Abstract
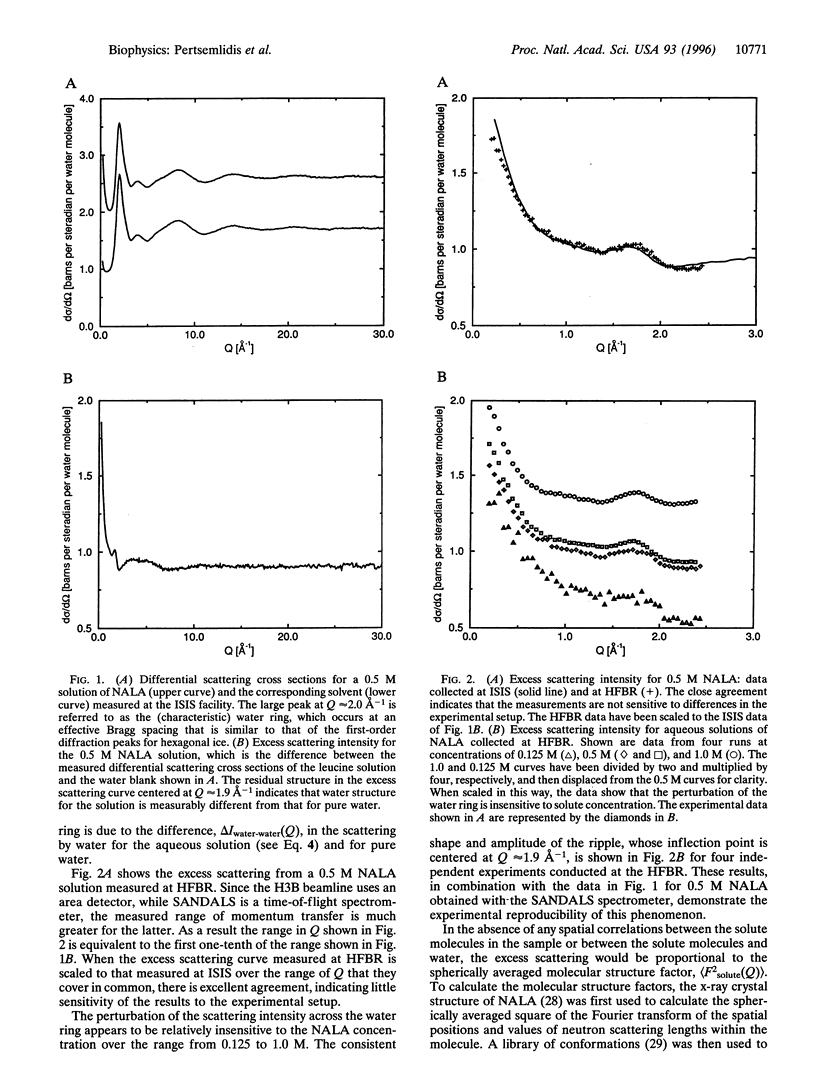
Neutron scattering experiments are used to determine scattering profiles for aqueous solutions of hydrophobic and hydrophilic amino acid analogs. Solutions of hydrophobic solutes show a shift in the main diffraction peak to smaller angle as compared with pure water, whereas solutions of hydrophilic solutes do not. The same difference for solutions of hydrophobic and hydrophilic side chains is also predicted by molecular dynamics simulations. The neutron scattering curves of aqueous solutions of hydrophobic amino acids at room temperature are qualitatively similar to differences between the liquid molecular structure functions measured for ambient and supercooled water. The nonpolar solute-induced expansion of water structure reported here is also complementary to recent neutron experiments where compression of aqueous solvent structure has been observed at high salt concentration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dill K. A. Dominant forces in protein folding. Biochemistry. 1990 Aug 7;29(31):7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- Dunbrack R. L., Jr, Karplus M. Conformational analysis of the backbone-dependent rotamer preferences of protein sidechains. Nat Struct Biol. 1994 May;1(5):334–340. doi: 10.1038/nsb0594-334. [DOI] [PubMed] [Google Scholar]
- Head-Gordon T. Is water structure around hydrophobic groups clathrate-like? Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8308–8312. doi: 10.1073/pnas.92.18.8308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Keener C. R., Fullerton G. D., Cameron I. L., Xiong J. Solution nonideality related to solute molecular characteristics of amino acids. Biophys J. 1995 Jan;68(1):291–302. doi: 10.1016/S0006-3495(95)80187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone J. R., Spolar R. S., Record M. T., Jr Contribution to the thermodynamics of protein folding from the reduction in water-accessible nonpolar surface area. Biochemistry. 1991 Apr 30;30(17):4237–4244. doi: 10.1021/bi00231a019. [DOI] [PubMed] [Google Scholar]
- Murphy K. P., Privalov P. L., Gill S. J. Common features of protein unfolding and dissolution of hydrophobic compounds. Science. 1990 Feb 2;247(4942):559–561. doi: 10.1126/science.2300815. [DOI] [PubMed] [Google Scholar]
- Stillinger F. H. Water revisited. Science. 1980 Jul 25;209(4455):451–457. doi: 10.1126/science.209.4455.451. [DOI] [PubMed] [Google Scholar]


