Abstract
The development of type 2 diabetes is accompanied by decreased immune function and the mechanisms are unclear. We hypothesize that oxidative damage and mitochondrial dysfunction may play an important role in the immune dysfunction in diabetes. In the present study, we investigated this hypothesis in diabetic Goto-Kakizaki rats by treatment with a combination of four mitochondrial-targeting nutrients, namely, R-α-lipoic acid, acetyl-L-carnitine, nicotinamide and biotin. We first studied the effects of the combination of these four nutrients on immune function by examining cell proliferation in immune organs (spleen and thymus) and immunomodulating factors in the plasma. We then examined, in the plasma and thymus, oxidative damage biomarkers, including lipid peroxidation, protein oxidation, reactive oxygen species, calcium and antioxidant defence systems, mitochondrial potential and apoptosis-inducing factors (caspase 3, p53 and p21). We found that immune dysfunction in these animals is associated with increased oxidative damage and mitochondrial dysfunction and that the nutrient treatment effectively elevated immune function, decreased oxidative damage, enhanced mitochondrial function and inhibited the elevation of apoptosis factors. These effects are comparable to, or greater than, those of the anti-diabetic drug pioglitazone. These data suggest that a rational combination of mitochondrial-targeting nutrients may be effective in improving immune function in type 2 diabetes through enhancement of mitochondrial function, decreased oxidative damage, and delayed cell death in the immune organs and blood.
Keywords: alpha-lipoic acid, acetyl-L-carnitine, nicotinamide, biotin, oxidative damage, mitochondrial dysfunction
Introduction
Pancreatic β-cell dysfunction and insulin resistance are the hallmark of type 2 diabetes. Various inflammatory cytokines and oxidative stress produced by islet-infiltrating immune cells have been proposed to play an important role in mediating the destruction of β cells [1, 2]. Diabetes has also been considered as an oxidative inflammatory disorder due to malnutrition characterized by an increased intake of total and n-6 fats and decreased intake of n-3 fats and antioxidants. Malnutrition in the modern diet induces oxidative stress and activates the immune system resulting in immune suppressive, pro-inflammatory and thrombogenic lipid metabolites [3].
Improving immune function has been used as a strategy to improve diabetes. For example, immuno modulators, or cytokine-inducers have been tried to prevent insulin-dependent diabetes mellitus (IDDM) and also non-insulin-dependent diabetes mellitus (NIDDM) in animal models [4]. Both exercise and caloric restriction, known to be able to improve diabetes, have been shown to affect the phagocytic activity of macrophages in the Zucker rat (fa/fa), a model for the study of immune function in type 2 diabetes mellitus [5].
The non-obese Goto-Kakizaki (GK) rat is a model of type 2 diabetes with an early manifestation of symptoms at 4–6 weeks after birth. This model was established from normal Wistar rats by selectively breeding the animals with signs of impaired glucose tolerance and is characterized by hyperglycaemia, impaired glucose tolerance, abnormal hepatic glucose production, insulin resistance, defects in insulin secretion [6, 7], decreased exploratory activity and learning impairments [8], mitochondrial dysfunction [9, 10] and increased mitochondrial susceptibility to oxidative stress [11]. However, no studies have been carried out in this diabetic model either to investigate the immune dysfunction or to improve immune function with mitochondrial-targeting nutrients. We have investigated the preventive effects of a combination of four mitochondrial-targeting nutrients [12], namely, R-α-lipoic acid, acetyl-L-carnitine, nicotinamide and biotin, in GK rats. It was found that this nutrient treatment improved glucose tolerance, insulin release, fatty acid metabolism and mitochondrial biogenesis and function in the skeletal muscles of the diabetic rats (Shen et al. submitted for publication). Based on these results, we propose that oxidative damage and mitochondrial dysfunction may play an important role in immune dysfunction in this diabetic model and that these mitochondrial nutrients may improve immune function through the mechanisms of decreasing oxidative damage and improving mitochondrial function.
In the present study, we first compared the immune functions of Wistar rats and GK rats by examining cell proliferation in the immune organs spleen and thymus, and by measuring the immunomodulating factors adiponectin, C-reactive protein (CRP) and tumour necrosis factor-α (TNF)-α in plasma. We then compared, in plasma and/or thymus, oxidative damage biomarkers, antioxidant defence parameters, mitochondrial membrane potential (MMP) and apoptosis-related factors. The oxidative damage biomarkers include lipid peroxidation, protein oxidation, reactive oxygen species (ROS) and calcium. The antioxidant defence parameters include total antioxidant capacity (T-AOC), glutathione (GSH), glutathione S-transferase (GST) and superoxide dismutase (SOD). The apoptosis-related factors include caspase 3, p53 and p21.
Materials and methods
Animals and treatments
Four-week-old male diabetic GK rats together with age-matched male non-diabetic Wistar rats were purchased from SLAC Laboratory Animal Co. Ltd (Shanghai, China). All animals were housed at 23 ± 2°C under 12 hrs light and dark cycles, and allowed access to food and water ad libitum. The experiments were performed in accordance with the Guidelines for Animal Experiments of the Institute for Nutritional Sciences, Chinese Academy of Sciences.
Five groups of rats (10 animals in each group) consisted of two control groups, Wistar and GK, and three GK treatment groups. A pioglitazone group received pioglitazone 20 mg/kg/day by gavage; a low-dose nutrient group received a combination of R-LA 50 mg/kg/day, ALC 100 mg/kg/day, biotin 0.1 mg/kg/day and nicotinamide 15 mg/kg/day by gavage; and a high-dose nutrient group received the same combination of nutrients as the low-dose group, but at a 10 times higher dosage. The control groups received the same gavage volume of phosphate buffered saline (PBS) alone. The treatments were started at 6 weeks of age and continued for 3 months. At approximately 18 weeks of age, animals were anaesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg) and sacrificed for obtaining plasma, spleen and thymus.
Sample preparation and maintenance
Blood samples were collected by cardiac puncture, with anticogulating agent ethylenediaminetetraacetic acid (EDTA). Each blood sample was centrifuged at 1200 g for 10 min. The plasma was taken out and stored at −80°C until analysis. Spleen and thymus were aseptically removed gently squashed between two glass slides, and the erythrocytes were lysed with Tris-NH4Cl (17 mM Tris/136 mM NH4Cl, pH 7.2) for 5 min. at room temperature, then centrifuged at 1200 g at 4°C for 10 min. The pelleted lymphocytes were re-suspended with RPMI 1640 medium (Invitrogen, GIBCO, Carlsbad, CA, USA) with 10% foetal bovine serum and prepared as a single-cell suspension. One part of the lymphocytes was placed in complete RPMI 1640 medium with foetal bovine serum for assaying proliferation, ROS and MMP. The other part was washed and homogenized in ice-cold isotonic saline. The homogenates were then centrifuged at 10,000 for 10 min. at 4°C. The supernatant was collected for assaying the biochemical parameters. Protein concentrations were determined using the BCA™ Protein Assay kit with bovine serum albumin (BSA) as the standard.
Assessment of splenocyte and thymocyte proliferation and thymus index
The proliferation ability of lymphocytes extracted from spleen and thymus was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) method [13]. We used the MTT assay because the results can be quickly read on a multi-well scanning spectrophotometer (ELISA reader) and show a high degree of precision without using radioisotopes [14]. In brief, lymphocytes from spleen and thymus were prepared aseptically immediately after isolation. A single-cell suspension was prepared and adjusted to 2 × 106 cells/ml. Aliquots (100 μl) of lymphocytes were seeded into a 96-well microplate in the presence of the mitogen concanavalin A (10 μg/ml; Sigma, St Louis, MO, USA). The plate was incubated for 48 hrs at 37°C in a humidified 5% CO2/air mixture. After the addition of 10 μl of MTT per well, the plate was incubated for an additional 4 hrs, and then 100 μl of 20% (w/v) SDS (Sigma) was pipetted into each well. After overnight incubation at 37°C in 5% CO2 atmosphere, the microplate was read at a wavelength of 570 nm. The thymus index was expressed as the thymus weight relative to body weight (mg/g).
Detection of plasma immunomodulating parameters
Plasma adiponectin was determined by enzyme-linked immunosorbent assay (ELISA) with a commercially available rat Adiponectin ELISA Kit (ADL, USA) using a plasma volume of 100 μl; a rat TNF-α ELISA kit (BioSource, Camarillo, CA, USA) using a plasma volume of 50 μl diluted twofold, and a rat CRP ELISA kit (RapidBio, CA, USA) using a plasma volume of 10 μl diluted 50,000-fold. All were processed according to the manufactures' instructions.
Estimation of lipid peroxidation in plasma and thymocytes
Malondialdehyde (MDA) was determined as a measurement of lipid peroxidation using a spectrophotometric assay for thiobarbituric acid-reactive substances [15] Samples were freshly prepared from plasma and thymus and then reacted with the mixture from the malondialdehyde (MDA) Detection Kit (Jiancheng Biochemical Inc., Nanjing, China). After incubation at 95°C for 40 min., the colour of the reaction turned pink. Absorbance at 532 nm was read with a microplate reader after the reaction mixture had cooled.
Detection of protein carbonyls in plasma and thymocytes
Protein carbonyls were assayed with the Oxyblot protein oxidation detection kit (Chemicon International, CA, USA). The carbonyl groups in the protein side chains were derivatized to form the 2,4,-dinitrophenylhydrazone (DNP-hydrazone) by reaction with 2,4-dinitrophenylhydrazine (DNPH). The DNP-derivatized protein samples were separated by polyacrylamide gel electrophoresis followed by Western blotting [16]. Polyacrylamide resolving gels (12%, w/v) were stained with Coomassie Brilliant Blue R250, and processed immediately for Western blotting.
Assessment of ROS production of thymocytes
ROS production by mitochondria was monitored by 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFH-DA) [17] and analysed by flow cytometry (FACS Calibur, Becton Dickinson). Freshly isolated thymocytes (2 × 106 cells) were incubated with 10 μM DCFH2-DA for 30 min., then washed with PBS. ROS levels were analysed by flow cytometry (FACSAria™, Becton Dickinson) [18].
Assay of intracellular calcium levels in thymocytes
Intracellular calcium levels were measured by a commercially available assay kit (Jiancheng Biochemical Inc.). Calcium reacted with methyl thymol blue to form a blue complex. Optical density was measured at 610 nm with a microplate reader.
Assay of total antioxidant capacity in plasma and thymocytes
The T-AOC was assayed with a commercially available kit (Jiancheng Biochemical Inc.). The principle of the test is to measure the change in colour following the reduction of Fe3+ to Fe2+ by the reducing components in the samples. The reducing components may include enzymes, and non-enzymatic molecules such as lipid-soluble antioxidant vitamin E and water-soluble antioxidants vitamin C, uric acid, bilirubin, thiols, gluthathione, etc. The optical density was measured at 520 nm with a microplate reader.
Assay of reduced glutathione content in plasma and thymocytes
Reduced GSH content was determined in both thymocytes and plasma using a commercially available GSH Detection Kit (Jiancheng Biochemical Inc.) using an assay based on the reaction with the thiol-specific reagent dithionitrobenzoic acid. The adduct was measured spectrophotometrically at 412 nm with a plate reader.
Glutathione S-transferase activity assay in plasma and thymocytes
Activities of the enzyme in plasma and thymocytes were measured by combining 1 μl plasma or 2 μg protein with 1 mM GSH, 1 mM chloro-2, 4-dinitrobenzene, 3 mg/ml BSA in 10 mM sodium phosphate buffer. The mixture was scanned at 340 nm for 5 min. at 25°C [19].
Assay of superoxide dismutase in plasma and thymocytes
Total superoxide dismutase (SOD; E.C.: 1.15.1.1) activity was assayed using a commercially available SOD Detection Kit (Jiancheng Biochemical Inc.). The reaction is based on a xanthine and xanthine oxidase system that produces superoxide, which in turn oxidizes hydroxylamine to nitrite, forming a carmine coloured solution. The optical density at 550 nm was measured by a microplate reader.
Assessment of mitochondrial membrane potential (MMP) of thymocytes using JC-1
Determination of MMP was carried out using the fluorescent dye JC-1 (5,5′,6,6′-tetrachloro-1,1,3,3-tetraethylbenzimidazolylcarbocyanine iodide) [20]. The green fluorescent JC-1 exists as a monomer (with emission at 529 nm) at low concentrations or at low membrane potential. However, at higher concentrations (aqueous solutions above 0.1 mM) JC-1 forms red fluorescent ‘J-aggregates’ that exhibit a broad excitation spectrum and an emission maximum at 590 nm. Both components of the dye are known to be sensitive to MMP and changes in the ratio between green and red fluorescence can provide information regarding the MMP. To stain cells with JC-1, 2 × 106 cells were incubated with 10 μg/ml of JC-1 for 15 min at 37°C, then washed twice with PBS, and analysed by a dual-wavelength/double-beam recording spectrophotometer (Flex Station 384, Molecular Devices, USA).
Western blot analysis of the apoptosis related factors p53, p21 and caspase-3 in thymocytes
These soluble proteins were subjected to 10% SDS-PAGE and detected with primary antibodies against p53 (1:1000, Mouse mAb No.12506, Santa Cruz, CA, USA), p21(1:1000, Mouse mAb No.2946, Cell Signaling, USA), Caspase-3 (1:1000, Rabbit mAb No. 9665, Cell Signaling, USA) and a-tubulin (1:5000). Western blots were developed using Enhanced Chemiluminescence (ECL) (Roche, Mannheim, Germany) and quantified by scanning densitometry (Bio-Rad, Hercules, CA, USA) after incubation with horseradish peroxidase-conjugated secondary antibody.
Statistical analysis
Data are presented as mean ± SE. Statistical significance was calculated by SPSS 10.0 software using one-way ANOVA, and P-values <0.05 were considered significant.
Results
Splenocyte and thymocyte proliferation and thymus index
The proliferation of thymocytes and splenocytes of GK rats was significantly decreased compared to that of Wistar rats (Fig. 1A and B). At the end of the nutrient treatment, the low-dose group showed elevated proliferation of splenocytes and thymocytes (versus GK rats, P < 0.05 and P < 0.01, respectively), but the pioglitazone and the high-dose groups showed no significant effect.
1.
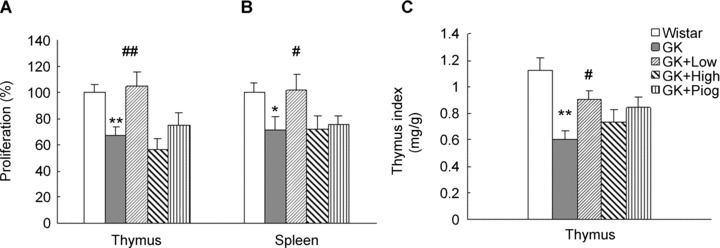
Effects of treatments on thymus and spleen cell proliferation and thymus index. (A) Proliferation of thymocytes; (B) Proliferation of splenocytes and (C) Thymus index. Data are means ± SEM of six animals (n = 6) in each group. *P < 0.05 and **P < 0.01 versus Wistar group; #P < 0.05, ##P < 0.01 versus Goto-Kakizaki (GK) untreated group.
The thymus index of GK rats was also significantly reduced, compared with that of the Wistar rats (Fig. 1C). We observed that the cortical layer of the thymus in diabetic GK rats became clearly reduced, showing apparent atrophy. Both the low-dose group and the pioglitazone group demonstrated significantly higher thymus indices than that of the non-treated GK rats (P< 0.05). These results suggest that low-dose nutrients and pioglitazone could ameliorate or partly ameliorate the immune function of lymphocytes of GK rats.
Immunomodulating parameters in plasma
As shown in Fig. 2, the plasma levels of TNF-α and CRP in GK rats were significantly higher than those in Wistar rats; the level of plasma adiponectin in GK rats was significantly lower than that in Wistar rats. These results indicate an increase in inflammation in GK rats. The low-dose nutrient treatment significantly decreased the levels of TNF-a and CRP and elevated the level of adiponectin in the GK rats; the pioglitazone treatment significantly decreased only the level of CRP, but had no effect on the levels of TNF-α and adiponectin. The high-dose nutrient treatment had no effect on improving the changes of any of the immunomodulating parameters.
2.
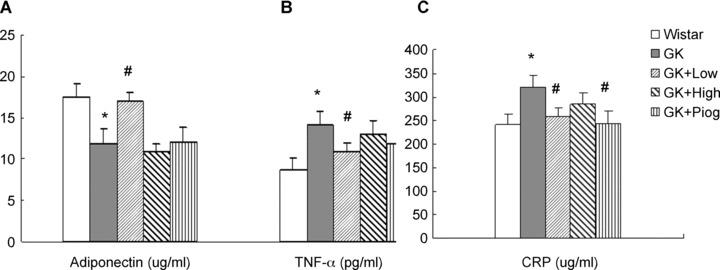
Effects of treatments on the anti-inflammatory and inflammatory factor levels in rat plasma. (A) Adiponectin; (B) Tumour necrosis factor-α (TNF-α), and (C) C-reactive protein (CRP). Data are means ± SEM (n= 10). *P < 0.05 versus Wistar group; #P < 0.05 versus GK untreated group.
Oxidative damage to lipids and proteins in plasma and thymocytes
To test whether decreased immune function is associated with an increase in oxidative damage, we first examined the level of lipid peroxidation and then detected protein oxidation. The levels of MDA in the plasma (Fig. 3A) and thymocytes (Fig. 3B) of GK rats were significantly higher than those of Wistar rats. The low-dose nutrient treatment showed significant reduction in the levels of MDA in both plasma and thymocytes, while the high-dose nutrient and pioglitazone treatments merely showed a non-significant tendency to reduce MDA.
3.

Effects of treatments on lipid peroxidation (MDA) and protein oxidation (carbonyls). (A) Malondialdehyde (MDA) in plasma; (B) MDA in thymus; (C) Western blotting for protein carbonyls (upper) and Coommassie blue staining for protein levels (lower) in plasma; (D) Quantitative results of protein carbonyls in plasma; (E) Western blotting for protein carbonyls (upper) and coommassie blue staining for protein levels (lower) in thymus and (F) Quantitative results of protein carbonyls in thymus. Data are means ± SEM (n= 8). *P < 0.05 and **P < 0.01 versus Wistar group; #P < 0.05, ##P < 0.01 versus GK untreated group.
Western blots of protein carbonyls in plasma (Fig. 3C, upper) and thymus (Fig. 3E, upper), (standardized with Coommassie blue stained gels as an equal protein reference in plasma (Fig. 3C, lower) and thymocytes (Fig. 3E, lower)), and the quantitative results (Fig. 3D for plasma and 3F for thymocytes) showed that GK rats had significantly increased protein oxidation in plasma and thymus. All of the treatments – low dose, high dose and pioglitazone – inhibited the increase in protein carbonyls in plasma; while in the thymus, both low and high-dose nutrient treatments, but not the pioglitazone treatment, significantly inhibited the increase in protein carbonyls.
ROS production and intracellular calcium levels in thymocytes
As shown in Fig. 4A, ROS levels in the diabetic GK rats were more than 1.5 times the Wistar rat levels (GK 150.19 ± 14.94%versus Wistar 100%, P< 0.05). Consistent with the increase in ROS, calcium levels in the diabetic GK rats were more than twice the Wistar rat levels (GK 79.09 ± 2.92 versus Wistar 36.03 ± 2.58 μmol/g protein, P < 0.01) (Fig. 4B). All treatments significantly inhibited the increase in calcium in GK rats, and all also tended to inhibit the ROS increases. However, only the low-dose nutrient treatment significantly reduced the ROS levels in GK rats.
4.
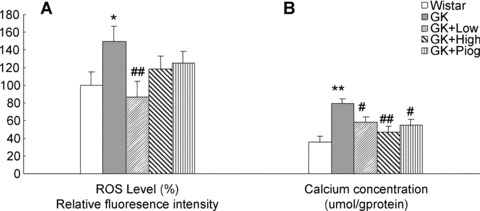
Effect of treatments on reactive oxygen species (ROS) and calcium levels in the thymus. (A) ROS levels, and (B) Calcium levels. Data are means ± SEM (n= 10). *P < 0.05 and **P < 0.01 versus. Wistar group; #P < 0.05, ##P < 0.01 versus GK untreated group.
T-AOC, GSH, and the activities of antioxidant enzymes SOD and GST in plasma and thymocytes
T-AOC levels in plasma (Fig. 5A) and thymocytes (Fig. 5B) were significantly decreased in the GK rats, compared with the Wistar rats. The low-dose nutrient treatment significantly inhibited the decrease in T-AOC in both plasma and thymocytes while the high-dose nutrient and pioglitazone treatments did not show significant inhibition.
5.
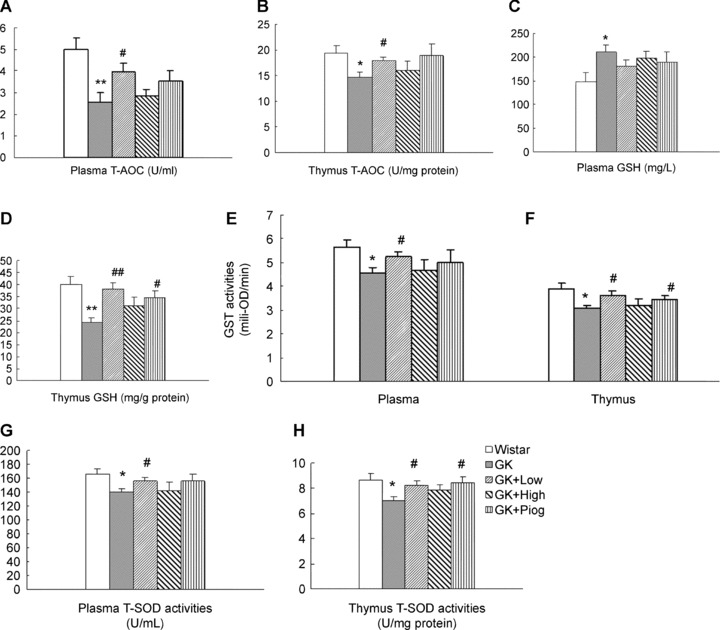
Effect of treatments on the total antioxidant capacities (T-AOC), glutathione (GSH), glutathione S-transferase (GST), and superoxide dismutase (SOD) in plasma and thymus. (A) T-AOC in plasma, n= 6; (B) T-AOC in thymus, n= 6; (C) GSH in plasma, n= 10; (D) Glutathione (GSH) in thymus, n= 8; (E) glutathione S-transferase (GST) in plasma, n= 10; (F) GST in thymus, n= 10; (G) Superoxide dismutase (SOD) in plasma, n= 10 and (H) SOD in thymus, n= 10. Data are means ± SEM. *P < 0.05 and **P < 0.01 versus Wistar group; #P < 0.05, ##P < 0.01 versus GK untreated group.
Compared with the GSH levels in Wistar rats, those in the thymocytes of GK rats were significantly decreased (Fig. 5D); however, GSH levels were unexpectedly increased in the plasma of GK rats (Fig. 5C). All treatments seemed to normalize the GSH to the levels in Wistar rats. The low-dose nutrients and pioglitazone both normalized GSH significantly in thymocytes (Fig. 5D). The low-dose nutrient treatment also showed a non-significant trend toward normalization of GSH in GK rat plasma (Fig. 5C).
Members of the phase 2 GST enzyme family catalyse the conjugation of reduced GSH via its sulfhydryl group, to electrophilic centers on a wide variety of substrates. The activation of GSTs is in the detoxification of endogenous compounds such as peroxidized lipids as well as in the metabolism of xenobiotics. GST activity in the plasma (Fig. 5E) and thymocytes (Fig. 5F) of GK rats was significantly lower than that in Wistar rats. The low-dose nutrient treatment significantly prevented the decrease in GST enzyme activity in both plasma and thymocytes; the pioglitazone treatment only showed protection in thymocytes, while the high-dose nutrient treatment did not show protection in either plasma or thymocytes.
The enzyme SOD catalyses the dismutation of superoxide into oxygen and hydrogen peroxide and provides an important antioxidant defence in nearly all cells exposed to oxygen. Similar to the changes in GST enzyme levels, GK rats, compared with Wistar rats, had a significant decrease in SOD activity in both plasma (Fig. 5G) and thymocytes (Fig. 5H). Similar to its effect on GST, the low-dose nutrient treatment, like the pioglitazone treatment, significantly inhibited the reduction in SOD activity in both plasma and thymocytes in the GK rats. The high-dose nutrient treatment did not show any protection.
Mitochondrial membrane potential in thymocytes
We examined the MMP by JC-1 fluorescence. As shown in Fig. 6, GK rats demonstrated a significant decrease in MMP, to about half the level in Wistar rats (51.54 ± 5.90%versus Wistar 100%, P< 0.01). Among the three treatments, only the low-dose nutrient treatment significantly inhibited the decrease in MMP in GK rats.
6.
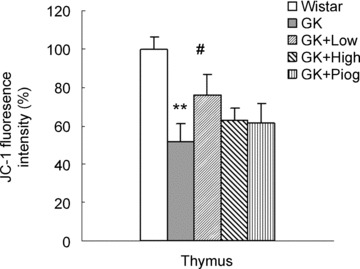
Effect of treatments on mitochondrial membrane potential detected by the fluorescent dye JC-1. Results are expressed as percentage of control (set to 100%). Data are means ± SEM (n= 10). *P < 0.05 and **P < 0.01 versus Wistar group; #P < 0.05 versus GK untreated group.
Apoptosis-related factors p53, p21 and caspase-3 in thymocytes
P53 protein expression was significantly increased in the thymocytes of GK rats, compared with that in Wistar rats and the increase in p53 was accompanied by an increased expression of p21 protein (Western blotting, Fig. 7A; quantitative results, Fig. 7B) and also caspase 3 (Western blotting, Fig. 7C; quantitative results, Fig. 7D). The treatment with low-dose nutrients significantly inhibited the increase in p53, p21 and caspase-3 while the treatments with either pioglitazone or the high-dose nutrients only significantly inhibited the increase in p21, but not p53 nor caspase-3.
7.
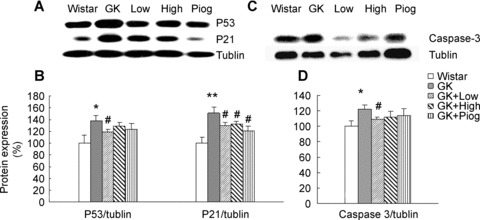
Effect of treatments on apoptosis related factors: caspase-3, p53 and p21. (A) Western blotting images of p53 and p21; (B) Quantitative results of p53 and p21 expressions; (C) Western blotting image of caspase-3, and (D) Quantitative results of caspase-3 expression. Ratios of the densities of the respective proteins to a-tubulin are expressed as a percentage of the control ratio (set to 100%). Data are means ± SEM (n= 8). *P < 0.05 and **P < 0.01 versus Wistar group; #P < 0.05 versus GK untreated group.
Discussion
In the present study, we found that GK rats showed decreased immune function, decreased mitochondrial function and an increase in oxidative damage and apoptosis factors. Low-dose nutrient treatment effectively improved immune function, decreased oxidative damage, enhanced mitochondrial function and inhibited the elevation of apoptosis factors. We will discuss the significance of these changes in relation to type 2 diabetes and also the relations among these changes.
Immune dysfunction in type 2 diabetic GK rats
Concanavalin A (Con A)-induced mitogen responses of rat splenocytes and thymocytes are important indices of immune function, since the proliferation of lymphocytes correlates well with the capacity of the immune system to fend off infections in vivo[21]. This proliferative process leads to an increase in the number of antigen-specific lymphocytes and is a key contributor to the regulation, amplification and memory capabilities of the cell-mediated immune response [21]. The proliferation of lymphocytes stimulated by mitogen has been applied to test immune function in diabetic animal models [22] and in patients [23, 24]. Our results on splenocyte and thymocyte proliferation clearly demonstrate that diabetic GK rats have a significant decrease in proliferation of thymocytes and splenocytes. In addition, the plasma inflammatory factors TNF-α and CRP were significantly higher while the anti-inflammatory factor adiponectin was significantly lower in the untreated GK rats compared to the Wistar rats. These results, combined with the observed decrease in thymus index, demonstrate that GK rats have decreased immune function. These results are consistent with those observed in another diabetic animal model [22] and in clinical diabetic patients [23, 24].
Pioglitazone, a drug approved for treatment of type 2 diabetes, is an agonist of the peroxisome proliferator activated receptor γ (PPARγ) and acts as an insulin sensitizer. Pioglatazone and other thiazolidinediones have recently been implicated as regulators of cellular inflammatory and immune responses and are thought to exert anti-inflammatory effects by negatively regulating the expression of pro-inflammatory genes [25]. In our study, pioglitazone showed beneficial effects on immune function in GK rats. The nutrient treatments, especially at the lower dose, demonstrated greater beneficial effects than pioglitazone. These results are consistent with our previous observation that lipoic acid and acetyl-L-carnitine acted as PPARγ agonists in adipocytes [26].
Mitochondrial dysfunction in type 2 diabetic GK rats
Mitochondria participate in intermediary metabolism, calcium signalling and apoptosis. Therefore, it is possible that mitochondrial dysfunction would give rise to a predictable set of defects in all tissues in aging, stress, and age-associated diseases [27–29]. The GK rat model has been shown to have mitochondrial dysfunction in the liver [9]. In the present study, we also found GK rats to have a significant decrease in mitochondrial function in thymocytes, compared with Wistar rats. This mitochondrial dysfunction is consistent with the increase in ROS and oxidative damage, calcium abnormality, and decrease in antioxidant defence observed in the GK thymocytes. Pioglitazone did not significantly improve thymocyte mitochondrial function; however, the lower dose of nutrient treatment effectively improved mitochondrial function in thymocytes, suggesting these mitochondrial nutrients could directly target mitochondria.
Increased oxidants and oxidative damage in type 2 diabetes
Increased oxidative damage due to oxidant attack on lipids, proteins and nucleic acids has been attributed to immune dysfunction. The oxidant/antioxidant balance is an important determinant of immune and other cell functions under normal or stress conditions [28, 30]. We have shown that lipid peroxidation and protein oxidation were significantly increased in the plasma and the thymocytes of GK rats, compared to Wistar rats. Oxidative damage to biomolecules such as lipids and proteins is caused by increased ROS due to abnormally increased levels of intracellular calcium. It was found that the GK rats do have both increased ROS and calcium in their thymocytes.
A second consequence of increased ROS may be the weakening of the antioxidant defence system by decreasing antioxidants and inactivating antioxidant enzymes. We, therefore examined T-AOC, levels of the highly important endogenous antioxidant GSH and the antioxidant enzymes GST and SOD. All of these parameters, except GSH, confirmed a significant decrease in the antioxidant defence system.
The increase in GSH in plasma of GK rats is unexpected but is not unusual nor unexplainable. It has been shown that GK rat liver mitochondria show a decreased ATP/ADP ratio, accompanied by an increase in both respiratory function and complex activity [9, 10, 31, 32]. The increased respiratory activity in liver mitochondria is considered a metabolic adaptation or adjustment to glucose injury (glucose toxicity) in hepatocytes due to a decrease in ATP synthesis. The metabolic adaptation/adjustment of liver mitochondria to glucose toxicity is further indicated by the fact that GK rats, compared to Wistar rats, have a higher level of the antioxidant coenzyme Q in their liver mitochondria [33]. Consistent with these alterations in liver mitochondria, we have also found that GK rats, compared to Wistar rats, show a significant increase in GSH in liver mitochondria (GK 112%versus Wistar 100%, P < 0.05, unpublished). The increase in liver GSH provides an explanation for the increased GSH level in plasma. Nevertheless, it is interesting to note that the low-dose nutrient treatment restores GSH levels in both plasma and thymus. This suggests that the nutrient treatment may strengthen liver mitochondrial function and antioxidant defences, and thus make the cells more resistant to environmental challenges.
Oxidative damage, mitochondrial dysfunction and apoptosis may be a cause of immune dysfunction
The development of type 2 diabetes is accompanied by decreased immune function, but the mechanisms are unclear. We propose that mitochondrial dysfunction may be one of the major causes of immune dysfunction in GK rats. Oxidative stress induces mitochondrial dysfunction and also apoptosis. Activation of caspase-3 as a consequence of mitochondrial membrane depolarization has been shown to result from both in vitro and in vivo cytotoxic treatments [34]. Apoptosis causes immune suppression by inducing depletion of various immune cells, resulting in the loss of key anti-microbial functions, and impairing immunity by inducing immunosuppressive effects in the surviving cells [35]. The p53 tumour suppressor gene product plays an important role in the regulation of apoptosis through either caspase-dependent or independent pathways and concomitant upregulation of cyclin-dependent kinase inhibitor p21 [36]. The enhanced expression of p53 and the accompanying increases in p21 and caspase-3 suggest that the decreased immune function and the suppression of cell proliferative mitogenic response in GK rats are mediated by mitochondrial caspase-dependent apoptosis in the thymus. Nevertheless, a caspase-independent pathway may also be operative since the increases in intracellular calcium, ROS, oxidative damage to lipids and proteins and mitochondrial impairment all favour a possible release of apoptosis inducing factor [34].
It was shown that in activated, proliferative T cells, inhibition of apoptosis mediated by NF-KB–prevents destruction of the proliferating T cells. However, mitochondrial membrane depolarization initiates apoptosis, and ROS vitiate NF-kB's ability to inhibit it [37], resulting in T-cell destruction and a weakened proliferative capacity. This provides direct evidence linking the depressed MMP and increased ROS levels in the GK rat thymocytes and splenocytes with their decreased proliferative capacity.
Therefore, increased oxidants cause mitochondrial dysfunction, which causes more production of oxidants and oxidative damage. This vicious cycle may ultimately cause cell death and lead to dysfunction of the immune system. This proposed mechanism is tested by the nutrient treatments as stated below.
Rationale for choosing the mitochondrial nutrients
The rationale for choosing for choosing these four mitochondrial nutrients is briefed as below. LA is a mitochondrial nutrient able to scavenge free radicals, chelate iron to prevent the generation of free radicals, induce phase 2 enzymes, stimulate mitochondrial biogenesis and act as a cofactor of pyruvate dehydrogenase and lipoamide dehydrogenase [12, 38]. Lipoic acid has been used for treatment of diabetes complications, such as neuropathy [39]. Acetyl-L-carnitine has been shown to be effective for improving insulin-mediated glucose disposal either in healthy subjects or in type 2 diabetic patients [40, 41]. We [26] have recently found that lipoic acid and acetyl-L-carnitine, individually and in combination, stimulate mitochondrial biogenesis in 3T3-L1 adipocytes. The combination is about 10–100 times as potent as either compound administered alone, suggesting a potent synergistic action [26]. Finally, an important factor in choosing lipoic acid and acetyl-L-carnitine in combination was their synergistic ability to improve mitochondrial function, ambulatory activity and cognition in old rats [42–44] and beagles [45].
Two reasons were behind our inclusion of biotin. First, four of the five biotin-dependent carboxylases are in the mitochondria. Second, a high intake of biotin may exert effects on β cells, liver and skeletal muscle, that favour good glucose tolerance [46].
Pancreatic islet dysfunction is an important feature of GK pathogenesis [6, 47]. Niacin appears to protect against the loss of β cell function in type 1 diabetes [48, 49]. The other main value of niacin supplementation is to increase the availability of this B vitamin in its role as component of the coenzymes NADH and NADPH, both of which are essential for proper mitochondrial function. NAD(P)H acts as a donor of hydrogen anion in a variety of enzymatic processes, such as the reduction of lipoic acid to dihydro-lipoic acid [50]. In addition, Kirsch and De Groot [51] have proposed that NAD(P)H may also act as a directly operating antioxidant.
Based on the above facts, we have proposed that the rational combination of mitochondria-targeted nutrients may complementarily promote mitochondrial synthesis and adipocyte metabolism and possibly prevent and treat insulin resistance in type 2 diabetes, and our results for the treatments with these four nutrients do support this hypothesis. Consistent with the effects of low-dose treatment on glucose tolerance, fatty acid metabolism, and muscle mitochondrial biogenesis and function in GK rats, the effects of treatment at this dosage seem comparable to, or even more effective than, pioglitazone treatment. Conversely, the 10-fold higher dose of these same mitochondrial nutrients, though not toxic, loses most of the beneficial effects of the low dose, except for the improvements in thymus MDA, plasma and thymus protein carbonyls, thymus calcium, and p21 expression. The decreased effectiveness of the high dose is possibly because it is an overdose that overshoots the optimal point in a bell-shaped dose–effect curve. These data suggest that a rational combination of mitochondrial-targeting nutrients in the proper dose may be used clinically for ameliorating immune dysfunction in patients with type 2 diabetes.
Although we have termed these nutrients mitochondrial-targeting agents and focused on the their effects in mitochondria, it should be borne in mind that the four nutrients used are essential for non-mitochondrial cell functions as well. For example, although lipoic acid is a mitochondrial cofactor and mainly located in mitochondria, it is also available to and influences many activities in other parts of the cell when supplied exogenously.
Conclusion
In conclusion, we have shown that GK rats, compared to Wistar rats, have a decrease in immune function and an increase in oxidative damage, mitochondrial dysfunction and apoptosis-related factors in the plasma, spleen and thymus. A 12-week-long treatment with a combination of mitochondrial-targeting nutrients in the GK rats effectively ameliorated their immune and mitochondrial dysfunction, inhibited oxidative damage, and reversed the enhanced apoptosis process. The significant beneficial effects of mitochondrial-targeting nutrients on diabetes-associated immune dysfunction suggest that this modern nutrition-related disease possibly may be controlled by appropriate manipulation with nutrients, rather than exclusively by drugs.
Acknowledgments
This study was supported by the Pujiang Talent Award and the Diabetes Research Grant from the Shanghai Municipal Committee of Science and Technology and a grant by the Chinese Academy of Sciences.
References
- 1.Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaneto H, Katakami N, Kawamori D, Miyatsuka T, Sakamoto K, Matsuoka TA, Matsuhisa M, Yamasaki Y. Involvement of oxidative stress in the pathogenesis of diabetes. Antioxid Redox Signal. 2007;9:355–66. doi: 10.1089/ars.2006.1465. [DOI] [PubMed] [Google Scholar]
- 3.Raheja BS. Diabetes and atherosclerosis as immune-inflammatory disorders: options for reversal of disease processes. J Assoc Physicians India. 1994;42:385–390. 395–6. [PubMed] [Google Scholar]
- 4.Zhu XP, Satoh J, Muto G, Muto Y, Sagara M, Takahashi K, Seino H, Hirai S, Masuda T, Tanaka S, Ishida H, Seino Y, Toyota T. Improvement of glucose tolerance with immunomodulators on type 2 diabetic animals. Biotherapy. 1996;9:189–97. doi: 10.1007/BF02620732. [DOI] [PubMed] [Google Scholar]
- 5.Plotkin BJ, Paulson D. Zucker rat (fa/fa), a model for the study of immune function in type-II diabetes mellitus: effect of exercise and caloric restriction on the phagocytic activity of macrophages. Lab Anim Sci. 1996;46:682–4. [PubMed] [Google Scholar]
- 6.Portha B. Programmed disorders of beta-cell development and function as one cause for type 2 diabetes? The GK rat paradigm. Diabetes Metab Res Rev. 2005;21:495–504. doi: 10.1002/dmrr.566. [DOI] [PubMed] [Google Scholar]
- 7.Rosen P, Wiernsperger NF. Metformin delays the manifestation of diabetes and vascular dysfunction in Goto-Kakizaki rats by reduction of mitochondrial oxidative stress. Diabetes Metab Res Rev. 2006;22:323–30. doi: 10.1002/dmrr.623. [DOI] [PubMed] [Google Scholar]
- 8.Moreira T, Malec E, Ostenson CG, Efendic S, Liljequist S. Diabetic type II Goto-Kakizaki rats show progressively decreasing exploratory activity and learning impairments in fixed and progressive ratios of a lever-press task. Behav Brain Res. 2007;180:28–41. doi: 10.1016/j.bbr.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira FM, Palmeira CM, Seica R, Santos MS. Alterations of liver mitochondrial bioenergetics in diabetic Goto-Kakizaki rats. Metabolism. 1999;48:1115–9. doi: 10.1016/s0026-0495(99)90124-5. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira FM, Palmeira CM, Seica R, Moreno AJ, Santos MS. Diabetes and mitochondrial bioenergetics: alterations with age. J Biochem Mol Toxicol. 2003;17:214–22. doi: 10.1002/jbt.10081. [DOI] [PubMed] [Google Scholar]
- 11.Santos MS, Santos DL, Palmeira CM, Seica R, Moreno AJ, Oliveira CR. Brain and liver mitochondria isolated from diabetic Goto-Kakizaki rats show different susceptibility to induced oxidative stress. Diabetes Metab Res Rev. 2001;17:223–30. doi: 10.1002/dmrr.200. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Ames BN. Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer's disease, and Parkinson's disease. Nutr Neurosci. 2005;8:67–89. doi: 10.1080/10284150500047161. [DOI] [PubMed] [Google Scholar]
- 13.Messa C, Notarnicola M, Russo F, Cavallini A, Pallottini V, Trentalance A, Bifulco M, Laezza C, Gabriella Caruso M. Estrogenic regulation of cholesterol biosynthesis and cell growth in DLD-1 human colon cancer cells. Scand J Gastroenterol. 2005;40:1454–61. doi: 10.1080/00365520510024007. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical biochemistry. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Meth Enzymol. 1994;233:346–63. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 17.Voloboueva LA, Liu J, Suh JH, Ames BN, Miller SS. (R)-alpha-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci. 2005;46:4302–10. doi: 10.1167/iovs.04-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shamoto-Nagai M, Maruyama W, Kato Y, Isobe K, Tanaka M, Naoi M, Osawa T. An inhibitor of mitochondrial complex I, rotenone, inactivates proteasome by oxidative modification and induces aggregation of oxidized proteins in SH-SY5Y cells. J Neurosci Res. 2003;74:589–97. doi: 10.1002/jnr.10777. [DOI] [PubMed] [Google Scholar]
- 19.Pabst MJ, Habig WH, Jakoby WB. Glutathione S-transferase A. A novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. J Biol Chem. 1974;249:7140–7. [PubMed] [Google Scholar]
- 20.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Jr, Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA. 1991;88:3671–5. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbas AK, Litchtman AH, Pober JS, editors. Cellular and molecular immunology. Philadelphia, PA: WB Saunders; [Google Scholar]
- 22.Moriguchi S, Kato M, Sakai K, Yamamoto S, Shimizu E. Exercise training restores decreased cellular immune functions in obese Zucker rats. J Appl Physiol. 1998;84:311–7. doi: 10.1152/jappl.1998.84.1.311. [DOI] [PubMed] [Google Scholar]
- 23.Chang FY, Shaio MF. Decreased cell-mediated immunity in patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1995;28:137–46. doi: 10.1016/0168-8227(95)00168-8. [DOI] [PubMed] [Google Scholar]
- 24.Foss-Freitas MC, Foss NT, Donadi EA, Foss MC. Effect of metabolic control on the in vitro proliferation of peripheral blood mononuclear cells in type 1 and type 2 diabetic patients. Sao Paulo Med J. 2006;124:219–22. doi: 10.1590/S1516-31802006000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belvisi MG, Hele DJ, Birrell MA. Peroxisome proliferator-activated receptor gamma agonists as therapy for chronic airway inflammation. Eur J Pharmacol. 2006;533:101–9. doi: 10.1016/j.ejphar.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 26.Shen W, Liu K, Tian C, Yang L, Li X, Ren J, Packer L, Cotman CW, Liu J. R-alpha-Lipoic acid and acetyl-L: -carnitine complementarily promote mitochondrial biogenesis in murine 3T3-L1 adipocytes. Diabetologia. 2008;51:165–74. doi: 10.1007/s00125-007-0852-4. [DOI] [PubMed] [Google Scholar]
- 27.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–8. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Mori A. Stress, aging, and brain oxidative damage. Neurochem Res. 1999;24:1479–97. doi: 10.1023/a:1022597010078. [DOI] [PubMed] [Google Scholar]
- 29.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–52. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 30.De la Fuente M, Hernanz A, Vallejo MC. The immune system in the oxidative stress conditions of aging and hypertension: favorable effects of antioxidants and physical exercise. Antioxid Redox Signal. 2005;7:1356–66. doi: 10.1089/ars.2005.7.1356. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira FM, Seica R, Santos MS, Palmeira CM. Age-related alterations in liver mitochondrial bioenergetics of diabetic Goto-Kakizaki rats. Acta Diabetol. 1999;36:173–7. doi: 10.1007/s005920050163. [DOI] [PubMed] [Google Scholar]
- 32.Palmeira CM, Ferreira FM, Santos DL, Ceica R, Suzuki K, Santos MS. Higher efficiency of the liver phosphorylative system in diabetic Goto-Kakizaki (GK) rats. FEBS Lett. 1999;458:103–6. doi: 10.1016/s0014-5793(99)01144-8. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira FM, Seica R, Oliveira PJ, Coxito PM, Moreno AJ, Palmeira CM, Santos MS. Diabetes induces metabolic adaptations in rat liver mitochondria: role of coenzyme Q and cardiolipin contents. Biochim Biophys Acta. 2003;1639:113–20. doi: 10.1016/j.bbadis.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Pathak N, Khandelwal S. Role of oxidative stress and apoptosis in cadmium induced thymic atrophy and splenomegaly in mice. Toxicol Lett. 2007;169:95–108. doi: 10.1016/j.toxlet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–22. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 36.Fanzo JC, Reaves SK, Cui L, Zhu L, Lei KY. p53 protein and p21 mRNA levels and caspase-3 activity are altered by zinc status in aortic endothelial cells. Am J Physiol Cell Physiol. 2002;283:C631–8. doi: 10.1152/ajpcell.00248.2001. [DOI] [PubMed] [Google Scholar]
- 37.Gronski MA, Weinem M. Death pathways in T cell homeostasis and their role in autoimmune diabetes. Rev Diabet Stud. 2006;3:88–95. doi: 10.1900/RDS.2006.3.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J. The effects and mechanisms of mitochondrial Nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: an overview. Neurochem Res. 2008;33:194–203. doi: 10.1007/s11064-007-9403-0. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler D, Hanefeld M, Ruhnau KJ, Meissner HP, Lobisch M, Schutte K, Gries FA. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multi-centre randomized controlled trial (ALADIN Study) Diabetologia. 1995;38:1425–33. doi: 10.1007/BF00400603. [DOI] [PubMed] [Google Scholar]
- 40.Mingrone G. Carnitine in type 2 diabetes. Ann N Y Acad Sci. 2004;1033:99–107. doi: 10.1196/annals.1320.009. [DOI] [PubMed] [Google Scholar]
- 41.Giancaterini A, De Gaetano A, Mingrone G, Gniuli D, Liverani E, Capristo E, Greco AV. Acetyl-L-carnitine infusion increases glucose disposal in type 2 diabetic patients. Metabolism. 2000;49:704–8. doi: 10.1053/meta.2000.6250. [DOI] [PubMed] [Google Scholar]
- 42.Hagen TM, Liu J, Lykkesfeldt J, Wehr CM, Ingersoll RT, Vinarsky V, Bartholomew JC, Ames BN. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci USA. 2002;99:1870–5. doi: 10.1073/pnas.261708898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci USA. 2002;99:2356–61. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Killilea DW, Ames BN. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci USA. 2002;99:1876–81. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milgram NW, Araujo JA, Hagen TM, Treadwell BV, Ames BN. Acetyl-L-carnitine and alpha-lipoic acid supplementation of aged beagle dogs improves learning in two landmark discrimination tests. FASEB J. 2007;21:3756–62. doi: 10.1096/fj.07-8531com. [DOI] [PubMed] [Google Scholar]
- 46.McCarty MF. Nutraceutical resources for diabetes prevention–an update. Med Hypotheses. 2005;64:151–8. doi: 10.1016/j.mehy.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 47.Janssen U, Vassiliadou A, Riley SG, Phillips AO, Floege J. The quest for a model of type II diabetes with nephropathy: the Goto Kakizaki rat. J Nephrol. 2004;17:769–73. [PubMed] [Google Scholar]
- 48.McCaman RE, McCaman MW, Stafford ML. Carnitine acetyltransferase in nervous tissue. J Biol Chem. 1966;241:930–4. [PubMed] [Google Scholar]
- 49.Shima K, Zhu M, Kuwajima M. A role of nicotinamide-induced increase in pancreatic beta-cell mass on blood glucose control after discontinuation of the treatment in partially pancreatectomized OLETF rats. Diabetes Res Clin Pract. 1998;41:1–8. doi: 10.1016/s0168-8227(98)00061-8. [DOI] [PubMed] [Google Scholar]
- 50.Haramaki N, Han D, Handelman GJ, Tritschler HJ, Packer L. Cytosolic and mitochondrial systems for NADH- and NADPH-dependent reduction of alpha-lipoic acid. Free Radic Biol Med. 1997;22:535–42. doi: 10.1016/s0891-5849(96)00400-5. [DOI] [PubMed] [Google Scholar]
- 51.Kirsch M, De Groot H. NAD(P)H, a directly operating antioxidant? FASEB J. 2001;15:1569–74. doi: 10.1096/fj.00-0823hyp. [DOI] [PubMed] [Google Scholar]


