Abstract
At the cellular level, the activation and transdifferentiation of quiescent hepatic stellate cells (HSC) into myofibroblasts is the key process involved in hepatic fibrogenesis that is associated with an increased and altered deposition of extracellular matrix components in the liver. The temporal sequence of molecular events associated with stellate cell activation turned out to be appropriately mimicked when HSC isolated from normal livers are cultured on uncoated plastic surface. Therefore, cultured primary cells isolated from rodents and human beings are common in vitro models in investigations addressing these issues of hepatic stellate biology and function. However, the limited supply, cost-effective isolation procedure and the ever growing need have resulted in efforts to establish immortalized stellate cell lines having the advantage of virtually unlimited access. They allow rapid screening for disease-associated factors and restrict the necessary number of animal experiments. From the first description of an immortal HSC line in 1986, a huge number of studies were conducted with these established cell lines. However, differences in morphology, growth characteristics and anomalies of chromosome number and structure make the applications of these models questionable. Here, we summarize the history and cellular characteristics of respective cell lines and discuss the differences of continuous HSC lines and their primary counterparts.
Keywords: hepatic stellate cells, immortalization, cell culture, liver, hepatic fibrogenesis
Introduction
Protocols for the isolation of hepatic stellate cells (HSC) from rodents were first established in 1984 [1]. In most of the published protocols, the liver is digested with collagenase and pronase followed by subsequent fractionation of the resulting heterogenous cell suspension through various density media [2]. Based on their high lipid content and concomitant low density, HSC effectively float away from other hepatic cells allowing cellular enrichment that can be further increased by centrifugal elutriation or scatter-activated cell sorting [2, 3]. Alternatively, human cells of HSC origin are obtained as outgrowths from (diseased) human liver explants that were taken for diagnostic reasons [4, 5].
As a general hallmark, primary HSC characteristically contain (i) numerous, large lipid droplets that are composed of retinoids, (ii) a cytoplasm that is typically equipped with a large Golgi apparatus, a well developed endoplasmic reticulum, small numbers of mitochondria and lysosomes, rare peroxisomes, a few glycogen particles and a distinct centriole, (iii) a cytoplasmic matrix containing bundles of microfilaments and microtubules, including actin and intermediate filaments, and express (iv) specific neuronal markers such as glial fibrillary acidic protein (GFAP) and synaptophysin, (v) intermediate filamental proteins (e. g. desmin, nestin and vimentin) and (vi) increase the expression of _-smooth muscle actin (_-SMA) and collagens during culturing thereby increasing their overall cellular contractility resulting in a myofibroblastic (MFB) phenotype [for review see 6–8]. Most important, the cells are quiescent in normal liver and once activated in culture they have a limited life span and enter senescence after a limited number of cell divisions.
Based on their pivotal role in the initiation and progression of liver fibrogenesis, some major issues including the regulation of extracellular matrix (ECM) components, retinoid metabolism, aspects of contractility and mechanisms of intracellular signalling are addressed in primary HSC cultures in which the cellular status execute a highly dynamic programme from quiescence to activation ending in a transdifferentiated phenotype (MFB).
However, the preparation of these primary cells is time-consuming and animal studies and experimental work using human tissues require ethical and institutional approval by respective national and animal legislation committees, wasteful equipment and skilful assistants. Moreover, the overall costs for isolation of primary HSC including manpower, animals and reagents (collagenase, pronase, DNAse, gradient material and buffers) are extremely high. When assuming that respective protocols yield approximately 35 _ 106 HSC from one rat liver with a plating efficiency of 60%, one eurocent (∼∼0.013 USD) is equivalent to ∼1715 HSC (see Table 1). More drastically, one eurocent is necessary to obtain ∼80 (C57 BL/6) or ∼530 (Balb/c) murine HSC.
1.
Estimated costs for isolation of primary rat HSC*
| Rat | Materials | Wage | |||||||||||
| 1 Rat | 20.00 € | Technician | |||||||||||
| Pronase E | 4.00 € | (3.5 hrs) | 70.00 € | ||||||||||
| Collagenase H | 15.00 € | ||||||||||||
| DNase I | 7.00 € | ||||||||||||
| Nycodenz | 3.00 € | ||||||||||||
| HBSS | 4.00 € | Total Costs | 123.00 € | ||||||||||
| ———– | (w/o equipment & labour expenses) | ||||||||||||
| 53.00 € | |||||||||||||
| ∼ 35 × 106 HSC (60% Plating efficiency) | 1 Cent for ∼ 1715 HSC | ||||||||||||
| Mouse | Materials | Wage | |||||||||||
| 5 Mice | 140.00 € | Technician | |||||||||||
| Pronase E | 8.75 € | (5 hrs) | 100.00 € | ||||||||||
| Collagenase H | 36.00 € | ||||||||||||
| DNase I | 2.50 € | ||||||||||||
| Nycodenz | 2.75 € | ||||||||||||
| HBSS | 4.00 € | Total costs | 294.00€ | ||||||||||
| ———– | (w/o equipment & labour expenses) | ||||||||||||
| 194.00 € | |||||||||||||
| ∼ 3 × 106 HSC (C57 BL/6) | 1 Cent for ∼ 80 HSC (C57 BL/6) | ||||||||||||
| ∼ 20 × 106 HSC (Balb/c) | 1 Cent for ∼ 533 (Balb/c) | ||||||||||||
| 60% Plating efficiency) | |||||||||||||
The costs are calculated based on the actual prices for animals, chemicals, buffers, and salary of personal in Germany. Based on the chosen protocols we use one rat or five mice for HSC preparation. In our hands, the outcome of total HSC from mouse is strongly dependent on animal's genetic background.
In addition, it becomes more and more evident that HSC are a heterogenous cell population [9–13] and it is likely that there will be batch-to-batch variations and differences in cultures from the same origin. Moreover, a significant perturbation of genes coding for transcription factors, antioxidant enzymes and nitric oxide metabolism is observed after prolonged culture times suggesting that safe use and proper analysis in primary cultured HSC is possibly limited to the first days in culture.
Therefore, spontaneous- or experimentallyderived HSC cell lines have at first glance several advantages. They grow continuously, have almost an unlimited lifespan, allow the performance of longterm experiments, and based on their clonal origin have apparently a homogenous and specific phenotype. In addition, they are easily available and culture conditions are simple and easily standardized among different laboratories. The extensive molecular characterization suggest that several of these lines are presumably attractive models to pursue issues of retinoid metabolism, ECM biology, gene regulation, cytokine production and signalling, pharmaceutical issues or other multifaceted research topics. Like primary HSC/MFB, most of these continuous cell lines have well developed _-smooth muscle actin (_-SMA) filaments, produce a multitude of connective tissue markers and some are able to take up and esterify retinol. Finally, several lines respond to transforming growth factor-_1 (TGF-_1) and plateletderived growth factor (PDGF), the major mediators of liver fibrogenesis. Consequently, many investigators argue that these cells resemble primary-cultured HSC and are valuable and useful to address issues of HSC biology and function. As a result, the number of studies performed with immortal HSC lines has increased drastically during the last years (Fig. 1A). Especially several lines established from rat (e. g. HSC-T6, CFSC) and human beings (e. g.LI90, LX-2) are presently attracting many researchers and are enrolled in many studies that were published in various peer-reviewed journals (Fig. 1B and C).
1.
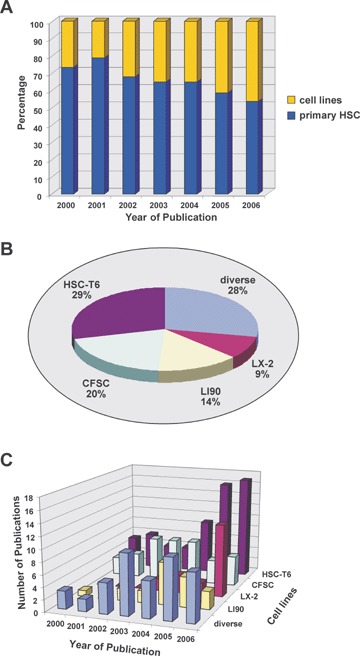
Comparison of studies performed with primary and immortal HSC during the last 6 years. (A) The distribution (in%) of studies performed with primary (in blue) and immortal HSC cell lines (in yellow) that are published in peer reviewed journals during 2000–2006. (B) Relative frequencies of cell lines used in studies with permanent lines during the last 6 years. (C) Numbers of studies performed with individual cell lines during the last 6 years.
However, although widely used in hepatology research, cell lines in general are prone to genotypic, karyotypic and phenotypic drift during prolonged culture time. In addition, sub-populations may arise by the selection of specific, more rapidly growing sub-clones that may produce intralaboratory cell line heterogeneity. Therefore, cell lines used as models in a meaningful way must be intensively characterized before planting on a programme of research. To estimate the advantages/disadvantages, we will first provide a short summary of general and special aspects of immortal HSC cell lines established from mouse, rat and human.
General aspects of immortal HSC
Primary HSC provide characteristics very close to that observed in vivo, but have some significant disadvantages. First, there may be considerable variation in the cellular features of cells prepared in different laboratories or from different operators. In addition, there are a number of plausible reports describing the occurrence of heterogeneity within HSC cultures. Secondly, cells originating from one preparation have only limited utility because of their limited life span. Thirdly, sub-cultivation of primary HSC can be achieved only for a limited number of passages associated with modified characteristics, apoptosis, altered cytokine susceptibility and variations in gene expression [14]. Fourthly, experiments requiring large numbers of cells or long-term growth are rarely possible. Moreover, there is considerable pressure on scientists to reduce or even eliminate the use of animals in research.
To overcome these limitations, many investigators have transferred established concepts to develop permanent HSC cell lines. Presently, immortal HSC cell lines were derived from primary HSC that were (i) transformed with the simian virus 40 large T-antigen (SV40 T) which has pleiotropic effects on cell division achieving this by binding to the transcription factor E2, p53 or pRB, (ii) manipulated by ectopic expression of telomerase reverse transcriptase (TERT) activity, (iii) isolated from experimentally diseased livers, (iv) subjected to UV light or alternatively were (v) spontaneously immortalized during culturing.
As a general feature, the ‘immortalized’ cells escape from normal cell cycle control and grow continuously enabling the preparation of bulk stocks called ‘cell banks’. It is noteworthy but often ignored that cell lines harbouring the SV40 T oncogene or an ectopic TERT are genetically modified and are considered to be genetically modified organisms (GMOs) that must be handled according to the general and national safety guidelines relating to GMOs. Moreover, mammalian cells carrying the SV40 T are classified as a Hazard Group 2 pathogen when the transgene is cloned behind an active promoter directing gene expression in mammals. In addition, several studies have implicated SV40 T in the etiology of tumour formation showing that special handling is advisable. To overcome this safety problem, some HSC cell lines were generated by transfection with a temperature-sensitive (thermolabile) mutant of the SV40 T (tsSV40 T) which is only functional at 33_C, but inactive at 39_C. Therefore, respective cells are reversible immortalized and proliferation only occurs at the permissive temperature while the cells become more differentiated at the nonpermissive temperature.
Although the general process of immortalization in which the successive growth arrest of a cell needs to be overcome parallels to some extent the process of tumourigenesis, none of the published cell lines is reported to have tumourigenic capacity. They are density-inhibited, require serum for their growth, do not grow in soft agar and do not form tumours when implanted or injected into nude mice.
By use of the outlined methodologies, several permanent HSC cell lines were established from mice, rat and human beings. In the following, we summarized aspects of origin, methodology used for immortalization, and cellular characteristics for each of this cell lines.
Immortalized murine HSC lines
GRX
The historically oldest immortal HSC line was obtained from livers of C3 H/HeN mice that were infected by transcutaneous penetration of cercarias from the Schistosoma mansoni BH strain [15]. Cell cultures were initiated from cells that were isolated either by enzymatic digestion or by spontaneous migration from resulting fibrotic granulomas. Individual continuous cell clones with highly proliferative growth characteristics were obtained by sub-culturing having a fibroblastic morphology with stellate, polygonal or elongate shape when cultured at low density on plastic, glass or collagen substrate. The cells are contact-inhibited, anchorage-dependent and accumulate lipids. The GRX cells display a MFB cell morphology including (i) nucleated sub-spherical nuclei, which are elongated and indented in cells that grew in multilayers, (ii) enrichment in ribosomes that are most often grouped in polyribosomes, (iii) extensive rough endoplasmic reticulum, (iv) an extremely rich Golgi system in the perinuclear region, (v) a distinct layer of contractile fibres with local densifications that is characteristic for smooth muscle cells and (vi) cell membranes that are covered with irregular, thick basement membranes.They secrete collagen types I, III and IV, contain laminin, fibronectin, and synthesize a multitude of glycosaminoglycans (i.e.heparitin- and chrondroitin-sulphate). Noteworthy, GRX cells produce viral particles of retrovirus type which can be observed in the cytoplasm, inside small vesicles or in spaces belonging apparently to a system of deep furrows in the cell cytoplasm. Most interestingly, retinol treatment induces an active reorganization of the cytoskeleton in GRX cells. During this process major stress fibres collapse and form a polygonal meshwork [16, 17]. Subsequently, they fragment and generate diffuse or granular actin in the perinuclear area, a thin continuous layer around lipid droplets and, in fully converted lipocytes, a peripheral layer of thin actin fibres. However, both the lipocyte and the myofibroblast phenotypes should be considered ‘activated states’ of HSC.
SV68 c-IS
For establishment of this immortalized cell line, primary HSC from a 6-week-old male ICR mouse were isolated using the collagenase perfusion and pronase- E digestion method and transfected with the wild-type simian virus 40 gene using the lipofectin reagent [18]. Individual clones were selected by limiting dilution culture. The phenotype of the resulting clone SV68 c-IS corresponds to the activated phenotype of HSC having an active growth potential and possessing a MFB shape. The cells express procollagen type III, _-SMA, desmin, GFAP and have a few vitamin A-containing lipid droplets that can be observed after oil red O staining. In addition, the cells show expression of the SV40 T in the nuclei requiring proper and stringent safety precautions when handled.
A640-IS
This immortalized HSC line originated from a male ICR mouse and carries a temperature-sensitive mutant of the SV40 large T-antigen that controls the growing properties of these cells. At the permissive temperature (33_C) the viral protein is expressed and directs active cell growth. Contrarily, under the nonpermissive condition (39_C) the large T-antigen is not expressed, and the cells do not grow. Furthermore, a shift from this non-permissive to the permissive condition restores T-antigen expression and proliferation [19]. In addition, the morphology is strictly dependent on the temperature. At 33_C the cells show a fibroblastic feature, whereas at 39_C they differentiate into a stellate-like HSC phenotype that is associated with an increase in lipid droplets, vitamin A fluorescence and desmin expression. In contrast, the expression of vimentin, collagen types I, III, IV, fibronectin and laminin is independent from the temperature. Interestingly, expression of _-SMA reported to be a good marker of HSC activation is observed under both conditions but is strongly dependent on cell density. It was speculated that at high cell density, contact inhibition induces cell differentiation explaining why expression of _-SMA expression ceased under these conditions [19].
M1–4HSC
This immortalized, non-tumourigenic cell line was established by long-term cultivation exceeding more than 100 cell doublings from primary murine HSC that were isolated by liver perfusion from a 4-month-old male mice that was deficient for p19ARF. This protein is encoded by the INK4 a/ARF tumour suppressor gene that restrains cell growth by modifying the functions of the retinoblastoma protein and p53 [20]. The cell line M1–4HSC displays an epithelial-like phenotype, is devoid of lipid droplets, expresses _-SMA, fibulin-2, GFAP, desmin and vimentin, grew in monolayers and shows a heterogeneous phenotype with regard to cell size and shape. Interestingly, albeit basically activated, M1–4HSC undergo a further activation/ transdifferentiation to a more pronounce spindle- shaped MFB phenotype during a 3-week treatment with TGF-_1. The resulting phenotype (M-HT) grows in polylayers and shows decreased expression of desmin, while the expression of other HSC markers (i.e._-SMA, GFAP) is not affected.
A7
This immortal murine cell line was established from the transgenic mouse strain H-2 kb- ts-A58 harbouring a temperature-sensitive SV40 T variation under the control of the ubiquitous H-2Kb promoter [21]. The cell clone A7 grows only under the permissive conditions (33_C) and not under the non-permissive conditions (37_C). Based on the properties to accumulate and esterify retinol it was suggested that this line is an ideal tool for studying retinoid metabolism.
Immortalized rat HSC lines
HSC-T6
For establishment of this cell line, primary HSC isolated from male Sprague–Dawley rats were transiently transfected at day 15 of primary culture with the SV40 T that was expressed under control of the Rous Sarcoma virus promoter [22]. Individual clones were harvested and plated to limiting dilution. The expansion of one clone resulted in a stable phenotype exhibiting an activated phenotype with a fibroblast- like shape and high proliferation activity. Typical cytoskeletal markers of activated HSC including _-SMA, desmin, GFAP, as well as vimentin are expressed in HSC-T6 and, when cultured in media containing high concentrations of retinol, the cells form cytosolic lipid droplets and accumulate retinyl esters that can be enhanced by enrichment of the media with exogenous fatty acids. In addition, the cells express the retinoid nuclear receptors RAR_, RAR_, RAT_, RXR_, RXR_ and RXR_ as well as the cellular retinol-binding protein type I (CRBP) that is up-regulated in the presence of retinol or all-transretinoic acid.
NFSC, CFSC and derivatives
In 1991, Greenwel and co-workers reported about the spontaneous immortalization of HSC from normal (NFSC) and cirrhotic livers (CFSC) from male Wistar rats [23]. Cirrhosis was induced by intraperitoneal administration of CCl4 for 5 weeks. Isolation of HSC from livers was performed by pronase perfusion following centrifugation on a Percoll gradient. In the initial characterization, it was found that NFSC proliferated more slowly than CFSC and that both cell fractions contained approximately 90% cells that were positive for desmin and vimentin. After 10 passages, the cells were again purified through percoll or metrizamide gradients resulting in NFSC cells that were smaller than those obtained from the cirrhotic livers. Both cell types are mononucleated, have fusiform appearances and the cytoplasm is rich in free ribosomes and contain an abundant rough endoplasmic reticulum that in some areas is dilated and contain granular material. Moreover, CFSC and NFSC express collagen types I and III, as well as fibronectin, laminin and TGF-_. However, the CFSC contain less fat droplets and a more pronounced fibrillar ECM. A further difference is the expression of IL-6 that is only detectable in NFSC but not in CFSC. Subsequent cell cloning by limiting dilution allowed the selection of four different clones that were named CFSC-8B, CFSC- 2G, CFSC-3 H and CFSC-5 H [24]. The individual lines show a striking clonal heterogeneity. They significantly differ in their proliferation properties, expression of ECM components and Gap junction proteins (i.e. connexion 43), and expression/responsiveness toward cytokines (i.e.IL-6, TGF-_).
PAV-1
This continuous rat HSC line was isolated by amplification of a colony that was obtained by spontaneous immortalization of isolated primary HSC isolated from an over 8-month-old male Wistar rat using the pronase-collagenase perfusion/Nycodenz methodology [25]. The phenotypic characteristics of PAV-1 cells resembled that of activated HSC, for example, they express _-SMA, vimentin, desmin, fibronectin, laminin and collagen types I and IV but are negative for GFAP. In addition, the PAV-1 cells do not express cytokeratin19 and von Willebrand factors representing marker proteins of hepatocytes, bile duct cells, or endothelial cells, respectively. The cells were reported to be highly appropriate for studies on retinol uptake and metabolisms since they possess all necessary pathways for retinol esterification [26].
HSC-PQ
This cell line originates from primary rat HSC that were separated from an adult male Sprague–Dawley rat by in situperfusion with collagenase IV/pronase E and purified on a single-step Nycodenz density gradient. The resulting HSC cultures were grown to confluence and subjected to ultraviolet light [27]. Sub-culturing resulted in the isolation of a permanent cell clone that showed a contractile, myofibroblast-like, starshaped or spindled shaped morphology. Light and transmission electron microscopy demonstrated that the respective cells have a low nucleus/cytoplasm ratio, long cytoplasmic protuberance, well developed bundles and filaments, one or two nucleoli with lowdense and separated chromatin, loss of fat droplet, dilated rough endoplasmic reticulum containing electron-densed material. Immunohistochemical analysis further revealed that the cells express collagen types I and III as well as _-SMA, desmin, laminin, fibronectin but lack collagen type IV. The originators further reported that the cell line stably maintains its characteristics in long-term cultures without supplementation of any growth factors.
BSC
This HSC line derived from a male Wistar rat that was subjected to experimental biliary liver fibrosis rendered by obstruction of the bile duct for 18 days. The primary cells were purified by sequential liver digestion with pronase/collagenase and subsequent purification in an arabinogalactan gradient [28, 29]. Individual clones that were obtained by spontaneous immortalization were isolated by serial dilution after 60 passages. One of the resulting clones, BSC-c10, was shown to express GFAP, synaptophysin, fibulin-2, desmin, _1(I) procollagen and _-SMA. When stimulated with TGF-_1, the cells significantly decrease in DNA synthesis and increase collagen production. Like other cell lines, BSC are easily transfectable with plasmid DNA.
MFBY2
For establishment of MFBY2 cells, a 6-week-old male Wister rat was intraperitoneally injected with a 1:1 mixture of CCl4:olive oil twice a week for 1 month and MFB-like cells were isolated [30]. The cells have a MFB-like morphology, express the neural cell adhesion molecule (N-CAM), _-SMA, TIMP-1, _1 collagen type I and type III. Most interestingly, the infection with an adenovirus expressing the amino terminal latency-associated peptide (LAP) of TGF-_1 resulted in the reverse transformation of the MFB-like into a phenotype which is more similar to HSC that was associated with a loss of _-SMA expression, expression of GFAP and the capacity to take up retinoic acid similar to primary HSC.
T-HSC/Cl-6
Starting from primary HSC that were isolated from the Sprague–Dawley strain and transfected at day 12 after initial plating with a plasmid carrying the SV40 T and a neomycin-resistant cassette, a cell line derived that were positive for _-SMA, desmin, GFAP, collagen type I and TGF-_ mRNA [31].
Other rat HSC lines
Features suggestive of HSC/MFB origin were reported for other cell lines [32, 33]. However, the cellular and biochemical properties of these lines are presently only marginal characterized (Table 2).
2.
Biochemical characteristics of HSC lines from mouse, rat and human*
| Origin | Cell line | Desmin | α-SMA | GFAP | Vimentin | Fibronectin | Collagen I | Collagen III | Collagen IV | Laminin | MMP2 | TIMP-1 | Ret.-Uptake | Esterification | TGFβ_1 | IL-6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rrt | CFSC* | + | N | N | + | + | + | + | - | + | N | N | + | N | + | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NFSC | + | N | N | + | + | + | + | - | + | N | N | + | N | + | + | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HSC-T6 | + | + | + | + | + | + | + | + | + | N | + | + | + | + | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PAV-1 | + | + | - | + | + | + | N | + | + | N | N | + | + | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HSC-PQ | + | + | N | + | + | + | + | - | + | N | N | - | N | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BSC | + | + | + | N | N | + | N | N | N | N | N | N | N | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MFBY2 | N | + | + | N | N | + | + | N | N | N | + | N | N | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MFBY2 (+LAP) | N | - | - | N | N | + | + | N | N | N | + | N | N | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T-HSC/CI6 | + | + | + | N | N | + | N | N | N | N | N | N | N | + | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Human | LI90 | - | + | N | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GREF-X | N | + | N | + | + | + | N | + | + | + | N | + | + | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LX-1 | N | + | + | + | N | + | N | N | N | + | + | + | + | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LX-2 | N | + | + | + | N | + | N | N | N | + | (+) | + | + | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| hTERT-HSC | N | + | + | N | + | + | N | N | + | N | N | + | N | N | + | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HSC 180 | N | + | N | N | N | + | N | N | N | + | + | N | N | + | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mouse | GRX | + | + | + | + | + | + | + | + | + | N | N | + | + | N | N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SV68-IS | + | + | + | N | N | N | + | N | N | N | N | + | + | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A640-IS (39_C) | + | + | N | + | + | + | + | + | + | N | N | + | N | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A640-IS (33_C) | - | + | N | + | + | + | + | + | + | N | N | + | N | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| M1–4HSC | + | + | + | + | N | N | N | N | N | N | N | - | - | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| M-HT | (+) | + | + | + | N | N | N | N | N | N | N | - | - | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A7 | N | N | N | N | N | N | N | N | N | N | N | + | + | N | N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cytoskeletal proteins | Matrix and adhesion proteins | MMPs and TIMPs | Retinol | Cytokines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The expression of individual markers was shown at protein or mRNA level. Abbreviations used are: _-SMA, _-smooth muscle actin, GFAP, glial fibrillary acidid protein; IL-6, interleukin-6, MMP2, matrix metalloproteinase 2; N, presently not tested; TIMP-1, tissue inhibitor of metalloproteinase-1, TGF-_1, transforming growth factor-_1.
Immortalized human HSC lines
LI90 and derivatives
The first human HSC line reported was establishedfrom an epithelioid hemangioendothelioma that was found in the right liver lobe of a 55-year-old Japanese female during cholecystectomy [34]. Outgrowths of the primary tumour contained large polygonal cells admixed with spindle-shaped cells. During passage, large polygonal cells that had abundant cytoplasm with well developed intracytoplasmic filaments and oval nuclei became predominant over spindle-shaped cells. Detailed analysis revealed that the growth properties of the cells were similar to that of cultured smooth muscle cells having a doubling time of approximately 60 hrs when cultured in medium containing 10% fetal calf serum (FCS) which allows serially passaging (at a dilution of 1:3) every 2–3 weeks. Typically, LI90 cells form a hills-and-valleys structure when over-confluently cultured. The karyotype analysis of 20 metaphase nuclei revealed that only 10% of cells analysed had minor chromosomal abnormalities suggesting that LI90 cells are not transformed and might have originated from non-neoplastic progenitor cells present in the original tumour. Typical markers that are expressed in these cells are vimentin, _-SMA, collagen types I, III, IV, V and VI, fibronectin and laminin, while they are negative for desmin and lack markers of endothelial cells or cells of the monocyte/macrophage-lineage. When the cells are cultured in the presence of retinol, they form many fat droplets in their cytoplasm and become able to take up, store and esterify vitamin A. Furthermore, LI90 cells produce TGF-_. Since the first report describing the isolation of this HSC line in 1995, the cell line has been used in a number of studies, but, however, subsequent studies revealed that parental LI90 enter replicative senescence that can be overcome by retrovirally introducing human telomerase reverse transcriptase resulting in a novel HSC line (TWNT-4) that is less prone to senescence [35]. In another derivative, TWNT-1, the LI90 cells were transduced with a retroviral vector expressing hTERT and the green fluorescent protein cDNA flanked by a pair of loxP sites [36].
GREF-X
For establishment of this cell line, human myofibroblasts that were isolated from explants of histologically normal liver were transfected with a plasmid containing the coding sequence of polyoma virus large T antigen expressed under the control of the early promoter of cytomegalovirus (CMV) [37]. These cells were repeatedly sub-cultured in medium containing 10% FCS resulting in a morphology resembling that of primary liver myofibroblasts and expression of _-SMA, collagens type I, IV, V and VI, MMP-2, laminin, vimentin as well as fibronectin. In addition, the cells are negative for indicative markers (i.e. cytokeratin, von Willebrand factor and CD68) of other hepatic cell populations. In addition, GREF-X cells were shown to incorporate and esterify retinol suggesting that they originated from HSC. The originators further reported that the doubling time of GREF-X cells is 3 days when grown in DMEM supplemented with 10% FCS. Although this cell line shows several anomalies of chromosome number and structure that are characteristic for a transformed phenotype, the cells are density-inhibited, require serum, do not grow in soft agar and are not able to induce subcutaneous tumours in nude mice. It is noteworthy to mention that the established cell line is negative for large T-antigen as demonstrated by immunofluorescence, Southern blotting and PCR allowing the usage of this cell line in laboratories without special biohazard precautions. Unfortunately, since the first description in 1997, there are presently no subsequent reports available studying this immortalized human cell line.
hTERT-HSC
To establish this HSC line, primary HSC were isolated from surgical specimens of normal liver and infected with a retrovirus expressing human telomerase reverse transcriptase (hTERT) driven by the CMV promoter and a neomycin-selectable gene cassette [38].Two individual cell clones were selected by incubation with G418 for 3 weeks. Using a telomeric repeat amplification protocol one cell clone was positively tested for telomerase activity demonstrating that the transgene maintains telomere integrity, while no colony formation in soft agar was found showing that the extended life span is not mediated by oncogenic transformation. The doubling time of this cell line is specified with 4 days. Detailed microarray analysis and RT-PCR further revealed that the gene expression pattern of this cell line is very similar to that of activated human HSC, both expressing mRNA for IL-6, IL-8, IL-10, PDGFR_, PDGFR_, GFAP, collagen _1(I) and _-SMA. In addition, the telomerasepositive cell line was capable to take up and store retinol. Most interestingly, these cells revert to a more quiescent phenotype by culturing in a basement membrane-like matrix indicating that this cell line is in principal a valuable tool for investigation of the transition from a quiescent to an activated phenotype.
LX-1 and LX-2
The human HSC lines most utilized (cf. Fig. 1C) were generated by either transformation with a plasmid encoding the SV40 large T antigen under the control of a Rous sarcoma virus promoter (LX-1) or by spontaneous immortalization of a subset of early passaged LX-1 cells that were grown in low serum conditions (LX-2) [39]. Immunohistochemical analysis revealed that the SV40 large T antigen was mostly localized in the nucleus of LX-1 cells, while the LX-2 cells were negative for viral proteins. Both cell lines express _-SMA, vimentin and GFAP, as well as the type _ receptor for platelet-derived growth (PDGFR_, discoidin domain receptor 2 (DDR2) and the leptin receptor OB-RL that are key components in fibrogenic response demonstrating that the phenotype of both LX lines is most similar to that of activated HSC. In addition, both LX cell lines secrete _1(I) procollagen and matrix degrading complexes comprising of pro- MMP-2, MT1-MMP and TIMP-2. Moreover, they retain key features of HSC in absorbing, accumulating and converting retinol to retinyl ester. Comparative microarray profiling further demonstrate that LX lines and primary HSC show a strong similarity in expression of a multitude of neuronal genes underscoring the notion that these lines are of HSC origin. Particularly, the ample characterization promoted these cell lines as starting material for many experimental studies addressing special aspects of the cell biology of human HSC and hepatic fibrogenesis.
HSC 180
Recently, a novel human HSC line was derived initially from a cirrhotic liver. This line was shown express _-SMA, MMP-2, MMP-3, MMP-9 and several molecules involved in the plasminogen activator pathway [40]. In addition, the cells were found to have capacity to synthesize the mRNAs for TGF-_1, TIMP-1 and collagen type I.
Qualities of primary and immortalized HSC
Several features make HSC lines potentially appropriate for investigations analysing general cellular and biochemical aspects of HSC biology. They exhibit many HSC/MFB-specific features and express almost all relevant marker genes indicating that they originated from HSC. Moreover, comparison of individual lines with primary cells by microarray analysis revealed that the gene expression patterns are striking similar (up to 98.7%) with expression of multiple neuronal and ECM genes, again suggesting that the cells originate from HSC [39, 41]. However, there are pronounced differences that are summarized in the following.
Culture and growth characteristics
Generally, primary HSC are maintained under standard conditions (humidified atmosphere with 5% CO2, 95% air at 37_C) in medium containing 10% FCS. Under these conditions, they have an overall low doubling time of several days and can be passaged only two or three times before going through a crisis, entering senescence and ending in an enlarged and flattened phenotype. The underlying mechanisms of this replicative senescence are most likely inactivation of proliferation-promoting genes and/or activation of anti-proliferative genes or the switch from a fibrogenic to an inflammatory phenotype leading to apoptosis [42]. Established HSC lines, however, grow faster with typical duplication times between 24 and 72 hrs and can be continuously passaged demonstrating that these cells have lost many characteristics of the primary cultures from which they are derived. In several lines, these changes of growth characteristic are accompanied by confounding genetic events introduced by an aneuploid chromosome complement that is characterized by additions or deletions of whole chromosomes from the expected balanced diploid number of chromosomes [15, 29, 42].
Differences in cellular morphology
As outlined above, the culturing of primary HSC induces a remarkably phenotypic gradually response ending in a MFB phenotype in which the responsiveness to cytokines and other local stimuli is temporarily modified [8, 43, 44]. Therefore, it is conceivable that the cellular phenotype of primary HSC and MFB is highly dynamic, time- and culture-dependent. This gradual phenotypic change under constant culture conditions from a non-proliferating, retinoid-storing cell to a proliferating, retinoid loosing phenotype, which increasingly expresses _-SMA can easily be detected (Fig. 2A–C). Contrarily, the morphology of most immortal HSC lines is a constant and more resembles that of a ‘MFB-like’ than a ‘HSC-like’ phenotype (Fig. 2D–F). The overall biochemical and morphological characteristics of these lines further suggest that most are indeed counterparts of fully transdifferentiated MFB (Fig. 3). Although some of these lines can be somewhat reverted into a ‘activated HSC’ phenotype by treatment with the latency-associated peptide (LAP) of TGF-_, high concentration of retinol, or culturing on basement membrane-like matrix components, none is capable to revert into the quiescent state. Contrarily, it has been reported that primary human MFB obtained from cirrhotic livers were reverted to _-SMA-negative, lipid droplet-positive quiescent morphology when re-cultured in type I collagen gels or MatrigelTM [45, 46]. Collectively, the morphological and biochemical features argue against the usage of these cell lines when critical issues of quiescence or modulated cellular events during activation and transdifferentiation should be experimentally addressed.
2.
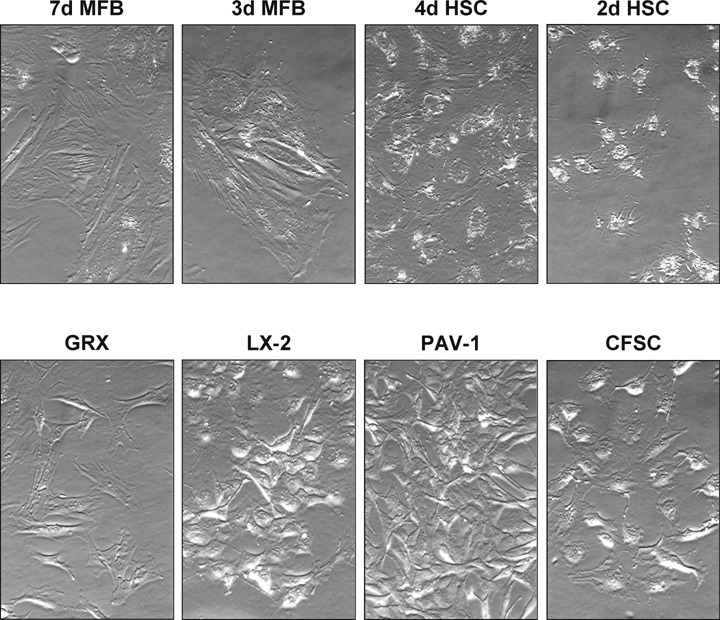
Morphology of primary and immortal HSC. Appearance of primary rat HSC and MFB cultured for indicated times (upper panel) and immortal GRX, LX-2, PAV-1 and CFSC cell lines (lower panel) in differential interference contrast (DIC)- Normaski microscopy (original magnification _ 320).The cells were cultured in DMEM supplemented with 10% FCS (HSC; MFB; CFSC; PAV-1 and GRX) or 2% FCS (LX-2), non-essential amino acids (CFSC), glutamine and antibiotics.
3.
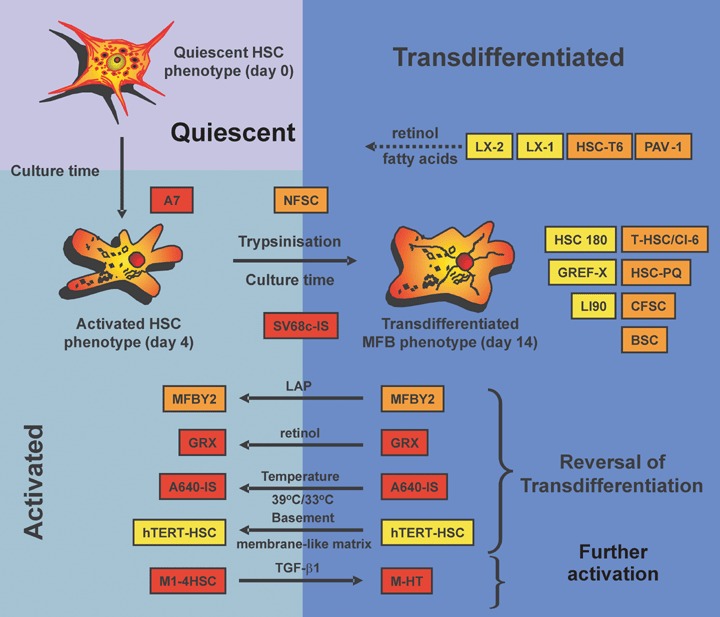
Classification of permanent HSC cell lines in regard to activation and transdifferentiation. Human (in yellow), rat (in orange) and murine cell lines (in red) were grouped depending on their biochemical characteristics into activated HSC or transdifferentiated MFB. The cell lines MFBY2, GRX, A640-IS and hTERT-HSC can be converted from a MFB-like into an activated HSC phenotype by treatment with LAP, retinol, temperature or culturing on a basement membrane-like matrix, respectively. Conversely, long-term treatment of M1–4HSC with TGF-_1 results in conversion into M-HT which more resembles the MFB phenotype. In addition, the cell lines LX-2, LX-1, HSC-T6 and PAV-1 accumulate cytosolic lipid droplets when exposed to media containing higher concentrations of retinol (1 and 5 μM).
Differences in gene expression
Gene expression profiling is providing novel insights into differences in primary and immortal HSC [39, 42]. The comparison of LX-1 and LX-2 with primary HSC resulted in the isolation of eighteen genes that were differentially expressed [39]. Consistent with the immortalized phenotype, the two cell lines showed reduced expression of the cyclin-dependent kinase inhibitor-1 A (p21). An increased expression was found for the cell division cycle 2 (CDC2), thymidine kinase 1, TGF-_1-induced 68 kDa protein, chromosome segregation 1-like and survivin, genes that were previously reported to either promote proliferation or include anti-apoptotic capacity. Another comparison of 9000 genes in activated human HSCs with immortal HSC generated by ectopic telomerase expression revealed that hundred fifty-two (1.8%) were detected at higher expression levels whereas 87 genes (1%) were found at lower levels in activated human HSCs [42]. Taken together, these two studies demonstrate that although primary and established HSC are substantially similar, distinct but significant differences in gene expression are present between primary and immortal HSCs.
Most immortal HSC cells are highly transfectable
It has been demonstrated that primary HSC have very low transfection efficiencies [47]. This has imposed significant limitations of this cell type when transient transfections are required during a study. In contrast, the transfection efficiency of many cell lines is rather good when commonly used reagent transfection agents (e. g. FuGENE 6TM, LipofectaminTM) are taken. It has been reported that the efficiency of transfection is ∼30% in LX-2 cells [39]. Although a similar degree of transfectability is also found in other lines (e. g. CFSC, GRX) in our laboratory, it is not a general feature of all immortal HSCs. LX-1 cells for example are reported to have efficiencies of less than 1%[39].
Response to growth factors
The growth of primary HSC is serum-dependent since serum starvation results in a significant decrease of cell proliferation. Although established lines are also widely cultured in medium containing FCS, it has been reported that LX-2 cells withstand serum deprivation [39] making it an effective tool for investigation requiring low serum conditions (e. g. zymographic or cytokine stimulation assays).
Interestingly, most (if not all) immortal HSC lines resembling a MFB-like phenotype sensitively respond to TGF-_1. In initial experiments it has been shown that the addition of TGF-_1 to LI90 cultures results in slight increases of _-SMA, hyaluronic acid, laminin, whereas the production of the hepatocyte growth factor (HGF) was significantly reduced [48]. Similarly, small increases in fibronectin, collagen type III and laminin were detected after a 24-hrs stimulation period with TGF-_1 [48]. Likewise, the treatment of both LX-1 and LX-2 cells resulted in a strong upregulation of (1_I) procollagen mRNA demonstrating that these lines are responsive to TGF-_1 [48, 39]. In addition, leptin increased collagen mRNA and protein expression, possibly by induction of the TGF- _ type II receptors [49]. Contrarily, fully transdifferentiated MFB generated from primary HSC cultures were not inhibited in proliferation activity on treatment with TGF-_1 and, furthermore, stimulation of collagen, Smad7 messenger RNA (mRNA) expression or stimulation of TGF-_ reporter assays by TGF- _1 were achieved in early HSC cultures but not in MFB [50, 51]. Concerning the highly dynamic conveniences in regard to TGF-_ responsiveness it is therefore essential to define the precise conditions (i.e. culture time) when reporting aspects of TGF-_- dependent gene expression or signalling.
Platelet-derived growth factor is the most effective mitogen for cultured primary HSC and stimulated cell proliferation even under low serum conditions [52, 53]. However, this cytokine has only marginal stimulatory effects on some immortal HSC [23]. Also the finding that primary HSC grow better in the presence of TGF-_ and EGF [54] is not true for some of the established cell lines [23]. Collectively, these data clearly indicate several differences in response to growth factors between immortal HSC and their primary counterpart. Anyhow, several insights in regard to activities of cytokines or other stimuli were uncovered in immortal HSC lines. A summary of functional aspects for selected HSC lines are listed in Table 3.
3.
Functional aspects of selected HSC lines
| Cell line | Effector | Response | References | |||||
|---|---|---|---|---|---|---|---|---|
| CFSC | PDGF | Proliferation (+) | [23] | |||||
| LTBP-1 (+) | [55] | |||||||
| TGF-β | α2(I)Collagen (+) | [56] | ||||||
| α1(I) Collagen (+) | [57] | |||||||
| CRP2 (+) | [58] | |||||||
| IL-6 | α1(VI)Procollagen (+) | [24] | ||||||
| Fibronectin (+) | [24] | |||||||
| IL-1β | TIMP-1 (+) | [59] | ||||||
| IFN-α | α2(I)Collagen (-) | [60] | ||||||
| IFN-γ | α2(I)Collagen (-) | [60] | ||||||
| TNF-α | α1(I)Collagen (-) | [61] | ||||||
| HSC-T6 | PDGF | Proliferation (+) | [62] | |||||
| TGF-β | α1(I)Collagen (+) | [63] | ||||||
| Fibronectin (+) | [64] | |||||||
| PAI-1 (+) | [64] | |||||||
| TIMP-1 (+) | [65] | |||||||
| IL-6 | HGF (+) | [66] | ||||||
| IFN-γ | Collagen I (-) | [67] | ||||||
| Collagen III (-) | [67] | |||||||
| Cathepsin S (+) | [68] | |||||||
| Angiotensin ll | α1(I)Procollagen (+) | [69] | ||||||
| Aldostrone | α1(I)Procollagen (+) | [69] | ||||||
| PDGF-B (+) | [70] | |||||||
| Leptin | α2(I)Collagen (+) | [71] | ||||||
| Retinol | CRBP-I (+) | [72] | ||||||
| GRX | Retinol | Proliferation (-) | [72] | |||||
| ECM synthesis (-) | [72] | |||||||
| Cell adherence (-) | [72] | |||||||
| Cytoskeletal reorganisation | [17] | |||||||
| Intermediate filament proteins (-) | [73] | |||||||
| LX-2 | PDGF | Proliferation (+) | [39] | |||||
| TGF-β | α1(I)Collagen (+) | [39] | ||||||
| PAI-1 (+) | [74] | |||||||
| Proliferation (+) | [75] | |||||||
| MMP-2 (+) | [75] | |||||||
| TIMP-1 (+) | [75] | |||||||
| Collagen III (+) | [75] | |||||||
| α-SMA (+) | [75] | |||||||
| Leptin | TIMP-1 (+) | [76] | ||||||
| α1(I) Collagen (+) | [77] | |||||||
| MMP-1 (-) | [78] | |||||||
| Li-90 | PDGF | Proliferation (+) | [79] | |||||
| TGF-β | Calponin-h1 (+) | [48] | ||||||
| Hyaluronan (+) | [80] | |||||||
| IL-4 | α1(I)Collagen (+) | [81] | ||||||
| IL-13 | Proliferation (-) | [81] | ||||||
| Angiotensin II | RhoA (+) | [82] | ||||||
| MCP-1 (+) | [82] | |||||||
| TNF-β | MMP-9 (+) | [83] | ||||||
| HGF | MMP-1 (+) | [84] | ||||||
Abbreviations: _-SMA, _-smooth muscle actin; CRBP-I, Cellular retinol-binding protein type I; CRP2, Cysteine- and glycine-rich protein 2; HGF, Hepatocyte growth factor; IFN-_/-_, Interferon-_/-_; IL-1_/-4/-6/-13, Interleukine-1_/-4/-6/-13; LTBP-1, Latent TGF-_ binding protein-1; MCP-1, Monocyte chemoattractant protein-1; MMP-1/-2/-9, Matrix-Metalloproteinase-1/-2/-9; PAI-1, Plasminogen activator inhibitor-1; PDGF, Platelet-derived growth factor; T3, Triiodothyronine; TGF-_, Transforming growth factor-_; TIMP-1, Tissue inhibitor of metalloproteinases-1; TNF-_, Tumor necrosis factor-_.
Conclusions
The introduction of protocols for isolation of animal and human HSC has enabled systematic in vitro studies on biology and function of this versatile hepatic cell type. Cultured on uncoated plastic surfaces, these primary cells undergo a gradual, highly dynamic change from a quiescent, fat- and retinoidstoring phenotype into ECM producing, contractile MFB. Therefore, the outcome of studies performed with these primary cells is strongly dependent on the time point at which the cells are investigated. However, due to many reasons a growing attention is given to immortal HSC lines. In such continuous populations, most phenotypic features remain constant during many passages. Undoubtedly, immortalized HSC cell lines have a number of advantages; they (i) represent an unlimited self-replicating source that can be grown easily in almost infinite quantities, (ii) potentially exhibit a relatively high degree of homogeneity, (iii) are susceptible to transient gene transfer, (iv) can be stored as frozen stocks for long term, and, moreover and (v) restrict animal experimentation. During the last years, they have been key reagents for the discovery of many fundamental, clearly defined basic approaches of HSC function and biology like studies on retinoid storage and metabolism, regulatory and signalling pathways, cellular targeting and proof-of-principle testing of therapeutically effective substances. However, these cell lines are prone to genotypic and phenotypic drift at higher passage numbers and are not appropriate to mimic the cellular dynamics of HSC in primary culture. In addition, these cell lines are inappropriate when general studies on cellular dynamics (e. g. activation, transdifferentiation, senescence) or its sustainable modulation (e. g. abrogation, reversal) in HSC/MFB should be performed. In addition, studies aiming to analyse mechanisms of HSC apoptosis are senseless when performed in these immortal lines. These studies that are only realizable in primary cells are definitely more complex and need more sophisticated methodologies, equipment and technicians aggravating experimental, high-throughput work.
Keeping in mind that immortalized cells are potentially vulnerable to artefacts careful planning at the outset of a study is demanded when deciding if primary or immortal HSC best fit the purpose during experimentation.
Acknowledgments
The authors thanks R. Borojevic (Departamento de Histologia e Embriologia, Instituto de Ciencias Biomedicas, Universidade Federal do Rio de Janeiro, RJ, Brazil), M. Rojkind (Marion Bessin Liver Research Center, Albert Einstein College of Medicine, Bronx, New York, USA) and S. L. Friedman (Division of Liver Diseases, Mount Sinai School of Medicine, New York, NY 10029, USA) for kindly providing cell lines GRX, CFSC and LX-2, respectively.
References
- 1.De Leeuw AM, McCarthy SP, Geerts A, Knook DL. Purified rat liver fat-stroing cells in culture divide and contain collagen. Hepatology. 1984;4:392–403. doi: 10.1002/hep.1840040307. [DOI] [PubMed] [Google Scholar]
- 2.Weiskirchen R, Gressner AM. Isolation and culture of hepatic stellate cells. Methods Mol Med. 2005;117:99–113. doi: 10.1385/1-59259-940-0:099. [DOI] [PubMed] [Google Scholar]
- 3.Geerts A, Niki T, Hellemans K, De Craemer D, Van Den Berg K, Lazou JM, Stange G, Van De Winkel M, De Bleser P. Purification of rat hepatic stellate cells by side scatter-activated cell sorting. Hepatology. 1998;27:590–8. doi: 10.1002/hep.510270238. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992;15:234–43. doi: 10.1002/hep.1840150211. [DOI] [PubMed] [Google Scholar]
- 5.Blazejewski S, Preaux AM, Mallat A, Brocheriou I, Mavier P, Dhumeaux D, Hartmann D, Schuppan D, Rosenbaum J. Human myofibroblastlike cells obtained by outgrowth are representative of the fibrogenic cells in the liver. Hepatology. 1995;22:788–97. [PubMed] [Google Scholar]
- 6.Kmiec Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol. 2001;161:1–151. doi: 10.1007/978-3-642-56553-3. [DOI] [PubMed] [Google Scholar]
- 7.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311–35. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 8.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-_ as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbst H, Frey A, Heinrichs O, Milani S, Bechstein WO, Neuhaus P, Schuppan D. Heterogeneity of liver cells expressing procollagen types I and IV in vivo. Histochem Cell Biol. 1997;107:399–409. doi: 10.1007/s004180050126. [DOI] [PubMed] [Google Scholar]
- 10.Zou Z, Ekataksin W, Wake K. Zonal and regional differences identified from precision mapping of vitamin A-storing lipid droplets of the hepatic stellate cells in pig liver: a novel concept of addressing the intralobular area of heterogeneity. Hepatology. 1998;27:1098–108. doi: 10.1002/hep.510270427. [DOI] [PubMed] [Google Scholar]
- 11.Knittel T, Kobold D, Saile B, Grundmann A, Neubauer K, Piscaglia F, Ramadori G. Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology. 1999;117:1205–21. doi: 10.1016/s0016-5085(99)70407-5. [DOI] [PubMed] [Google Scholar]
- 12.Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology. 2004;40:1151–9. doi: 10.1002/hep.20427. [DOI] [PubMed] [Google Scholar]
- 13.Kordes C, Sawitza I, Müller-Marbach A, Ale-Agha N, Keitel V, Klonowski-Stumpe H, Häussinger D. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun. 2007;352:410–7. doi: 10.1016/j.bbrc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen DB, Kawada N, Imamura K, Miyamoto Y, Tateno C, Seki S, Kuroki T, Yoshizato K. Proteome analysis of rat hepatic stellate cells. Hepatology. 2000;32:268–77. doi: 10.1053/jhep.2000.9322. [DOI] [PubMed] [Google Scholar]
- 15.Borojevic R, Monteiro AN, Vinhas SA, Domont GB, Mourao PA, Emonard H, Grimaldi G, Jr, Grimaud JA. Establishment of a continuous cell line from fibrotic schistosomal granulomas in mice livers. In vitro Cell Dev Biol. 1985;21:382–90. doi: 10.1007/BF02623469. [DOI] [PubMed] [Google Scholar]
- 16.Guma FCR, Mello TG, Mermelstein CS, Fortuna VA, Wofchuk ST, Gottfried C, Guaragna RM, Costa ML, Borojevic R. Intermediate filaments modulation in an in vitro model of the hepatic stellate cell activation or conversion into the lipocyte phenotype. Biochem Cell Biol. 2001;79:409–17. [PubMed] [Google Scholar]
- 17.Mermelstein CS, Guma FC, Mello TG, Fortuna VA, Guaragna RM, Costa ML, Borojevic R. Induction of the lipocyte phenotype in murine hepatic stellate cells: reorganisation of the actin cytoskeleton. Cell Tissue Res. 2001;306:75–83. doi: 10.1007/s004410100428. [DOI] [PubMed] [Google Scholar]
- 18.Horie S, Kitamura Y, Kawasaki H, Terada T. Inhibitory effects of antisense oligonucleotides on the expression of procollagen type III gene in mouse hepatic stellate cells transformed by simian virus 40. Pathol Int. 2000;50:937–44. doi: 10.1046/j.1440-1827.2000.01146.x. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura Y, Tanigawa T, Katsumoto T, Tomita K, Wang HR, Hirai K, Ichihara K, Terada T. Cell growth and differentiation of a novel mouse Ito (fat-storing) cell line transformed by a temperature-sensitive mutant of simian virus 40. Hepatology. 1997;26:323–9. doi: 10.1002/hep.510260211. [DOI] [PubMed] [Google Scholar]
- 20.Proell V, Mikula M, Fuchs E, Mikulits W. The plasticity of p19 ARF null hepatic stellate cells and the dynamics of activation. Biochim Biophys Acta. 2005;1744:76–87. doi: 10.1016/j.bbamcr.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Matsuura T, Kawada M, Sujino H, Hasumura S, Nagamori S, Shimizu H. Vitamin A metabolism of immortalized hepatic stellate cells in the bioreactor. In: Wisse E, Knook DL, De Zanger R, Arthur MJP, editors. Cells of the Hepatic Sinusoid 7. Leiden: Kupffer Cell Foundation; 1999. pp. 88–89. [Google Scholar]
- 22.Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, Blaner WS. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882–93. [PubMed] [Google Scholar]
- 23.Greenwel P, Schwartz M, Rosas M, Peyrol S, Grimaud JA, Rojkind M. Characterization of fatstoring cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin- 6. Lab Invest. 65:644–53. [PubMed] [Google Scholar]
- 24.Greenwel P, Rubin J, Schwartz M, Hertzberg EL, Rojkind M. Liver fat-storing cell clones obtained from a CCl4-cirrhotic rat are heterogeneous with regard to proliferation, expression of extracellular matrix components, interleukin-6, and connexin 43. Lab Invest. 1993;69:210–6. [PubMed] [Google Scholar]
- 25.Sauvant P, Sapin V, Abergel A, Schmidt CK, Blanchon L, Alexandre-Gouabau MC, Rosenbaum J, Bommelaer G, Rock E, Dastugue B, Nau H, Azais-Braesco V. PAV-1, a new rat hepatic stellate cell line converts retinol into retinoic acid, a process altered by ethanol. Int J Biochem Cell Biol. 2002;34:1017–29. doi: 10.1016/s1357-2725(02)00023-7. [DOI] [PubMed] [Google Scholar]
- 26.Sauvant P, Abergel A, Partier A, Alexandre-Gouabau MC, Rock E, Sion B, Motta C, Sapin V, Azais-Bresco V. Treatment of the rat hepatic stellate cell line, PAV-1, by retinol and palmitic acid leads to a convenient model to study retinoids metabolism. Biol Cell. 2002;94:401–8. doi: 10.1016/s0248-4900(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 27.Pan Q, Li DG, Lu HM, Wang YQ, Zhang WZ, Xu QF. A new immortalized rat cell line, hepatic stellate cell-PQ, exhibiting characteristics of hepatic stellate cell. Hepatobiliary Pancreat Dis Int. 2005;4:281–4. [PubMed] [Google Scholar]
- 28.Xiong S, Yavrom S, Hazra S, Wu D, She H. Spontaneously immortalized cell line from rat liver fibrosis with phenotypes of both hepatic stellate cells and myofibroblasts. Hepatology. 2001;34:520A. [Google Scholar]
- 29.Sung SK, She H, Xiong S, Tsukamoto H. Tumor necrosis factor-_ inhibits peroxisome proliferator-activated receptor _ activity at a posttranslational level in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2003;286:G722–9. doi: 10.1152/ajpgi.00411.2003. [DOI] [PubMed] [Google Scholar]
- 30.Isono M, Soda M, Inoue A, Akiyoshi H, Sato K. Reverse transformation of hepatic myofibroblast-like cells by TGF_1/LAP. Biochem Biophys Res Commun. 2003;311:959–65. doi: 10.1016/j.bbrc.2003.10.093. [DOI] [PubMed] [Google Scholar]
- 31.Kim JY, Kim KM, Nan JX, Zhao YZ, Park PH, Lee SJ, Sohn DH. Induction of apoptosis in transhinone I via cytochrome c release in activated hepatic stellate cells. Pharmacol & Toxicol. 2003;92:195–200. doi: 10.1034/j.1600-0773.2003.920410.x. [DOI] [PubMed] [Google Scholar]
- 32.Tahashi Y, Matsuzaki K, Date M, Yoshida K, Furukawa F, Sugano Y, Matsushita M, Himeno Y, Inagaki Y, Inoue K. Differential regulation of TGF-_ signal in hepatic stellate cells between acute and chronic rat liver injury. Hepatology. 2002;35:49–61. doi: 10.1053/jhep.2002.30083. [DOI] [PubMed] [Google Scholar]
- 33.Rao HY, Wei L, Fei R, Wang JH, Jiang D, Zhang Q, Cong X. Inhibitory effect of interferon-_ on the activation of LX-2 and rHSC-99 hepatic stellate cells in culture. Zhonghua Gan Zang Bing Za Zhi. 2006;14:550–2. [PubMed] [Google Scholar]
- 34.Murakami K, Abe T, Miyazawa M, Yamaguchi M, Masuda T, Matsuura T, Nagamori S, Takeuchi K, Abe K, Kyogoku M. Establishment of a new human cell line, LI90, exhibiting characteristics of hepatic Ito (fat-storing) cells. Lab Invest. 1995;72:731–9. [PubMed] [Google Scholar]
- 35.Shibata N, Watanabe T, Okitsu T, Sakaguchi M, Takesue M, Kunieda T, Omoto K, Yamamoto S, Tanaka N, Kobayashi N. Establishment of an immortalized human hepatic stellate cell line to develop antifibrotic therapies. Cell Transplant. 2003;12:499–507. doi: 10.3727/000000003108747064. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe T, Shibata N, Westerman KA, Okitsu T, Allain JE, Sakaguchi M, Totsugawa T, Maruyama M, Matsumura T, Noguchi H, Yamamoto S, Hikida M, Ohmori A, Reth M, Weber A, Tanaka N, Leboulch P, Kobayashi N. Establishment of immortalized human hepatic stellate scavenger cells to develop bioartificial livers. Transplantation. 2003;75:1873–80. doi: 10.1097/01.TP.0000064621.50907.A6. [DOI] [PubMed] [Google Scholar]
- 37.Weill FX, Blazejewski S, Blanc JF, Huet S, Gauthier JM, Neaud V, Olaso E, Dubuisson L, Azais-Braesco V, Vidal-Vanaclocha F, Balabaud C, Bioulac-Sage P, Rosenbaum J. Characterization of a new human liver myofibroblast cell line: transcriptional regulation of plasminogen activator inhibitor type I by transforming growth factor beta 1. Lab Invest. 1997;77:63–70. [PubMed] [Google Scholar]
- 38.Schnabl B, Choi YH, Olsen JC, Hagedorn CH, Brenner DA. Immortal activated human hepatic stellate cells generated by ectopic telomerase expression. Lab Invest. 2002;82:323–33. doi: 10.1038/labinvest.3780426. [DOI] [PubMed] [Google Scholar]
- 39.Xu L, Hui AY, Albanis E, Arthur MJ, O’Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–51. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González-Cuevas J, Bueno-Topete M, Armendariz-Borunda Urokinase plasminogen activator stimulates function of active forms of stromelysin and gelatinases (MMP-2 and MMP-9) in cirrhotic tissue. J Gastroenterol Hepatol. 2006;21:1544–54. doi: 10.1111/j.1440-1746.2006.04398.x. [DOI] [PubMed] [Google Scholar]
- 41.Schnabl B, Purbeck CA, Choi YH, Hagedorn CH, Brenner D, Schnabl B, Purbeck CA, Choi YH, Shibata N, Watanabe T, Okitsu T, Sakaguchi M, Takesue M, Kunieda T, Omoto K, Yamamoto S, Tanaka N, Kobayashi N. Establishment of an immortalized human hepatic stellate cell line to develop antifibrotic therapies. Cell Transplant. 2003;12:499–507. doi: 10.3727/000000003108747064. [DOI] [PubMed] [Google Scholar]
- 42.Schnabl B, Purbeck CA, Choi YH, Hagedorn CH, Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–64. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- 43.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 44.Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-_ in hepatic fibrosis. Front Biosci. 2002;7:d793–807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- 45.Senoo H, Imai K, Sato M, Kojima N, Miura M, Hata RI. Three-dimensional structure of extracellular matrix reversibly regulates morphology, proliferation, and collagen metabolism of perisinusoidal stellate cells (Vitamin A-storing cells) Cell Biol Int. 1996;20:501–12. doi: 10.1006/cbir.1996.0065. [DOI] [PubMed] [Google Scholar]
- 46.Sohara N, Znoyko I, Levy MT, Trojanowska M, Reuben A. Reversal of activation of human myofibroblast- like cells by culture on a basement membrane- like substrate. J Hepatol. 2002;37:214–21. doi: 10.1016/s0168-8278(02)00103-4. [DOI] [PubMed] [Google Scholar]
- 47.Weiskirchen R, Kneifel J, Weiskirchen S, Van De Leur E, Kunz D, Gressner AM. Comparative evaluation of gene delivery devices in primary cultures of rat hepatic stellate cells and rat myofibroblasts. BMC Cell Biol. 2000;1:4. doi: 10.1186/1471-2121-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueki N, Ohkawa T, Yamamura H, Takahashi K, Tsutsui T, Kawai Y, Yokoyama Y, Amuro Y, Hada T, Higashino K. Induction of calponin-h1 by transforming growth factor-_1 in cultured human Ito cells, LI90. Biochim Biophys Acta. 1998;1403:28–36. doi: 10.1016/s0167-4889(98)00015-9. [DOI] [PubMed] [Google Scholar]
- 49.Tang M, Potter JJ, Mezey E. Leptin enhances the effect of transforming growth factor _ in increasing type I collagen formation. Biochem Biophys Res Commun. 2002;297:906–11. doi: 10.1016/s0006-291x(02)02300-8. [DOI] [PubMed] [Google Scholar]
- 50.Dooley S, Delvoux B, Lahme B, Mangasser-Stephan K, Gressner AM. Modulation of transforming growth factor beta response and signaling during transdifferentiation of rat hepatic stellate cells to myofibroblasts. Hepatology. 2000;31:1094–106. doi: 10.1053/he.2000.6126. [DOI] [PubMed] [Google Scholar]
- 51.Dooley S, Delvoux B, Streckert M, Bonzel L, Stopa M, Ten Dijke P, Gressner AM. Transforming growth factor beta signal transduction in hepatic stellate cells via Smad2/3 phosphorylation, a pathway that is abrogated during in vitro progression to myofibroblasts. TGF_ signal transduction during transdifferentiation of hepatic stellate cells. FEBS Lett. 2001;502:4–10. doi: 10.1016/s0014-5793(01)02656-4. [DOI] [PubMed] [Google Scholar]
- 52.Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989;84:1786–93. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borkham-Kamphorst E, Van Roeyen CR, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol. 2007;46:1064–74. doi: 10.1016/j.jhep.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 54.Bachem MG, Riess U, Gressner AM. Liver fat-storing cell proliferation is stimulated by epidermal growth factor/transforming growth factor β and inhibited by transforming growth factor β. Biochem Biophys Res Commun. 1989;162:708–14. doi: 10.1016/0006-291x(89)92368-1. [DOI] [PubMed] [Google Scholar]
- 55.Westhoff JH, Sawitza I, Keski-Oja J, Gressner AM, Breitkopf K. PDGF-BB induces expression of LTBP-1 but not TGF-β 1 in a rat cirrhotic fat storing cell line. Growth Factors. 2003;21:121–30. doi: 10.1080/08977190310001637224. [DOI] [PubMed] [Google Scholar]
- 56.Inagaki Y, Truter S, Greenwel P, Rojkind M, Unoura M, Kobayashi K, Ramirez F. Regulation of the alpha 2(I) collagen gene transcription in fat-storing cells derived from a cirrhotic liver. Hepatology. 1995;22:573–9. [PubMed] [Google Scholar]
- 57.Garcia-Trevijano ER, Iraburu MJ, Fontana L, Dominguez-Rosales JA, Auster A, Covarrubias-Pinedo A, Rojkind M. Transforming growth factor beta1 induces the expression of alpha1(I) procollagen mRNA by a hydrogen peroxide-C/EBPbetadependent mechanism in rat hepatic stellate cells. Hepatology. 1999;29:960–70. doi: 10.1002/hep.510290346. [DOI] [PubMed] [Google Scholar]
- 58.Herrmann J, Borkham-Kamphorst E, Haas U, Van de Leur E, Fraga MF, Esteller M, Gressner AM, Weiskirchen R. The expression of CSRP2encoding the LIM domain protein CRP2 is mediated by TGF-_ in smooth muscle and hepatic stellate cells. Biochem Biophys Res Commun. 2006;345:1526–35. doi: 10.1016/j.bbrc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 59.Zhang YP, Yao XX, Zhao X. Interleukin-1 _ up-regulates tissue inhibitor of matrix metalloproteinase-1 mRNA and phosphorylation of c-jun N-terminal kinase and p38 in hepatic stellate cells. World J Gastroenterol. 2006;12:1392–6. doi: 10.3748/wjg.v12.i9.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inagaki Y, Nemoto T, Kushida M, Sheng Y, Higashi K, Ikeda K, Kawada N, Shirasaki F, Takehara K, Sugiyama K, Fujii M, Yamauchi H, Nakao A, De Crombrugghe B, Watanabe T, Okazaki I. Interferon alfa down-regulates collagen gene transcription and suppresses experimental hepatic fibrosis in mice. Hepatology. 2003;38:890–9. doi: 10.1053/jhep.2003.50408. [DOI] [PubMed] [Google Scholar]
- 61.Iraburu MJ, Dominguez-Rosales JA, Fontana L, Auster A, Garcia-Trevijano ER, Covarrubias-Pinedo A, Rivas-Estilla AM, Greenwel P, Rojkind M. Tumor necrosis factor alpha down-regulates expression of the alpha1(I) collagen gene in rat hepatic stellate cells through a p20 C/EBPbeta- and C/EBPdelta-dependent mechanism. Hepatology. 2000;31:1086–93. doi: 10.1053/he.2000.5981. [DOI] [PubMed] [Google Scholar]
- 62.Zhang M, Zhang JP, Ji HT, Wang JS, Qian DH. Effect of six flavonoids on proliferation of hepatic stellate cells in vitro. Acta Pharmacol Sin. 2000;21:253–6. [PubMed] [Google Scholar]
- 63.Song YH, Zhou XM, Xue XN, Liu NZ, Tian DA, Kong XJ, Wu XL, Lin JS, Jin YX. Effect of ribozyme against transforming growth factor _1 on biological character of activated HSCs. IUBMB Life. 2005;57:31–9. doi: 10.1080/15216540400024470. [DOI] [PubMed] [Google Scholar]
- 64.Liu X, Wang W, Hu H, Tang N, Zhang C, Liang W, Wang M. Smad3 specific inhibitor, naringenin, decreases the expression of extracellular matrix induced by TGF-beta1 in cultured rat hepatic stellate cells. Pharm Res. 2006;23:82–9. doi: 10.1007/s11095-005-9043-5. [DOI] [PubMed] [Google Scholar]
- 65.Gui M, Zhang YF, Xiao ZY, Sun P, Dai JF, Wang SF, Rui YC, Zhang JP. Inhibitory effect of emodin on tissue inhibitor of metalloproteinases-1 (TIMP-1) expression in rat hepatic stellate cells. Dig Dis Sci. 2007;52:200–7. doi: 10.1007/s10620-006-9321-z. [DOI] [PubMed] [Google Scholar]
- 66.Kariv R, Enden A, Zvibel I, Rosner G, Brill S, Shafritz DA, Halpern Z, Oren R. Triiodothyronine and interleukin-6 (IL-6) induce expression of HGF in an immortalized rat hepatic stellate cell line. Liver Int. 2003;23:187–93. doi: 10.1034/j.1600-0676.2003.00827.x. [DOI] [PubMed] [Google Scholar]
- 67.Ma H, Ma XM, Yin CH, Jia JD, Wang BE. Effects of gamma-interferon on gene expression of collagen I, III and on the tissue inhibitor of metalloprotenase 1 in HSC-T6 cells. Zhonghua Gan Zang Bing Za Zhi. 2005;13:528–30. [PubMed] [Google Scholar]
- 68.Maubach G, Lim MC, Kumar S, Zhuo L. Expression and upregulation of cathepsin S and other early molecules required for antigen presentation in activated hepatic stellate cells upon IFN-gamma treatment. Biochim Biophys Acta. 2007;1773:219–31. doi: 10.1016/j.bbamcr.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Meng Y, Cai SX, Yang XS, Zhang YJ, Wu PS. Angiotensin II and aldosterone stimulate alpha1-(I) procollagen mRNA expression in hepatic stellate cells via activation of ERK1/2 and AP-1. Zhonghua Yi Xue Za Zhi. 2005;85:1831–5. [PubMed] [Google Scholar]
- 70.Li X, Meng Y, Yang XS, Wu PS, Zhang ZS. Aldosterone stimulating PDGF-B expression in HSC via activation of EGR-1. Zhonghua Gan Zang Bing Za Zhi. 2005;13:567–70. [PubMed] [Google Scholar]
- 71.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–71. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Margis R, Pinheiro-Margis M, Da Silva LC, Borojevic R. Effects of retinol on proliferation, cell adherence and extracellular matrix synthesis in a liver myofibroblast or lipocyte cell line (GRX) Int J Exp Pathol. 1992;73:125–35. [PMC free article] [PubMed] [Google Scholar]
- 73.Guma FCR, Mello TG, Mermelstein CS, Fortuna VA, Wofchuk ST, Gottfried C, Guaragna RM, Costa ML, Borojevic R. Intermediate filaments modulation in an in vitro model of the hepatic stellate cell activation or conversion into the lipocyte phenotype. Biochem Cell Biol. 2001;79:409–17. [PubMed] [Google Scholar]
- 74.Zhao C, Chen W, Yang L, Chen L, Stimpson SA, Diehl AM. PPAR_ agonists prevent TGF_1/Smad3- signaling in human hepatic stellate cells. Biochem Biophys Res Commun. 2006;350:385–91. doi: 10.1016/j.bbrc.2006.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi YF, Zhang Q, Cheung PY, Shi L, Fong CC, Zhang Y, Tzang CH, Chan BP, Fong WF, Chun J, Kung HF, Yang M. Effects of rhDecorin on TGF-_1 induced human hepatic stellate cells LX-2 activation. Biochim Biophys Acta. 2006;1760:1587–95. doi: 10.1016/j.bbagen.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 76.Cao Q, Mak KM, Ren C, Lieber CS. Leptin stimulates tissue inhibitor of metalloproteinase-1 in human hepatic stellate cells: respective roles of the JAK/STAT and JAK-mediated H2 O2-dependant MAPK pathways. J Biol Chem. 2004;279:4292–304. doi: 10.1074/jbc.M308351200. [DOI] [PubMed] [Google Scholar]
- 77.Cao Q, Mak KM, Lieber CS. Leptin enhances _1(I) collagen gene expression in LX-2 human hepatic stellate cells through JAK-mediated H2 O2-dependent MAPK pathways. J Cell Biochem. 2006;97:188–97. doi: 10.1002/jcb.20622. [DOI] [PubMed] [Google Scholar]
- 78.Cao Q, Mak KM, Lieber CS. Leptin represses matrix metalloproteinase-1 gene expression in LX2 human hepatic stellate cells. J Hepatol. 2007;46:124–33. doi: 10.1016/j.jhep.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 79.Hui AY, Cheng AS, Chan HL, Go MY, Chan FK, Sakata R, Ueno T, Sata M, Sung JJ. Effect of prostaglandin E2 and prostaglandin I2 on PDGFinduced proliferation of LI90, a human hepatic stellate cell line. Prostaglandins Leukot Essent Fatty Acids. 2004;71:329–33. doi: 10.1016/j.plefa.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Ueki N, Taguchi T, Takahashi M, Adachi M, Ohkawa T, Amuro Y, Hada T, Higashino K. Inhibition of hyaluronan synthesis by vesnarinone in cultured human myofibroblasts. Biochim Biophys Acta. 2000;1495:160–7. doi: 10.1016/s0167-4889(99)00161-5. [DOI] [PubMed] [Google Scholar]
- 81.Sugimoto R, Enjoji M, Nakamuta M, Ohta S, Kohjima M, Fukushima M, Kuniyoshi M, Arimura E, Morizono S, Kotoh K, Nawata H. Effect of IL-4 and IL-13 on collagen production in cultured LI90 human hepatic stellate cells. Liver Int. 2005;25:420–8. doi: 10.1111/j.1478-3231.2005.01087.x. [DOI] [PubMed] [Google Scholar]
- 82.Kanno K, Tazuma S, Nishioka T, Hyogo H, Chayama K. Angiotensin II participates in hepatic inflammation and fibrosis through MCP-1 expression. Dig Dis Sci. 2005;50:942–8. doi: 10.1007/s10620-005-2669-7. [DOI] [PubMed] [Google Scholar]
- 83.Migita K, Maeda Y, Abiru S, Nakamura M, Komori A, Yokoyama T, Takii Y, Mori T, Yatsuhashi H, Eguchi K, Ishibashi H. Immunosuppressant FK506 inhibits matrix metalloproteinase-9 induction in TNF- _-stimulated human hepatic stellate cells. Life Sci. 2006;78:2510–5. doi: 10.1016/j.lfs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Ozaki I, Zhao G, Mizuta T, Ogawa Y, Hara T, Kajihara S, Hisatomi A, Sakai T, Yamamoto K. Hepatocyte growth factor induces collagenase (matrix metalloproteinase-1) viathe transcription factor Ets-1 in human hepatic stellate cell line. J Hepatol. 2002;36:169–78. doi: 10.1016/s0168-8278(01)00245-8. [DOI] [PubMed] [Google Scholar]


