/F12 containing 10% serum replacement (SR)) in suspension using non-tissue culture-treated Petri dishes. After 5 days of growth in suspension, EBs were transferred to gelatinecoated plates and cultured in hEB culture medium. Under these conditions, cells attached to the bottom of the plates, where they were left to differentiate for an additional 15 days, changing the medium as required.
BrdU incorporation
hESCs were grown on matrigel–coated 4-well LabTec chamber slides for 4 days for the 5-bromo-2-deoxyuridine (BrdU; BD Pharmingen, San Diego, CA, USA) incorporation assays (Supporting Information ‘Materials and methods’).
Growth efficiency test
hESCs grown for 7 days were dissociated into uniform sized, square clumps (100 × 100 μm; approximately 70–90 cells per clump) using a Mcllwain tissue chopper [20]. The same number of hESC clumps (approximately 5500 to 7000 clumps per dish) was seeded evenly on a 35 mm culture dish coated with Matrigel in a final volume of 2 ml. The plated cells were allowed to grow for 6 days under described culture conditions. To determine the number of cells, the cells were washed with PBS and trypsinized. The cell suspension was mixed with a 0.4% (wt/vol) trypan blue solution, and the number of live cells was determined using a haemocytometer. Each trial was performed in triplicate.
Results
hESCs and MEFs express NPY and NPY receptors
Consistent with a meta-analysis of 38 different array experiments [21] (http://amazonia.montp.inserm.fr/), the expression of the NPY, NPY1R and NPY5R transcripts were primarily detected in undifferentiated hESCs (H9 and HUES-7), while the mRNAs encoding Y2 and Y4 were not detected (Fig. 1A). The expression of the mRNAs encoding NPY, Y1, Y2 and Y5 were confirmed in MEF feeders using RT-PCR (Fig. 1A). However, the NPY4R transcript was undetectable in MEFs. A sequence analysis confirmed that the sequence of the RT-PCR products was 100% identical to the published human NPY, NPY1R and NPY5R receptor sequences (data not shown). The expression of the mature NPY proteins in undifferentiated hESCs and MEFs was further evaluated by immunocytochemical analysis. The NPY proteins were found in both the nuclei and cytoplasm of undifferentiated hESCs and MEFs (Fig. 1B). To test whether the expression of NPY and its receptors are influenced by the differentiation status of the hESCs, the relative mRNA expression level of NPY and its receptors were compared between undifferentiated hESCs, retinoic acid (RA)-differentiated hESCs and differentiating hEBs using qRT-PCR. hESCs were triggered to differentiate by treatment with 1 μM RA for 5 days. The differentiation of the hESCs was confirmed by the down-regulation of the hESC-specific markers OCT4 and NANOG (Fig. 1C). The mRNA expression of NPY, NPY1R and NPY5R was altered upon hESC differentiation. Compared to undifferentiated hESCs, the expression of both the NPY and NPY1R mRNAs was down-regulated in RA-differentiated hESCs, but their expression was increased in differentiating hEBs. We also observed that the expression of the NPY5R receptor was down-regulated both in RA-differentiated hESCs and differentiating hEBs to a different extent (Fig. 1C). Similar results were obtained from two independent hESC lines. These results indicate that changes in the level of NPY and/or its receptor may be associated with the different differentiation stages of hESCs.
Fig 1.
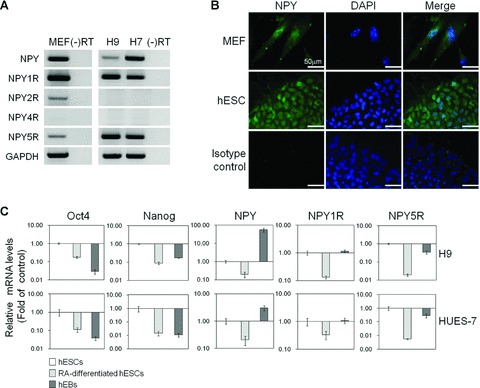
Expression of NPY and its receptor subtypes on hESCs and MEF feeder cells. (A) Semi-quantitative RT-PCR analysis of mRNA expression for NPY, NPY1R, NPY2R, NPY4R and NPY5R in hESCs (H9 and HES-7) and MEFs. GAPDH was used as a loading control. (B) Immunofluorescent analysis of NPY protein expression in H9 cells and MEFs. Cell nuclei were counterstained with DAPI. Bar π 50 μm. (C) Real-time qRT-PCR analysis of mRNA expression of NPY, NPY1R and NPY5R in undifferentiated and differentiated H9 hESCs (RA-differentiated hESCs and differentiating hEBs). The results are displayed as the relative mRNA level with the level in undifferentiated hESCs cultured in MEF-CM referred set to 1 and are presented as the mean ± S.E. (n= 3).
Exogenous NPY supports the maintenance of undifferentiated hESCs and the selective inhibition and activation of NPY Y1 and Y5 receptors affects the undifferentiated state
We next determined whether activation or inhibition of NPY signalling in hESCs influences on hESC self-renewal and pluripotency. In general, hESCs were maintained in an undifferentiated state either on feeders or feeder-free conditions with daily change of fresh medium, otherwise they underwent differentiation. Based on general hESC feeder-free culture condition, we added 0–3 μM NPY to fresh culture medium on a daily basis and monitored the changes in the morphology of the hESCs and the expression of hESC-specific markers.
The hESCs cultured in UM for 5–7 days lost their undifferentiated state and underwent the early-phases of hESC differentiation, as indicated by morphological changes (Fig. 2A) and the down-regulation of hESC markers including ALP, OCT4, SOX2, human telomerase reverse transcriptase (hTERT), teratocarcinoma-derived growth factor (TDGF) and SSEA-4 (Fig. 2B and C). In contrast, hESCs cultured in UM plus NPY (> 0.1 μM) significantly maintained their undifferentiated state after one and two consecutive passages, as characterized by the typical hESC morphology (Fig. 2A) and the positive expression of the hESC markers ALP, SOX2, hTERT and SSEA-4 (Fig. 2B and C). The addition of 0.5 μM NPY to UM (NPY medium) effectively supported the maintenance of undifferentiated hESCs, almost to a similar extent as hESCs cultured in MEF-CM (CM-hESCs) or UM plus high amounts of bFGF (≥40 ng/ml) while PBS control had no effects (Fig. 2A–C). However, hESCs cultured in UM plus a low concentration of NPY (≤0.1 μM) failed to retain their undifferentiated state after more than one passage. We also tested two different random-sequence peptides (SC-10 [10 amino acids] and SC-36 [36 amino-acids]) as specificity controls of NPY treatments and observed that they had no supportive effects on hESC cultures, confirmed by morphological changes, ALP staining and the expression of the hESC markers ALP, SOX2, hTERT and SSEA-4 (Figs 2A, B and S1B).
Fig 2.
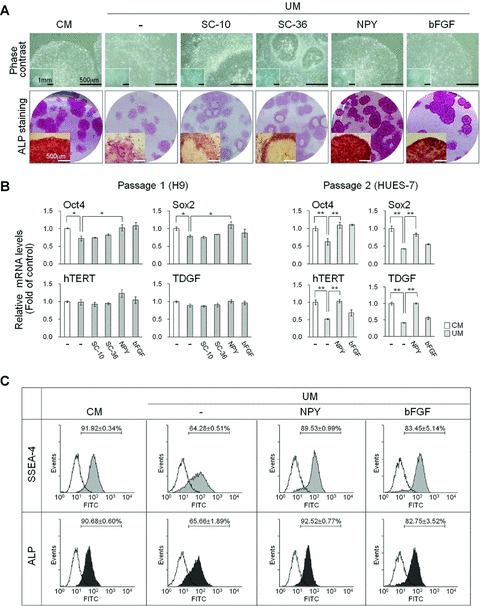
Feeder-free hESC culture in NPY medium. H9 hESCs were maintained in MEF-CM or UM containing either PBS (control; -), 0.5 μM scrambled control SC-10, 0.5 μM scrambled control SC-36, 0.5 μM NPY or 40 ng/ml bFGF for three passages. Peptides were added to fresh medium daily. (A) Representative morphology of H9 hESCs cultured under various medium conditions as indicated. Upper panels: phase contrast images. Bar = 500 μm or 1 μm (inset images). Lower panels: scanned images of 35 mm round culture dishes and inverted macroscopic images were acquired after ALP staining. Bar = 500 mm (inset images). (B) Real-time qRT-PCR analysis for the expression of OCT4, SOX2, hTERT and TDGF. H9 and HES-7 hESCs were cultured using the indicated media for one or two passages. The results are displayed as the relative mRNA level with the level detected in undifferentiated hESCs cultured in CM set to 1 and are presented as the mean ± S.E. **P < 0.01, *P < 0.05, by t-test. (C) FACS analysis of SSEA-4 and ALP expression on H9 hESCs. Representative plots following flow cytometry from three independent experiments are shown.
We further tested whether of the effects of NPY on the maintenance of undifferentiated hESCs are mediated through the NPY1R and/or NPY5R, which are primarily expressed on hESCs, using a selective Y1 and Y5 antagonists and agonists. In the presence of either Y1 (BIBP3226; 3 μM) or Y5 antagonist (L152804; 3 μM), hESCs cultured in NPY medium lost their self-renewal capacity and underwent differentiation within 4 days, as confirmed by morphological changes (Fig. 3A) and the diminished expression of hESC markers (Fig. 3A and B). Y1 or Y5 antagonist alone had no notable effects compared to DMSO controls (Fig. S1B). NPY antagonists (3 μM) had no cytotoxic effects in hESCs as determined by cell counting Kit-8 (CCK-8) (Fig. S1A). Consistently, continuous exposure to either Y1 or Y5 receptor selective peptide agonist markedly blocked differentiation and growth inhibition of hESCs cultured in UM under feeder-free condition, confirmed by hESC morphology, hESC-specific marker expression and BrdU staining (Figs 3C and 4). Differentiated hESC colonies determined by morphological assessment were significantly lower in hESCs cultured with UM plus Y1 or Y5 agonist compared to PBS control groups. Combined treatment with Y1 and Y5 agonist showed a better effect of blocking the differentiation of hESC cultured in UM and showed higher ALP activity than single treatment groups. These results indicate that NPY Y1 and Y5 receptors are both involved in NPY-mediated maintenance of hESCs in an undifferentiated state.
Fig 3.
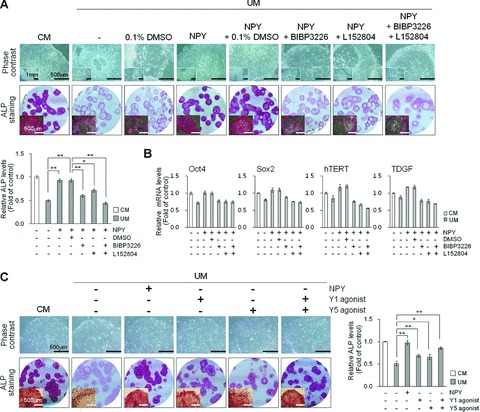
Effect of selective NPY Y1 and Y5 receptor antagonists and agonists on hESC cultures. (A and B) Effects of combined Y1 and Y5 antagonists on hESC cultures. H9 hESCs were cultured for 5 days under feeder-free conditions using CM or UM containing 0.5 mM NPY, 3 mM Y1 receptor antagonist (BIBP3226), or/and 3 μM Y5 receptor antagonist (L152804) in comparison with drug vehicle control (0.1% DMSO) or no treatment as indicated. Peptides and antagonists were added to fresh medium daily. Upper panels: representative phase contrast and ALP-stained images of H9 hESCs. Bar = 500 μm or 1 mm (inset images; upper row). Scanned images of 35 mm round culture dishes and macroscopic images were acquired after ALP staining. Bar = 500 mm (inset images; bottom row). Lower panels: relative ALP activity of the cell lysate measured at 405 nm. (B) Real-time qRT-PCR analysis for the expression of OCT4, SOX2, hTERT and TDGF. The results are displayed as the relative mRNA level with the level detected in undifferentiated hESCs cultured in MEF-CM set to 1 and are presented as the mean ± S.E. (n= 3). (C) Effects of combined Y1 and Y5 agonists on hESC cultures. H9 hESCs were cultured for 5 days under feeder-free conditions using CM or UM containing 0.5 μM NPY, 0.5 μM Y1 receptor agonist ([Leu31, Pro34]-NPY), or/and 0.5 μM Y5 receptor agonist ([cPP1–7, NPY19–23, Ala31, Aib32, Gln34]–hPP) in comparison with drug vehicle control (PBS) as indicated. Peptides and antagonists were added to fresh medium daily. Left panels: representative phase contrast and ALP-stained images of H9 hESCs. Bar = 500 μm (upper row). Scanned images of 35 mm round culture dishes and macroscopic images were acquired after ALP staining. Bar = 500 μm (inset images; bottom row). Right panels: relative ALP activity of the cell lysate measured at 405 nm. Relative ALP activity results are expressed as fold increase in ALP level as compared with that of MEF-CM. The data are presented as the mean ± S.E. (n= 3). **P < 0.01, *P < 0.05, by t-test.
Fig 4.
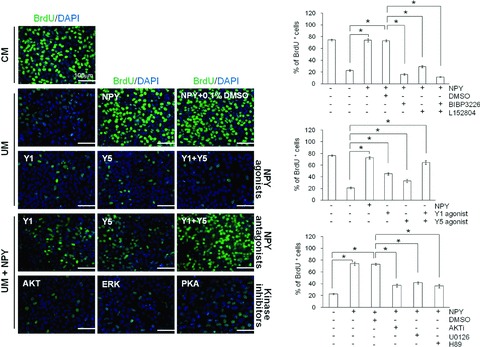
Effect of NPY on hESC proliferation. H9 hESCs were cultured for 4 days under feeder-free conditions using CM or UM containing 0.5 μM NPY, 0.5 μM NPY agonists, 3 μM NPY antagonists (BIBP3226 or L152804), 10 μM kinase inhibitors (AKT inhibitor, ERK1/2 inhibitor (U0126), or PKA inhibitor (H89)) in comparison with drug vehicle control (0.1% DMSO) or no treatment as indicated. Peptides and antagonists were added to fresh medium daily. The proliferation rate of H9 hESCs cultured using the indicated media was measured using BrdU incorporation. Left panels: representative images of BrdU+ cells. Bar = 100 mm. Right panels: quantification of BrdU+ cells. The relative number of BrdU+ cells per field of vision was quantified and is presented as the percentage of the total number of cells counted. The data are presented as the mean ± S.E. (n= 3). *P < 0.01, by t-test.
Proliferation of undifferentiated hESCs maintained in NPY medium
To better understand the role of NPY on hESCs, we tested the direct effect of NPY on hESC proliferation by assessing BrdU incorporation. hESCs cultured in UM, which lost their undifferentiated state, displayed a lower rate of BrdU incorporation (24.5 ± 2.3%) when compared with undifferentiated hESCs cultured in MEF-CM (72.4 ± 2.9%; Fig. 4). The addition of 0.5 μM NPY to the UM resulted in a significant increase in the BrdU incorporation by the hESCs, reaching levels comparable to hESCs cultured in MEF-CM (Fig. 4). No significant difference was seen in the number of BrdU+ cells between hESCs cultured in NPY medium (73.4 ± 2.9%) and hESCs cultured in MEF-CM (72.4 ± 2.9%; Fig. 4). We further evaluated the contribution of the NPY1R and NPY5R in NPY-mediated hESC proliferation using their selective antagonists BIBP3226 and L152804. As expected, the number of BrdU+ cells was significantly lower in NPY medium with 3 μM BIBP3226 (16.3 ± 1.7%) or 3 μM L152804 (29.1 ± 1.8%) than in NPY medium alone (73.4 ± 2.9%; Fig. 4A). The addition of Y1 and Y5 antagonists together had an additive effect on the suppression of hESC proliferation (11.7 ± 0.6% BrdU+ cells).
Involvement of Y1 and Y5 receptors in the regulation of hESC proliferation by NPY was also determined by using the selective Y1 and Y5 agonists. Y1 (45.0 ± 2.1%) or Y5 agonist-treated cells (33.1 ± 2.6%) showed a marked increase in the BrdU+ cells compared to PBS control groups (21.1 ± 1.7%) (Fig. 4). Moreover, combined treatment of Y1 and Y5 agonist (61.9 ± 3.9%) showed higher percentage of BrdU+ cells than single treatment groups, but failed to fully reverse to the same extent as NPY treatment (72.8 ± 2.0%) (Fig. 4). These results indicate that the use of NPY medium in the feeder-free culture of hESCs effectively maintains undifferentiated hESC proliferation, and the effect of NPY on hESC proliferation appears to be mediated by the activation of both the Y1 and Y5 receptors.
Increasing data have suggested that the proliferative effects of NPY are regulated by cross-talk among multiple signalling pathways including PI3K/AKT, MAPK and PKA [11, 12]. Therefore, we further explored whether PI3K/AKT, MAPK/ERK1/2 and PKA signalling is involved in mediating the proliferative effect of NPY on hESCs using pharmacological inhibitors of the respective signalling cascades. As shown in Fig. 4, the inhibitors of AKT (AKTi), ERK1/2 (U0126) and PKA (H89) were potent in suppressing NPY-stimulated BrdU incorporation. These results suggest that the proliferative effect of NPY on hESCs is mediated through the Y1 and Y5 receptors that trigger multiple intracellular signalling pathways including AKT, ERK1/2 and PKA.
Exogenous NPY supports long-term, feeder-free culture of undifferentiated hESCs
We subsequently evaluated whether NPY supports the long-term, continuous culture of undifferentiated hESCs without feeder cells. Two different hESC lines were maintained in an undifferentiated state in NPY medium (UM supplemented with 0.5 μM NPY) without feeders for more than 15 passages over 4 months, as confirmed by the normal expression of hESC markers (Fig. 5A) and a normal karyotype (Fig. 5B). hESCs cultured in NPY medium (NPY-hESCs) had the same growth rate during the cultivation period as hESCs cultured in MEF-CM.
Fig 5.
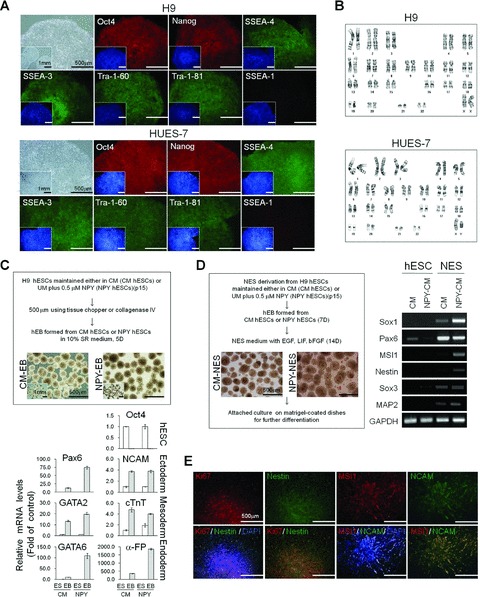
Long-term, feeder-free hESC culture in NPY medium. (A) Morphology of a representative hESC colony and immunohistochemical analysis of OCT4, NANOG, SSEA-4, SSEA-3, TRA-1–60 and TRA-1–80. H9 and HES-7 cells were cultured in NPY medium for 15 passages. Nuclei were visualized by DAPI staining (insets). Bar = 500 mm or 1 mm (inset images). (B) Karyotype analysis of hESCs cultured in NPY medium for 15 passages. (C) hEB formation and differentiation. hEBs were derived from H9 cells cultured in CM or NPY medium for 15 passages. Upper panel; representative images of hEBs. Bar = 500 mm or 1 mm (inset images). Lower panel; real-time RT-PCR analysis for the expression of OCT4 and differentiation markers characteristic of the three germ layers; ectoderm (PAX6 and NCAM), mesoderm (GATA2 and cTnT) and endoderm (α-FP and GATA6). The data are presented as the mean ± S.E. of three independent experiments. (D) NESs derived from hESCs. Left panel; representative images of NESs. Right panel; RT-PCR analysis of neural stem cell marker expression in NESs. (E) Representative images of NESs stained either for Ki67 (cell proliferation marker), nestin, MSI1 and/or NCAM. Bar = 500 mm.
The pluripotency of the NPY-hESCs was confirmed by evaluating their differentiation capacity. hESCs cultured in NPY medium for 15 passages were harvested and plated on non-tissue culture-treated Petri dishes to form hEBs in suspension culture (Fig. 5C). After 5 days in suspension, the hEBs were transferred to gelatine-coated dishes and cultured for 15 additional days for further differentiation. Quantitative RT-PCR demonstrated that the markers specific for ectoderm (PAX6, NCAM), mesoderms (GATA2, cTnT) and endoderm (GATA6, α-FP) were highly expressed in NPY-hESC-derived hEBs (NPY-hEB), which was similar to the CM-hESC-derived hEBs (CM-hEBs; Fig. 5C). A marked reduction in the expression of the hESC marker OCT4 was observed in both CM-hEBs and NPY-hEBs (Fig. 5C). These results indicate that the hESCs maintained in NPY medium retain the potential to form derivatives of all three embryonic germ layers and are pluripotent.
We further tested whether directed differentiation of hESCs cultured in NPY medium (NPY-hESCs) into the neuroectodermal lineage could be induced. NPY-hEBs were continuously cultivated in neuroectodermal sphere (NES)-culture medium. After 7–10 days of incubation, the hEBs displayed morphological changes and a rosette structure, a characteristic of neural stem cells (Fig. 5D). Their neural identity was confirmed by the expression of neural precursor markers, such as SOX1, PAX6, MSI1, NES, SOX3 and MAP2, using semi-quantitative RT-PCR (Fig. 5D). The majority of the differentiating NESs expressed the proliferation marker Ki67 and the neural precursor markers, NES, MSI1 and NCAM (Fig. 5E). These results indicate that hESCs cultured in NPY medium can be successfully differentiated into neural cells and are pluripotent.
A novel chemically defined medium with NPY supplement supports the maintenance of undifferentiated hESCs
The defined N2/B27 supplement, bFGF and TGF-β1 have been used to replace KSR and to develop a chemically defined medium for hESC culture [19, 22–24]. We further optimized the serum- and feeder-free culture conditions based on combinations of N2/B27, bFGF and TGF-β1 with or without addition of NPY. In our conditions, hESCs cultured in N2/B27-based medium containing 20 ng/ml bFGF (N2/B27 medium) failed to maintain an undifferentiated state, proliferated less and differentiated to a greater extent. However, the addition of 1 mM NPY to N2/B27 medium containing 20 ng/ml bFGF clearly improved the undifferentiated colony morphology, growth efficiency and expression of the hESC markers in hESCs (Fig. S2C and D). Under such conditions, hESCs retained their undifferentiated state for more than six passages, appeared similar way to hESCs cultured in MEF-CM in terms of cell morphology, maintained normal hESC marker expression (Fig. S2A) and retained a normal karyotype (Fig. S2B).
Defined N2/B27 medium containing 1 μM NPY, but not TGF-β1, was able to support undifferentiated hESC expansion for 6–10 passages; however, addition of 1 ng/ml TGF-β1 to defined N2/B27 medium containing 1 μM NPY was required for long-term maintenance of undifferentiated hESCs (≥15 passages) (Fig. S3A and B). The optimum and minimum concentration of NPY required for hESC culture was largely dependent upon the medium composition, but its effect on hESC self-renewal was dose dependent at a range of 0.005–3 mM (data not shown). Our results suggest that NPY functions as a positive regulator of hESC self-renewal and that the use of NPY in the culture medium supports the long-term culture of hESCs without feeder cells and serum.
NPY activates multiple pathways including AKT, ERK1/2, PKA and CREB in hESCs
In an attempt to elucidate how NPY regulates hESC self-renewal and proliferation, we evaluated the AKT, ERK1/2, PKA and CREB signal transduction cascades by Western blot using phospho-specific antibodies. In response to NPY, a transient increase in the level of phospho-AKT (pAKT) and phospho-ERK (pERK) in hESCs was observed within 1 min., while the total expression of AKT and ERK was not changed compared to PBS control groups (Fig. 6A). The phosphorylation of AKT peaked 0.5 min. after the application of 1 μM NPY and this activation decreased to the basal level by 60 min. The phosphorylation of ERK1/2 peaked 5 min. after the addition of NPY and dropped to the basal level by 30 min. (Fig. 6A). In contrast, both the total and phosphorylated p38 and c-Jun N-terminal kinase (JNK) protein levels remained stable following NPY addition (data not shown).
Fig 6.
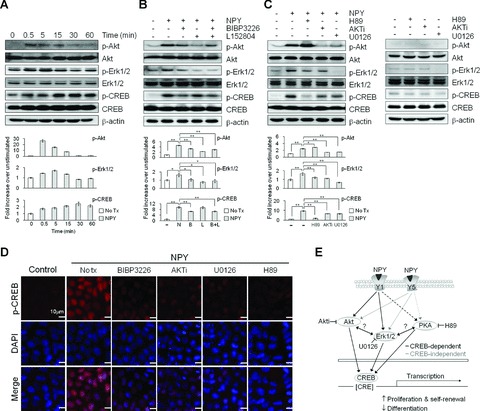
Effect of NPY on intracellular signalling in hESCs. (A) Western blot of H9 cells treated with 1 μM NPY (N) for the indicated times. (B) Western blot of H9 cells treated with 1 μM NPY for 5 min. with or without a 1 hr pre-treatment with 3 μM BIBP3226 (B) or 3 μM L152804 (L). (C) Western blot of H9 cells treated with 1 μMNPY for 5 min. with or without a 1 hr pre-treatment with 10 μM AKTi, 10 μM U0126 or 10 μM H89 (upper panel), and kinase inhibitors only (lower panel). The membranes were probed with the indicated antibodies, and β-actin was used as a loading control. For protein quantification, the blots were scanned, and the bands were quantified using densitometry (A–C, lower panels). The data are presented as the mean ± S.E. (n= 3). **P < 0.01, *P < 0.05, by t-test. (D) Immunohistochemical analysis of hESCs treated with 1 μM NPY for 5 min. with or without a 1 hr pre-treatment with 3 μM BIBP3226, 10 μM AKTi, 10 μM U0126 or 10 μM H89. (E) Simplified schematic of possible intracellular signalling pathways activated by NPY and the Y1 and Y5 receptors in hESCs.
NPY-mediated AKT and ERK activation was significantly reduced by a 30 min. pre-treatment with an Y1 or Y5 receptor antagonist compared to NPY treatments alone. However, co-treatment with the Y1 and Y5 antagonists showed no additive or synergistic effects (Fig. 6B). The NPY-mediated AKT activation was inhibited by pre-treatment of with an AKT or ERK1/2 inhibitor, but not with a PKA inhibitor. In contrast, the NPY-mediated ERK activation was blocked by all three inhibitors (Fig. 6C). We further investigated whether CREB signalling is involved in the NPY-mediated signal transduction by determining the expression level of CREB and phospho-CREB (p-CREB). In response to 1 μM NPY, hESCs exhibited a rapid increase of p-CREB in 15 min. without a significant change in the total CREB protein level (Fig. 6A). The p-CREB was predominantly localized to the nucleus in NPY-treated hESCs (Fig. 6D). The pre-treatment with the Y1 antagonist, but not the Y5 antagonist, partially blocked the NPY-mediated up-regulation of p-CREB (Fig. 6B and D). Furthermore, pre-treatment of hESCs with inhibitors specific for AKT, ERK1/2 or PKA blocked the NPY-mediated CREB phosphorylation; however, their ability to inhibit CREB phosphorylation appeared to be incomplete (Fig. 6C). Without NPY treatment, kinase inhibitors alone had no notable effects on the expression levels of AKT, ERK and CREB proteins compared to DMSO control groups. PKA, AKT, or ERK1/2 inhibitor induced the decrease in basal levels of p-CREB compared to DMSO control groups. Under our condition, ERK1/2 inhibitor alone induced decrease in basal levels of p-ERK1/2 compared to 0.1% DMSO control groups (Fig. 6C). These data suggest that NPY-mediated CREB activation is mediated through AKT, ERK1/2 and PKA. Our results indicate that NPY mediates its effects through the Y1 and Y5 receptors in hESCs and that these effects are tightly associated with the concerted activation of the AKT, ERK1/2, PKA and CREB pathways. The cross-talk between these signalling pathways may be important in the NPY-mediated regulation of hESC function.
Discussion
The present data demonstrate that supplementary NPY supports the long-term, feeder-free culture of undifferentiated hESCs both in the presence and absence of serum in vitro. The data obtained with various antagonists indicate that not only did NPY stimulate hESC self-renewal, it also caused an increase in cell proliferation through activation of the NPY Y1 and Y5 receptors.
The potential roles of NPY and its receptors on hESCs were demonstrated by their expression in hESCs. Significant amounts of NPY mRNA and protein are present in undifferentiated hESCs, and among the NPY receptor subtypes, the NPY Y1 and Y5 receptors appear to be expressed, but the NPY2R and NPY4R mRNAs were not detected (Fig. 1A and B). Therefore, NPY most likely mediates its biological effects by interacting with the Y1 and Y5 receptors in hESCs. NPY has shown to possess a virtually identical affinity for both the Y1 and Y5 receptors [25, 26]. Changes in the mRNA expression of NPY, NPY1R and NPY5R receptor mRNA expression level upon hESC differentiation were observed, suggesting that the expression levels appear to be influenced by the differentiation state of the hESC s (Fig. 1C). NPY is highly conserved, and the sequence of human and mouse NPY is identical [2]. Of note, we were able to detect the NPY, NPY1R, NPY2R and NPY5R mRNAs in MEF feeder cells, but failed to detect the NPY4R mRNA. Using the commercially available NPY ELISA kit (Phoenix Pharmaceuticals, Belmont, CA, USA), we were able to detect a very low amount of NPY in culture medium of hESCs cultured with MEF feeders and MEF-CM at concentrations of 80 ± 2 pg/ml and 60 ± 2 pg/ml, respectively, but we failed to detect NPY in UM (data not shown). This indicates that NPY derived from hESCs and MEFs could modulate hESC functions by activating the Y1 and Y5 receptors expressed on hESCs via an autocrine/paracrine loop.
Although a role for NPY in hESCs has not been previously demonstrated, reports have suggested that its expression could be modulated by neurotrophins, including nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, neurotrophin-4/5 [27] and bFGF [28–30], all of which have been shown to affect hESC self-renewal and survival [31–33]. Additionally, we have confirmed that while hESCs cultured in UM, which lacks MEF-derived factors, undergo differentiation, this differentiation is attenuated by the addition of neurotrophins (data not shown). Furthermore, we found that hESCs cultured in UM containing neurotrophins had a significantly higher mRNA level of NPY than hESCs cultured in UM (data not shown). A direct or modulatory role of NPY receptors on cellular proliferation and differentiation have been demonstrated in a number of cell types including neural precursor/progenitor cells [4, 34–36]. Decressac et al. showed that neuroproliferative effects observed in neural precursors was mediated by the NPY Y1 receptor in the mice sub-ventricular zone [34]. Neural-like differentiation of bone marrow derived from mesenchymal stem cells mediated by amyloid b was related to NPY Y1 receptor via phosphatidylinositol-3-OH kinase-dependent activation of the MAPK/ERK1/2 [37].
Those observations led us to speculate that NPY may act as growth factor to support hESC self-renewal. To address this, we examined a feeder-free hESC culture system with or without the addition of exogenous NPY to the culture medium by varying NPY supplementation frequency (one to three times per day or week) and concentrations (0.005–3 mM). We determined that NPY levels in culture medium were rapidly depleted by 23.6% within a day compared to zero time measurements (Fig. S3C). Supportive effect of NPY on the maintenance of undifferentiated hESCs maintained with UM was supplementation frequency-dependent and dose-dependent (Fig. S3A and B). Under our condition, a daily addition of 0.5 μM NPY to hESC cultures with UM was sufficient to maintain the properties of the undifferentiated hESCs. We have successfully maintained undifferentiated hESCs in NPY medium for more than 4 months without feeder cell-derived factors. The long-term expanded hESCs in NPY medium retain a stable normal karyotype and the potential to differentiate into multiple lineages.
Substantially higher concentrations of bFGF in defined N2/B27-based medium were required for the maintenance of undifferentiated hESCs for serial passages and expansion without other factors [22, 38, 39]. It is widely accepted that exogenous bFGF and the FGF signalling pathway are required, but not sufficient, for the maintenance of hESC self-renewal and pluripotency when used in a serum-free, chemically defined medium [22, 40–42]. In our defined conditions, the addition of 1 mM NPY to N2/B27 medium containing low concentration of bFGF (20 ng/ml) clearly minimized the spontaneous differentiation of hESCs and was sufficient to maintain hESCs in an undifferentiated state for 6–10 passages. Combinatorial treatment with NPY and TGF-β1 tend to be more efficient to achieve relatively long-term maintenance of undifferentiated hESCs over 15 passages (Fig. S2A and B).
The addition of exogenous NPY to hESC culture likely inhibits or attenuates the differentiation of hESCs and also induced undifferentiated proliferation of hESCs (Fig. 4). Requirement of both Y1 and Y5 receptor signalling on hESC maintenance was determined by using the selective NPY receptor antagonist and agonists. Similar to NPY, both NPY Y1 and Y5 agonist treatment had a supportive effect on the maintenance of undifferentiated hESCs cultured with UM (Fig. 3C). Addition of either Y1 or Y5 agonists to UM markedly blocked hESC differentiation as confirmed by hESC morphology, and the expression of hESC marker. Combined treatments of Y1 and Y5 agonists produced the additive effects (Fig. 3C). In correlation, Y1 and Y5 antagonists fully blocked the NPY’s supportive effects on the maintenance of undifferentiated hESCs (Fig. 3A). However, we cannot rule out a possibility that other NPY receptors may contribute to these responses because agonists used in these studies could not fully replace the NPY’s actions in hESCs under our experimental conditions.
Our results supports that the supportive effect of NPY on hESC maintenance is mediated through the Y1 and Y5 receptors that activate the AKT, ERK1/2 and PKA intracellular signalling pathways (Figs 4 and 6). The importance of these pathways on NPY-mediated signalling was examined by determining the activation status of the signalling components. NPY directly activates AKT, ERK1/2, PKA and CREB, as determined by the rapid increase of AKT, ERK1/2, PKA and CREB phosphorylation (Fig. 6A). Both the NPY Y1 and Y5 receptor antagonists prevented NPY-induced activation of AKT and ERK1/2 (Fig. 6B), suggesting possible interactions between the NPY receptors and the intracellular signalling pathways. More importantly, the NPY-mediated activation of the NPY Y1 and Y5 receptors appears to be associated with the AKT and ERK1/2 signalling pathways. However, these NPY receptors seem to trigger different downstream events. Activation of CREB by NPY appears to be NPY Y1 receptor dependent, while it is independent of NPY Y5 receptor (Fig. 6E). Furthermore, inhibitors of AKT, ERK1/2 and PKA partially blocked the NPY-mediated increase in CREB phosphorylation (Fig. 6C). These results suggest that the NPY-mediated activation of CREB in hESCs is AKT, ERK and PKA-dependent (Fig. 6E). Because the AKT, ERK1/2 and PKA inhibitors did not completely attenuate the NPY-mediated activation of CREB, cross-talk between the pathways in regulating NPY-mediated hESC self-renewal is highly probable (Fig. 6E). Based on our observations, CREB is phosphorylated after activation of the PI3K/AKT and MAPK pathway by NPY. This phosphorylation, in turn, can lead to the regulation of genes that contain CREs, which are important for hESC function.
It has been demonstrated that NPY signalling interacts with other known signalling elements, which is important for the regulation of hESC self-renewal and pluripotency. Interaction between bFGF and NPY has been shown by confirming the bFGF-mediated increase of NPY production in neural cells foetal brain cells and dorsal root ganglion neurons [43, 44]. Platelet-derived growth factor (PDGF) promotes intracellular S1P signalling by activating sphingosine kinase, which in turn converts sphingosine to S1P. Pebay et al. showed that extracellular S1P and PDGF blocked spontaneous differentiation of hESCs under serum-free culture condition [45]. In correlation, we determined that activation of NPY signalling differentially modulated the mRNA expression levels of key components of bFGF, TGF-β/bone morphogenetic protein (BMP) and PDGF signalling pathways (Fig. S4). These results suggest that interaction of NPY and other signalling pathways including bFGF, TGF-β/BMP and PDGF may be required for their function on the regulation of hESC states. However, future efforts should elucidate how these signalling cascades may regulate one another and contribute to NPY signalling and define the intermediate steps involved in discrete contexts.
Inactivation of NPY receptor signalling stimulated the hESC differentiation as confirmed by morphological changes and down-regulation of hESC marker expression (Fig. S5). Thus, we also determined the differentiation potential of hESCs induced by inactivation of NPY receptor signalling by examining the mRNA expression levels of marker genes for the three germ layers including the ectoderm (Pax6 and GFAP), endoderm (Amylase and Albumin) and mesoderm (GATA2 and Tbx20). All three germ layer markers were detected at variable levels (1.2–21.1 fold) in differentiated hESCs induced by Y1 or/and Y5 antagonists, suggesting that those cells were composed of a heterogeneous mixture of cells from different lineages at different levels. Such patterns were also observed in differentiated hESCs by bFGF-depleted condition (Fig. S5).
In conclusion, NPY supports hESC proliferation and self-renewal. We have demonstrated that NPY directly activates Akt, ERK1/2 and the CREB pathway through the NPY Y1 and Y5 receptors, and that this is ultimately coupled to the control of hESC proliferation and self-renewal. Our results suggest that NPY could be a potent and widely effective supplement for hESC culture. Further studies examining the mode of action of NPY on hESCs will further our understanding of the signalling pathways required for hESC growth and maintenance and allow for the development of a defined media for the culture of hESCs without xeno-contamination for clinical use.
Acknowledgments
We thank Dr. Hyung Min Chung for kindly providing CF1 mouse for MEF preparation. This work was supported by the MEST/KOSEF Stem Cell Research Program (2010-0020272) and the KRIBB/KRCF Research Initiative Program.
Supporting Information
(A) Cytotoxicity assay of NPY and NPYantagonists using CCK-8. H9 hESCs were cultivated in CM with orwithout BIBP3226 or L152804 (0.5–80 mM) and their cytotoxicactivity were determined using a cell counting kit-8 (CCK-8) asdescribed in Supporting Information ‘Materials andmethods’ at different times as indicated. The data arepresented as the mean 6 S.E. (n 5 3). (B) Effects of NPYantagonists or kinase inhibitors alone on hESC culture. H9 hESCswere cultured in CM or UM containing 3 mM NPY antagonists((BIBP3226 or L152804) or 10 mM kinase inhibitors (AKT[AKTi], PKA [H89] or ERK1/2inhibitor [U0126]) for 5 days in comparison withdrug vehicle control (0.1% DMSO) or no treatment. NPYantagonists and kinase inhibitors were added to fresh medium daily.Upper panels: Representative phase contrast and ALP-stained imagesof H9 hESCs. Bar 5 500 mm. Lower panels: Scanned images of 35 mmround culture dishes and macroscopic images were acquired after ALPstaining. Bar 5 500 mm (inset images). (C) Effects ofpeptide scramble control, NPY antagonists or kinase inhibitorsalone on hESC proliferation. HESCs were cultivated in CM or UMcontaining 0.5 mM scrambled peptide control (SC-36), 3 mM NPYantagonists (BIBP3226 or L152804), 10 mM kinase inhibitors (AKT[AKTi], PKA [H89] or ERK1/2inhibitor [U0126]) for 5 days in comparison withdrug vehicle control (0.1% DMSO) or no treatment. Theproliferation rate of H9 hESCs cultured using the indicated mediawas measured using BrdU incorporation. Left panels: Representativeimages of BrdU1 cells. Bar 5 100 mm. Right panels:Quantification of BrdU1 cells. The relative number ofBrdU1 cells per field of vision was quantified and is presented as the percentage of the total number of cells counted. The data are presented as the mean 6 S.E. (n 5 3).
Feeder-free, serum-free culture of hESCs inNPY-N2/B27 medium. (A) Morphology of a representative hESCcolony and immunohistochemical analysis of OCT4, NANOG, SSEA-1,SSEA-3, SSEA-4, TRA-1–60 and TRA-1–80. H9 cells weremaintained in serum-free, feeder-free NPY N2/B27 medium containing13DMEM/F12, 13N2/B27, 1 mM NPY and 20 ng/ml bFGF for six passages.Nuclei were visualized with DAPI staining. Bar 5 500 mm or 1 mm(inset images). (B) Karyotype analysis of H9 cells culturedin NPY N2/B27 medium for six passages. (C) Comparison of thegrowth efficiency of H9 cells cultured using the indicated mediafor 6 days by measuring the cell number as described in‘Materials and methods’. The data are presented as themean 6 S.E. (n 5 3). **P, 0.01, *P, 0.02,by t-test. (D) Comparison of different hESC cultureconditions. hESCs cultured in different media were evaluated basedon morphology, ALP staining and growth efficiency.
(A) Dose-dependent and (B)supplementation frequency-dependent effects of NPY on hESCcultures. H9 hESCs were cultured either in MEF-CM or UM containingNPY (0–0.1 mM) for one passage. NPY was added to freshculture medium one (13) to two times (23) per day as indicated.(A) Representative morphology of H9 hESCs. Phase contrastimages. Bar 5 500 mm. (B) Real-time qRT-PCR analysis for theexpression of OCT4, SOX2, hTERT and TDGF. The results are displayedas the relative mRNA level with the level detected inundifferentiated hESCs cultured in CM set to 1 and are presented asthe mean 6 S.E. (C) Relative amount of NPY in culturemedium. H9 hESCs were maintained in UM containing 0.5 mM NPYwithout medium change. The supernatant of culture medium of H9hESCs were collected at indicated times (0–72 hrs) and NPYlevels were measured using NPY ELISA kit as described in SupportingInformation ‘Materials and methods’. The amount of NPYat each of the times was calculated relative to that of zero timewhich was taken as 100. Statistically significant change comparedwith the zero time control. **P, 0.01, *P,0.05, by t-test.
Expression changes of genes associated with FGF, PDGF and BMP/TGFb signalling pathway after NPY treatment in hESCs. H9 hESCs were cultured in CM or UM with or without 0.5 mM NPY for one passage. NPY was added to fresh culture medium daily. Expression levels of the indicated genes were analysed by semi-quantitative RT-PCR. GAPDH expression was used as internal control.
Expression pattern of differentiation makers in hESCs treated with NPY antagonists. H9 hESCs were cultured in CM, CM without bFGF (CM-bF), CM with 3 mM Y1 antagonist (CM 1 B), CM with 3 mM Y5 antagonist (CM1L), CM with both Y1 and Y5 antagonists (CM 1 BL), or UM for one passage. Expression levels of the indicated genes were analysed by real-time PCR. GAPDH expression was used as internal control. Results are expressed as a fold difference in gene expression relative to control (undifferentiated hESCs cultured with CM). The data are presented as the mean 6 S.E. of three independent experiments Primers for hESC markers (OCT4 and NANOG) and differentiation makers including ectoderm (PAX6 and GFAP), mesoderm (GATA2 and Tbx20) and endoderm (Amylase and Albumin) markers were listed in Table S2.
List of peptides and antagonists used in this study
List of primers used in this study
References
- 1.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y–a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–60. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 2.Tatemoto K. Neuropeptide Y: complete amino acid sequence of the brain peptide. Proc Natl Acad Sci USA. 1982;79:5485–9. doi: 10.1073/pnas.79.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corder R, Emson PC, Lowry PJ. Purification and characterization of human neuropeptide Y from adrenal-medullary phaeochromocytoma tissue. Biochem J. 1984;219:699–706. doi: 10.1042/bj2190699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanic D, Paratcha G, Ledda F, et al. Peptidergic influences on proliferation, migration, and placement of neural progenitors in the adult mouse forebrain. Proc Natl Acad Sci USA. 2008;105:3610–5. doi: 10.1073/pnas.0712303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howell OW, Doyle K, Goodman JH, et al. Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus. J Neurochem. 2005;93:560–70. doi: 10.1111/j.1471-4159.2005.03057.x. [DOI] [PubMed] [Google Scholar]
- 6.Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–4. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- 7.Movafagh S, Hobson JP, Spiegel S, et al. Neuropeptide Y induces migration, proliferation, and tube formation of endothelial cells bimodally via Y1, Y2, and Y5 receptors. FASEB J. 2006;20:1924–6. doi: 10.1096/fj.05-4770fje. [DOI] [PubMed] [Google Scholar]
- 8.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, et al. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–95. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
- 9.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Fisher TA, et al. Mechanisms of vascular growth-promoting effects of neuropeptide Y: role of its inducible receptors. Regul Pept. 1998:75–76. doi: 10.1016/s0167-0115(98)00073-1. 231–8. [DOI] [PubMed] [Google Scholar]
- 10.Erlinge D, Brunkwall J, Edvinsson L. Neuropeptide Y stimulates proliferation of human vascular smooth muscle cells: cooperation with noradrenaline and ATP. Regul Pept. 1994;50:259–65. doi: 10.1016/0167-0115(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 11.Pons J, Kitlinska J, Jacques D, et al. Interactions of multiple signaling pathways in neuropeptide Y-mediated bimodal vascular smooth muscle cell growth. Can J Physiol Pharmacol. 2008;86:438–48. doi: 10.1139/y08-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang K, Guan H, Arany E, et al. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J. 2008;22:2452–64. doi: 10.1096/fj.07-100735. [DOI] [PubMed] [Google Scholar]
- 13.Inui A. Neuropeptide Y feeding receptors: are multiple subtypes involved? Trends Pharmacol Sci. 1999;20:43–6. doi: 10.1016/s0165-6147(99)01303-6. [DOI] [PubMed] [Google Scholar]
- 14.Nie M, Selbie LA. Neuropeptide Y Y1 and Y2 receptor-mediated stimulation of mitogen-activated protein kinase activity. Regul Pept. 1998:75–76. doi: 10.1016/s0167-0115(98)00070-6. 207–13. [DOI] [PubMed] [Google Scholar]
- 15.Pellieux C, Sauthier T, Domenighetti A, et al. Neuropeptide Y (NPY) potentiates phenylephrine-induced mitogen-activated protein kinase activation in primary cardiomyocytes via NPY Y5 receptors. Proc Natl Acad Sci USA. 2000;97:1595–600. doi: 10.1073/pnas.030533197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li AJ, Ritter S. Functional expression of neuropeptide Y receptors in human neuroblastoma cells. Regul Pept. 2005;129:119–24. doi: 10.1016/j.regpep.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Kim MS, Kim J, Han HW, et al. Microfabricated embryonic stem cell divider for large-scale propagation of human embryonic stem cells. Lab Chip. 2007;7:513–5. doi: 10.1039/b617760n. [DOI] [PubMed] [Google Scholar]
- 18.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–4. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 19.Yao S, Chen S, Clark J, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci USA. 2006;103:6907–12. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joannides A, Fiore-Heriche C, Westmore K, et al. Automated mechanical passaging: a novel and efficient method for human embryonic stem cell expansion. Stem Cells. 2006;24:230–5. doi: 10.1634/stemcells.2005-0243. [DOI] [PubMed] [Google Scholar]
- 21.Assou S, Le Carrour T, Tondeur S, et al. A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells. 2007;25:961–73. doi: 10.1634/stemcells.2006-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Song Z, Zhao Y, et al. A novel chemical-defined medium with bFGF and N2B27 supplements supports undifferentiated growth in human embryonic stem cells. Biochem Biophys Res Commun. 2006;346:131–9. doi: 10.1016/j.bbrc.2006.05.086. [DOI] [PubMed] [Google Scholar]
- 23.Tseng SY, Nishimoto KP, Silk KM, et al. Generation of immunogenic dendritic cells from human embryonic stem cells without serum and feeder cells. Regener Med. 2009;4:513–26. doi: 10.2217/rme.09.25. [DOI] [PubMed] [Google Scholar]
- 24.Peerani R, Rao BM, Bauwens C, et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–55. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blomqvist AG, Herzog H. Y-receptor subtypes–how many more? Trends Neurosci. 1997;20:294–8. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- 26.Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38:189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Lindsay RM. Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: an overview. Philos Trans R Soc Lond. 1996;351:365–73. doi: 10.1098/rstb.1996.0030. [DOI] [PubMed] [Google Scholar]
- 28.Kerekes NA, Landry M, Lundmark K, et al. Effect of NGF, BDNF, bFGF, aFGF and cell density on NPY expression in cultured rat dorsal root ganglion neurones. J Auton Nerv Syst. 2000;81:128–38. doi: 10.1016/s0165-1838(00)00115-6. [DOI] [PubMed] [Google Scholar]
- 29.Allen JM, Martin JB, Heinrich G. Neuropeptide Y gene expression in PC12 cells and its regulation by nerve growth factor: a model for developmental regulation. Brain Res. 1987;427:39–43. doi: 10.1016/0169-328x(87)90042-8. [DOI] [PubMed] [Google Scholar]
- 30.Carnahan J, Nawa H. Regulation of neuropeptide expression in the brain by neurotrophins. Potential role in vivo. Mol Neurobiol. 1995;10:135–49. doi: 10.1007/BF02740672. [DOI] [PubMed] [Google Scholar]
- 31.Soh BS, Song CM, Vallier L, et al. Pleiotrophin enhances clonal growth and long-term expansion of human embryonic stem cells. Stem Cells. 2007;25:3029–37. doi: 10.1634/stemcells.2007-0372. [DOI] [PubMed] [Google Scholar]
- 32.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24:344–50. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 33.Xu RH, Peck RM, Li DS, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–90. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 34.Decressac M, Prestoz L, Veran J, et al. Neuropeptide Y stimulates proliferation, migration and differentiation of neural precursors from the subventricular zone in adult mice. Neurobiol Dis. 2009;34:441–9. doi: 10.1016/j.nbd.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Doyle KL, Karl T, Hort Y, et al. Y1 receptors are critical for the proliferation of adult mouse precursor cells in the olfactory neuroepithelium. J Neurochem. 2008;105:641–52. doi: 10.1111/j.1471-4159.2007.05188.x. [DOI] [PubMed] [Google Scholar]
- 36.Howell OW, Silva S, Scharfman HE, et al. Neuropeptide Y is important for basal and seizure-induced precursor cell proliferation in the hippocampus. Neurobiol Dis. 2007;26:174–88. doi: 10.1016/j.nbd.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Jin HK, Bae JS, Furuya S, et al. Amyloid beta-derived neuroplasticity in bone marrow-derived mesenchymal stem cells is mediated by NPY and 5-HT2B receptors via ERK1/2 signalling pathways. Cell Prolif. 2009;42:571–86. doi: 10.1111/j.1365-2184.2009.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu XQ, Graichen R, Soo SY, et al. Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation. 2008;76:958–70. doi: 10.1111/j.1432-0436.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 39.Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–7. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Gonzalo FR, Belmonte JC. Albumin-associated lipids regulate human embryonic stem cell self-renewal. PLoS ONE. 2008;3:e1384. doi: 10.1371/journal.pone.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Powell S, Brunette E, et al. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol Bioeng. 2005;91:688–98. doi: 10.1002/bit.20536. [DOI] [PubMed] [Google Scholar]
- 42.Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–21. doi: 10.1016/j.ydbio.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 43.Barnea A, Cho G. Basic fibroblast growth factor selectively amplifies the functional state of neurons producing neuropeptide Y but not somatostatin in cultures of fetal brain cells: evidence for a cooperative interaction with insulin-like growth factor-I. Endocrinology. 1993;133:1895–8. doi: 10.1210/endo.133.4.8104779. [DOI] [PubMed] [Google Scholar]
- 44.Kerekes N, Landry M, Lundmark K, et al. Effect of NGF, BDNF, bFGF, aFGF and cell density on NPY expression in cultured rat dorsal root ganglion neurones. J Auton Nerv Syst. 2000;81:128–38. doi: 10.1016/s0165-1838(00)00115-6. [DOI] [PubMed] [Google Scholar]
- 45.Pebay A, Wong RC, Pitson SM, et al. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells. 2005;23:1541–8. doi: 10.1634/stemcells.2004-0338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Cytotoxicity assay of NPY and NPYantagonists using CCK-8. H9 hESCs were cultivated in CM with orwithout BIBP3226 or L152804 (0.5–80 mM) and their cytotoxicactivity were determined using a cell counting kit-8 (CCK-8) asdescribed in Supporting Information ‘Materials andmethods’ at different times as indicated. The data arepresented as the mean 6 S.E. (n 5 3). (B) Effects of NPYantagonists or kinase inhibitors alone on hESC culture. H9 hESCswere cultured in CM or UM containing 3 mM NPY antagonists((BIBP3226 or L152804) or 10 mM kinase inhibitors (AKT[AKTi], PKA [H89] or ERK1/2inhibitor [U0126]) for 5 days in comparison withdrug vehicle control (0.1% DMSO) or no treatment. NPYantagonists and kinase inhibitors were added to fresh medium daily.Upper panels: Representative phase contrast and ALP-stained imagesof H9 hESCs. Bar 5 500 mm. Lower panels: Scanned images of 35 mmround culture dishes and macroscopic images were acquired after ALPstaining. Bar 5 500 mm (inset images). (C) Effects ofpeptide scramble control, NPY antagonists or kinase inhibitorsalone on hESC proliferation. HESCs were cultivated in CM or UMcontaining 0.5 mM scrambled peptide control (SC-36), 3 mM NPYantagonists (BIBP3226 or L152804), 10 mM kinase inhibitors (AKT[AKTi], PKA [H89] or ERK1/2inhibitor [U0126]) for 5 days in comparison withdrug vehicle control (0.1% DMSO) or no treatment. Theproliferation rate of H9 hESCs cultured using the indicated mediawas measured using BrdU incorporation. Left panels: Representativeimages of BrdU1 cells. Bar 5 100 mm. Right panels:Quantification of BrdU1 cells. The relative number ofBrdU1 cells per field of vision was quantified and is presented as the percentage of the total number of cells counted. The data are presented as the mean 6 S.E. (n 5 3).
Feeder-free, serum-free culture of hESCs inNPY-N2/B27 medium. (A) Morphology of a representative hESCcolony and immunohistochemical analysis of OCT4, NANOG, SSEA-1,SSEA-3, SSEA-4, TRA-1–60 and TRA-1–80. H9 cells weremaintained in serum-free, feeder-free NPY N2/B27 medium containing13DMEM/F12, 13N2/B27, 1 mM NPY and 20 ng/ml bFGF for six passages.Nuclei were visualized with DAPI staining. Bar 5 500 mm or 1 mm(inset images). (B) Karyotype analysis of H9 cells culturedin NPY N2/B27 medium for six passages. (C) Comparison of thegrowth efficiency of H9 cells cultured using the indicated mediafor 6 days by measuring the cell number as described in‘Materials and methods’. The data are presented as themean 6 S.E. (n 5 3). **P, 0.01, *P, 0.02,by t-test. (D) Comparison of different hESC cultureconditions. hESCs cultured in different media were evaluated basedon morphology, ALP staining and growth efficiency.
(A) Dose-dependent and (B)supplementation frequency-dependent effects of NPY on hESCcultures. H9 hESCs were cultured either in MEF-CM or UM containingNPY (0–0.1 mM) for one passage. NPY was added to freshculture medium one (13) to two times (23) per day as indicated.(A) Representative morphology of H9 hESCs. Phase contrastimages. Bar 5 500 mm. (B) Real-time qRT-PCR analysis for theexpression of OCT4, SOX2, hTERT and TDGF. The results are displayedas the relative mRNA level with the level detected inundifferentiated hESCs cultured in CM set to 1 and are presented asthe mean 6 S.E. (C) Relative amount of NPY in culturemedium. H9 hESCs were maintained in UM containing 0.5 mM NPYwithout medium change. The supernatant of culture medium of H9hESCs were collected at indicated times (0–72 hrs) and NPYlevels were measured using NPY ELISA kit as described in SupportingInformation ‘Materials and methods’. The amount of NPYat each of the times was calculated relative to that of zero timewhich was taken as 100. Statistically significant change comparedwith the zero time control. **P, 0.01, *P,0.05, by t-test.
Expression changes of genes associated with FGF, PDGF and BMP/TGFb signalling pathway after NPY treatment in hESCs. H9 hESCs were cultured in CM or UM with or without 0.5 mM NPY for one passage. NPY was added to fresh culture medium daily. Expression levels of the indicated genes were analysed by semi-quantitative RT-PCR. GAPDH expression was used as internal control.
Expression pattern of differentiation makers in hESCs treated with NPY antagonists. H9 hESCs were cultured in CM, CM without bFGF (CM-bF), CM with 3 mM Y1 antagonist (CM 1 B), CM with 3 mM Y5 antagonist (CM1L), CM with both Y1 and Y5 antagonists (CM 1 BL), or UM for one passage. Expression levels of the indicated genes were analysed by real-time PCR. GAPDH expression was used as internal control. Results are expressed as a fold difference in gene expression relative to control (undifferentiated hESCs cultured with CM). The data are presented as the mean 6 S.E. of three independent experiments Primers for hESC markers (OCT4 and NANOG) and differentiation makers including ectoderm (PAX6 and GFAP), mesoderm (GATA2 and Tbx20) and endoderm (Amylase and Albumin) markers were listed in Table S2.
List of peptides and antagonists used in this study
List of primers used in this study


