Abstract
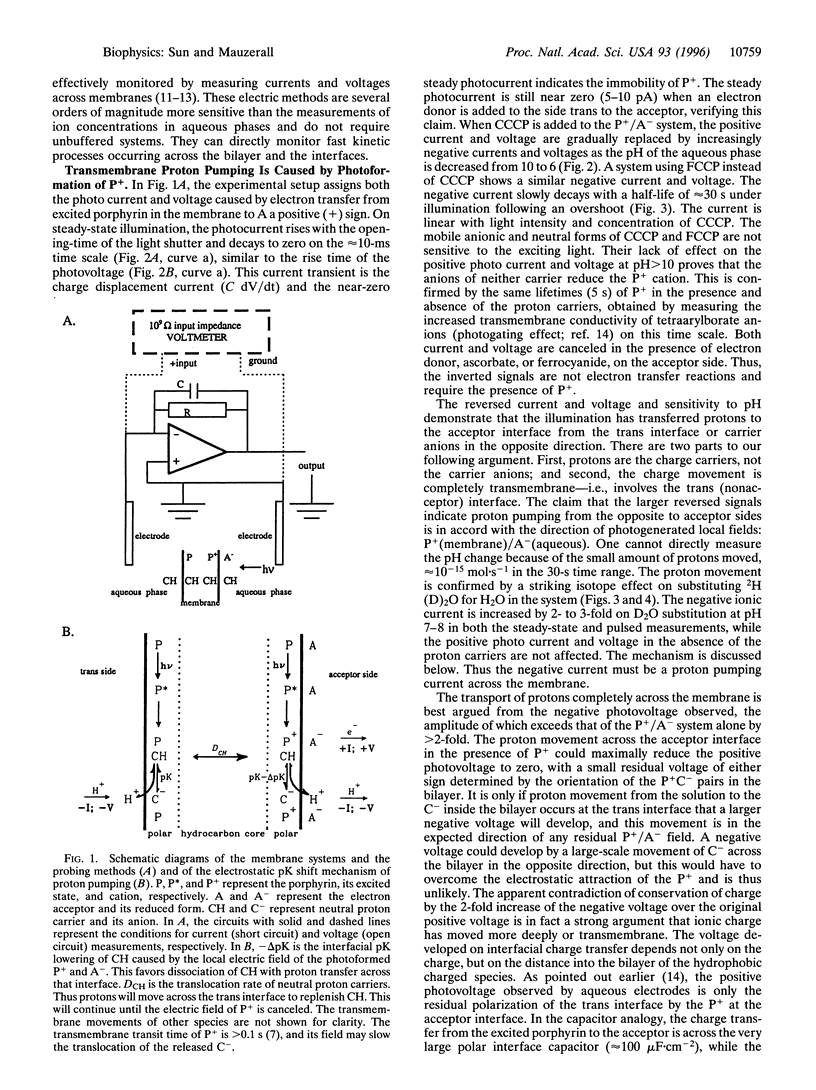
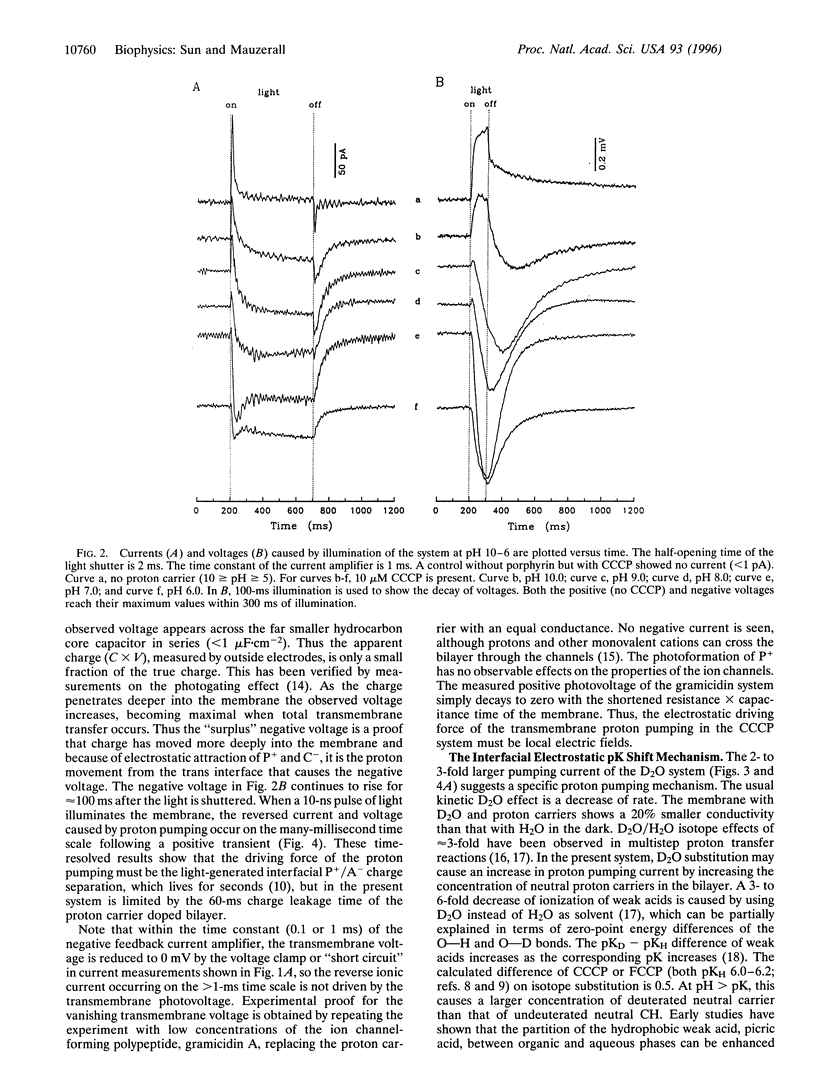
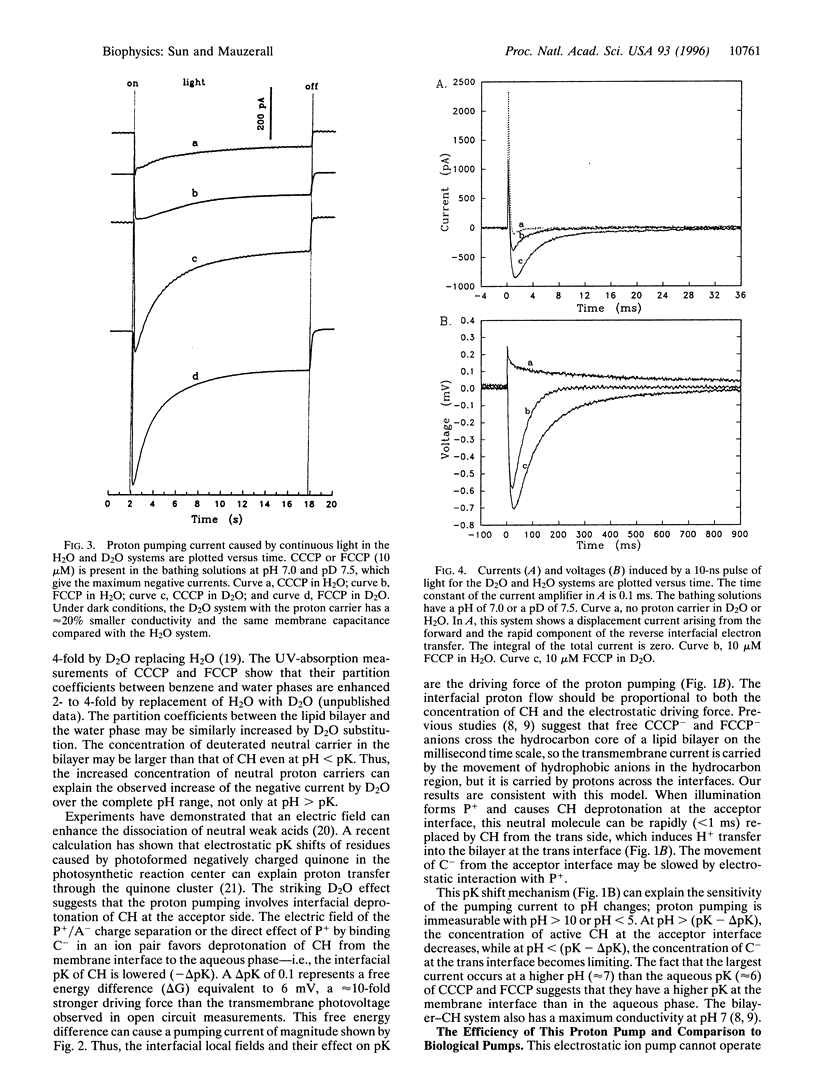
Light-induced lipophilic porphyrin/aqueous acceptor charge separation across a single lipid-water interface can pump protons across the lipid bilayer when the hydrophobic weak acids, carbonylcyanide m-chlorophenylhydrazone and its p-trifluoromethoxyphenyl analogue, are present. These compounds act as proton carriers across lipid bilayers. In their symmetric presence across the bilayer, the positive currents and voltages produced by the photogeneration of porphyrin cations are replaced by larger negative currents and voltages. The maximum negative current and voltage occur at the pH of maximum dark conductance. The reversed larger current and voltage show a positive ionic charge transport in the same direction as the electron transfer. This transport can form an ion concentration gradient. The movement of protons is verified by an unusual D2O isotope effect that increases the negative ionic current by 2- to 3-fold. These effects suggest that an interfacial pK shift of the weak acid caused by the local electric field of photoformed porphyrin cations/acceptor anions functions as the driving force. The estimated pumping efficiency is 10-30%. Time-resolved results show that proton pumping across the bilayer occurs on the millisecond time scale, similar to that of biological pumps. This light-driven proteinless pump offers a simple model for a prebiological energy transducer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberg E., Butt H. J., Eisenrauch A., Fendler K. Charge transport of ion pumps on lipid bilayer membranes. Q Rev Biophys. 1993 Feb;26(1):1–25. doi: 10.1017/s0033583500003942. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Tittor J., Oesterhelt D. Light-driven proton or chloride pumping by halorhodopsin. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):639–643. doi: 10.1073/pnas.90.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. S., Lanyi J. K. Determination of the transiently lowered pKa of the retinal Schiff base during the photocycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1731–1734. doi: 10.1073/pnas.93.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickopf S., Alexiev U., Krebs M. P., Otto H., Mollaaghababa R., Khorana H. G., Heyn M. P. Proton transport by a bacteriorhodopsin mutant, aspartic acid-85-->asparagine, initiated in the unprotonated Schiff base state. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11519–11523. doi: 10.1073/pnas.92.25.11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A., Andersen O. S. The gramicidin A channel: a review of its permeability characteristics with special reference to the single-file aspect of transport. J Membr Biol. 1981 Apr 30;59(3):155–171. doi: 10.1007/BF01875422. [DOI] [PubMed] [Google Scholar]
- Kasianowicz J., Benz R., McLaughlin S. The kinetic mechanism by which CCCP (carbonyl cyanide m-chlorophenylhydrazone) transports protons across membranes. J Membr Biol. 1984;82(2):179–190. doi: 10.1007/BF01868942. [DOI] [PubMed] [Google Scholar]
- Lancaster C. R., Michel H., Honig B., Gunner M. R. Calculated coupling of electron and proton transfer in the photosynthetic reaction center of Rhodopseudomonas viridis. Biophys J. 1996 Jun;70(6):2469–2492. doi: 10.1016/S0006-3495(96)79820-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Bacteriorhodopsin as a model for proton pumps. Nature. 1995 Jun 8;375(6531):461–463. doi: 10.1038/375461a0. [DOI] [PubMed] [Google Scholar]
- Läuger P. Thermodynamic and kinetic properties of electrogenic ion pumps. Biochim Biophys Acta. 1984 Sep 3;779(3):307–341. doi: 10.1016/0304-4157(84)90015-7. [DOI] [PubMed] [Google Scholar]
- Mauzerall D. C., Drain C. M. Photogating of ionic currents across lipid bilayers. Electrostatics of ions and dipoles inside the membrane. Biophys J. 1992 Dec;63(6):1544–1555. doi: 10.1016/S0006-3495(92)81738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Morowitz H. J. Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Mauzerall D. Charge transfer across a single lipid-water interface causes ion pumping across the bilayer. Biophys J. 1996 Jul;71(1):309–316. doi: 10.1016/S0006-3495(96)79226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Mauzerall D. Evidence for ion chain mechanism of the nonlinear charge transport of hydrophobic ions across lipid bilayers. Biophys J. 1996 Jul;71(1):295–308. doi: 10.1016/S0006-3495(96)79225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada H. The interaction of highly active uncouplers with mitochondria. Biochim Biophys Acta. 1981 Dec 30;639(3-4):225–242. doi: 10.1016/0304-4173(81)90011-2. [DOI] [PubMed] [Google Scholar]
- Woodle M. C., Mauzerall D. Photoinitiated ion movements in bilayer membranes containing magnesium octaethylporphyrin. Biophys J. 1986 Sep;50(3):431–439. doi: 10.1016/S0006-3495(86)83479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodle M., Zhang J. W., Mauzerall D. Kinetics of charge transfer at the lipid bilayer-water interface on the nanosecond time scale. Biophys J. 1987 Oct;52(4):577–586. doi: 10.1016/S0006-3495(87)83247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]



