Abstract
The Gas6/Axl pathway regulates many cell functions and is implicated in hypertension. In this study we aimed to investigate the role of Axl in immune cells on initiation and progression of salt-dependent hypertension. Deoxycorticosterone-acetate (DOCA; 75mg/60days release) and salt hypertension was induced for 1week or 6weeks in Axl chimeras generated by bone marrow (BM) transplant to restrict Axl deficiency to hematopoietic or non-hematopoietic compartments. Depletion of Axl in hematopoietic cells (Axl−/− →Axl+/+) reduced (133±2mmHg) increase in systolic blood pressure (BP) compared to other Axl chimeras (~150mmHg) 1week after DOCA-salt. Urine protein and renal oxidative stress were lowest in Axl−/− →Axl+/+ at 1week after DOCA-salt. Compensatory increase in Gas6 in kidneys of recipient Axl−/− may affect kidney function and BP in early phase of hypertension. Flow cytometry on kidneys from Axl−/− →Axl+/+ showed increase in total leukocytes, B and dendritic cells and decrease in macrophages compared to Axl+/+ →Axl+/+. These immune changes were associated with decrease in pro-inflammatory gene expression, in particular interferon gamma. Systolic BP returned to baseline in Axl−/− →Axl+/+ and Axl−/− →Axl−/− but remained increased in Axl+/+ →Axl+/+ and Axl+/+ →Axl−/− chimeras after 6weeks of DOCA-salt. Vascular apoptosis was increased in the global Axl−/− chimeras in the late phase of hypertension. In summary, we found that expression of Axl in hematopoietic cells is critical for kidney pathology in early phase of salt-dependent hypertension. However, Axl in both hematopoietic and non-hematopoietic lineages contributes to the late phase of hypertension.
Keywords: Axl, hypertension, bone marrow transplant, immunity, DOCA-salt, kidney
Introduction
The immune system has been increasingly considered as an important player in the progression of hypertension with a major role for angiotensin II (AngII)-dependent pathways1. More experimental evidence indicates that subsets of T lymphocytes accumulate in the renal interstitial tissues and alter glomerular filtration rate leading to further elevation of blood pressure (BP)2. Consistent with these observations, inhibitory studies using immunosuppressant agents or genetic manipulation with T cells ameliorate BP elevation3, 4. However participation of other immune cells in the pathogenesis of salt-induced hypertension is not well understood. Axl is a receptor tyrosine kinase that belongs to a TAM family of tyrosine receptors including Tyro3 and Mertk5. Growth arrest–specific protein 6 (Gas6) is a common ligand for the TAM family 6. The Gas6/Axl pathway is highly regulated in chronic disorders such as cancer, autoimmunity and cardiovascular diseases7. Transcriptomic and genomic analyses of kidneys from Sabra rats showed that Axl is a candidate gene for salt-induced hypertension8. We9 and others10 confirmed in global knockout mice the critical role for the Gas6/Axl pathway in deoxycorticosterone-acetate (DOCA)-salt hypertension. Specifically, we showed that Axl affects vascular dysfunction and contributes to late phase of DOCA-salt hypertension9. In contrast, Gas6 is involved in kidney remodeling without any effect on BP levels in the same mouse model10. Leukocytes contribute to development of hypertension in the salt sensitive Sabra rat11. However, the role of Axl in the immune system in the development of hypertension is not clear. This study was aimed to investigate the role of Axl in hematopoietic (bone marrow-derived (BM) cells) vs. non-hematopoietic cells in the pathological processes during salt-dependent hypertension. Our results suggest that Axl plays distinct roles in immune cells at early vs. late phases of DOCA-salt hypertension.
Materials and Methods
Materials and Methods are available in the online-only Supplements
Results
Hypertension development in Axl chimeras
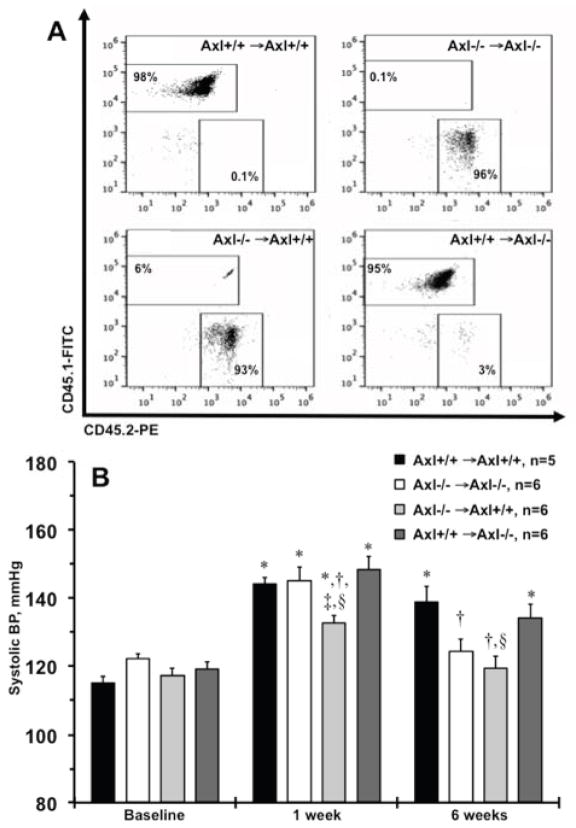
In our previous studies we showed that global deletion of the Axl gene protects from elevation of systolic BP at the late phase of DOCA-salt hypertension9. Axl is expressed in immune cells and is important for multiple functions12. To address the role of Axl in immune cells in the development of hypertension we generated Axl chimeras by bone marrow transplant (BMT). Representative flow cytometry plots of CD45.1+/CD45.2+ staining on peripheral blood confirmed successful generation of Axl chimeras 6weeks after BMT (Fig. 1A): Axl−/− →Axl+/+, Axl-deficient hematopoietic compartment; Axl+/+ →Axl−/−, Axl-deficient non-hematopoietic compartment and respective controls Axl+/+ →Axl+/+ and Axl−/− →Axl−/−. Baseline systolic BP was ~120mmHg and was similar among Axl chimeras (Fig. 1B). As we reported in global Axl−/− mice9, systolic BP rose significantly in Axl+/+ →Axl+/+ and Axl−/− →Axl−/− chimeras at the early phase (1week) of DOCA-salt (Fig. 1B). However, chimeric mice that lacked Axl only in hematopoietic cells (Axl−/− →Axl+/+) exhibited significantly lower systolic BP compared to all other chimeras at week 1 (Fig. 1B). As we reported in global Axl−/− mice9, systolic BP was significantly reduced in Axl−/− →Axl−/− compared to Axl+/+ →Axl+/+ chimeras at the late phase (6week) of DOCA-salt (Fig. 1B). Again, systolic BP was significantly lower in Axl−/− →Axl+/+ compared to Axl+/+ →Axl+/+ chimeras and was similar to that in Axl−/− →Axl−/− chimeras after 6weeks of DOCA-salt (Fig. 1B). Engraftment of wild type BM cells increased systolic BP in Axl+/+ →Axl−/− chimeras at week 6 compared to global deletion, Axl−/− →Axl−/− chimeras (Fig. 1B). Taken together our data suggest that Axl in the hematopoietic compartment is essential for initiation of early BP changes and also for the late maintenance of salt-dependent hypertension.
Figure 1.
Development of salt-dependent hypertension in Axl chimeras. A. Representative double staining CD45.1 (Axl+/+) and CD45.2 (Axl−/−) of peripheral blood from four Axl chimeric mice. Numbers inside quadrants shows engraftment (%) following 6weeks of bone marrow transplant. B. Systolic blood pressure (BP) across Axl chimeras at the baseline, after 1week and 6weeks of DOCA-salt. Black bars show Axl+/+ →Axl+/+. Open - Axl−/− →Axl−/−. Light gray - Axl−/− →Axl+/+. Dark gray - Axl+/+ →Axl−/−. Values are mean±SEM. *, p<0.05 vs. Baseline. †, p<0.05 vs. Axl+/+ →Axl+/+. ‡, p<0.05 vs. Axl−/− →Axl−/−. §, p<0.05 vs. Axl+/+ →Axl−/−. n, Number per group.
Kidney function in Axl chimeras in early phase of hypertension
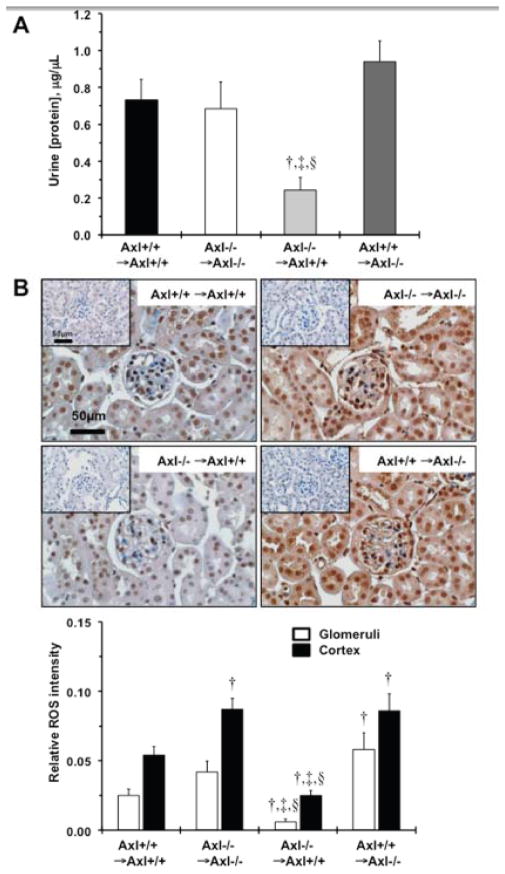
A central role for immune cells in an increase in oxidative stress has been shown in development of renal disease and elevation of BP3. Therefore, we examined kidney structure and function 1week after DOCA-salt. The absence of Axl in the hematopoietic compartment significantly attenuated the kidney dysfunction associated with DOCA-salt. We observed that the total concentration of protein in urine was significantly reduced (>3-fold) in the Axl−/− →Axl+/+ compared to other Axl chimeras after 1week of DOCA-salt (Fig. 2A). In addition, albumin levels in the urine tended to be lower (p=0.06) in this group (7.5±3.5μg/mL vs. ~15μg/mL). However, higher levels of reactive oxygen species (ROS) were noted in the glomeruli and cortex region (~2-fold) of the kidneys from Axl−/− →Axl−/− and Axl+/+ →Axl−/− compared to Axl+/+ →Axl+/+ chimeras (Fig. 2B). We found that relative ROS expression was significantly reduced in glomeruli (>5-fold) and the cortex (>3-fold) of the kidneys from Axl−/− →Axl+/+ chimeras (Fig. 2B). The latter observation suggests that the lack of Axl in kidneys leads to compensatory mechanisms that increase ROS production in early phase of hypertension. Given the known roles for Gas6 in kidney pathology10 we examined Gas6 and Axl levels in the kidneys from Axl chimeras (Fig. S1). We found that Axl expression was dramatically reduced in Axl−/− recipients: Axl−/− →Axl−/− and Axl+/+ →Axl−/− (Fig. S1A–B). However, Gas6 levels were slightly elevated in these chimeras after 1week of DOCA-salt (Fig. S1A vs. S1B). These findings suggest that Axl in hematopoietic cells contributes to early phases of hypertension probably via affecting kidney function that leads to the initial increase in systolic BP. Also, global deletion of Axl may result in an increase in renal Gas6 that might cause greater ROS production in the kidneys and a compensatory increase in BP.
Figure 2.
Kidney function in Axl chimeras 1week after DOCA-salt. A. Changes in total urine protein concentration, μg/μL. †, p<0.05 vs. Axl+/+ →Axl+/+. ‡, p<0.05 vs. Axl−/− →Axl−/−. §, p<0.05 vs. Axl+/+ →Axl−/−. B. Representative images and quantitative analysis represent the levels of ROS in kidneys from Axl chimeras. Positive cells are dark brown. Negative controls as shown as insets. Magnification bar is 50μm. Open bars represent relative expression of ROS in glomeruli. Black bars – ROS in cortical region. Values are mean±SEM. †, p<0.05 vs. Axl+/+ →Axl+/+. §, p<0.05 vs. Axl+/+ →Axl−/−. #, p<0.05 vs. Axl−/− →Axl+/+. n=10 per each group.
Characterization of immune changes in Axl chimeras
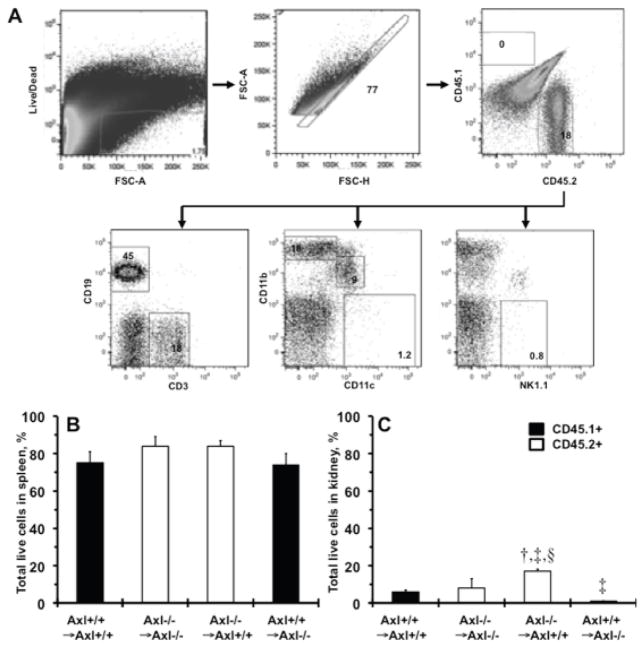
To determine how Axl may alter immune function we analyzed immune cell subsets in spleens and kidneys of Axl chimeras following 1week of DOCA-salt (Fig. 3). Analysis of the spleen provided the assessment of the immune changes in the chimeras before specific analysis of changes in the kidney. Total leukocytes (CD45.1+ vs. CD45.2+) in the spleens were not significantly different but tended to be slightly higher (p=0.07) in Axl−/− compared to Axl+/+ genotypes (Fig. 3B). These findings indicate that lack of Axl in the hematopoietic compartment does not affect immune cell re-population compared to Axl+/+ chimeras.
Figure 3.
Accumulation of donor bone marrow cells in Axl chimera’s 1week after DOCA-salt. A. Schematic representation of the gating strategy used to analyze the immune cell subsets in the spleen from Axl+/+ →Axl+/+ mice. B. Total live immune cells (%) in spleens. C. Total live immune cells (%) in kidneys. Black bars show Axl+/+ (CD45.1+). Open bars - Axl−/− (CD45.2+). Values are mean±SEM. †, p<0.05 vs. Axl+/+ →Axl+/+. ‡, p<0.05 vs. Axl−/− →Axl−/−. §, p<0.05 vs. Axl+/+ →Axl−/−. n=3 per each group.
Role of Axl in accumulation of immune cells in kidneys in early phase of hypertension
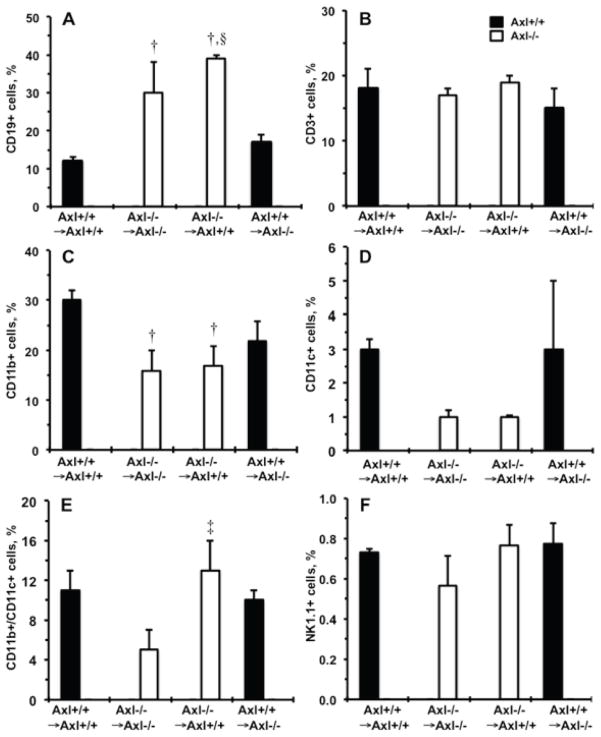
Expression of Axl dramatically affected accumulation of leukocytes in kidneys after 1week of DOCA-salt (Fig. 3C). Specifically, we found that Axl−/− →Axl+/+ mice had a significantly higher percentage of donor BM-derived cells compared to other Axl chimeras 1week after DOCA-salt (Fig. 3C). The percentage of CD19+ B cells was greater and CD11b+ macrophages were lower in Axl−/− →Axl−/− and Axl−/− →Axl+/+ compared to Axl+/+ →Axl+/+ chimeras (Fig. 4A,C). Interestingly, a double-positive (CD11b+/CD11c+) subset of dendritic cells was increased in the kidney only when Axl deficiency was restricted to the immune cells, Axl−/− →Axl+/+ vs. Axl−/− →Axl−/− mice (Fig. 4E). Finally, kidney populations of T cell, NK cells and mature dendritic cells (CD11c+) did not differ across Axl chimeras 1week after DOCA-salt (Fig. 4B,D,F). Taken together, these data suggest that expression of Axl in BM-derived cells affects the presence of populations of innate and adaptive immune cells and may determine kidney dysfunction during early phase of hypertension.
Figure 4.
Immune cell subsets in the kidneys from Axl chimeras 1week after DOCA-salt. Percentage of donor CD45+ shows: A. CD19+, B cells. B. CD3+, T cells. C. CD11b+, Macrophages. D. CD11c+, Dendritic cells. E. CD11b+/CD11c+ cells. F. NK1.1+, NK cells. Black bars show Axl+/+ cells. Open - Axl−/− cells. Values are mean±SEM. †, p<0.05 vs. Axl+/+ →Axl+/+. ‡, p<0.05 vs. Axl−/− → Axl−/−. §, p<0.05 vs. Axl+/+ →Axl−/−. n=3 per each group.
Cytokine and chemokine expression in kidneys from Axl chimeras
To gain insight into the potential mechanisms by which Axl regulates kidney inflammation we evaluated cytokine/chemokine and their receptors expression after 1week of DOCA-salt (Fig. 5, Table S1). We found that an equal number of genes were down- or up-regulated in the kidneys from Axl−/− →Axl−/− vs. Axl+/+ →Axl+/+ chimeras (Fig. 5A). However, there were more down-regulated genes in the kidneys from Axl−/− →Axl+/+ vs. Axl−/− →Axl−/− or Axl+/+ →Axl+/+ chimeras (Fig. 5B–C). We performed pathway analyses to dissect possible immune cell functions based on the lists of differentially expressed genes across Axl chimeras (Tables S2–S4). Evaluation of the up-regulated pathways showed no differences between global Axl−/− and Axl−/− →Axl+/+ chimeras (Table S2). We found a large number of common pathways down-regulated in Axl−/− →Axl+/+ than compared to Axl−/− →Axl−/− or Axl+/+ →Axl+/+ chimeras (Table S3). These pathways were also down-regulated in Axl−/− →Axl−/− vs. Axl+/+ →Axl+/+ chimeras. However, we identified 14 unique pathways, which were down-regulated in Axl−/− →Axl+/+ chimeras (Table S4). Importantly, down-regulation of four genes (interferon gamma (IFNγ), complement C3 (C3), interleukin 3 (Il3), CD40 ligand (CD40lg)) might explain the protective effects of Axl−/− in BM-derived cells on kidney dysfunction in early phase of hypertension (Table S4, Fig. 5B–C). Thus, we conclude that Axl expression is critical in immune cells for the up-regulation of several inflammatory pathways in the kidneys during the early phase of hypertension.
Figure 5.
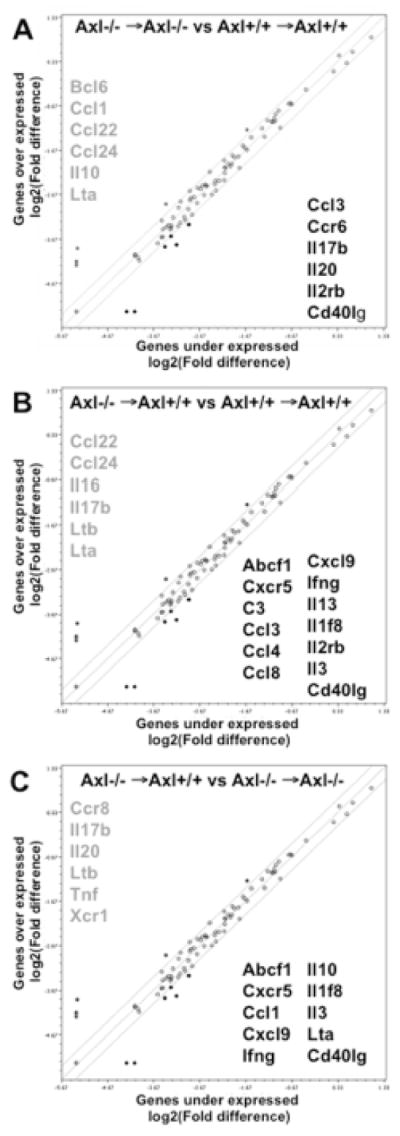
Cytokine and chemokine gene profiling in the kidneys from Axl chimeras 1week after DOCA-salt. A. Changes in Axl−/− →Axl−/− compared to Axl+/+ →Axl+/+ chimeras. B. Axl−/− →Axl+/+ compared to Axl+/+ →Axl+/+. C. Axl−/− →Axl+/+ compared to Axl−/− →Axl−/−. Individual genes are represented as dots on the scattered plot. The thinner lines show 2-fold cut offs. Up-regulated genes are listed in gray color, down-regulated – in black color.
Vascular changes in Axl chimeras during late phase of hypertension
Previously we showed that Axl−/− mice had lower systolic BP at 6weeks after DOCA-salt due to decrease in vascular remodeling via increase in vascular apoptosis9. Morphological evaluation of the arteries from Axl chimeras is shown in Table S5. Media area of thoracic aorta was significantly decreased in Axl−/− →Axl+/+ compared to Axl+/+ →Axl+/+ or Axl−/− →Axl−/− chimeras. Axl−/− →Axl+/+ and Axl−/− →Axl−/− mice exhibited lower values of media area compared to other chimeras (p=0.6–0.9) in the mesenteric artery (Table S5). The mesenteric artery remodeling index (media:lumen ratio) was significantly decreased in Axl−/− →Axl+/+ and Axl−/− →Axl−/− compared to Axl+/+ →Axl+/+ or Axl+/+ →Axl−/− chimeras (Fig. 6A). Despite these similarities in vascular remodeling between Axl−/− →Axl+/+ and Axl−/− →Axl−/− chimeras, relative numbers of apoptotic cells were significantly lower in the media from Axl−/− →Axl+/+ vs. Axl−/− →Axl−/− mice (Fig. 6B). These findings demonstrate an additional role of Axl in the non-hematopoietic compartment in the late phase of hypertension.
Figure 6.
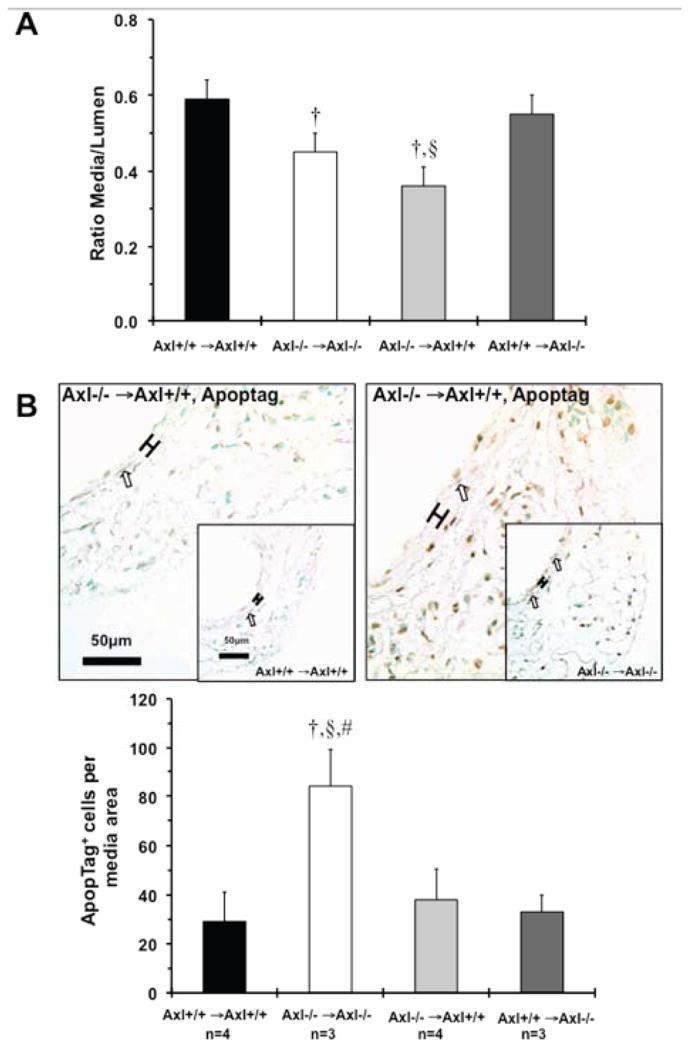
Vascular changes in Axl chimeras 6weeks after DOCA-salt. A. Mesenteric artery remodeling. B. Images and quantitative analyses of apoptotic cells in the media of mesenteric artery. Apoptotic cells stained in dark brown. Open arrows indicate positive cells. Brackets show area between internal and external elastic lamina. Magnification bar is 50μm. Values are mean±SEM. †, p<0.05 vs. Axl+/+ →Axl+/+. §, p<0.05 vs. Axl+/+ →Axl−/−. #, p<0.05 vs. Axl−/− →Axl+/+. n, Number per group.
Discussion
This is the first study that shows differences in immune-specific mechanisms controlled by Axl during early vs. late phases of salt-dependent hypertension. Here we report that the expression of Axl in the hematopoietic compartment is critical for initiation of DOCA-salt hypertension and for altered kidney function in the early phase of hypertension. We also found that global Axl−/− may lead to compensation of Gas6 in the kidneys that “mask” beneficial effect of Axl deletion during early phase of hypertension. Axl regulates the frequencies of immune cells, innate (macrophages and dendritic cells) and adaptive (B cells) during the early phase of DOCA-salt hypertension in the kidney. These immune cell changes are associated with altered kidney function and a change in inflammatory cytokines. Most importantly, expression of Axl is critical for up-regulation of the pro-inflammatory cytokine, IFNγ that regulates many immune pathways in the kidneys during early hypertension. Finally, expression of Axl in both, hematopoietic and non-compartment cells controls vascular changes and BP during late phase of DOCA-salt hypertension. Taken together, we uncovered a dual role of Axl in immune and non-immune cells in initiation and progression of salt-dependent hypertension (Fig. S2).
Genetic mapping studies in rat salt-sensitive models (Dahl and Sabra) have identified a number of blood pressure-related genes13. Axl is one of the candidate genes for salt-induced hypertension in the Sabra rat8. It was shown in mouse experiments that the Gas6/Axl pathway is critical for salt-dependent hypertension9, 10. Previously we confirmed a pathogenic role for Axl in DOCA-salt hypertension using global knockout mice9 where the lack of Axl reduced late phase of systolic BP elevation by decrease in vascular remodeling. In the present study the BP time-course and kidney remodeling in Axl−/− →Axl−/− chimeras was very similar to that in Axl−/− mice suggesting that the BMT procedure has no effect on progression of DOCA-salt hypertension in Axl mice. The Gas6/Axl pathway has been implicated in pathogenesis of several kidney diseases14. Proliferation of the mesangial cells is induced by Gas6 in the rat experimental model of glomerulonephritis15. Studies in knockout mice suggested that Gas6 plays a crucial role in the early stage of diabetic nephropathy16. It has been also shown that Gas6−/− mice had reduced kidney remodeling without any effect on systolic BP in DOCA-salt model10. We observed that the relative right kidney weight to body weight tended to be lower (p=0.06) in Axl−/− →Axl+/+ vs. Axl−/− →Axl−/− mice after 6weeks of DOCA-salt (data not shown). Our new findings in Axl chimeras might explain the phenotypic differences between Gas6−/− and Axl−/− mice in progression of salt-dependent hypertension. Up-regulation of Gas6 and Axl in the kidneys was evident in patients with chronic inflammatory renal diseases17. In vitro stimulation of vascular smooth muscle cells or immortalized human mesangial cells with AngII induced Gas6 and Axl expression via NADPH-oxidase17. A more recent clinical study18 demonstrated that circulating Gas6 is associated with renal disease severity and Gas6 levels were inversely correlated with kidney function in patients with end-stage renal disease. Likewise, in recipient Axl−/− chimeras the increases in kidney Gas6 mRNA levels showed greater ROS in kidneys during early phase of DOCA-salt. Thus, activation of the Gas6/Axl pathway is necessary in salt-dependent hypertension but might have distinct pathophysiological roles in the kidney vs. other tissues (e.g., arteries) and requires further clarification.
Over the past several years immune cells have been increasingly implicated in pathogenesis of salt-sensitive hypertension by altering kidney’s glomerular filtration2. Although it is recognized that inflammation in renal tissues is responsible for hypertension, the exact contribution of specific subsets of immune cells in hypertension is still unclear19. The majority of data emphasize the role of T lymphocytes in hypertension1. Seminal studies in RAG1−/− mice showed that lack of T cells prevented AngII or DOCA-salt hypertension4. Involvement of innate cells has also been indicated in DOCA-salt hypertension in rats20. Neutralization of polymorphonuclear leukocytes significantly reduced hypertension in Sabra rat11. Interestingly, we showed here that the balance of the monocyte/macrophage subsets appears to be altered in the absence of Axl. Thus, innate and adaptive immunity contributes to progression of salt-dependent hypertension.
The Gas6/TAM pathways are involved in differentiation and function of innate immune cells and are implicated in autoimmune disorders12. Conversely, we found an increase in the accumulation of B and dendritic cells with decreased macrophages in chimeras that lack Axl in BM-derived cells. These immune changes were coupled with reduction in systolic BP and proteinuria during the early phase of hypertension in Axl−/− →Axl+/+ chimeras. Further, Axl in the hematopoietic compartment regulates IFNγ in early hypertensive kidneys. IFNγ has been implicated in regulation of major interactions between the innate and adaptive immunity in AngII-induced cardiac remodeling21. Recent mouse studies documented the importance of cell specificity in IFNγ signaling on kidney injury after AngII infusion22, 23. Future investigations will be required to evaluate Axl-dependent mechanisms across immune cell populations in the kidneys during the early phase of salt-induced hypertension.
We further confirmed the importance of the Axl signaling in anti-apoptotic mechanisms in the arteries during the late phase of hypertension. Findings in Axl+/+ →Axl−/− and Axl−/− →Axl+/+ chimeras suggested that both, hematopoietic and non-compartment cells participate in late phase of DOCA-salt hypertension. Similar to the role of Axl in non-hematopoietic cells in carotid remodeling in response to low blood flow24, 25. We also found that Axl can affect immune activation of vascular cells by IFNγ25. In contrast to a recent report22 we found that Axl in immune cells regulates early DOCA-salt hypertension and kidney changes without any effect on the frequency of T lymphocytes, although we did not assess the function of the T cells that could be modified by the presence or absence of Axl. Taken together, our data suggest that initiation of salt-dependent hypertension depends on the distribution of innate and adaptive immune cells in the kidneys and is regulated by Axl. In addition, Axl-dependent interactions of immune cells with the vasculature are critical in the late phase of hypertension.
Perspective
Expression of Axl in the hematopoietic compartment affects accumulation of several subsets of immune cells and pro-inflammatory cytokines that determine kidney function during early phase of salt-dependent hypertension. These early changes in the kidney that have been revealed with Axl deletion only in the immune system suggested that some compensatory mechanisms must exist in the global Axl−/− mice, that might be linked to enhanced Gas6 expression. We provide new insights on immune-driven mechanisms during early vs. late phases of salt-dependent hypertension. Future studies will help to clarify the role of Axl in interactions among distinct immune cell types in salt-dependent hypertension.
Supplementary Material
Novelty and Significance.
What Is New?
Expression of Axl affects accumulation of macrophages, dendritic and B cells in the kidneys and determines pathogenesis of early phase of DOCA-salt hypertension
Expression of Axl in both, hematopoietic and non-hematopoietic compartments controls vascular changes during the late phase of DOCA-salt hypertension
Global Axl knockout leads to compensation of Gas6 in the kidneys that “mask” beneficial effect of Axl deletion during early phase of hypertension
What Is Relevant?
The Gas6/Axl pathway is critical in DOCA-salt hypertension
Axl is important for development and function of several subsets of the immune cells
Infiltration of immune cells are shown to alter kidney function in salt-dependent hypertension
Summary
In this study we demonstrated that lack of Axl only in hematopoietic compartment regulates the percentage of total immune cells, innate (macrophages and dendritic cells) and adaptive (B cells) in the kidney that reduced oxidative stress, pro-inflammatory cytokines and attenuated proteinuria and BP increases during the early phase of DOCA-salt. Interestingly, deletion of Axl in both immune and non-immune cells prevented late phase of DOCA-salt hypertension via vascular changes. Gas6 compensation in the kidney may explain early hypertension in global Axl knockouts. Collectively out data suggest that immune cell subsets are critical for renal pathology during the early phase of hypertension, while immune and non-immune cells are involved in the late phase of salt-dependent hypertension.
Acknowledgments
We would like to thank Michelle Zanche (Functional Genomics Core) for assistance with gene expression assays.
Sources of Funding
This study was supported by NIH grant HL105623 to V.A.K. and by NIAID A1072690 to D.J.F.
Footnotes
Conflict of interest disclosure
None.
References
- 1.Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Frontiers in physiology. 2012;3:128. doi: 10.3389/fphys.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson RJ. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clinical and experimental pharmacology & physiology. 2012;39:96–103. doi: 10.1111/j.1440-1681.2011.05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in dahl salt-sensitive rats. American journal of physiology, Regulatory, integrative and comparative physiology. 2010;298:R1136–1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. The Journal of experimental medicine. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa Rd, Le Beau MM, Earp HS, Liu ET. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for axl, sky, and mer receptor tyrosine kinases. J Biol Chem. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 7.Korshunov VA. Axl-dependent signalling: A clinical update. Clin Sci (Lond) 2012;122:361–368. doi: 10.1042/CS20110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yagil C, Hubner N, Monti J, Schulz H, Sapojnikov M, Luft FC, Ganten D, Yagil Y. Identification of hypertension-related genes through an integrated genomic-transcriptomic approach. Circulation research. 2005;96:617–625. doi: 10.1161/01.RES.0000160556.52369.61. [DOI] [PubMed] [Google Scholar]
- 9.Korshunov VA, Daul M, Massett MP, Berk BC. Axl mediates vascular remodeling induced by deoxycorticosterone acetate salt hypertension. Hypertension. 2007;50:1057–1062. doi: 10.1161/HYPERTENSIONAHA.107.096289. [DOI] [PubMed] [Google Scholar]
- 10.Park JK, Theuer S, Kirsch T, Lindschau C, Klinge U, Heuser A, Plehm R, Todiras M, Carmeliet P, Haller H, Luft FC, Muller DN, Fiebeler A. Growth arrest specific protein 6 participates in doca-induced target-organ damage. Hypertension. 2009;54:359–364. doi: 10.1161/HYPERTENSIONAHA.109.129460. [DOI] [PubMed] [Google Scholar]
- 11.Mazor R, Kristal B, Cohen-Mazor M, Yagil C, Yagil Y, Sela S. The polymorphonuclear leukocyte contributes to the development of hypertension in the sabra rat. J Hypertens. 2007;25:2249–2256. doi: 10.1097/HJH.0b013e3282dd79b6. [DOI] [PubMed] [Google Scholar]
- 12.Lemke G, Rothlin CV. Immunobiology of the tam receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowley AW., Jr The genetic dissection of essential hypertension. Nature reviews. Genetics. 2006;7:829–840. doi: 10.1038/nrg1967. [DOI] [PubMed] [Google Scholar]
- 14.Yanagita M. The role of the vitamin k-dependent growth factor gas6 in glomerular pathophysiology. Current opinion in nephrology and hypertension. 2004;13:465–470. doi: 10.1097/01.mnh.0000133981.63053.e9. [DOI] [PubMed] [Google Scholar]
- 15.Yanagita M, Arai H, Ishii K, Nakano T, Ohashi K, Mizuno K, Varnum B, Fukatsu A, Doi T, Kita T. Gas6 regulates mesangial cell proliferation through axl in experimental glomerulonephritis. The American journal of pathology. 2001;158:1423–1432. doi: 10.1016/S0002-9440(10)64093-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagai K, Arai H, Yanagita M, Matsubara T, Kanamori H, Nakano T, Iehara N, Fukatsu A, Kita T, Doi T. Growth arrest-specific gene 6 is involved in glomerular hypertrophy in the early stage of diabetic nephropathy. J Biol Chem. 2003;278:18229–18234. doi: 10.1074/jbc.M213266200. [DOI] [PubMed] [Google Scholar]
- 17.Fiebeler A, Park JK, Muller DN, Lindschau C, Mengel M, Merkel S, Banas B, Luft FC, Haller H. Growth arrest specific protein 6/axl signaling in human inflammatory renal diseases. Am J Kidney Dis. 2004;43:286–295. doi: 10.1053/j.ajkd.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Lee IJ, Hilliard B, Swami A, Madara JC, Rao S, Patel T, Gaughan JP, Lee J, Gadegbeku CA, Choi ET, Cohen PL. Growth arrest-specific gene 6 (gas6) levels are elevated in patients with chronic renal failure. Nephrology, dialysis, transplantation. 2012;27:4166–4172. doi: 10.1093/ndt/gfs337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison DG, Guzik TJ. Studies of the t-cell angiotensin receptor using cre-lox technology: An unan-t-cellpated result. Circulation research. 2012;110:1543–1545. doi: 10.1161/CIRCRESAHA.112.271411. [DOI] [PubMed] [Google Scholar]
- 20.Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with doca-salt hypertension. American journal of physiology. Renal physiology. 2009;297:F740–748. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han YL, Li YL, Jia LX, Cheng JZ, Qi YF, Zhang HJ, Du J. Reciprocal interaction between macrophages and t cells stimulates ifn-gamma and mcp-1 production in ang ii-induced cardiac inflammation and fibrosis. PloS one. 2012;7:e35506. doi: 10.1371/journal.pone.0035506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang JD, Patel MB, Song YS, Griffiths R, Burchette J, Ruiz P, Sparks MA, Yan M, Howell DN, Gomez JA, Spurney RF, Coffman TM, Crowley SD. A novel role for type 1 angiotensin receptors on t lymphocytes to limit target organ damage in hypertension. Circulation research. 2012;110:1604–1617. doi: 10.1161/CIRCRESAHA.111.261768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marko L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, Muller DN. Interferon-gamma signaling inhibition ameliorates angiotensin ii-induced cardiac damage. Hypertension. 2012;60:1430–1436. doi: 10.1161/HYPERTENSIONAHA.112.199265. [DOI] [PubMed] [Google Scholar]
- 24.Korshunov VA, Mohan AM, Georger MA, Berk BC. Axl, a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circulation research. 2006;98:1446–1452. doi: 10.1161/01.RES.0000223322.16149.9a. [DOI] [PubMed] [Google Scholar]
- 25.Gerloff J, Korshunov VA. Immune modulation of vascular resident cells by axl orchestrates carotid intima-media thickening. The American journal of pathology. 2012;180:2134–2143. doi: 10.1016/j.ajpath.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.