Abstract
Mitochondrial biogenesis is a critical metabolic adaptation to aerobic exercise training that results in enhanced mitochondrial size, content, number, and activity. Recent evidence has shown that dietary manipulation can further enhance mitochondrial adaptations to aerobic exercise training, which may delay skeletal muscle fatigue and enhance exercise performance. Specifically, studies have demonstrated that combining carbohydrate restriction (endogenous and exogenous) with a single bout of aerobic exercise potentiates the beneficial effects of exercise on markers of mitochondrial biogenesis. Additionally, studies have demonstrated that high-quality protein supplementation enhances anabolic skeletal muscle intracellular signaling and mitochondrial protein synthesis following a single bout of aerobic exercise. Mitochondrial biogenesis is stimulated by complex intracellular signaling pathways that appear to be primarily regulated by 5′AMP-activated protein kinase and p38 mitogen-activated protein kinase mediated through proliferator-activated γ receptor co-activator 1 α activation, resulting in increased mitochondrial DNA expression and enhanced skeletal muscle oxidative capacity. However, the mechanisms by which concomitant carbohydrate restriction and dietary protein supplementation modulates mitochondrial adaptations to aerobic exercise training remains unclear. This review summarizes intracellular regulation of mitochondrial biogenesis and the effects of carbohydrate restriction and protein supplementation on mitochondrial adaptations to aerobic exercise.
Introduction
Skeletal muscle is highly adaptive and sensitive to a variety of external stimuli, particularly exercise (1). Skeletal muscle adaptations to exercise training (i.e., multiple bouts of exercise over time) are dependent on the frequency, intensity, duration, and mode (i.e., resistance vs. aerobic) of exercise performed (2). One pronounced i.m. adaptive response to aerobic exercise training is mitochondrial biogenesis, which increases mitochondria mRNA expression, protein content, number, and oxidative activity (2–4). The peroxisome proliferator-activated γ receptor co-activator 1 α (PGC-1α)3, which is activated in response to a single bout of aerobic exercise through 5′AMP-activated protein kinase (AMPK) and p38 mitogen-activated protein kinase (p38 MAPK) intracellular signaling, is thought to be the primary regulator of mitochondria biogenesis (5, 6). Upregulated PGC-1α activity and subsequent increases in mitochondrial biogenesis may enhance aerobic capacity by increasing fatty acid β-oxidation and attenuating muscle glycogenolysis, thus delaying the onset of muscle fatigue and improving aerobic exercise performance (7–9).
Dietary intake modulates skeletal muscle adaptations to aerobic training (10). It is generally accepted that dietary carbohydrate intake and endogenous glycogen availability must be adequate to sustain aerobic performance and delay the onset of muscle fatigue (11, 12). However, evidence now suggests that periodic restriction of carbohydrate intake prior to aerobic exercise can influence skeletal muscle oxidative capacity by enhancing mitochondrial biogenesis (13, 14). Although deliberate carbohydrate restriction may potentiate metabolic adaptations to aerobic exercise, likely to improve physical performance, combining glycogen-depleting aerobic exercise with dietary carbohydrate restriction can also increase skeletal muscle proteolysis, resulting in negative muscle protein balance (15). Additionally, although consuming a low-carbohydrate (<2.5 g · kg−1·d−1), high-fat (65–70% kcal · d−1) diet may improve lipid oxidation, manipulating dietary carbohydrate and fat intake to that extent may not necessarily translate to improved aerobic exercise performance (16). However, increasing dietary protein intake at the expense of carbohydrate, while maintaining dietary fat at recommended levels (∼35% kcal · d−1), is perhaps the more appropriate dietary manipulation. Recently, several investigations have demonstrated that combining high-quality protein supplementation with aerobic exercise increases mixed muscle protein synthesis, mitigating proteolysis associated with carbohydrate restriction and resulting in positive protein balance (17, 18). However, whether increased mixed muscle protein synthesis in response to aerobic exercise and protein consumption results from enhanced mitochondrial protein synthesis is not well described.
This manuscript provides a contemporary review of mitochondrial biogenesis and the mitochondrial adaptive responses to aerobic exercise training. This manuscript will also highlight dietary strategies to optimize aerobic exercise–induced mitochondrial biogenesis. Specifically, the mechanistic advantages by which carbohydrate restriction modulates skeletal muscle oxidative capacity and the effects of protein supplementation on i.m. regulators of mitochondrial biogenesis will be explored.
Intracellular Signaling and the Regulation of Mitochondrial Biogenesis
Mitochondria are often described as the “powerhouse” of the cell given their ability to generate chemical energy in the form of ATP through fatty acid β-oxidation, the tricarboxylic acid cycle, and oxidative phosphorylation. Continuous ATP generation is essential to maintain function, particularly in response to cellular stress, such as exercise (10). Mitochondrial adaptations to aerobic exercise training are salient to the metabolic plasticity of skeletal muscle. The biosynthesis of mitochondria enhances skeletal muscle oxidative capacity, allowing for greater generation of ATP, thereby delaying muscle time to fatigue and improving aerobic exercise performance. This dramatic phenotypic alteration is known as mitochondrial biogenesis, which results in increased mitochondrial size, content, number, and function in response to changes in energy status, contractile activity, and metabolic stress.
Regulation of mitochondrial biogenesis appears to be mediated at the level of transcription initiation by a complex intracellular signaling cascade. Central to the activation of this signaling cascade is PGC-1α, often referred to as the master regulator of mitochondrial biogenesis (19, 20). The expression of PGC-1α regulates interaction and coactivation of nuclear respiratory factor-1 (NRF-1) and NRF-2, which control the expression of genes involved in oxidative phosphorylation through the electron transport chain by encoding cytochrome c (COX) and COX oxidase subunit IV (COX IV), mitochondrial DNA transcription and replication, protein import machinery, and protein assembly (21–23). The activity of PGC-1α also modulates the activity of various nuclear transcription factors, including the PPARs and estrogen-related receptors (ERRs) involved in the regulation of mitochondrial fatty acid β-oxidation, the tricarboxylic acid cycle, and the electron transport chain (24).
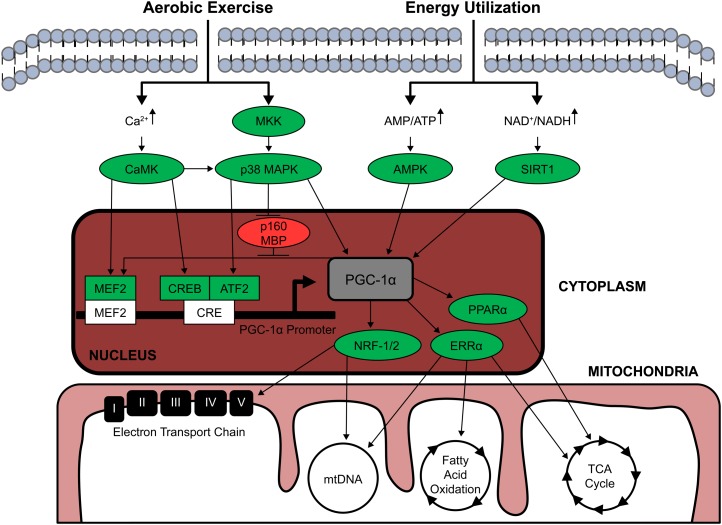
Activation of PGC-1α occurs at both the transcriptional and post-translational levels (Fig. 1) (23). Transcriptional PGC-1α expression is regulated through PGC-1α promoter binding activity of transcription factors myocyte enhancer factor 2 (MEF2), cAMP response element-binding protein (CREB), and activating transcription factor 2 (ATF-2) (25, 26). Interestingly, although MEF2 enhances PGC-1α transcription, it is also a target of PGC-1α, which is indicative of an autoregulatory loop by which PGC-1α regulates PGC-1α expression (27). Post-translational activation of PGC-1α is regulated through direct phosphorylation by AMPK and p38MAPK as well as deacetylation through silent mating type information regulation 2 homolog 1 (SIRT1) (26, 28).
FIGURE 1.
PGC-1α regulation of mitochondrial biogenesis. Aerobic exercise and energy utilization initiate mitochondrial biogenesis. This process is centrally regulated by PGC-1α, which can be activated at the transcriptional level through promoter binding activity and at the post-translational level via direct phosphorylation and deacetylation. PGC-1α controls mitochondrial biogenesis through interaction and coactivation of NRF-1, NRF-2, PPARα, and ERRα, which are regulators of mitochondrial DNA expression, fatty acid β-oxidation, the tricarboxylic acid cycle, and the electron transport chain. Stimulators of mitochondrial biogenesis are shown in green. Inhibitors of mitochondrial biogenesis are depicted in red. AMPK, 5′AMP-activated protein kinase; ATF-2, activating transcription factor 2; CaMK, Ca2+/calmodulin-dependent protein kinase; CRE, cAMP response element; CREB, cAMP response element-binding protein; ERRα, estrogen-related receptor α MBP, myelin basic protein; MEF2, myocyte enhancer factor 2; MKK, mitogen-activated protein kinase kinase; mtDNA, mitochondrial DNA; NRF-1/2, nuclear respiratory factor-1/2; p38 MAPK, p38 mitogen-activated protein kinase; PGC-1α, proliferator-activated γ receptor co-activator; SIRT1, silent mating type information regulation 2 homolog 1; TCA, tricarboxylic acid cycle.
Effects of Aerobic Exercise on the Regulation of Mitochondrial Biogenesis
The cumulative effects of aerobic training generally increase skeletal muscle mitochondria amount and activity with concomitant increases in PGC-1α mRNA expression and protein content (26, 29–31). The mechanism by which aerobic exercise training modulates mitochondrial biogenesis is dependent on disruption of cellular homeostasis. Contractile-induced increases in cytosolic Ca2+ and increased ratios of AMP:ATP and NAD+:NADH regulates PGC-1α activity by triggering intracellular signaling.
Contractile-induced Ca2+ release from the sarcoplasmic reticulum results in increased cytosolic Ca2+ concentrations, which upregulate PGC-1α expression and mitochondrial biogenesis through activation of Ca2+/calmodulin-dependent protein kinase (CaMK) (32, 33). CaMK may indirectly activate PGC-1α by phosphorylating the transcription factors CREB and MEF2, thereby enabling binding of these factors to the PGC-1α promoter site, which enhances PGC-1α transcription (26, 27). Increased intracellular Ca2+ concentrations may also mediate upregulation of p38 MAPK through CaMK activation (34). Similar to CaMK, p38 MAPK may also indirectly stimulate PGC-1α activity by phosphorylating the transcription factors ATF-2 and MEF2 and inhibiting the repressor p160 myb binding protein (p160 MBP), which blocks PGC-1α and MEF2 autoregulation (26, 35–38). Additionally, p38 MAPK directly phosphorylates PGC-1α (36) and although p38 MAPK signaling occurs downstream of CaMK, p38 MAPK appears to activate PGC-1α through a CaMK-independent mechanism (6). CaMK-independent, upregulated p38 MAPK phosphorylation may be attributed to aerobic exercise–induced expression of the upstream regulatory signaling proteins mitogen-activated protein kinase kinase 3 (MKK3) and MKK6. Investigations have shown that aerobic exercise upregulates MKK3 and MKK6 phosphorylation (39), which in turn directly phosphorylates p38 MAPK (40).
In addition to muscle contraction, cellular energy status is also a critical regulator of mitochondrial biogenesis. Prolonged aerobic exercise accelerates ATP utilization, increasing i.m. AMP:ATP ratios (41). Elevated cellular AMP initiates AMPK activation, which maintains cellular energy balance by inhibiting energy-utilizing anabolic pathways and upregulating ATP-yielding catabolic pathways (28, 42). The metabolic demand associated with sustained aerobic exercise increases AMPK phosphorylation, which appears to be an upstream intracellular regulator of PGC-1α activity (43, 44), because AMPK directly phosphorylates PGC-1α (45). Increased energy utilization during aerobic exercise also activates SIRT1 due to elevations in the cellular ratio of NAD+:NADH (46). The activation of SIRT1 results in PGC-1α deacetylation, which in turn activates PGC-1α and subsequent mitochondrial biogenesis (46). The phosphorylation status of AMPK indirectly regulates SIRT1, because AMPK controls the activation of signaling proteins involved in the catabolic energy yielding process, such as acetyl-CoA carboxylase and 6-phosphofructo-2-kinase, which result in increased NAD+:NADH levels (47). Together, these findings clearly illustrate the complexity associated with aerobic exercise–induced modulation of mitochondrial biogenesis, with multiple convergent signaling pathways sensitive to contractile force and cellular energy status regulating PGC-1α activity and mitochondrial biogenesis. Ultimately, aerobic training-induced alterations in intracellular signaling enhances mitochondrial content, number, size, and activity.
Effects of Carbohydrate Restriction on Aerobic Training#x2013Induced Mitochondrial Biogenesis
Maintaining carbohydrate availability can sustain and perhaps enhance aerobic exercise performance by delaying time to exhaustion (48). However, recent evidence now suggests that periodic reductions in glycogen stores by dietary carbohydrate restriction combined with short-term aerobic exercise training periods (3–10 wk) enhances mitochondrial biogenesis to a greater extent than when aerobic exercise is performed in a glycogen-replete state (13). Specifically, dietary carbohydrate restriction increases markers of mitochondrial activity, including citrate synthase and β-hydroxyacyl-CoA dehydrogenase activity, enhances COX IV total protein content, upregulates whole-body fat oxidation, and improves exercise time to exhaustion (14, 49). Furthermore, periods of reduced glycogen stores alter the activity of signaling proteins integral to intracellular lipid and glucose metabolism, including carnitine palmitoyltransferase-I, pyruvate dehydrogenase kinase-4, and glucose transporter protein 4 (50–53).
The mechanism by which skeletal muscle oxidative capacity is upregulated in response to aerobic exercise when dietary carbohydrate intake is restricted appears to occur upstream of PGC-1α and is dependent on AMPK and p38 MAPK activation. Phosphorylation of AMPK and p38 MAPK is higher when exogenous carbohydrate availability is restricted following a bout of glycogen-depleting aerobic exercise compared with phosphorylation levels when carbohydrate intake is adequate during recovery (53, 54). Recent reports demonstrate that increased AMPK and p38 MAPK phosphorylation in response to carbohydrate restriction upregulates PGC-1α activity following aerobic exercise (30).
However, not all studies support the link between carbohydrate availability and PGC-1α activity. In 2 recent studies, restricting carbohydrate availability with aerobic exercise increases markers of mitochondrial activity compared with aerobic exercise alone, but carbohydrate restriction had no effect on PGC-1α mRNA expression (48, 52). These data suggest that although PGC-1α is the central regulator of mitochondrial biogenesis in response to aerobic exercise, the mechanism by which carbohydrate restriction influences mitochondrial adaptations to aerobic exercise is not clear.
The tumor suppressor protein, p53, which is sensitive to carbohydrate availability, has recently been identified as a potential regulator of mitochondrial biogenesis (55). Studies have demonstrated that p53 is phosphorylated by AMPK (56) and p38 MAPK (57) and stimulates the expression of genes that promote and maintain mitochondrial function (58, 59). Bartlett et al. (60) demonstrated upregulation in p53, AMPK, and p38 MAPK phosphorylation in glycogen-depleted human skeletal muscle following 50 min of continuous aerobic exercise or high-intensity, interval-type exercise. The same researchers demonstrated that p53 phosphorylation, mitochondrial transcription factor A (Tfam), and COX IV mRNA expression were greater during recovery from 50 min of high-intensity interval cycling when volunteers were restricted from consuming carbohydrate compared with volunteers who consumed carbohydrate before, during, and after exercise (61). This investigation also observed greater PGC-1α mRNA expression during carbohydrate restriction. It is important to note that a glycogen depletion protocol was utilized the evening prior to the experimental session to elicit the low-carbohydrate state. As such, the higher PGC-1α mRNA expression observed during baseline and recovery from the 50-min aerobic exercise bout may have been a carryover effect from the glycogen depletion protocol (61). However, because this investigation utilized a glycogen depletion protocol combined with dietary carbohydrate restriction, it is difficult to interpret the influence on PGC-1α mRNA expression. Thus, although periodic carbohydrate restriction potentiates aerobic exercise–induced mitochondrial biogenesis, whether the increase in mitochondrial biogenesis was due to activation of p53 or PGC-1α remains unclear.
Effects of Protein Supplementation on Aerobic Training#x2013Induced Mitochondrial Biogenesis
Although carbohydrate restriction may augment mitochondrial adaptations to exercise, it may also impair skeletal muscle repair and recovery from aerobic exercise. Howarth et al. (15) reported that performing aerobic exercise under conditions of limited muscle glycogen availability increases skeletal muscle proteolysis and reduces muscle protein synthesis during recovery compared with responses when aerobic exercise was performed in a glycogen-replete state. However, it might be possible to use carbohydrate restriction to augment mitochondrial adaptations to exercise but offset those negative effects on muscle protein turnover by supplementing with dietary protein. Recent evidence has demonstrated that consuming dietary protein during or immediately following aerobic exercise increases mixed muscle protein synthesis, resulting in positive net protein balance (17, 18). Furthermore, increasing extracellular amino acid levels upregulate mitochondrial protein synthesis (62), suggesting that protein supplementation with aerobic exercise during carbohydrate restriction may not only maintain skeletal muscle protein balance but may also contribute to mitochondrial adaptations to aerobic exercise.
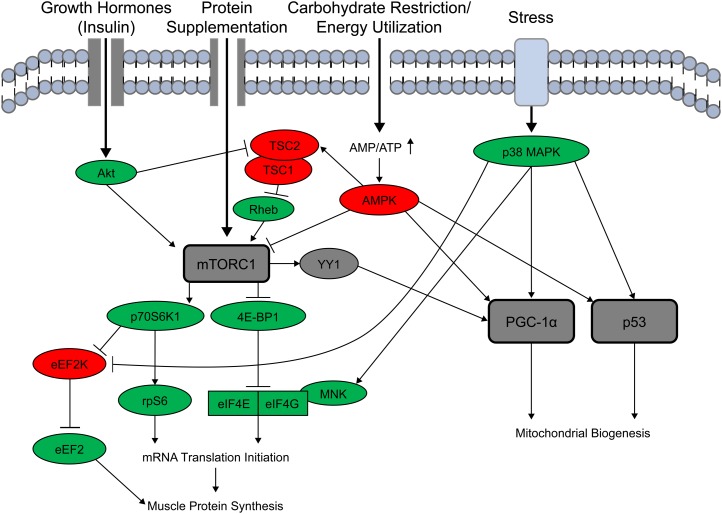
The mechanism by which dietary protein modulates skeletal muscle protein synthesis through the mammalian target of rapamycin complex 1 (mTORC1) is well described (63, 64). Activation of the mTORC1 complex triggers downstream signaling through p70 S6 kinase (p70 S6K1), ribosomal protein S6 (rpS6), eukaryotic elongation factor 2 kinase (eEF2), and eukaryotic initiation factor 4E-binding protein (4E-BP1) that increases mRNA translational efficiency and ultimately muscle protein synthesis (65). Although it was generally accepted that activation of the mTORC1 and AMPK-PGC-1α signaling pathways require different stimuli, with mTORC1 activated by primarily by resistance exercise and AMPK-PGC-1α activated by mainly by aerobic exercise (43), recent investigations indicate potential interactions between the pathways (Fig. 2) (66–68). For example, p38 MAPK phosphorylation can inhibit eEF2 kinase (eEF2K), thereby activating eEF2 and stimulating muscle protein synthesis (66). Also, p38 MAPK phosphorylation activates mitogen and stress activated kinase (MNK), which catalyzes the phosphorylation eukaryotic initiation factor 4E (eIF4E), an important regulator of translation initiation (67). Additionally, it has been reported that the amino acid leucine, a potent stimulator of mTORC1 signaling, may increase mitochondria size via SIRT1 and subsequent activation of PGC-1α (69). The interaction of these regulatory pathways also operates in the other direction. Inhibition of mTOR decreases activation of PGC-1α, resulting in decreased expression of mitochondrial genes and mitochondrial DNA via an inhibition of yin yang 1 (YY1) (68). This finding suggests a potential mechanism of crosstalk between intracellular pathways such that mTOR balances anabolic activity and energy metabolism through transcriptional control of mitochondrial biogenesis (68).
FIGURE 2.
Integrated muscle protein synthesis and mitochondrial biogenesis intracellular signaling. Muscle protein synthesis and mitochondrial biogenesis require activation of divergent intracellular signaling cascades for initiation; however, individual signaling proteins interact, indicating a convergence between the 2 signaling pathways. Muscle protein synthetic stimulators are depicted in green and inhibitors shown in red. Akt, protein kinase B; AMPK, AMP-activated protein kinase; 4E-BP1, eukaryotic initiation factor 4E-binding protein; eEF2, eukaryotic elongation factor 2; eEF2K, eukaryotic elongation factor 2 kinase; eIF4E/eIF4G, eukaryotic initiation factor; MNK, mitogen and stress activated kinase; mTORC1, mammalian target of rapamycin complex 1; p38 MAPK, p38 mitogen-activated protein kinase; p53, tumor suppressor protein; p70S6K, p70 S6 kinase; PGC-1α, proliferator-activated γ receptor co-activator; Rheb, ras homolog enriched in brain; rpS6, ribosomal protein S6; YY1, yin yang 1; TSC, tuberous sclerosis complex.
In addition to the observed overlap in signaling of muscle protein synthesis and mitochondrial biogenesis, similar upregulation in mTOR and AMPK-PGC-1α signaling cascades can be achieved in response to resistance and aerobic exercise, particularly when supplemental protein is consumed (70–72). Camera et al. (70) reported that phosphorylation of protein kinase B (Akt) and mTOR in the fasted state are similar with aerobic and resistance-type exercise. However, AMPK was phosphorylated only in response to aerobic exercise. On the other hand, when participants consume a mixed-meal containing 20 g of high-quality protein before, during, and after exercise, phosphorylation of Akt, mTOR, p70S6K, and AMPK were all similar in response to aerobic and resistance-type exercise (72). Furthermore, PGC-1α mRNA expression was 2-fold higher with combined aerobic and resistance exercise compared with performing only aerobic exercise (71). Concomitant phosphorylation of AMPK and mTOR suggests both cellular growth and mitochondrial biogenesis may occur in response to combined training.
Several studies have observed that consumption of supplemental protein following aerobic exercise stimulates mitochondrial protein synthesis (72, 73). However, studies have reported no differences in postaerobic exercise mitochondrial protein synthesis when volunteers consumed a combined carbohydrate and protein supplement compared with a noncaloric placebo (74) or carbohydrate alone (75), nor was there a difference in the phosphorylation of AMPK or PGC-1α mRNA expression immediately and 3 h postexercise (76). Furthermore, dietary leucine may also suppress phosphorylation of AMPK (77). Conversely, Hill et al. (78) reported greater PGC-1α mRNA expression when participants consumed a carbohydrate-protein supplement compared with carbohydrate alone 6 h postexercise.
Despite the conflicting results, protein supplementation does not appear to further enhance aerobic exercise–induced mitochondrial biogenesis when carbohydrate is restricted. However, it is important to recognize that protein supplementation does not hinder the activation of intracellular signaling proteins associated with mitochondrial biogenesis, nor does protein supplementation impede mitochondrial protein synthesis. Additionally, protein supplementation increased myofibrillar protein synthesis and phosphorylation of mTOR, p70S6K, and rpS6 following aerobic exercise (74, 75). Thus, although protein supplementation may not elevate mitochondrial biogenesis per se, consuming high-quality protein during or after aerobic exercise promotes skeletal muscle recovery, especially when aerobic exercise is performed with concomitant carbohydrate restriction.
In conclusion, mitochondrial biogenesis is a critical metabolic adaptation to aerobic exercise training. The activity of PGC-1α appears central to aerobic training-induced mitochondrial adaptations. Emerging evidence suggests that the mitochondrial adaptive response to aerobic exercise can be further potentiated by restricting carbohydrate availability, although the underlying mechanism has not been determined. The synergistic effect of carbohydrate restriction with aerobic exercise training may elicit greater aerobic exercise–induced adaptations, thereby delaying the onset of muscle fatigue and improving aerobic performance. Additionally, consuming supplemental protein during or in recovery from aerobic exercise, especially during periods of carbohydrate restriction, may facilitate the maintenance of skeletal muscle integrity and support mitochondrial biogenesis, although standardized dietary carbohydrate and protein recommendations are not possible at this time. Further study is warranted to determine dietary recommendations by assessing the isolated effects of supplemental protein on mitochondrial biogenesis following aerobic exercise and whether habitual dietary carbohydrate and protein intake modulates skeletal muscle mitochondrial adaptive response to chronic aerobic training.
Acknowledgments
The authors thank Dr. Andrew J. Young for his critical review in the development of this manuscript. Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: Akt, protein kinase B; AMPK, AMP-activated protein kinase; ATF-2, activating transcription factor 2; CaMK, Ca2+/calmodulin-dependent protein kinase; COX; cytochrome c; COX IV, cytochrome c oxidase subunit IV; CREB, cAMP response element-binding protein; 4E-BP1, eukaryotic initiation factor 4E-binding protein; eEF2, eukaryotic elongation factor 2; eEF2K, eukaryotic elongation factor 2 kinase; eIF4E/eIF4G, eukaryotic initiation factor 4E/4G; ERRα estrogen-related receptor α MEF2, myocyte enhancer factor 2; MKK3/MKK6, mitogen-activated protein kinase kinase 3/6; MNK, mitogen and stress activated kinase; mTORC1, mammalian target of rapamycin complex 1; NRF-1/2, nuclear respiratory factor-1/2; p38 MAPK, p38 mitogen-activated protein kinase; p53; tumor suppressor protein; p70 S6K, p70 S6 kinase; p160 MBP, p160 myb binding protein; PGC-1α, proliferator-activated γ receptor co-activator; rpS6, ribosomal protein S6; SIRT1, silent mating type information regulation 2 homolog 1; Tfam, mitochondrial transcription factor A; YY1; yin yang 1.
Literature Cited
- 1.Hawley JA, Burke LM, Phillips SM, Spriet LL. Nutritional modulation of training-induced skeletal muscle adaptations. J Appl Physiol. 2011;110:834–45 [DOI] [PubMed] [Google Scholar]
- 2.Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol. 2006;209:2265–75 [DOI] [PubMed] [Google Scholar]
- 3.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–82 [PubMed] [Google Scholar]
- 4.Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1271–8 [DOI] [PubMed] [Google Scholar]
- 5.Baar K. Involvement of PPAR gamma co-activator-1, nuclear respiratory factors 1 and 2, and PPAR alpha in the adaptive response to endurance exercise. Proc Nutr Soc. 2004;63:269–73 [DOI] [PubMed] [Google Scholar]
- 6.Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol. 2009;106:929–34 [DOI] [PubMed] [Google Scholar]
- 7.Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104:1304–12 [DOI] [PubMed] [Google Scholar]
- 8.Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O. Skeletal muscle-specific expression of PGC-1alpha-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS ONE. 2011;6:e28290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wende AR, Schaeffer PJ, Parker GJ, Zechner C, Han DH, Chen MM, Hancock CR, Lehman JJ, Huss JM, McClain DA, et al. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J Biol Chem. 2007;282:36642–51 [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez NR, Di Marco NM, Langley S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med Sci Sports Exerc. 2009;41:709–31 [DOI] [PubMed] [Google Scholar]
- 11.Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the "crossover" concept. J Appl Physiol. 1994;76:2253–61 [DOI] [PubMed] [Google Scholar]
- 12.Coyle EF, Coggan AR, Hemmert MK, Ivy JL. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol. 1986;61:165–72 [DOI] [PubMed] [Google Scholar]
- 13.Hawley JA, Burke LM. Carbohydrate availability and training adaptation: effects on cell metabolism. Exerc Sport Sci Rev. 2010;38:152–60 [DOI] [PubMed] [Google Scholar]
- 14.Yeo WK, Paton CD, Garnham AP, Burke LM, Carey AL, Hawley JA. Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J Appl Physiol. 2008;105:1462–70 [DOI] [PubMed] [Google Scholar]
- 15.Howarth KR, Phillips SM, MacDonald MJ, Richards D, Moreau NA, Gibala MJ. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. J Appl Physiol. 2010;109:431–8 [DOI] [PubMed] [Google Scholar]
- 16.Burke LM, Kiens B. "Fat adaptation" for athletic performance: the nail in the coffin? J Appl Physiol. 2006;100:7–8 [DOI] [PubMed] [Google Scholar]
- 17.Howarth KR, Moreau NA, Phillips SM, Gibala MJ. Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. J Appl Physiol. 2009;106:1394–402 [DOI] [PubMed] [Google Scholar]
- 18.Pasiakos SM, McClung HL, McClung JP, Margolis LM, Andersen NE, Cloutier GJ, Pikosky MA, Rood JC, Fielding RA, Young AJ. Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am J Clin Nutr. 2011;94:809–18 [DOI] [PubMed] [Google Scholar]
- 19.Andersson U, Scarpulla RC. Pgc-1-related coactivator, a novel, serum-inducible coactivator of nuclear respiratory factor 1-dependent transcription in mammalian cells. Mol Cell Biol. 2001;21:3738–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta. 2002;1576:1–14 [DOI] [PubMed] [Google Scholar]
- 21.Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25:1354–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarpulla RC. Nucleus-encoded regulators of mitochondrial function: integration of respiratory chain expression, nutrient sensing and metabolic stress. Biochim Biophys Acta. 2012;1819:1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenz T. Regulation of mitochondrial biogenesis and PGC-1alpha under cellular stress. Mitochondrion. 2013;13:134–42 [DOI] [PubMed] [Google Scholar]
- 24.Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23:459–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akimoto T, Li P, Yan Z. Functional interaction of regulatory factors with the Pgc-1alpha promoter in response to exercise by in vivo imaging. Am J Physiol Cell Physiol. 2008;295:C288–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–93 [DOI] [PubMed] [Google Scholar]
- 27.Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci USA. 2003;100:7111–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–86 [DOI] [PubMed] [Google Scholar]
- 30.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588:4795–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright DC. Mechanisms of calcium-induced mitochondrial biogenesis and GLUT4 synthesis. Appl Physiol Nutr Metab. 2007;32:840–5 [DOI] [PubMed] [Google Scholar]
- 33.Irrcher I, Adhihetty PJ, Sheehan T, Joseph AM, Hood DA. PPARgamma coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol Cell Physiol. 2003;284:C1669–77 [DOI] [PubMed] [Google Scholar]
- 34.Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:18793–9 [DOI] [PubMed] [Google Scholar]
- 35.Knutti D, Kressler D, Kralli A. Regulation of the transcriptional coactivator PGC-1 via MAPK-sensitive interaction with a repressor. Proc Natl Acad Sci USA. 2001;98:9713–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8:971–82 [DOI] [PubMed] [Google Scholar]
- 37.Zetser A, Gredinger E, Bengal E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J Biol Chem. 1999;274:5193–200 [DOI] [PubMed] [Google Scholar]
- 38.Fan M, Rhee J, St-Pierre J, Handschin C, Puigserver P, Lin J, Jaeger S, Erdjument-Bromage H, Tempst P, Spiegelman BM. Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1alpha: modulation by p38 MAPK. Genes Dev. 2004;18:278–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boppart MD, Asp S, Wojtaszewski JF, Fielding RA, Mohr T, Goodyear LJ. Marathon running transiently increases c-Jun NH2-terminal kinase and p38 activities in human skeletal muscle. J Physiol. 2000;526:663–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keesler GA, Bray J, Hunt J, Johnson DA, Gleason T, Yao Z, Wang SW, Parker C, Yamane H, Cole C, et al. Purification and activation of recombinant p38 isoforms alpha, beta, gamma, and delta. Protein Expr Purif. 1998;14:221–8 [DOI] [PubMed] [Google Scholar]
- 41.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase–development of the energy sensor concept. J Physiol. 2006;574:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda). 2006;21:48–60 [DOI] [PubMed] [Google Scholar]
- 43.Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–8 [DOI] [PubMed] [Google Scholar]
- 44.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA. 2002;99:15983–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:12017–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morton JP, Croft L, Bartlett JD, Maclaren DP, Reilly T, Evans L, McArdle A, Drust B. Reduced carbohydrate availability does not modulate training-induced heat shock protein adaptations but does upregulate oxidative enzyme activity in human skeletal muscle. J Appl Physiol. 2009;106:1513–21 [DOI] [PubMed] [Google Scholar]
- 49.Hansen AK, Fischer CP, Plomgaard P, Andersen JL, Saltin B, Pedersen BK. Skeletal muscle adaptation: training twice every second day vs. training once daily. J Appl Physiol. 2005;98:93–9 [DOI] [PubMed] [Google Scholar]
- 50.Arkinstall MJ, Tunstall RJ, Cameron-Smith D, Hawley JA. Regulation of metabolic genes in human skeletal muscle by short-term exercise and diet manipulation. Am J Physiol Endocrinol Metab. 2004;287:E25–31 [DOI] [PubMed] [Google Scholar]
- 51.Civitarese AE, Hesselink MK, Russell AP, Ravussin E, Schrauwen P. Glucose ingestion during exercise blunts exercise-induced gene expression of skeletal muscle fat oxidative genes. Am J Physiol Endocrinol Metab. 2005;289:E1023–9 [DOI] [PubMed] [Google Scholar]
- 52.Cluberton LJ, McGee SL, Murphy RM, Hargreaves M. Effect of carbohydrate ingestion on exercise-induced alterations in metabolic gene expression. J Appl Physiol. 2005;99:1359–63 [DOI] [PubMed] [Google Scholar]
- 53.Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E813–22 [DOI] [PubMed] [Google Scholar]
- 54.Chan MH, McGee SL, Watt MJ, Hargreaves M, Febbraio MA. Altering dietary nutrient intake that reduces glycogen content leads to phosphorylation of nuclear p38 MAP kinase in human skeletal muscle: association with IL-6 gene transcription during contraction. FASEB J. 2004;18:1785–7 [DOI] [PubMed] [Google Scholar]
- 55.Saleem A, Carter HN, Iqbal S, Hood DA. Role of p53 within the regulatory network controlling muscle mitochondrial biogenesis. Exerc Sport Sci Rev. 2011;39:199–205 [DOI] [PubMed] [Google Scholar]
- 56.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–93 [DOI] [PubMed] [Google Scholar]
- 57.She QB, Bode AM, Ma WY, Chen NY, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 2001;61:1604–10 [PubMed] [Google Scholar]
- 58.Donahue RJ, Razmara M, Hoek JB, Knudsen TB. Direct influence of the p53 tumor suppressor on mitochondrial biogenesis and function. FASEB J. 2001;15:635–44 [DOI] [PubMed] [Google Scholar]
- 59.Saleem A, Adhihetty PJ, Hood DA. Role of p53 in mitochondrial biogenesis and apoptosis in skeletal muscle. Physiol Genomics. 2009;37:58–66 [DOI] [PubMed] [Google Scholar]
- 60.Bartlett JD, Hwa Joo C, Jeong TS, Louhelainen J, Cochran AJ, Gibala MJ, Gregson W, Close GL, Drust B, Morton JP. Matched work high-intensity interval and continuous running induce similar increases in PGC-1alpha mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J Appl Physiol. 2012;112:1135–43 [DOI] [PubMed] [Google Scholar]
- 61.Bartlett JD, Louhelainen J, Iqbal Z, Cochran AJ, Gibala MJ, Gregson W, Close GL, Drust B, Morton JP. Reduced carbohydrate availability enhances exercise-induced p53 signaling in human skeletal muscle: implications for mitochondrial biogenesis. Am J Physiol Regul Integr Comp Physiol. 2013;304:R450–8 [DOI] [PubMed] [Google Scholar]
- 62.Bohé J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552:315–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol. 2009;106:1374–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Proud CG. Amino acids and mTOR signalling in anabolic function. Biochem Soc Trans. 2007;35:1187–90 [DOI] [PubMed] [Google Scholar]
- 66.Knebel A, Morrice N, Cohen P. A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38delta. EMBO J. 2001;20:4360–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waskiewicz AJ, Johnson JC, Penn B, Mahalingam M, Kimball SR, Cooper JA. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol. 1999;19:1871–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–40 [DOI] [PubMed] [Google Scholar]
- 69.Sun X, Zemel MB. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr Metab (Lond). 2009;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG. Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc. 2010;42:1843–52 [DOI] [PubMed] [Google Scholar]
- 71.Wang L, Mascher H, Psilander N, Blomstrand E, Sahlin K. Resistance exercise enhances the molecular signaling of mitochondrial biogenesis induced by endurance exercise in human skeletal muscle. J Appl Physiol. 2011;111:1335–44 [DOI] [PubMed] [Google Scholar]
- 72.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586:3701–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009;587:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coffey VG, Moore DR, Burd NA, Rerecich T, Stellingwerff T, Garnham AP, Phillips SM, Hawley JA. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur J Appl Physiol. 2011;111:1473–83 [DOI] [PubMed] [Google Scholar]
- 75.Breen L, Philp A, Witard OC, Jackman SR, Selby A, Smith K, Baar K, Tipton KD. The influence of carbohydrate-protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. J Physiol. 2011;589:4011–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor C, Bartlett JD, van de Graaf CS, Louhelainen J, Coyne V, Iqbal Z, Maclaren DP, Gregson W, Close GL, Morton JP. Protein ingestion does not impair exercise-induced AMPK signalling when in a glycogen-depleted state: implications for train-low compete-high. Eur J Appl Physiol. 2013. l113:1457–68. [DOI] [PubMed] [Google Scholar]
- 77.Wilson GJ, Layman DK, Moulton CJ, Norton LE, Anthony TG, Proud CG, Rupassara SI, Garlick PJ. Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am J Physiol Endocrinol Metab. 2011;301:E1236–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hill KM, Stathis CG, Grinfeld E, Hayes A, McAinch AJ. Co-ingestion of carbohydrate and whey protein isolates enhance PGC-1alpha mRNA expression: a randomised, single blind, cross over study. J Int Soc Sports Nutr. 2013;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]