Abstract
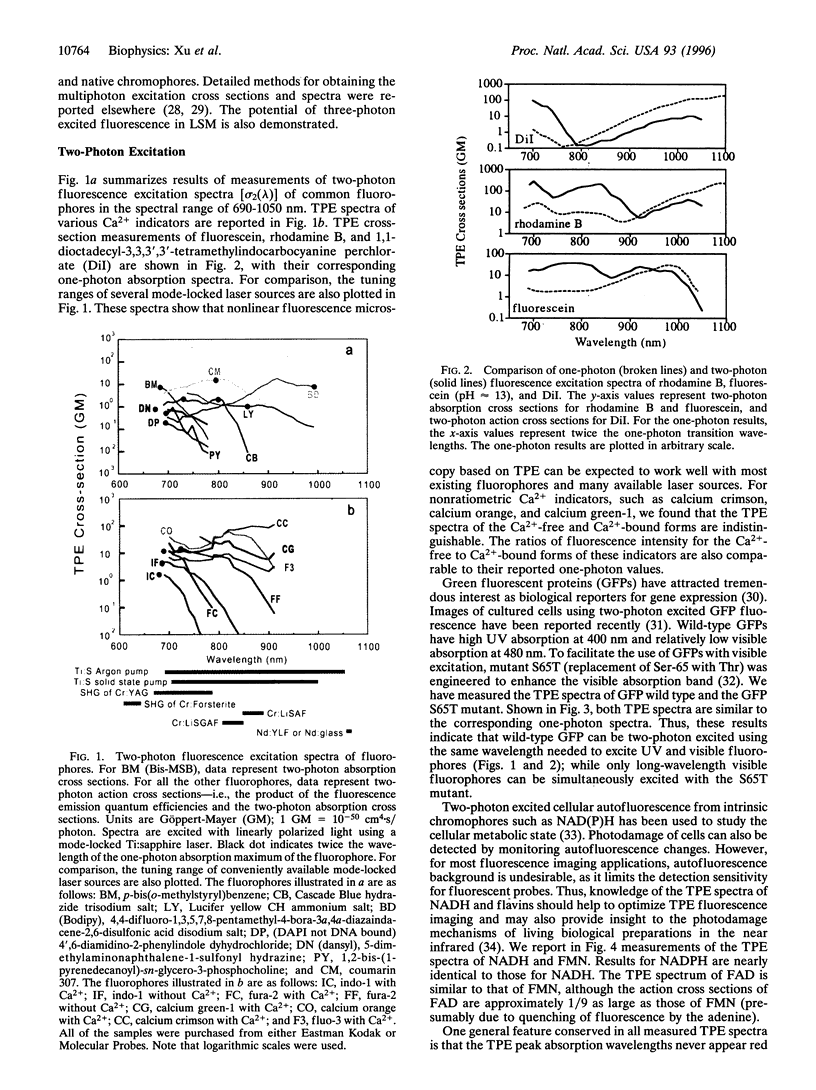
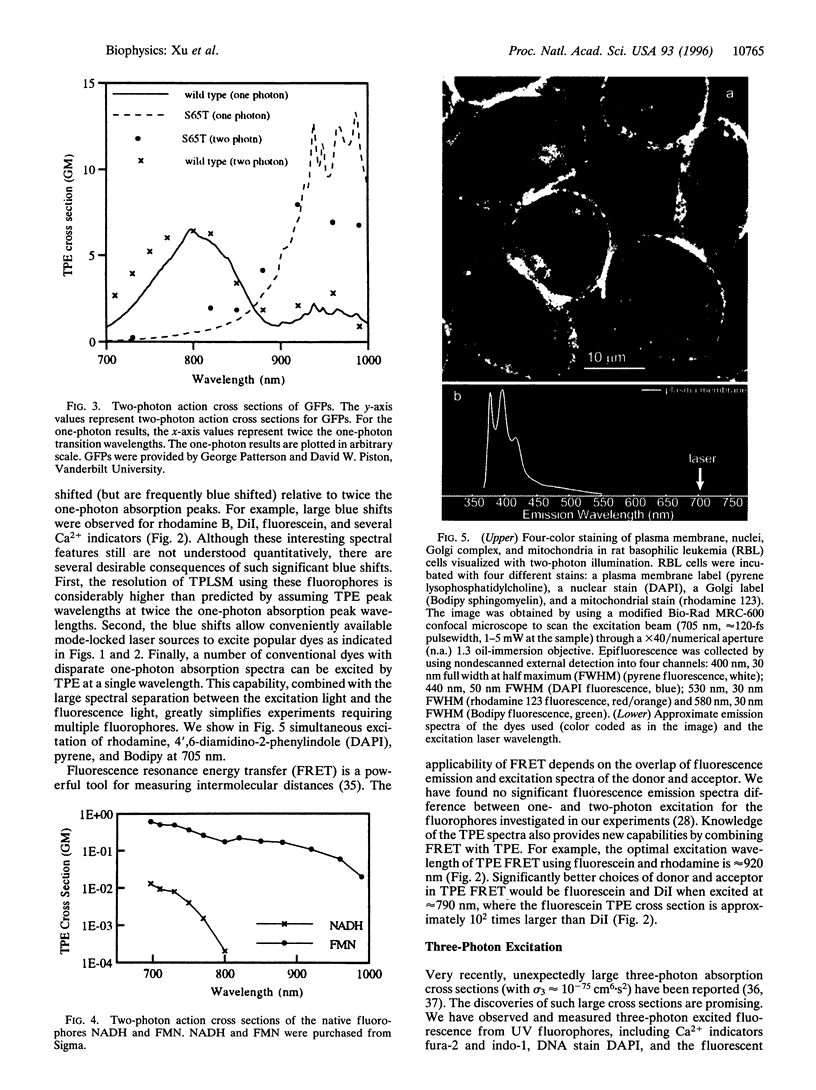
Intrinsic, three-dimensionally resolved, microscopic imaging of dynamical structures and biochemical processes in living preparations has been realized by nonlinear laser scanning fluorescence microscopy. The search for useful two-photon and three-photon excitation spectra, motivated by the emergence of nonlinear microscopy as a powerful biophysical instrument, has now discovered a virtual artist's palette of chemical indicators, fluorescent markers, and native biological fluorophores, including NADH, flavins, and green fluorescent proteins, that are applicable to living biological preparations. More than 25 two-photon excitation spectra of ultraviolet and visible absorbing molecules reveal useful cross sections, some conveniently blue-shifted, for near-infrared absorption. Measurements of three-photon fluorophore excitation spectra now define alternative windows at relatively benign wavelengths to excite deeper ultraviolet fluorophores. The inherent optical sectioning capability of nonlinear excitation provides three-dimensional resolution for imaging and avoids out-of-focus background and photodamage. Here, the measured nonlinear excitation spectra and their photophysical characteristics that empower nonlinear laser microscopy for biological imaging are described.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Cubitt A. B., Heim R., Adams S. R., Boyd A. E., Gross L. A., Tsien R. Y. Understanding, improving and using green fluorescent proteins. Trends Biochem Sci. 1995 Nov;20(11):448–455. doi: 10.1016/s0968-0004(00)89099-4. [DOI] [PubMed] [Google Scholar]
- Denk W., Strickler J. H., Webb W. W. Two-photon laser scanning fluorescence microscopy. Science. 1990 Apr 6;248(4951):73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Gryczynski I., Szmacinski H., Lakowicz J. R. On the possibility of calcium imaging using Indo-1 with three-photon excitation. Photochem Photobiol. 1995 Oct;62(4):804–808. doi: 10.1111/j.1751-1097.1995.tb08733.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- König K., Liu Y., Sonek G. J., Berns M. W., Tromberg B. J. Autofluorescence spectroscopy of optically trapped cells. Photochem Photobiol. 1995 Nov;62(5):830–835. doi: 10.1111/j.1751-1097.1995.tb09143.x. [DOI] [PubMed] [Google Scholar]
- Niswender K. D., Blackman S. M., Rohde L., Magnuson M. A., Piston D. W. Quantitative imaging of green fluorescent protein in cultured cells: comparison of microscopic techniques, use in fusion proteins and detection limits. J Microsc. 1995 Nov;180(Pt 2):109–116. doi: 10.1111/j.1365-2818.1995.tb03665.x. [DOI] [PubMed] [Google Scholar]
- Piston D. W., Masters B. R., Webb W. W. Three-dimensionally resolved NAD(P)H cellular metabolic redox imaging of the in situ cornea with two-photon excitation laser scanning microscopy. J Microsc. 1995 Apr;178(Pt 1):20–27. doi: 10.1111/j.1365-2818.1995.tb03576.x. [DOI] [PubMed] [Google Scholar]
- Schmitt J. M., Knüttel A., Yadlowsky M. Confocal microscopy in turbid media. J Opt Soc Am A Opt Image Sci Vis. 1994 Aug;11(8):2226–2235. doi: 10.1364/josaa.11.002226. [DOI] [PubMed] [Google Scholar]
- Shear J. B., Brown E. B., Webb W. W. Multiphoton-excited fluorescence of fluorogen-labeled neurotransmitters. Anal Chem. 1996 May 15;68(10):1778–1783. doi: 10.1021/ac960007s. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Stuart BC, Feit MD, Rubenchik AM, Shore BW, Perry MD. Laser-induced damage in dielectrics with nanosecond to subpicosecond pulses. Phys Rev Lett. 1995 Mar 20;74(12):2248–2251. doi: 10.1103/PhysRevLett.74.2248. [DOI] [PubMed] [Google Scholar]
- Williams R. M., Piston D. W., Webb W. W. Two-photon molecular excitation provides intrinsic 3-dimensional resolution for laser-based microscopy and microphotochemistry. FASEB J. 1994 Aug;8(11):804–813. doi: 10.1096/fasebj.8.11.8070629. [DOI] [PubMed] [Google Scholar]
- Yuste R., Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995 Jun 22;375(6533):682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]