Abstract
Pentraxins act as soluble pattern recognition receptors with a wide range of functions in various pathophysiological conditions. The long-pentraxin PTX3 shares the C-terminal pentraxin-domain with short-pentraxins C-reactive protein and serum amyloid P component and possesses an unique N-terminal domain. These structural features suggest that PTX3 may have both overlapping and distinct biological/ligand recognition properties when compared to short-pentraxins. PTX3 serves as a mechanism of amplification of inflammation and innate immunity. Indeed, vessel wall elements produce high amounts of PTX3 during inflammation and the levels of circulating PTX3 increase in several pathological conditions affecting the cardiovascular system. PTX3 exists as a free or extracellular matrix-associated molecule and it binds the complement fraction C1q. PTX3 binds also apoptotic cells and selected pathogens, playing a role in innate immunity processes. In endothelial cells and macrophages, PTX3 upregulates tissue factor expression, suggesting its action as a regulator of endothelium during thrombogenesis and ischaemic vascular disease. Finally, PTX3 binds the angiogenic fibroblast growth factor-2, thus inhibiting its biological activity. Taken together, these properties point to a role for PTX3 during vascular damage, angiogenesis, atherosclerosis, and restenosis.
Keywords: angiogenesis, atherosclerosis, bacteria, complement, cytokines, extracellular matrix, FGF, pentraxin, restenosis, vasculitis
Introduction
Pentraxins are a superfamily of evolutionarily conserved proteins originally named for their structural organization characterized by a radial pentameric structure. Pentraxins are divided into two subfamilies (short-pentraxins and long-pentraxins) sharing a C-terminal pentraxin domain that contains the HxCxS/TWxS pentraxin signature (where x is any amino acid) [1]. Long-pentraxins differ from short-pentraxins for the presence of an unrelated N-terminal domain coupled to the C-terminal domain [2] (Fig. 1).
1.
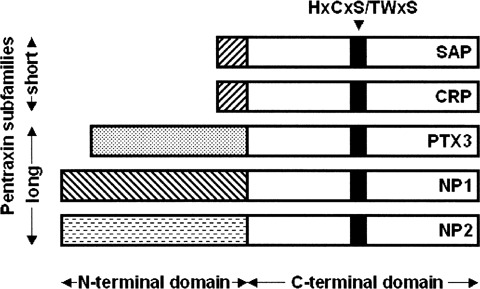
Schematic representation of the pentraxin superfamily. Short- and long-pentraxins show a significant homology in their C-terminal pentraxin domain whereas long-pentraxins are characterized by unique N-terminal extensions. The 8 amino acid-long pentraxin family signature is highlighted.
C-reactive protein (CRP) and serum amyloid P component (SAP) constitute the short-pentraxin arm of the superfamily. These “classical” pentraxins are acute phase proteins in man and mouse, respectively, and are produced in liver in response to inflammatory mediators, most prominently interleukin-6 (IL-6) [3, 4]. They are involved in the innate resistance to microbes and scavenging of cellular debris and extracellular matrix (ECM) components [1, 5–7].
Pentraxin 3 (PTX3, also called TSG-14) is the prototypic member of the long-pentraxin subfamily [8, 9] that includes also guinea pig apexin, rat, human, and murine neuronal pentraxins 1 (NP1 or NPTX1) and NP2 (also called Narp or NPTX2), and the putative integral membrane pentraxin NRP (see [1] for a recent review). NP1 and Narp may form mixed pentraxin assemblies that play a role in excitatory synaptic plasticity in developing and adult brain [10]. Long-pentraxins have been found in Xenopus(XL-PXN1) [2] and in Drosophila melanogaster[11]. Also, PTX3, NP1, NP2, and NPR orthologs have been identified in zebrafish [1].
PTX3 is a soluble pattern recognition receptor with unique non-redundant functions in various physiopathological conditions [12]. Unlike short-pentraxins, PTX3 is synthesized locally at the inflammatory site by mononuclear phagocytes [13], myeloid dendritic cells [14], fibroblasts [9], adipocytes [15], granulosa cells [16], mesangial cells [17, 18], smooth muscle cells (SMCs) [8] and endothelial cells [8]. PTX3 expression is upregulated in mice during inflammation [19, 20] and the levels of circulating PTX3 increase in several pathological conditions affecting the cardiovascular system, including vasculitides [21], acute myocardial infarction [22], rheumatoid arthritis [23], and systemic inflammatory response syndrome/septic shock [24].
Like “classical” pentraxins, PTX3 binds the complement component C1q, leading to activation of the classical complement pathway [25]. PTX3 binds also apoptotic cells, selected pathogens, ECM components and angiogenic growth factors (Table 1). PTX3 transgenic mice show improved survival to endotoxemia and sepsis [26]. Conversely, PTX3−/− mice show defective resistance against selected pathogens [27]. Also, PTX3−/− mice show an alteration in seizureinduced damage in the central nervous system [20] and deficient women are sub-fertile [16]. Taken together, the data indicate that PTX3 may serve as a mechanism of amplification of inflammation and innate immunity, tightly related to endothelial cell functions (Fig. 2).
1.
PTX3 ligands
| Tested ligands | Interaction with PTX3 | References | ||||||
|---|---|---|---|---|---|---|---|---|
| Complement fractions | C1q | yes | [25, 47, 53–55] | |||||
| C1s | no | [25] | ||||||
| Short-pentraxin ligands | phosphorylcoline | no | [25] | |||||
| phosphoethanolamine | no | [25] | ||||||
| high pyruvate agarose | no | [25] | ||||||
| histones | yes | [25, 44] | ||||||
| Isolated microbial components | zymosan | yes | [48] | |||||
| KpOmpA | yes | [51] | ||||||
| ECM components | hyaluronic acid | no | [35] | |||||
| TSG6 | yes | [35] | ||||||
| heparin/HSPGs | no | * | ||||||
| type IV collagen | no | [25, 44] | ||||||
| fibronectin | no | [25, 44] | ||||||
| vitronectin | no | [44] | ||||||
| Eukariotic cell surfaces/receptors | apoptotic cell extranuclear membrane | yes | [82] | |||||
| A-type K(+) channel | yes | [105] | ||||||
| integrins | no | * | ||||||
| gangliosides | no | * | ||||||
| HSPGs | no | * | ||||||
| Growth factors/cytokines/chemokines | FGF2 | yes | [44] | |||||
| FGF1 | no | [44] | ||||||
| FGF4 | no | [44] | ||||||
| FGF8 | weak | [44] | ||||||
| VEGF | weak | [44] | ||||||
| NGF | no | [44] | ||||||
| M-CSF | no | [44] | ||||||
| TNF | no | [44] | ||||||
| IL-1 | no | [44] | ||||||
| IL-4 | no | [44] | ||||||
| IL-6 | no | [44] | ||||||
| IL-8 | no | [44] | ||||||
| IL-10 | no | [44] | ||||||
| IL-12 | no | [44] | ||||||
| MCP-1 | no | [44] | ||||||
| lymphotactin | no | [44] | ||||||
M. Rusnati, unpublished observations.
2.
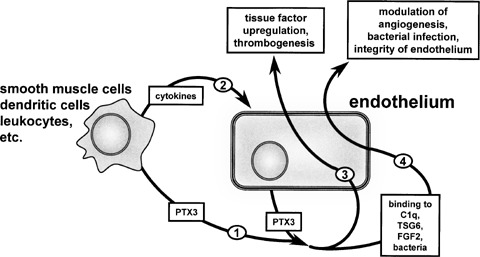
Cross-talk between PTX3 and endothelium. Different cell types produce PTX3 (1) and/or cytokines that stimulate PTX3 upregulation in endothelium (2). PTX3 may then stimulate directly endothelial cells in a paracrine/autocrine manner (3) or it can bind various endothelial effectors (4). This will affect endothelial cell functions and integrity.
Here, the biochemical and biological properties of PTX3 will be reviewed in the context of its role in vascular biology.
PTX3 gene and expression
The human PTX3 gene has been identified using differential screening of cDNA libraries constructed from IL-1β-stimulated human umbilical vein endothelial cells [8] and from transforming growth factorα (TGFα) and TNFα-simulated normal FS-4 fibroblasts [28]. Unlike CRP and SAP genes that map on chromosome 1, PTX3 gene is localized on chromosome 3, band q25. The PTX3 gene comprises three exons, the first exon extending to nucleotide 197, the second one covering nucleotides 198–599, and the third one extending from nucleotide 600 to the 3’-terminus. The first two exons encode for the signal peptide and the N-terminal domain of PTX3 protein whereas the third exon encodes for its C-terminal domain, matching exactly the second exon of the short-pentraxin genes [8].
The complete nucleotide sequence of the PTX3 transcript consists of a 5’-terminal untraslated region of 68-bp, an open reading frame of 1143 bp with a stop codon at position 1211, and a polyadenylation signal at position 1802. In addition, the 3’-terminal untranslated region contains two consensus sequences for mRNA instability [8]. The proximal 1317-bp promoter of the human PTX3 gene is responsive to TNFα and IL-1β but not to IL-6. Multiple binding sites for various transcription factors have been identified in this sequence, including NF-IL-6, AP-1, Pu1, PEA-3, Ets-1, and Sp1 sites. Also, NF-kB sites are essential for induction by IL-1 and TNFα[29, 30]. No TATA or CAAT consensus sequences have been identified.
As already mentioned, PTX3 is synthesized locally at the inflammatory site by several cell types upon exposure to different inflammatory signals. PTX3 expression in monocytes is stimulated by IL-1β, TNFα, bacterial lipopolysaccharide (LPS), and lipoarabinomannans [13, 31]. The induction is rapid and transient, with a peak at 4–6 hrs after stimulation. Other cytokines, including IL-6, monocyte chemoattractant protein-1 (MCP-1), monocyte colony stimulating factor (M-CFS), and granulocyte/macrophage colony stimulating factor (GM-CSF) do not induce PTX3 gene expression despite their capacity to induce other biological responses in these cells [13]. In human monocytes, interferon-γ (IFNγ) strongly inhibits PTX3 expression and protein secretion stimulated by IL-1β, TNFα, and LPS [32].
Human dendritic cells of myelomonocytic origin produce the highest amount of PTX3 in response to microbial ligands engaging different members of the Toll-like receptor (TLR) family [14]. Indeed, PTX3 production in these cells is induced by TLR and CD40 ligands, IL-1β and IL-10, being instead suppressed by dexamethasone, vitamin D3, prostaglandin E2, IFNγ, and IL-4 [33].
Recent data have shown that human synoviocytes from type B osteoarthritis produce PTX3 in response to TNFα, but not to IL-1β, whereas transforming growth factor β (TGFβ) partially inhibits TNFα- dependent PTX3 upregulation. On the other hand, synoviocytes from rheumatoid arthritis constitutively express high levels of PTX3 and this expression is not affected by anti-TNFα antibodies, IL-1β receptor antagonist, or by a combination of the two agents [23]. Similarly, a constitutive high expression of PTX3 is observed in human scleroderma fibroblasts that is inhibited by IFNα and TGFβ[34].
In differentiating murine adipocytes, but not in fully differentiated cells, TNFα induces a transient PTX3 mRNA upregulation. Also, in respect to control mice, genetically obese mice show an increased PTX3 expression in adipose tissue, even though the relative contribution of stromal vascular cells and adipocytes to PTX3 expression in this tissue remains unclear [15].
PTX3 expression is regulated by the growth differentiation factor-9 in murine granulosa cells of preovulatory follicles. Accordingly, PTX3 is produced in vivo by murine cumulus cells during cumulus expansion [16, 35].
Cultured human mesangial cells synthesize PTX3 when stimulated with TNFα or IgA. Interestingly, the specific binding of recombinant PTX3 to these cells has been reported, indicating that mesangial cells may be both a source of and a target for PTX3 [17]. In addition, stimulation of human proximal renal tubular epithelial cells with IL-17 and CD40 ligands, but not with IL-6 or IL-4, results in a strong increase in PTX3 production [18]. Finally, IL-1β and TNFα induce PTX3 expression in human lung epithelial cells via the JNK pathway [36, 37].
Vascular endothelial cells and SMCs produce abundant amounts of PTX3 in response to inflammatory signals. Beside IL-1β and LPS, oxidized and enzymatically degraded low density lipoproteins (LDL) promote PTX3 production by human primary vascular SMCs [38, 39] (see below for further details about PTX3 expression in atherosclerosis). Statins inhibit PTX3 gene expression in human umbilical vein endothelial cells and SMCs, but not in hepatocarcinoma cells [40]. Since PTX3 plays a pivotal role in thrombogenesis and ischaemic vascular disease (see below), PTX3 down-regulation may contribute to the reduction of the risk of cardiovascular diseases by statins [40, 41].
In vivo, PTX3 gene expression is induced by LPS in a variety of organs in mice. In particular, PTX3 is produced by the vascular endothelium of skeletal and cardiac muscle. Unlike neuronal long-pentraxins, PTX3 is not constitutively expressed by the central nervous system. However, injection into the cerebral ventricles of LPS, IL-1β and TNFα induces local expression of PTX3. Interestingly, LPS and IL-1β also induce high levels of PTX3 expression in the heart [19]. Finally, intratracheal instillation of LPS, that induces acute lung injury in mice, is associated with a significant increase of PTX3 expression in the bronchoalveolar lavage fluid and in the lung tissue [37].
PTX3 protein structure
The human PTX3 protomer is a 381 amino acid glycoprotein, including a 17 amino acid signal peptide for secretion. PTX3 primary sequence is highly conserved among animal species (human and murine PTX3 sharing 92% of conserved amino acid residues), suggesting a strong evolutionary pressure to maintain its structure-function relationships [1]. As the other members of the long-pentraxin subfamily, PTX3 is composed of an unique N-terminal domain (spanning amino acid residues 1–178) and of a Cterminal 203 amino acid domain highly homologous among the various members of the pentraxin family (57% of conserved amino acids with short-pentraxins CRP and SAP).
No crystallographic data are available for the Nterminal portion of long-pentraxins. We performed consensus secondary structure prediction of PTX3 N-terminus [42]. Four α-helix regions connected by short loops have been identified that span amino acid residues 55–75 (αA), 78–97 (αB), 109–135 (αC), and 144–170 (αD) (Fig. 3A). Moreover, αB contains the structural heptad repeat motif (abcdefg) spanning amino acid residues 85–91, where a and d are hydrophobic residues and e and g represent charged residues [43]. Also, hydrophobic residues repeated with a period of one each three or six amino acids are present within the primary sequence of αC and αD. Thus, αB, αC, and αD helices of the N-terminal PTX3 domain have propensity to be in a coiled-coil conformation (as predicted by Coils2; http://www.ch.embnet.org/software/COILS_form.html). Moreover, the occurrence of short loops between α-helices suggests an up-down topological distribution (Fig. 3B).
3.
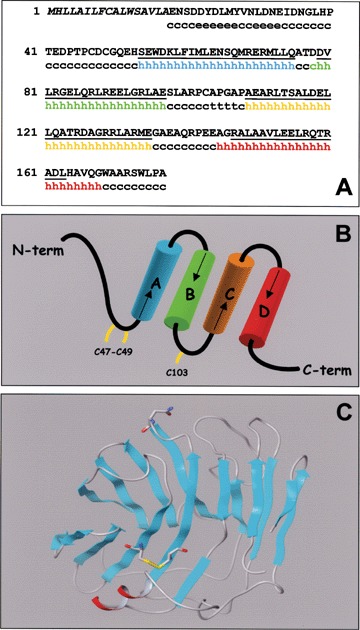
PTX3 protein structure. (A) - Amino acid sequence (single letter code) and prediction of the secondary structure of the PTX3 N-terminus (residues 1-178): h =α helix; e =β-sheet; c = random coil; t =β-turn. Coiled-coil regions are underlined and the signal sequence is in Italics. (B) - Topological representation of PTX3 N-terminus; cysteine residues are highlighted. (C) - Model of the PTX3 C-terminus obtained by homology modelling with the crystallographic structure of CRP. Residue Asn220 and the Cys210- Cys271 disulphide bridge are represented by sticks. The α-helix is shown in red.
As described below, PTX3 binds with high affinity the angiogenic polypeptide fibroblast growth factor-2 (FGF2) [44]. The putative minimal linear FGF2 binding region in PTX3 comprises amino acid residues 97–110 [42]. This binding domain is predicted in an exposed loop region that comprises the end of the αB-helix (Glu97), a β-turn on residues Ala104 -Pro105 -Gly106 -Ala107, and the first two residues of the αC-helix (Ala109-Glu110) [42].
The N-terminal sequence of PTX3 contains three cysteine residues in positions 47, 49, and 103. Three cysteine residues are present also in the N-terminal coiled-coil α-helices of the human long-pentraxins NP1 and NP2 in the positions 16, 26, 73 and 14, 26, 79, respectively [10]. These residues are engaged in inter-chain disulphide bonds, thus providing stability to the multimeric status of NP1 and NP2 [10]. The similar distribution of N-terminal cysteine residues in NP1, NP2, and PTX3 in terms of relative distances suggests that Cys47, 49, 103 within the PTX3 N-terminus may exert an analogous structural role. In spite of no obvious primary sequence similarity between PTX3 N-terminus and other known proteins, the occurrence of α-helices in coiled-coil conformation and the likelihood of cysteine residues engaged in inter-chain disulphide bonds suggest a structural similarity between the N-terminal region of PTX3 and the N-terminal domain of the members of the collectin family, including the mannose-binding protein (MBP) and the surfactant proteins A and D (SP-A and SP-D) [45]. This structural similarity well matches with the role of these collectins and of PTX3 as humoral components of the innate immune system [45].
The high similarity of the primary sequence of the PTX3 C-terminus with short-pentraxins allowed to produce a predicted structure for the C-terminal PTX3 region (residues 179–381) using the crystallographic structure of CRP as template (PDB code: 1B09). The model of PTX3 C-terminus presents a hydrophobic core composed by two anti-parallel β-sheets organised in a typical β-jelly roll topology (Fig. 3C). A similar model was described when PTX3 was modelled on the tertiary structure of SAP [46]. A single α-helix spanning amino acid residues 344–351 is located on the protein surface, whereas Cys210 and Cys271 are located on opposite sites of the two anti-parallel β-sheets. These cysteine residues are conserved among pentraxins and since they establish a disulphide bond in CRP and SAP, they were imposed also in the C-terminal model of PTX3 as covalently linked. The proximity of Cys179 and Cys357 within the model suggests that they are reciprocally engaged in another disulphide bond, thus potentially linking the N-terminal end of PTX3 to the tail of the C-terminal domain [46].
PTX3 contains a unique N-glycosylation site at Asn220 which is located on an exposed loop of the C-terminal domain [47]. The PTX3 protein is conjugated with complex-type oligosaccharides, mainly biantennary fucosylated and terminally sialylated structures [47]. It has been proposed that the negatively charged sialic acid residues may establish ionic interactions with polar and basic amino acids located far from the Asn220 N-glycosylation site [47]. As detailed below, the glycosidic moiety is involved in a fine tuning of the interaction of PTX3 with the complement fraction C1q [47].
PTX3 protomers form higher ordered multimeric structures that, at variance with classical pentraxins CRP and SAP, are stabilized by a network of interchain disulphide bonds [25]. Gel electrophoresis under native conditions has shown that PTX3 subunits form multimers with an apparent molecular weight of 440 kDa. However, PTX3 behaves anomalously in size exclusion chromatography being eluted as a 900 kDa protein [25]. No data are available to discriminate whether this chromatographic behaviour reflects the ability of PTX3 to form larger multimeric structures or whether PTX3 multimers have a high hydrodynamic volume.
Attempts to predict the quaternary structure of PTX3 were performed by replacing one of the subunits of the pentameric ring of SAP with the model of the PTX3 C-terminus [2]. The residue substitution pattern and primary sequence analysis of the modelled interfaces indicates the presence of several non-conservative substitutions. Thus, the quaternary structure of PTX3 may not be easily traced from the known structure of the other pentraxins. This limitation, together with the lack of information about the relative organization of the N-terminal and C-terminal domains, do not allow us to draw any ultimate conclusion about the quaternary structure of PTX3.
PTX3 ligands
Pentraxins act as soluble pattern recognition receptors with a wide range of functions in various pathophysiological conditions. As described above, PTX3 shares with short-pentraxins the C-terminal pentraxin- domain and possesses an unique N-terminal domain. These structural features suggest that PTX3 may have both overlapping and distinct biological/ligand recognition properties when compared to CRP and SAP (Table 1). Also, the simultaneous presence of multiple ligands may mutually affect their capacity to bind to PTX3 multimers with possible different consequences for the biology of blood vessels.
PTX3 binds selected pathogens, including Aspergillus fumigatus, Pseudomonas aeruginosa, Salmonella typhimurium, Paracoccidioides brasiliensis, Klebsiella pneumoniae, but not Escherichia coli, Burkholderia cepacia, Listeria monocytogenes, and Candida albicans[27]. The molecular determinants of these interactions have not been fully elucidated. Unlike CRP and SAP, PTX3 does not bind the classic short-pentraxin ligands phosphorylcoline, phosphoethanolamine, and high pyruvate agarose (see Table 1). However, PTX3 interaction with A. fumigatus is competed by galactomannan [27]. This is consistent with the capacity of PTX3 to interact with zymosan [48], a complex carbohydrate of the yeast cell wall composed mainly by mannan and β-glucan. Zymosan induces inflammatory signals in macrophages following recognition by dectin-1 and the Toll-like receptors TLR2 and TLR6 [49]. PTX3 interaction increases the clearance of zymosan particles by macrophages in a dectin-1-dependent manner [48]. Accordingly, PTX3 functions as an opsonin for the yeast cell form of the fungal pathogen Paracoccidioides brasiliensis[48]. Zymosan is known also to affect endothelial permeability in a complement- independent manner [50]. Thus, besides its role as a mediator of the innate immune response during fungal infection, PTX3 may modulate blood vessel permeability by regulating zymosan bioavailability. Recently, the bacterial outer membrane protein A of Klebsiella pneumoniae(KpOmpA) has been identified as a high affinity binder of PTX3 [51]. PTX3/KpOmpA interaction occurs in vitro with an association rate (Kon) equal to 1.77 × 104 M−1 s−1, a dissociation rate (Koff) equal to 8.57 × 10−4 s−1, and a dissociation constant (Kd) equal to 5.0 × 10−8 M [51]. As a consequence of this interaction, PTX3 may modulate the proinflammatory program triggered by K. pneumoniaein endothelial cells [52].
The complement fraction C1q was the first characterized PTX3 ligand [25]. More recently, the interaction of PTX3 with the whole C1 complex, but not with the fraction C1s [25], has been reported [53]. PTX3/C1q interaction requires multimer formation and occurs in vitro with a Kon equal to 2.4 × 105 M−1 s−1, a Koff equal to 4.0 × 10−4 s−1, and a Kd equal to 7.4 × 10−8 M. As anticipated, C1q binds the C-terminal pentraxin domain of PTX3 [25]. Conversely, the PTX3-binding domain has been localized in the globular head region of C1q [53, 54]. At variance with short-pentraxins, the interaction of PTX3 with C1q does not require calcium [25], but it is significantly affected by the glycosilation status of PTX3 [47]. Indeed, removal of the sialic acid residues or of the entire glycosidic moiety from the PTX3 glycoprotein enhances C1q binding and activation of the classical complement pathway [47].
At the functional level, the binding of C1q to substratum- immobilized PTX3 induces the activation of the classical complement pathway on the surface of apoptotic cells [53]. In contrast, the binding of C1q to PTX3 in the fluid phase prevents the formation of C1q/immunoglobulin complexes, complement activation, and hemolytic activity [53, 55]. Since a crosstalk exists between the complement system and endothelial cells in different physiological conditions and in vascular diseases [56], these observations suggest that PTX3 may differently regulate endothelial cell functions depending upon its free or immobilized status. Indeed, besides its presence in blood as a circulating protein, PTX3 can be detected in the ECM [35, 39], raising the question of the ECM component(s) binding to PTX3.
PTX3 does not bind type IV collagen, fibronectin, and gelatin, all ECM ligands for short-pentraxins [25]. In contrast, PTX3 binds the TNFα-induced protein 6 (TNFAIP6 or TSG6) that, in turn, binds hyaluronic acid (HA). This leads to the formation of multimolecular PTX3/TSG6/HA complexes, even though no direct interaction occurs between PTX3 and HA [35]. Through this mechanism, PTX3 plays a key role in the assembly of the ECM of the cumulus oophorus, essential for female fertility [35]. Interestingly, HA and TSG6 are also present in the endothelial microenvironment where they regulate different aspects of endothelial cell biology [57–59]. On the other hand, PTX3 is found in the subendothelial matrix of atherosclerotic plaques (see below). Further experiments are required to evaluate the presence of PTX3/TSG6/HA complexes in the subendothelial matrix and to assess their potential significance for blood vessel function and integrity.
When assessed for the capacity to interact with a variety of extracellular signaling polypeptides (Table 1), PTX3 was found to bind the angiogenic polypeptide FGF2 with high specificity [44]. Indeed, under the same experimental conditions, PTX3 does not bind to a wide panel of cytokines, chemokines, and growth factors representative of different classes of soluble polypeptide mediators, showing only a limited interaction with FGF8 (but not with FGF1 or FGF4, all members of the FGFs family) and with the angiogenic vascular endothelial growth factor (VEGF) [44]. PTX3/FGF2 interaction occurs with high affinity, with a Kon equal to 0.2 × 103 s−1 M−1, a Koff equal to 6.0 × 10−5 s−1, and a Kd value ranging between 3.0 x 10–7 and 3.0 x 10–8 M depending upon the experimental model adopted [42, 44].
In agreement with the incapacity of short-pentraxins to bind FGF2 [44], the FGF2-binding domain of PTX3 has been located in its N-terminal region [42]. Accordingly, C1q does not compete for FGF2 binding to PTX3 [7]. Also, glycosilation of PTX3, which is involved in PTX3/C1q interaction (see above), does not affect PTX3/FGF2 interaction (M. Camozzi, unpublished observations). An integrated approach that utilized PTX3-related synthetic peptides, monoclonal antibodies, and surface plasmon resonance analysis has identified the FGF2-binding domain in the PTX3(97–110) amino acid sequence within the PTX3 N-terminus (see above) [42]. These observations point to a novel unanticipated function for the Nterminal extension of PTX3.
At the functional level, PTX3/FGF2 interaction prevents the binding of the growth factor to its cell surface receptors, thus inhibiting its biological activity in target cells. Accordingly, PTX3, but not short-pentraxins, suppresses the pro-angiogenic and prorestenotic activity exerted by FGF2 on endothelial cells and SMCs, respectively (see below).
It has been demonstrated that PTX3 can directly stimulate endothelial cells and macrophages [41, 60], pointing to the presence of putative PTX3 receptors on the surface of these cells. Preliminary experiments have ruled out the possibility that integrins, gangliosides, heparin/heparan sulfate proteoglycans (HSPGs) may act as PTX3 receptors or co-receptors (M. Rusnati, unpublished observations), despite the role of these cell surface components in the interaction with a variety of extracellular molecules, including ECM components, growth factors, and proteases [61].
Taken together, experimental evidences indicate that PTX3 is a modular protein able to interact with various ligands via its N-terminal or C-terminal domains. Module exchange has played a major role during protein evolution in eukaryotic cells [62] and the identification of such modules in a given protein may give clues about its functional properties [63]. A significant module homology has been observed among short-pentraxins, laminin, and thrombospondin (TSP) [63]. Relevant to this point, PTX3 and TSP share the capacity to exist either as circulating or ECM-associated proteins and are both involved in the regulation of endothelial cell integrity and angiogenesis. Accordingly, they bind FGF2 and inhibit its biological activity with similar affinity and mechanism of action [64, 65]. Further studies are awaited to map the binding regions in the PTX3 molecule responsible for the interaction with its various ligands.
PTX3 in vascular pathology
PTX3 as a marker of vascular damage
Vessel wall elements produce high levels of PTX3 during inflammation. Thus, when compared to the liver-produced short-pentraxin CRP, PTX3 may represent a rapid marker for primary local activation of inflammation as well as of innate immunity. Indeed, blood levels of PTX3 rise dramatically in inflammatory conditions from <2 ng/ml up to 200–800 ng/ml during active disease [1].This occurs very rapidly when compared to the increase of CRP levels, consistent with the original identification of PTX3 as an immediate early gene [22].
Increased levels of PTX3 have been observed in diverse infectious disorders, including sepsis, A. fumigatus infections, tuberculosis, and dengue [24, 27], in the synovial fluid in rheumatoid arthritis [23], and in plasma of untreated psoriatic patients [66]. In some of these conditions, PTX3 levels correlate with disease activity or severity. PTX3 levels increase also in preeclampsia, possibly as a consequence of the endothelial dysfunction that characterizes this syndrome [67, 68]. PTX3 levels peak in plasma early after myocardial infarction (approximately after 6-8 hrs) [22], thus representing an independent predictor of mortality when measured within the first day from the onset of symptoms [69]. The presence of PTX3 in infarcted hearts and the higher degree of ischaemic injury in hearts of PTX3−/− mice support a pathophysiologic role of the protein in myocardial damage and repair [69].
Small-vessel vasculitides represent a group of disorders characterized by inflammatory damage of the vessel wall consequent to immunopathogenic mechanisms and primary inflammatory cytokines [70]. PTX3 is abundantly present in endothelial cells of skin biopsies from patients with active vasculitis [21]. Also, in keeping with the inhibitory effect exerted by PTX3 on the phagocytosis of late apoptotic polymorphonuclear leukocytes by macrophages [71], PTX3 is overexpressed at sites of leukocytoclastic vasculitis lesions [72]. Patients with active vasculitis have serum concentrations of PTX3 significantly higher than those of patients with quiescent disease or of healthy individuals [21]. Interestingly, PTX3 levels are decreased in patients who undergo immunosuppressive treatment.Taken together, these observations suggest that PTX3 may serve as an independent indicator of disease activity in small-vessel vasculitides.
Atherosclerosis
An increasing body of evidence point to the inflammatory nature of the atherosclerotic process [73]. Plasma levels of the classical short-pentraxin CRP correlate with increased risk of cardiovascular disease [74] and subendothelial deposition of CRP has been detected in atherosclerotic lesions, possibly as the consequence of its passive transudation from circulation to the extravascular space [75]. As stated above, two of the major cellular components of the atherosclerotic lesion, namely endothelial cells and macrophages, are potent producers of PTX3 in response to inflammatory stimuli. Accordingly, immunoistochemical staining has revealed a strong expression of PTX3 in advanced atherosclerotic lesions [39]. PTX3 immunoreactivity was observed in ECM, in endothelial cells and macrophages within the lesion, as well as in subendothelial SMCs and in foam cells within lipid-rich areas of the plaque. In keeping with these observations, IL-1 and LPS induce PTX3 production in cultured human SMCs [39]. As already mentioned, enzymatically degraded and oxidized LDL, but not native LDL, induce PTX3 upregulation in cholesterol-loaded human primary SMCs [38]. Relevant to this point, atherogenic lipoproteins may induce the local production of proinflammatory cytokines, including IL-1 and IL-6 [76, 77], that, in turn, will upregulate PTX3 expression by infiltrating monocytes, endothelium, and SMCs.
Adipocytokines, bioactive proteins produced by the adipose tissue, may play an important role in atherosclerosis among patients with insulin resistance [78]. Recent observations have shown that resistin, an adipocytokine whose expression tightly correlates with insulin resistance, triggers a potent NF-κBmediated proinflammatory response in endothelial cells, including PTX3 upregulation [79]. Interestingly, this response is inhibited by the anti-atherosclerotic adipocytokine adiponectin and by the vascular protective pitavastatin [79].
PTX3 deposition in the atherosclerotic lesion may lead to an in situ vascular acute-response by binding C1q and triggering complement activation via the classical pathway (see above). On the other hand, due do its FGF2 antagonist activity (see below), PTX3 may inhibit SMC migration and proliferation triggered by FGF2 produced by SMCs themselves [80] or by T lymphocytes that infiltrate the atherosclerotic plaque [81]. Also, PTX3-mediated opsonization of apoptotic cells may contribute to the clearance of lipid-loaded macrophages and foam cells by dendritic cells [82]. Finally, PTX3 may play a role in the progression of the advanced atherosclerotic lesions by stimulating the production of tissue factor by endothelium [60] and activated monocytes [41], thus favoring clotting activation and thrombogenesis (Fig. 2). More data are required to define the role of PTX3 in the modulation of the atherosclerosis disease process.
Angiogenesis
Angiogenesis is the process of generating new capillary blood vessels. Uncontrolled neovascularization occurs during tumor growth and atherosclerosis and plays a pivotal role in inflammation [83]. Angiogenesis is controlled by the balance between pro- and anti-angiogenic factors [84].
FGF2 is a prototypic heparin-binding angiogenic growth factor that induces cell proliferation, chemotaxis, and protease production in cultured endothelial cells by interacting with high affinity tyrosine-kinase receptors (FGFRs) [85]. FGF2 induces angiogenesis in vivo and modulates neovascularization during tumor growth, wound healing, inflammation, and atherosclerosis [86]. FGF2 is produced by various tumor and normal cell types, including cells involved in inflammation and immunity like mononuclear phagocytes [87], T lymphocytes [81], and endothelial cells [88]. Also, FGF2 production and release is modulated by inflammatory mediators (e.g. IL-2 [89] and nitric oxide [90]), hypoxia [87], and cell damage [91]. Thus, endothelial cells and other cell types of the vessel wall can express both FGF2 and PTX3 under inflammatory conditions.
Recent observations have shown that PTX3 binds FGF2 (see above). Accordingly, PTX3 inhibits FGF2- dependent endothelial cell proliferation in vitro and angiogenesis in vivo[44] at doses comparable to those measured in the blood of patients affected by inflammatory diseases [21, 22]. Moreover, due to its capacity to accumulate in the ECM, the local concentration of PTX3 at the site of inflammation can be significantly higher than that measured in the blood stream, supporting the possibility that PTX3/FGF2 interaction may occur and be biologically relevant in vivo. Thus, PTX3 produced by inflammatory and endothelial cells may affect the autocrine and paracrine activity exerted by FGF2 on endothelium. This should allow a fine tuning of the neovascularization process viathe production of both angiogenesis inhibitors and stimulators.
To this respect, it is interesting to note that PTX3 is produced by poorly-angiogenic LPS-activated myeloid dendritic cells [14] whereas myeloid dendritic cells alternatively matured in the presence of the anti-inflammatory molecules calcitriol or prostaglandin E2 produce the potent angiogenic polypeptide VEGF and are highly angiogenic in vivo[92]. Thus, depending upon the activation status and the cytokine milieu, PTX3 may contribute to the different impact on the VEGF-dependent neovascularization process exerted by different populations of dendritic cells.
Restenosis
Percutaneous transluminal coronary angioplasty is commonly used to repair occluded atherosclerotic blood vessels, but its long-term efficacy is limited by restenosis [93] that leads to recurrent acute coronary events and even death [94].
The process of restenosis starts with the disruption of the endothelial layer followed by SMC migration and proliferation [95]. The FGF/FGFR system plays an important role in SMC activation in vitro and in vivo following arterial injury. Indeed, FGF2 promotes survival, proliferation, and migration of SMCs [96–99] that express FGFRs [100]. FGFs are expressed in injured arteries and contribute to intimal thickening [101, 102]. Accordingly, arterial injury leads to FGFR upregulation in SMCs [101, 103]. PTX3 inhibits the biological activity exerted by FGF2 on SMCs in vitro and in vivo[104]. Indeed, PTX3 prevents the binding of FGF2 to FGFRs in human coronary artery SMCs (HCASMCs). This leads to inhibition of endogenous FGF2-dependent proliferation in HCASMCs and fully suppresses the mitogenic and chemotactic activity exerted by exogenous FGF2 on these cells. Accordingly, PTX3 overexpression following recombinant adeno-associated virus (rAAV)- PTX3 gene transfer fully inhibits HCASMC proliferation exerted by endogenous and exogenous FGF2. Consistently, a single local endovascular injection of AAV-PTX3 inhibits intimal thickening after balloon injury in rat carotid arteries [104]. Thus, experimental evidences indicate that PTX3 may represent a potent inhibitor of the autocrine and paracrine stimulation exerted by FGF2 on SMCs and point to a novel therapeutic role of PTX3 in the treatment of restenosis after angioplasty.
Concluding remarks
Experimental evidence demonstrates that PTX3 exerts both overlapping and distinct biological/ligand recognition properties when compared to short-pentraxins. PTX3 may serve as a mechanism of amplification of inflammation and innate immunity, tightly related to endothelial cell functions. Vessel wall elements produce high amounts of PTX3 during inflammation and the levels of circulating PTX3 increase in several pathological conditions affecting the cardiovascular system. The capacity to interact with various ligands, including microbial components, the complement fraction C1q, apoptotic cells, ECM components, and angiogenic growth factors, points to a role for PTX3 during vascular damage, angiogenesis, atherosclerosis, and restenosis (Fig. 2).
Besides its presence in blood as a circulating protein, PTX3 can be detected in the ECM. Thus, PTX3 may differently regulate endothelial cell functions depending upon its free or immobilized status. Also, the capacity of PTX3 to directly stimulate endothelial cells and macrophages points to the presence of putative PTX3 receptors on the surface of these cells. The identification of ECM and cell surface PTX3 ligands is of pivotal importance for a better understanding of the biology, bioavailability, and biological functions of this long-pentraxin.
Endothelial and inflammatory cells play an important role in tumor growth and metastasis by affecting tumor microenvironment. It is therefore conceivable that PTX3 may be implicated in these processes. Preliminary observations from our laboratory indicate that PTX3 overexpression may affect tumor-growth viaangiogenesis-dependent and independent mechanisms of action. Further experiments are required to clarify the impact of PTX3 on tumor progression and the possibility to design PTX3-derived anti-neoplastic and/or anti-angiogenic agents.
Acknowledgments
Limitations of space preclude extensive citation of the literature; we apologize with those whose work is not mentioned herein. This work was supported by grants from AIRC, MIUR (Centro di Eccellenza “IDET”, Cofin), ISS (Oncotechnological Program), Fondazione Berlucchi and NOBEL Project Cariplo “Genetic and functional genomics of myelomonocytic cells” to MP and from AIRC and ISS (AIDS Project) to MR.
References
- 1.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–66. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 2.Goodman AR, Cardozo T, Abagyan R, Altmeyer A, Wisniewski HG, Vilcek J. Long pentraxins: an emerging group of proteins with diverse functions. Cytokine Growth Factor Rev. 1996;7:191–202. doi: 10.1016/1359-6101(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 3.Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–8. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 4.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 5.Du Clos TW. The interaction of C-reactive protein and serum amyloid P component with nuclear antigens. Mol Biol Rep. 1996;23:253–60. doi: 10.1007/BF00351177. [DOI] [PubMed] [Google Scholar]
- 6.Gewurz H, Zhang XH, Lint TF. Structure and function of the pentraxins. Curr Opin Immunol. 1995;7:54–64. doi: 10.1016/0952-7915(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 7.Nauta AJ, Daha MR, Van Kooten C, Roos A. Recognition and clearance of apoptotic cells: a role for complement and pentraxins. Trends Immunol. 2003;24:148–54. doi: 10.1016/s1471-4906(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 8.Breviario F, D'Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, Saccone S, Marzella R, Predazzi V, Rocchi M, Della Valle G, Dejana E, Mantovani A, Introna M. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–7. [PubMed] [Google Scholar]
- 9.Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J Immunol. 1993;150:1804–12. [PubMed] [Google Scholar]
- 10.Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O'Brien RJ, Worley P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003;39:513–28. doi: 10.1016/s0896-6273(03)00463-x. [DOI] [PubMed] [Google Scholar]
- 11.Kretzschmar D, Hasan G, Sharma S, Heisenberg M, Benzer S. The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J Neurosci. 1997;17:7425–32. doi: 10.1523/JNEUROSCI.17-19-07425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani A, Garlanda C, Bottazzi B. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine. 2003;21(Suppl 2):S43–7. doi: 10.1016/s0264-410x(03)00199-3. [DOI] [PubMed] [Google Scholar]
- 13.Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–93. [PubMed] [Google Scholar]
- 14.Doni A, Peri G, Chieppa M, Allavena P, Pasqualini F, Vago L, Romani L, Garlanda C, Mantovani A. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur J Immunol. 2003;33:2886–93. doi: 10.1002/eji.200324390. [DOI] [PubMed] [Google Scholar]
- 15.Abderrahim-Ferkoune A, Bezy O, Chiellini C, Maffei M, Grimaldi P, Bonino F, Moustaid-Moussa N, Pasqualini F, Mantovani A, Ailhaud G, Amri EZ. Characterization of the long pentraxin PTX3 as a TNFalpha-induced secreted protein of adipose cells. J Lipid Res. 2003;44:994–1000. doi: 10.1194/jlr.M200382-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16:1154–67. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- 17.Bussolati B, Peri G, Salvidio G, Verzola D, Mantovani A, Camussi G. The long pentraxin PTX3 is synthesized in IgA glomerulonephritis and activates mesangial cells. J Immunol. 2003;170:1466–72. doi: 10.4049/jimmunol.170.3.1466. [DOI] [PubMed] [Google Scholar]
- 18.Nauta AJ, De Haij S, Bottazzi B, Mantovani A, Borrias MC, Aten J, Rastaldi MP, Daha MR, Van Kooten C, Roos A. Human renal epithelial cells produce the long pentraxin PTX3. Kidney Int. 2005;67:543–53. doi: 10.1111/j.1523-1755.2005.67111.x. [DOI] [PubMed] [Google Scholar]
- 19.Polentarutti N, Bottazzi B, Di Santo E, Blasi E, Agnello D, Ghezzi P, Introna M, Bartfai T, Richards G, Mantovani A. Inducible expression of the long pentraxin PTX3 in the central nervous system. J Neuroimmunol. 2000;106:87–94. doi: 10.1016/s0165-5728(00)00214-9. [DOI] [PubMed] [Google Scholar]
- 20.Ravizza T, Moneta D, Bottazzi B, Peri G, Garlanda C, Hirsch E, Richards GJ, Mantovani A, Vezzani A. Dynamic induction of the long pentraxin PTX3 in the CNS after limbic seizures: evidence for a protective role in seizure-induced neurodegeneration. Neuroscience. 2001;105:43–53. doi: 10.1016/s0306-4522(01)00177-4. [DOI] [PubMed] [Google Scholar]
- 21.Fazzini F, Peri G, Doni A, Dell'Antonio G, Dal Cin E, Bozzolo E, D'Auria F, Praderio L, Ciboddo G, Sabbadini MG, Manfredi AA, Mantovani A, Querini PR. PTX3 in small-vessel vasculitides: an independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum. 2001;44:2841–50. doi: 10.1002/1529-0131(200112)44:12<2841::aid-art472>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, Pizzetti F, Maggioni AP, Moccetti T, Metra M, Cas LD, Ghezzi P, Sipe JD, Re G, Olivetti G, Mantovani A, Latini R. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–41. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- 23.Luchetti MM, Piccinini G, Mantovani A, Peri G, Matteucci C, Pomponio G, Fratini M, Fraticelli P, Sambo P, Di Loreto C, Doni A, Introna M, Gabrielli A. Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA) Clin Exp Immunol. 2000;119:196–202. doi: 10.1046/j.1365-2249.2000.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller B, Peri G, Doni A, Torri V, Landmann R, Bottazzi B, Mantovani A. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29:1404–7. doi: 10.1097/00003246-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Bottazzi B, Vouret-Craviari V, Bastone A, De Gioia L, Matteucci C, Peri G, Spreafico F, Pausa M, D'Ettorre C, Gianazza E, Tagliabue A, Salmona M, Tedesco F, Introna M, Mantovani A. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem. 1997;272:32817–23. doi: 10.1074/jbc.272.52.32817. [DOI] [PubMed] [Google Scholar]
- 26.Dias AA, Goodman AR, Dos Santos JL, Gomes RN, Altmeyer A, Bozza PT, Horta MF, Vilcek J, Reis LF. TSG-14 transgenic mice have improved survival to endotoxemia and to CLP-induced sepsis. J Leukoc Biol. 2001;69:928–36. [PubMed] [Google Scholar]
- 27.Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, Maccagno A, Riva F, Bottazzi B, Peri G, Doni A, Vago L, Botto M, De Santis R, Carminati P, Siracusa G, Altruda F, Vecchi A, Romani L, Mantovani A. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–6. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 28.Lee GW, Goodman AR, Lee TH, Vilcek J. Relationship of TSG-14 protein to the pentraxin family of major acute phase proteins. J Immunol. 1994;153:3700–7. [PubMed] [Google Scholar]
- 29.Basile A, Sica A, D'Aniello E, Breviario F, Garrido G, Castellano M, Mantovani A, Introna M. Characterization of the promoter for the human long pentraxin PTX3. Role of NF-kappaB in tumor necrosis factor-alpha and interleukin-1beta regulation. J Biol Chem. 1997;272:8172–8. doi: 10.1074/jbc.272.13.8172. [DOI] [PubMed] [Google Scholar]
- 30.Altmeyer A, Klampfer L, Goodman AR, Vilcek J. Promoter structure and transcriptional activation of the murine TSG-14 gene encoding a tumor necrosis factor/interleukin-1-inducible pentraxin protein. J Biol Chem. 1995;270:25584–90. doi: 10.1074/jbc.270.43.25584. [DOI] [PubMed] [Google Scholar]
- 31.Vouret-Craviari V, Matteucci C, Peri G, Poli G, Introna M, Mantovani A. Expression of a long pentraxin, PTX3, by monocytes exposed to the mycobacterial cell wall component lipoarabinomannan. Infect Immun. 1997;65:1345–50. doi: 10.1128/iai.65.4.1345-1350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polentarutti N, Picardi G, Basile A, Cenzuales S, Rivolta A, Matteucci C, Peri G, Mantovani A, Introna M. Interferon-gamma inhibits expression of the long pentraxin PTX3 in human monocytes. Eur J Immunol. 1998;28:496–501. doi: 10.1002/(SICI)1521-4141(199802)28:02<496::AID-IMMU496>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 33.Doni A, Michela M, Bottazzi B, Peri G, Valentino S, Polentarutti N, Garlanda C, Mantovani A. Regulation of PTX3, a key component of humoral innate immunity in human dendritic cells: stimulation by IL-10 and inhibition by IFN-gamma. J Leukoc Biol. 2006;79:797–802. doi: 10.1189/jlb.0905493. [DOI] [PubMed] [Google Scholar]
- 34.Luchetti MM, Sambo P, Majlingova P, Svegliati Baroni S, Peri G, Paroncini P, Introna M, Stoppacciaro A, Mantovani A, Gabrielli A. Scleroderma fibroblasts constitutively express the long pentraxin PTX3. Clin Exp Rheumatol. 2004;22:S66–72. [PubMed] [Google Scholar]
- 35.Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, Doni A, Bastone A, Mantovani G, Beck Peccoz P, Salvatori G, Mahoney DJ, Day AJ, Siracusa G, Romani L, Mantovani A. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–86. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 36.Han B, Mura M, Andrade CF, Okutani D, Lodyga M, Dos Santos CC, Keshavjee S, Matthay M, Liu M. TNFalpha-induced long pentraxin PTX3 expression in human lung epithelial cells via JNK. J Immunol. 2005;175:8303–11. doi: 10.4049/jimmunol.175.12.8303. [DOI] [PubMed] [Google Scholar]
- 37.He X, Han B, Liu M. Long pentraxin PTX3 in pulmonary infection and acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1039–49. doi: 10.1152/ajplung.00490.2006. [DOI] [PubMed] [Google Scholar]
- 38.Klouche M, Peri G, Knabbe C, Eckstein HH, Schmid FX, Schmitz G, Mantovani A. Modified atherogenic lipoproteins induce expression of pentraxin- 3 by human vascular smooth muscle cells. Atherosclerosis. 2004;175:221–8. doi: 10.1016/j.atherosclerosis.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:e10–4. doi: 10.1161/01.atv.0000015595.95497.2f. [DOI] [PubMed] [Google Scholar]
- 40.Morikawa S, Takabe W, Mataki C, Wada Y, Izumi A, Saito Y, Hamakubo T, Kodama T. Global analysis of RNA expression profile in human vascular cells treated with statins. J Atheroscler Thromb. 2004;11:62–72. doi: 10.5551/jat.11.62. [DOI] [PubMed] [Google Scholar]
- 41.Napoleone E, Di Santo A, Peri G, Mantovani A, De Gaetano G, Donati MB, Lorenzet R. The long pentraxin PTX3 up-regulates tissue factor in activated monocytes: another link between inflammation and clotting activation. J Leukoc Biol. 2004;76:203–9. doi: 10.1189/jlb.1003528. [DOI] [PubMed] [Google Scholar]
- 42.Camozzi M, Rusnati M, Bugatti A, Bottazzi B, Mantovani A, Bastone A, Inforzato A, Vincenti S, Bracci L, Mastroianni D, Presta M. Identification of an antiangiogenic FGF2-binding site in the N terminus of the soluble pattern recognition receptor PTX3. J Biol Chem. 2006;281:22605–13. doi: 10.1074/jbc.M601023200. [DOI] [PubMed] [Google Scholar]
- 43.Berger B, Wilson DB, Wolf E, Tonchev T, Milla M, Kim PS. Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci U S A. 1995;92:8259–63. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rusnati M, Camozzi M, Moroni E, Bottazzi B, Peri G, Indraccolo S, Amadori A, Mantovani A, Presta M. Selective recognition of fibroblast growth factor-2 by the long pentraxin PTX3 inhibits angiogenesis. Blood. 2004;104:92–9. doi: 10.1182/blood-2003-10-3433. [DOI] [PubMed] [Google Scholar]
- 45.Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–78. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 46.Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, Peri G, Breviario F, Salmona M, De Gregorio L, Dragani TA, Srinivasan N, Blundell TL, Hamilton TA, Mantovani A. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862–72. [PubMed] [Google Scholar]
- 47.Inforzato A, Peri G, Doni A, Garlanda C, Mantovani A, Bastone A, Carpentieri A, Amoresano A, Pucci P, Roos A, Daha MR, Vincenti S, Gallo G, Carminati P, De Santis R, Salvatori G. Structure and function of the long pentraxin PTX3 glycosidic moiety: fine-tuning of the interaction with C1q and complement activation. Biochemistry. 2006;45:11540–51. doi: 10.1021/bi0607453. [DOI] [PubMed] [Google Scholar]
- 48.Diniz SN, Nomizo R, Cisalpino PS, Teixeira MM, Brown GD, Mantovani A, Gordon S, Reis LF, Dias AA. PTX3 function as an opsonin for the dectin-1- dependent internalization of zymosan by macrophages. J Leukoc Biol. 2004;75:649–56. doi: 10.1189/jlb.0803371. [DOI] [PubMed] [Google Scholar]
- 49.Brown GD, Gordon S. Fungal beta-glucans and mammalian immunity. Immunity. 2003;19:311–5. doi: 10.1016/s1074-7613(03)00233-4. [DOI] [PubMed] [Google Scholar]
- 50.Gupta N, Jacobs DL, Miller TA, Smith GS, Dahms TE. Hypoxia-reoxygenation potentiates zymosan activated plasma-induced endothelial injury. J Surg Res. 1998;77:91–8. doi: 10.1006/jsre.1998.5344. [DOI] [PubMed] [Google Scholar]
- 51.Jeannin P, Bottazzi B, Sironi M, Doni A, Rusnati M, Presta M, Maina V, Magistrelli G, Haeuw JF, Hoeffel G, Thieblemont N, Corvaia N, Garlanda C, Delneste Y, Mantovani A. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–60. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Noel RF, Jr, Sato TT, Mendez C, Johnson MC, Pohlman TH. Activation of human endothelial cells by viable or heat-killed gram-negative bacteria requires soluble CD14. Infect Immun. 1995;63:4046–53. doi: 10.1128/iai.63.10.4046-4053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nauta AJ, Bottazzi B, Mantovani A, Salvatori G, Kishore U, Schwaeble WJ, Gingras AR, Tzima S, Vivanco F, Egido J, Tijsma O, Hack EC, Daha MR, Roos A. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur J Immunol. 2003;33:465–73. doi: 10.1002/immu.200310022. [DOI] [PubMed] [Google Scholar]
- 54.Roumenina LT, Ruseva MM, Zlatarova A, Ghai R, Kolev M, Olova N, Gadjeva M, Agrawal A, Bottazzi B, Mantovani A, Reid KB, Kishore U, Kojouharova MS. Interaction of C1q with IgG1, C-reactive protein and pentraxin 3: mutational studies using recombinant globular head modules of human C1q A, B, and C chains. Biochemistry. 2006;45:4093–104. doi: 10.1021/bi052646f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baruah P, Dumitriu IE, Peri G, Russo V, Mantovani A, Manfredi AA, Rovere-Querini P. The tissue pentraxin PTX3 limits C1q-mediated complement activation and phagocytosis of apoptotic cells by dendritic cells. J Leukoc Biol. 2006;80:87–95. doi: 10.1189/jlb.0805445. [DOI] [PubMed] [Google Scholar]
- 56.Fischetti F, Tedesco F. Cross-talk between the complement system and endothelial cells in physiologic conditions and in vascular diseases. Autoimmunity. 2006;39:417–28. doi: 10.1080/08916930600739712. [DOI] [PubMed] [Google Scholar]
- 57.Cao TV, La M, Getting SJ, Day AJ, Perretti M. Inhibitory effects of TSG-6 Link module on leukocyteendothelial cell interactions in vitro and in vivo. Microcirculation. 2004;11:615–24. doi: 10.1080/10739680490503438. [DOI] [PubMed] [Google Scholar]
- 58.Gordon PB, Conn G, Hatcher VB. Glycosaminoglycan production in cultures of early and late passage human endothelial cells: the influence of an anionic endothelial cell growth factor and the extracellular matrix. J Cell Physiol. 1985;125:596–607. doi: 10.1002/jcp.1041250332. [DOI] [PubMed] [Google Scholar]
- 59.Fournier N, Doillon CJ. in vitro angiogenesis in fibrin matrices containing fibronectin or hyaluronic acid. Cell Biol Int Rep. 1992;16:1251–63. doi: 10.1016/s0309-1651(06)80042-1. [DOI] [PubMed] [Google Scholar]
- 60.Napoleone E, Di Santo A, Bastone A, Peri G, Mantovani A, De Gaetano G, Donati MB, Lorenzet R. Long pentraxin PTX3 upregulates tissue factor expression in human endothelial cells: a novel link between vascular inflammation and clotting activation. Arterioscler Thromb Vasc Biol. 2002;22:782–7. doi: 10.1161/01.atv.0000012282.39306.64. [DOI] [PubMed] [Google Scholar]
- 61.Rusnati M, Presta M. Extracellular angiogenic growth factor interactions: an angiogenesis interactome survey. Endothelium. 2006;13:93–111. doi: 10.1080/10623320600698011. [DOI] [PubMed] [Google Scholar]
- 62.Patthy L. Exon shuffling and other ways of module exchange. Matrix Biol. 1996;15:301–10. doi: 10.1016/s0945-053x(96)90131-6. [DOI] [PubMed] [Google Scholar]
- 63.Beckmann G, Hanke J, Bork P, Reich JG. Merging extracellular domains: fold prediction for laminin Glike and amino-terminal thrombospondin-like modules based on homology to pentraxins. J Mol Biol. 1998;275:725–30. doi: 10.1006/jmbi.1997.1510. [DOI] [PubMed] [Google Scholar]
- 64.Taraboletti G, Belotti D, Borsotti P, Vergani V, Rusnati M, Presta M, Giavazzi R. The 140-kilodalton antiangiogenic fragment of thrombospondin-1 binds to basic fibroblast growth factor. Cell Growth & Differentiation. 1997;8:471–9. [PubMed] [Google Scholar]
- 65.Margosio B, Marchetti D, Vergani V, Giavazzi R, Rusnati M, Presta M, Taraboletti G. Thrombospondin 1 as a scavenger for matrix-associated fibroblast growth factor 2. Blood. 2003;102:4399–406. doi: 10.1182/blood-2003-03-0893. [DOI] [PubMed] [Google Scholar]
- 66.Bevelacqua V, Libra M, Mazzarino MC, Gangemi P, Nicotra G, Curatolo S, Massimino D, Plumari A, Merito P, Valente G, Stivala F, La Greca S, Malaponte G. Long pentraxin 3: a marker of inflammation in untreated psoriatic patients. Int J Mol Med. 2006;18:415–23. [PubMed] [Google Scholar]
- 67.Rovere-Querini P, Antonacci S, Dell'Antonio G, Angeli A, Almirante G, Cin ED, Valsecchi L, Lanzani C, Sabbadini MG, Doglioni C, Manfredi AA, Castiglioni MT. Plasma and tissue expression of the long pentraxin 3 during normal pregnancy and preeclampsia. Obstet Gynecol. 2006;108:148–55. doi: 10.1097/01.AOG.0000224607.46622.bc. [DOI] [PubMed] [Google Scholar]
- 68.Cetin I, Cozzi V, Pasqualini F, Nebuloni M, Garlanda C, Vago L, Pardi G, Mantovani A. Elevated maternal levels of the long pentraxin 3 (PTX3) in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2006;194:1347–53. doi: 10.1016/j.ajog.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 69.Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, Pasqualini F, Signorini S, Soldateschi D, Tarli L, Schweiger C, Fresco C, Cecere R, Tognoni G, Mantovani A. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–54. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- 70.Sundy JS, Haynes BF. Pathogenic mechanisms of vessel damage in vasculitis syndromes. Rheum Dis Clin North Am. 1995;21:861–81. [PubMed] [Google Scholar]
- 71.Van Rossum AP, Fazzini F, Limburg PC, Manfredi AA, Rovere-Querini P, Mantovani A, Kallenberg CG. The prototypic tissue pentraxin PTX3, in contrast to the short pentraxin serum amyloid P, inhibits phagocytosis of late apoptotic neutrophils by macrophages. Arthritis Rheum. 2004;50:2667–74. doi: 10.1002/art.20370. [DOI] [PubMed] [Google Scholar]
- 72.Van Rossum AP, Pas HH, Fazzini F, Huitema MG, Limburg PC, Jonkman MF, Kallenberg CG. Abundance of the long pentraxin PTX3 at sites of leukocytoclastic lesions in patients with small-vessel vasculitis. Arthritis Rheum. 2006;54:986–91. doi: 10.1002/art.21669. [DOI] [PubMed] [Google Scholar]
- 73.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 74.Albert MA, Ridker PM. The role of C-reactive protein in cardiovascular disease risk. Curr Cardiol Rep. 1999;1:99–104. doi: 10.1007/s11886-999-0066-0. [DOI] [PubMed] [Google Scholar]
- 75.Torzewski J, Torzewski M, Bowyer DE, Frohlich M, Koenig W, Waltenberger J, Fitzsimmons C, Hombach V. C-reactive protein frequently colocalizes with the terminal complement complex in the intima of early atherosclerotic lesions of human coronary arteries. Arterioscler Thromb Vasc Biol. 1998;18:1386–92. doi: 10.1161/01.atv.18.9.1386. [DOI] [PubMed] [Google Scholar]
- 76.Klouche M, Gottschling S, Gerl V, Hell W, Husmann M, Dorweiler B, Messner M, Bhakdi S. Atherogenic properties of enzymatically degraded LDL: selective induction of MCP-1 and cytotoxic effects on human macrophages. Arterioscler Thromb Vasc Biol. 1998;18:1376–85. doi: 10.1161/01.atv.18.9.1376. [DOI] [PubMed] [Google Scholar]
- 77.Klouche M, Rose-John S, Schmiedt W, Bhakdi S. Enzymatically degraded, nonoxidized LDL induces human vascular smooth muscle cell activation, foam cell transformation, and proliferation. Circulation. 2000;101:1799–805. doi: 10.1161/01.cir.101.15.1799. [DOI] [PubMed] [Google Scholar]
- 78.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–8. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 79.Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314:415–9. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 80.Lindner V, Olson NE, Clowes AW, Reidy MA. Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. J Clin Invest. 1992;90:2044–9. doi: 10.1172/JCI116085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peoples GE, Blotnick S, Takahashi K, Freeman MR, Klagsbrun M, Eberlein TJ. T lymphocytes that infiltrate tumors and atherosclerotic plaques produce heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor: a potential pathologic role. Proc Natl Acad Sci USA. 1995;92:6547–51. doi: 10.1073/pnas.92.14.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rovere P, Peri G, Fazzini F, Bottazzi B, Doni A, Bondanza A, Zimmermann VS, Garlanda C, Fascio U, Sabbadini MG, Rugarli C, Mantovani A, Manfredi AA. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000;96:4300–6. [PubMed] [Google Scholar]
- 83.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 84.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–36. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 85.Gerwins P, Skoldenberg E, Claesson-Welsh L. Function of fibroblast growth factors and vascular endothelial growth factors and their receptors in angiogenesis. Crit Rev Oncol Hematol. 2000;34:185–94. doi: 10.1016/s1040-8428(00)00062-7. [DOI] [PubMed] [Google Scholar]
- 86.Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–78. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Kuwabara K, Ogawa S, Matsumoto M, Koga S, Clauss M, Pinsky DJ, Lyn P, Leavy J, Witte L, Joseph-Silverstein J, Furies MB, Torcia G, Cozzolino F, Kamada T, Stern DM. Hypoxia-mediated induction of acidic/basic fibroblast growth factor and platelet-derived growth factor in mononuclear phagocytes stimulates growth of hypoxic endothelial cells. Proc Natl Acad Sci USA. 1995;92:4606–10. doi: 10.1073/pnas.92.10.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dell'Era P, Presta M, Ragnotti G. Nuclear localization of endogenous basic fibroblast growth factor in cultured endothelial cells. Exp Cell Res. 1991;192:505–10. doi: 10.1016/0014-4827(91)90070-b. [DOI] [PubMed] [Google Scholar]
- 89.Cozzolino F, Torcia M, Lucibello M, Morbidelli L, Ziche M, Platt J, Fabiani S, Brett J, Stern D. Interferon-alpha and interleukin 2 synergistically enhance basic fibroblast growth factor synthesis and induce release, promoting endothelial cell growth. J Clin Invest. 1993;91:2504–12. doi: 10.1172/JCI116486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ziche M, Parenti A, Ledda F, Dell'Era P, Granger HJ, Maggi CA, Presta M. Nitric oxide promotes proliferation and plasminogen activator production by coronary venular endothelium through endogenous bFGF. Circ Res. 1997;80:845–52. doi: 10.1161/01.res.80.6.845. [DOI] [PubMed] [Google Scholar]
- 91.Gajdusek CM, Carbon S. Injury-induced release of basic fibroblast growth factor from bovine aortic endothelium. J Cell Physiol. 1989;139:570–9. doi: 10.1002/jcp.1041390317. [DOI] [PubMed] [Google Scholar]
- 92.Riboldi E, Musso T, Moroni E, Urbinati C, Bernasconi S, Rusnati M, Adorini L, Presta M, Sozzani S. Cutting edge: proangiogenic properties of alternatively activated dendritic cells. J Immunol. 2005;175:2788–92. doi: 10.4049/jimmunol.175.5.2788. [DOI] [PubMed] [Google Scholar]
- 93.Francis DJ, Parish CR, McGarry M, Santiago FS, Lowe HC, Brown KJ, Bingley JA, Hayward IP, Cowden WB, Campbell JH, Campbell GR, Chesterman CN, Khachigian LM. Blockade of vascular smooth muscle cell proliferation and intimal thickening after balloon injury by the sulfated oligosaccharide PI-88: phosphomannopentaose sulfate directly binds FGF-2, blocks cellular signaling, and inhibits proliferation. Circ Res. 2003;92:e70–7. doi: 10.1161/01.RES.0000071345.76095.07. [DOI] [PubMed] [Google Scholar]
- 94.Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316:701–6. doi: 10.1056/NEJM198703193161201. [DOI] [PubMed] [Google Scholar]
- 95.Faxon DP, Coats W, Currier J. Remodeling of the coronary artery after vascular injury. Prog Cardiovasc Dis. 1997;40:129–40. doi: 10.1016/s0033-0620(97)80005-9. [DOI] [PubMed] [Google Scholar]
- 96.Koyama H, Olson NE, Dastvan FF, Reidy MA. Cell replication in the arterial wall: activation of signaling pathway following in vivo injury. Circ Res. 1998;82:713–21. doi: 10.1161/01.res.82.6.713. [DOI] [PubMed] [Google Scholar]
- 97.Jackson CL, Reidy MA. Basic fibroblast growth factor: its role in the control of smooth muscle cell migration. Am J Pathol. 1993;143:1024–31. [PMC free article] [PubMed] [Google Scholar]
- 98.Miyamoto T, Leconte I, Swain JL, Fox JC. Autocrine FGF signaling is required for vascular smooth muscle cell survival in vitro. J Cell Physiol. 1998;177:58–67. doi: 10.1002/(SICI)1097-4652(199810)177:1<58::AID-JCP6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 99.Fox JC, Shanley JR. Antisense inhibition of basic fibroblast growth factor induces apoptosis in vascular smooth muscle cells [published erratum appears in J Biol Chem 1997; 272:17245] J Biol Chem. 1996;271:12578–84. doi: 10.1074/jbc.271.21.12578. [DOI] [PubMed] [Google Scholar]
- 100.Segev A, Aviezer D, Safran M, Gross Z, Yayon A. Inhibition of vascular smooth muscle cell proliferation by a novel fibroblast growth factor receptor antagonist. Cardiovasc Res. 2002;53:232–41. doi: 10.1016/s0008-6363(01)00447-3. [DOI] [PubMed] [Google Scholar]
- 101.Agrotis A, Kanellakis P, Kostolias G, Di Vitto G, Wei C, Hannan R, Jennings G, Bobik A. Proliferation of neointimal smooth muscle cells after arterial injury. Dependence on interactions between fibroblast growth factor receptor-2 and fibroblast growth factor-9. J Biol Chem. 2004;279:42221–9. doi: 10.1074/jbc.M408121200. [DOI] [PubMed] [Google Scholar]
- 102.Hanna AK, Fox JC, Neschis DG, Safford SD, Swain JL, Golden MA. Antisense basic fibroblast growth factor gene transfer reduces neointimal thickening after arterial injury. J Vasc Surg. 1997;25:320–5. doi: 10.1016/s0741-5214(97)70353-7. [DOI] [PubMed] [Google Scholar]
- 103.Casscells W, Lappi DA, Olwin BB, Wai C, Siegman M, Speir EH, Sasse J, Baird A. Elimination of smooth muscle cells in experimental restenosis: targeting of fibroblast growth factor receptors. Proc Natl Acad Sci USA. 1992;89:7159–63. doi: 10.1073/pnas.89.15.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Camozzi M, Zacchigna S, Rusnati M, Coltrini D, Ramirez-Correa G, Bottazzi B, Mantovani A, Giacca M, Presta M. Pentraxin 3 inhibits fibroblast growth factor 2-dependent activation of smooth muscle cells in vitro and neointima formation in vivo. Arterioscler Thromb Vasc Biol. 2005;25:1837–42. doi: 10.1161/01.ATV.0000177807.54959.7d. [DOI] [PubMed] [Google Scholar]
- 105.Duzhyy D, Harvey M, Sokolowski B. A secretorytype protein, containing a pentraxin domain, interacts with an A-type K+ channel. J Biol Chem. 2005;280:15165–72. doi: 10.1074/jbc.M500111200. [DOI] [PubMed] [Google Scholar]


