Abstract
Fructose-containing sugars, including fructose itself, high fructose corn syrup (HFCS), and sucrose have engendered considerable controversy. The effects of HFCS and sucrose in sugar-sweetened beverages, in particular, have generated intense scientific debate that has spilled over to the public. This controversy is related to well-known differences in metabolism between fructose and glucose in the liver. In addition, research studies have often been conducted comparing pure fructose and pure glucose even though neither is consumed to any appreciable degree in isolation in the human diet. Other evidence has been drawn from animal studies and epidemiologic or cohort studies. Few randomized controlled trials (RCTs) have compared HFCS with sucrose (the 2 sugars most commonly consumed in the human diet) at dosage amounts within the normal human consumption range. This review compares results of recently concluded RCTs with other forms of evidence related to fructose, HFCS, and sucrose. We conclude that great caution must be used when suggesting adverse health effects of consuming these sugars in the normal way they are consumed and at the normal amounts in the human diet, because RCTs do not support adverse health consequences at these doses when employing these sugars.
Introduction
Few items in the human diet have engendered as much controversy as fructose, high fructose corn syrup (HFCS)4, and sucrose (1–21). What links these 3 sugars is the presence of fructose, either by itself or as a component of HFCS and sucrose. Controversies related to fructose-containing sugars are typically based on the well-known differences between metabolism of fructose and glucose in the liver (22). Numerous investigators have suggested that fructose-containing sugars may be associated with a variety of metabolic diseases, including diabetes (23, 24), metabolic syndrome (MetS) (25, 26), heart disease (19), nonalcoholic fatty liver disease (NAFLD) (27, 28), and even certain cancers and dementia (29). These assertions are often based on epidemiologic studies (low evidentiary value) (23, 24, 30), animal studies (which often do not translate well to human metabolism) (31–34), theoretical constructs (25, 35), or experiments involving large doses of pure fructose compared with pure glucose, neither of which is consumed to any appreciable degree in isolation in the human diet (36, 37).
Challenges to fructose-containing sugars are not new. In the 1980s Reiser et al. (38, Hallfrisch et al. (39), Reaven (40), and Hwang et al. (41) and others alleged that fructose alters lipid, glucose, and uric acid metabolism, resulting in increased risk factors for cardiovascular disease (CVD) and elevated blood pressure. These issues and others were reviewed in a comprehensive monograph published in 1993 in the American Journal of Clinical Nutrition (42).
In retrospect, the modern challenge to fructose appears to have begun in 2004 with a publication by Bray et al. (43) that asserted that “the increase in consumption of HFCS has a temporal relation to the epidemic of obesity, and the overconsumption of HFCS in calorically sweetened beverages may play a role in the epidemic of obesity.”
Although the authors of this commentary were careful to point out that this temporal association did not establish cause and effect, it was widely misinterpreted and sparked a series of research trials and debate within the scientific community.
Subsequently, a broad consensus has emerged that there is nothing unique about HFCS compared with sucrose or other nutritive sweeteners when it comes to a potential association with obesity. This position has been supported both by the American Medical Association (11) and the Academy of Nutrition and Dietetics (12).
Although the authors of the original “HFCS hypothesis,” as it has come to be known, have acknowledged that their original hypothesis concerning HFCS did not intend to imply or establish causation, they and others have reiterated their concern that fructose-containing sugars such as HFCS or sucrose, particularly when consumed in sugar-sweetened beverages (SSBs), may be linked to a variety of adverse health consequences (44).
With this information as background, the current review will explore issues related to the metabolism of fructose, HFCS, and sucrose as well as evaluate the strengths and weaknesses of the evidence supporting putative links between the consumption of these sugars and health consequences. These issues will be addressed by raising a series of questions and presenting results from recent randomized controlled trials (RCTs) from my research laboratory and others utilizing various amounts of HFCS, sucrose, and fructose exposure ranging from the 25th to the 90th percentile population consumption level of fructose.
HFCS, sucrose, and fructose: the “perfect storm” for confusion and mistaken identity.
Confusion among these 3 fructose-containing sugars appears to be based on a confluence of factors that essentially created a “perfect storm” for a variety of misperceptions, not only in the scientific and medical communities but subsequently in the media and the public at large.
One factor that contributed to the confusion regarding fructose, HFCS, and sucrose is the failure to distinguish between association and cause and effect. Although it is clearly understood within the scientific community that epidemiologic studies raise questions and can only establish association and not cause and effect, oftentimes results of these studies have been reported in ways that blur this distinction. In retrospect, the confusion was probably exacerbated by the unfortunate choice of the name “high fructose” corn syrup, which was utilized early in the history of this product to distinguish it from the corn syrup it was derived from. The choice of this name led both the public and even the scientific community to believe that HFCS was high in fructose and thus comparable with fructose itself rather than the appropriate comparison between HFCS and sucrose, both of which contain ~50% glucose and 50% fructose. HFCS in its normal usage is available in 2 forms: HFCS-55, which contains 55% fructose and 45% glucose, and HFCS-42, which contains 42% fructose and 58% glucose. Both contain minor amounts of glucose polymers.
The confusion between fructose and HFCS was further exacerbated by research trials that compared pure fructose to pure glucose and suggested that there were a variety of metabolic differences between these 2 sugars (16, 20, 36, 37). Although these studies, many of which utilized very large doses of either pure fructose or pure glucose, provided interesting scientific comparisons, it is important to understand that neither pure fructose nor pure glucose is consumed to any appreciable degree in isolation in the human diet.
An additional contributor to the “perfect storm” for confusion and mistaken identity relates to the fact that linking added sugar consumption to obesity raised strong emotional feelings, given the rapidly increasing worldwide pandemic of obesity, and provoked the understandable but misguided desire to look for a simple solution to what is clearly a very complex problem.
Finally, these emotional responses were further stimulated when various scientists used inflammatory language such as “toxic” (45) or “threat to global health” or “pure, white and deadly” (5, 46) when referring to fructose-containing sugars.
Is there a link between added sugars and obesity?
The world is in the midst of a pandemic of obesity. Both childhood and adult obesity represent enormous global health problems for developed as well as underdeveloped nations. Recent estimates suggest that more than 66 million American adults are obese and an additional 74 million are overweight (47). The prevalence of obesity in the United States has grown a shocking 40% in the last 30 y (48). The obesity epidemic is truly global. In European countries it ranges from 20% to 30% and it is even higher in South America, Australia, and Polynesia. According to the WHO, there will be 1.5 billion obese individuals worldwide by 2015 if current trends continue (49).
The role of fructose-containing sugars in the worldwide epidemic of obesity is uncertain. A number of epidemiologic studies have suggested an association between SSB consumption and obesity, increased caloric intake, and poor dietary quality (50–52). In an area as complicated as obesity, however, it would appear unlikely that one component of the diet represents a major cause of this worldwide pandemic. A recent scientific statement from the ASN emphasized the complexity of energy regulation and weight and cautioned against isolating one component of the diet as a primary cause of weight gain and/or obesity (53).
RCTs do not offer much support for a causative link between SSB consumption and weight change. A meta-analysis of RCTs by Kaiser et al. (54) evaluated studies that explored increased SSB consumption and weight gain as well as studies that explored the strategy of reducing SSBs as a tool to reduce weight. These investigators reported that RCTs exploring the effect of increased consumption of SSB explained only 1.92% of variance in body weight or BMI change. Reducing consumption of SSB in persons of all weight categories explained only 0.09% of variance of body weight or BMI change. Among individuals who were overweight or obese at baseline reducing the consumption of SSB explained 1.54% of the variance in body weight or BMI change. These investigators concluded that although there was reasonable evidence to support the conjecture that SSBs might contribute to obesity, the currently available evidence from RCTs was small and equivocal (54).
Recent meta-analyses by Dolan et al. in normal weight (55) and obese individuals (56) did not support a link between fructose consumption and obesity at amounts up to the 90 percentile population consumption level for fructose. Moreover, a systematic review and meta-analysis by Sievenpiper et al. (57), which explored the effect of fructose on body weight in controlled feeding trials, concluded that fructose did not appear to cause weight gain when substituted for other carbohydrates in diets providing similar calories even at relatively high amounts of consumption of fructose. However, consumption of fructose at high amounts providing excess calories modestly increased body weight. They concluded that this latter effect was most likely due to extra calories rather than fructose (57).
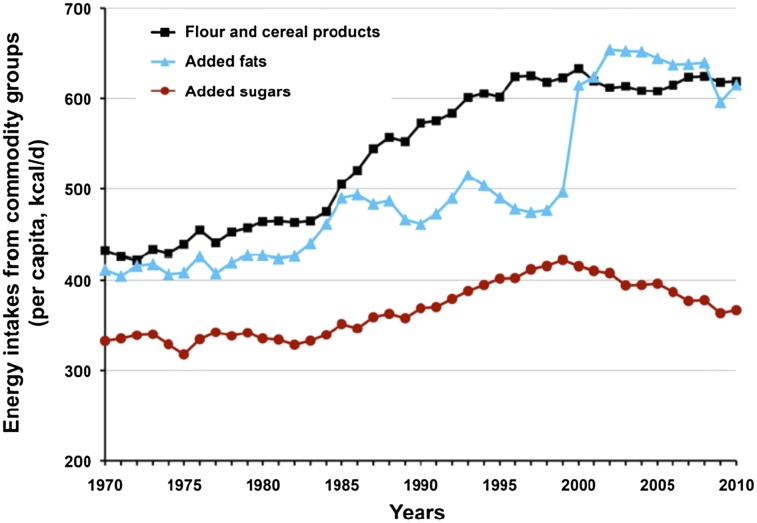
It is also important to note that sugar consumption has not increased disproportionately with the rise in core consumption of calories in the modern diet during the last 40 y (58). Moreover, both USDA historical trends and NHANES data demonstrate declines in the intake of added sugars in children of all ages and people of all ethnicities since 1999 (2). According to USDA data, there was a total increase of 449 kcal/d in mean intake in the United States between 1970 and 2010. During this 40-y period, only 8% of the calorie increase resulted from all added sugars combined, whereas flour-cereal products and added fats accounted for the vast majority, nearly 90%, as illustrated in Figure 1 (58).
FIGURE 1.
Energy intakes from commodity groups, 1970–2010, demonstrating that added sugars comprise a small portion of increased caloric consumption and have been in decline since 1998. Reproduced from (2) with permission. From: USDA Economic Research Service Average Daily per Capita Energy from the US Food Availability, adjusted for loss.
A study published by our research laboratory demonstrated that consumption of mean amounts of fructose-containing sugars did not result in increased body weight (59) during a 10-wk free-living trial. In a separate study, mean amounts of fructose-containing sugars were utilized as part of an overall hypocaloric diet and did not impede weight loss (60).
Thus, the impact of added sugars on obesity and weight gain or loss remains in dispute, with most of the RCTs suggesting that if any effect exists, it is small and/or equivocal.
The impact of fructose, glucose, HFCS, and sucrose on energy-regulating hormones.
Several studies have compared the effect of consuming fructose with that of glucose, often delivered at 25% of calories consumed, on energy-regulating hormones (16, 20, 36, 37). These studies have clearly demonstrated that differences in energy-regulating hormones can occur under artificial conditions, with glucose stimulating increased insulin production and a concomitant rise in leptin and suppression of ghrelin compared with fructose (16, 20, 36).
It has been argued that these differences in energy-regulating hormones could create an environment of increased hunger and appetite, resulting in increased consumption of calories. However, as already indicated, pure fructose and pure glucose are not typically consumed in isolation in the human diet to any appreciable degree. When these studies were repeated in analogous conditions with up to 30% of calories coming from either the more commonly consumed HFCS or sucrose, all differences in energy-regulating hormones disappeared (36, 61, 62).
Thus, experiments comparing pure fructose with pure glucose must be treated with great caution, because they represent an artificial condition and do not appear to accurately reflect the realities of human nutrition. Moreover, as recently reviewed by White (2), these studies have often used extreme fructose doses frequently exceeding even the 95th human population percentile intake by 1.5- to 3-fold. Fructose doses administered in animal studies have even been more extreme, often exceeding the 95th human percentile population intake by 4 or 5 times (2). Responses to such extreme doses should, therefore, be treated with appropriate caution because of the potential that such high dosages may distort normal metabolic responses.
Does dosage matter?
Several competing recommendations exist for appropriate upper limits of added sugars. Recently, the AHA recommended that the average adult male not consume >150 kcal/d and the average adult female no more than 100 kcal/d from all added sugars (63). It must be noted that these recommendations are exceeded by >90% of the adult population in the United States. Moreover, they are considerably more restrictive than recommendations published by the Institute of Medicine (IOM) (64) and the Dietary Guidelines for Americans (DGA) (65). These latter recommendations allow up to 25% of calories to be consumed from added sugars. The IOM/DGA guidelines are based on evidence suggesting that when amounts of added sugars exceed 25% of calories, dilution of important micronutrients may occur.
To explore the relation of various amounts of added sugars to health variables, our research group conducted a double-blind, prospective, RCT of 352 men and women between the ages of 20 and 60 y who consumed either HFCS or sucrose at 8% of calories (approximately the upper limit recommended by the AHA), 18% of calories (approximately the 50th percentile for fructose consumption in the United States), or 30% of calories (approximately the 90th percentile of fructose consumption). Individuals were studied at the beginning and end of a 10-wk, free-living trial consuming these sugars (66). A subset of individuals had spent 2 overnight stays in our Metabolic Unit where measurements of insulin, leptin, and ghrelin were obtained over a 24-h period. A subgroup of these individuals also underwent CT scanning of the liver to assess changes in liver fat and an MRI of the muscles to assess any potential impact of these various dosages on fatty infiltration of muscle. This study showed that there was no adverse impact on blood lipids (67), no changes in blood pressure (66), no changes in insulin, leptin, or ghrelin (68), no changes in liver fat or muscle fat, and no differences between HFCS and sucrose when comparing these 3 different doses of HFCS or sucrose on these experimental outcomes (69). These data support the IOM upper limits of intake and DGA conclusions that there are no adverse health effects of consuming up to 25% of calories from added sugars (64, 65).
Do Fructose-Containing Sugars Adversely Affect Lipids?
The issue of whether or not consumption of added sugars results in dyslipidemias remains controversial. The AHA has released a statement on TGs and CVD recommending that adults limit their consumption of fructose-containing sugars as a means of controlling TGs (70). This statement has, however, been challenged on a number of grounds (J. White, J. Rippe, J. Sievenpiper, unpublished data). Livesey and Taylor (71) published a meta-analysis that showed a lack of effect of fructose on fasting TGs at intakes ≤100 g/d, which is above the 95th percentage intake for even the most extreme users of fructose. A more recent systematic review and meta-analysis by Sievenpiper et al. (72), which included 13 isocaloric and 2 hypercaloric chronic feeding trials all of which were ≥7 d in duration, also showed no differences between fructose and any other carbohydrate source on TGs when fructose was isocalorically substituted into the diet. When fructose was hypercalorically substituted, increases in TGs were observed. These data suggest that the effects of fructose on TGs do not differ from other carbohydrate sources as long as the diets are matched for dose and energy. These data further suggest that the observed effect of fructose on cardiometabolic risk factors in hypercaloric trials appears to be more attributable to excess energy than to fructose per se.
Some investigators have reported results in which sugar consumption resulted in various dyslipidemias in human participants. In particular, Stanhope et al. (16), using a model in which 25% of energy consumption from fructose was compared to 25% of energy from glucose in acute experiments, showed increases in TGs in the fructose-consuming group. Other investigators, including Marckmann (73), Raben et al. (74), Maersk et al. (26), and Teff et al. (20), have also reported increases in cholesterol and/or LDL cholesterol in participants consuming either sucrose or HFCS.
In contrast, studies in our research laboratory at amounts 2–3 times the upper limit recommended by the AHA did not show any adverse impact on lipids (75). Furthermore, the trial in our research laboratory already mentioned, which involved 352 overweight or obese individuals who consumed up to the 90 percentile population consumption levels of fructose as part of a mixed nutrient eucaloric diet, did not show any effect on total cholesterol (P = 0.88) or LDL cholesterol (P = 0.85) (75). A significant 14% increase in TGs was noted in this study, although it must be noted that individuals gained ∼2 pounds during the course of the research trial, which may have contributed to increased TGs. It should also be noted that TG amounts remained within the normal range both before and after the 10-wk study period of sugars consumption. Thus, the impact of fructose-containing sugars on lipids remains in dispute. When large amounts of sugars (>20% of calories) are given, particularly in hypercaloric trials, TGs often increase (70, 76). The effect of sugars on other lipids remains in dispute and will require further RCTs to clarify.
Do Fructose-Containing Sugars Adversely Affect Risk Factors for Diabetes?
The issue of whether or not added sugars exert an adverse impact on risk factors for diabetes has been debated for many years. Fructose was initially viewed as a potentially useful substitute for glucose in individuals with diabetes, because it does not stimulate insulin response to the degree that glucose does. However, early research in this area suggested that increased consumption of fructose could result in increased TGs, thereby increasing the risk of CVD and negating any benefit that might be derived from lower insulin excursion (77). The American Diabetes Association recommends that individuals with diabetes should moderate their consumption of added sugars (78).
Recently, several investigators have argued, based on ecological studies, that either sugars in general or HFCS in particular may increase the risk of diabetes. Goran et al. (24) correlated production of HFCS by country with prevalence of diabetes. Their analysis concluded that countries where more HFCS was produced had a higher prevalence of diabetes, purportedly because of the incremental increase in fructose due to HFCS-55 and sucrose. Their analysis of the European Union was flawed, however, because only HFCS-42 is produced there; overall fructose intake will decrease when HFCS-42 is substituted for sucrose. Basu et al. (23) correlated sugars consumption by country with risk of diabetes and concluded that there was a positive association between sugars consumption and diabetes. It should be noted that data from this latter study showed a correlation between obesity and diabetes that was 20 times greater than sugars consumption and diabetes. Both of these research groups rightly cautioned that ecological studies are a low form of evidence and conceded that despite efforts to control confounding factors, multiple other factors could have contributed to the associations observed. Unfortunately, various media picked up on this work and reported that sugars consumption could represent a significant risk factor for diabetes.
Most studies reviewed do not support the contention that sugar consumption per se increases the risk of diabetes. The European Prospective Investigation into Cancer and Nutrition Study, a large, multi-national cohort study, did not find a correlation between consumption of sugars or other carbohydrates and the risk of diabetes (79). Research performed in our laboratory compared HFCS with sucrose at 8, 18, and 30% of calories in a 10-wk, free-living trial and did not find any differences between the 2 sugars and no increases in glucose, insulin, insulin resistance, AUC for glucose, or AUC for insulin (J. Lowndes, T. Angelopoulos, J. Rippe, unpublished data). A subsequent study conducted by our research group compared HFCS and sucrose at 18% of calories with fructose and glucose at 9% of calories as part of mixed nutrient nutrition plans. Once again, we did not find any differences in glucose, insulin, insulin resistance by the HOMA, or AUC for glucose or insulin when comparing the 3 fructose-containing sugars with the glucose control condition (J. Rippe, T. Angelopoulos, T. Lowndes, unpublished data).
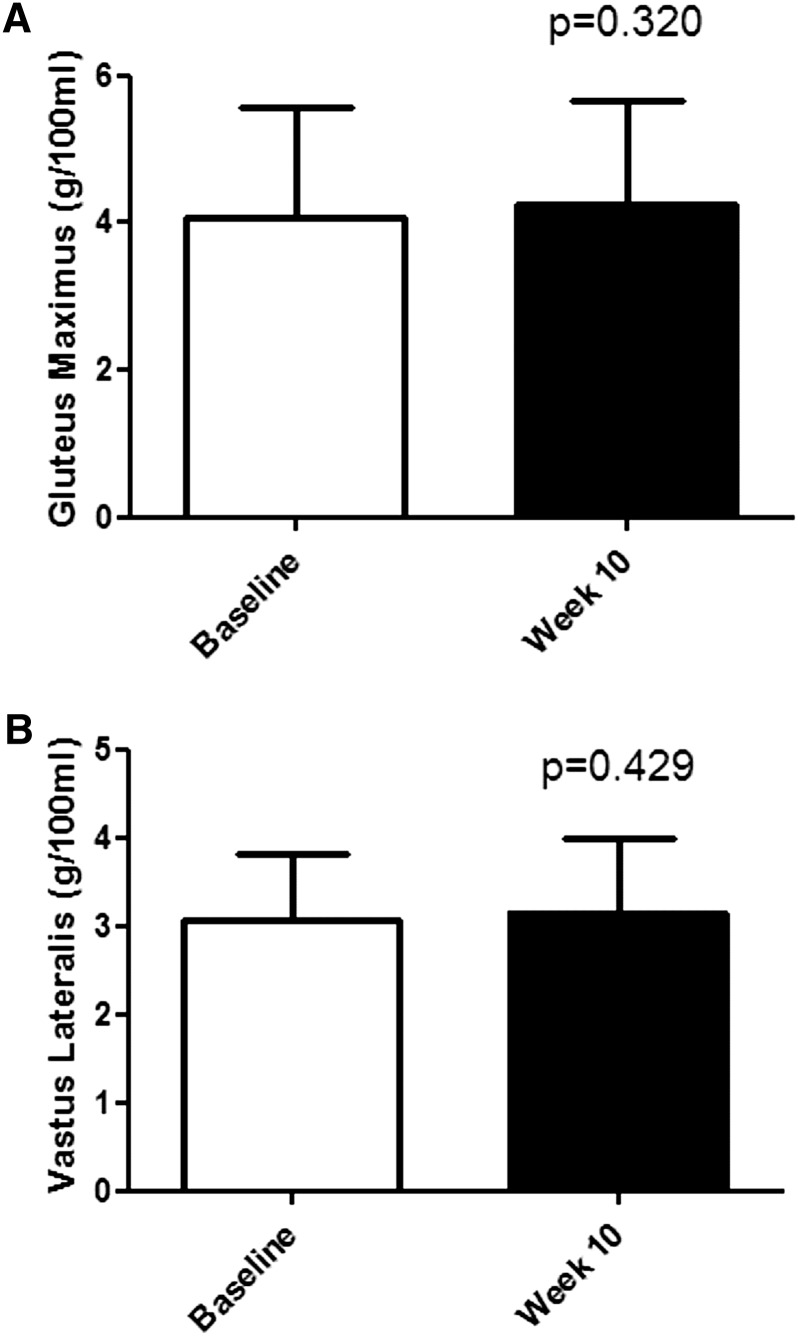
Insulin resistance typically precedes diabetes by 10–20 y (80). It has been reported that ectopic deposition of fat in skeletal muscle is responsible for up to 75% of all insulin resistance (81). A research study conducted by our group compared muscle fat before and after a 10-wk, free-living intervention where participants consumed either HFCS or sucrose at 8, 18, or 30% of calories. As illustrated in Figure 2, no changes were found in ectopic muscle fat in the gluteus maximus or vastus lateralis muscles when consuming these amounts of either sugar (69). Furthermore, the meta-analysis by Cozma et al. (82) found that participants consuming fructose demonstrated decreased hemoglobin A1C compared with those consuming other substituted sugars.
FIGURE 2.
Muscle fat determination (by MRI) on 68 participants who consumed either HFCS or sucrose to supply 8, 18, or 30% of energy in low-fat milk during a 10-wk, free-living trial. Reproduced from (69) with permission. (A) Gluteus maximus. (B) Vastus lateralis. HFCS, high fructose corn syrup.
Thus, there is considerable conflicting evidence concerning whether added sugars increase the risk of diabetes. Stronger studies, including cohort and RCTs, suggest that there is not a relation between sugars consumption and risk factors for diabetes. Because of the well-known and strong correlation between diabetes and obesity, any propensity for added sugars to increase weight might indirectly increase risk factors for diabetes. However, a direct link does not appear supported by the current literature.
Does Consumption of Fructose-Containing Sugars Increase Blood Pressure?
Some studies have suggested that consumption of added sugars may cause an increase in blood pressure (83, 84). However, results in human studies are equivocal (85). Some epidemiologic studies have suggested that increased consumption of sugars may increase blood pressure (83, 84, 86); however, other studies have not confirmed these findings (85).
Johnson et al. (25) have proposed a mechanism through which increased consumption of sugars might cause an increase in blood pressure. These investigators propose a model where fructose metabolism in the liver leads to consumption of ATP, which is ultimately degraded to uric acid. Uric acid in turn, according to this model, could cause endothelial dysfunction, leading to high blood pressure.
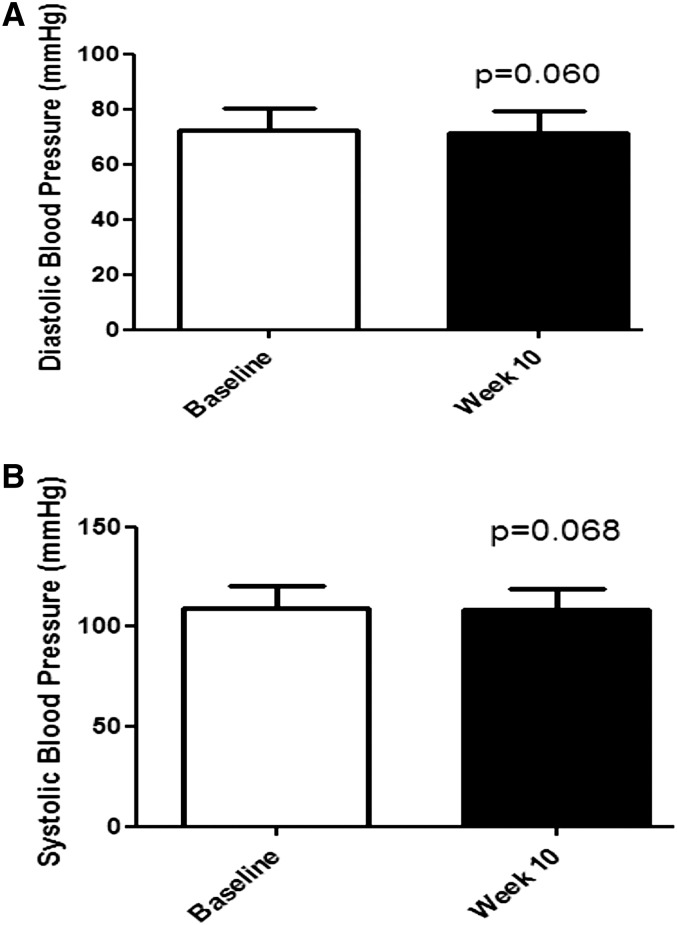
Research in our laboratory compared 8, 18, or 30% of calories from HFCS or sucrose during a 10-wk, free-living period in 352 normotensive individuals and found no increase in blood pressure (75) (Fig. 3). A subsequent research study by our group showed that HFCS consumed at 18% of calories compared with sucrose at 18% of calories, fructose at 9% of calories, or glucose at 9% of calories consumed during a 10-wk period in a free-living cohort of 123 individuals did not raise either systolic or diastolic blood pressure.
FIGURE 3.
Blood pressure response in 352 individuals who consumed either HFCS or sucrose to supply 8, 18, or 30% of calories in low-fat milk during a 10-wk, free-living trial. The white bars indicate baseline measurements and the black bars indicate measurements obtained following the 10-wk intervention. Adapted with permission from (75). (A) Diastolic blood pressure. (B) Systolic blood pressure. HFCS, high fructose corn syrup.
Sun et al. (87) looked at nationally representative data comparing fructose consumption with uric acid measurements and did not find a correlation. These data were corroborated by research in our laboratory showing that at multiple different dosages of HFCS, sucrose, or fructose, there was no increase in either fasting uric acid or uric AUC (88, 89).
Ha et al. (90) conducted a systematic review and meta-analysis of studies evaluating the effect of fructose on blood pressure. They reported that when fructose was isocalorically substituted for other carbohydrates, no increase in blood pressure occurred. Moreover, the same research group found that there was no correlation in published studies between fructose consumption and uric acid even at amounts higher than normal human consumption (91). Thus, the hypothesized relation between fructose-containing sugars and increase in blood pressure is not supported by recent RCTs or meta-analyses in studies involving humans.
Does Consumption of Fructose-Containing Sugars Increase the Risk of MetS?
The prevalence of MetS has considerably increased in the United States in the past 20 y. Some reports utilizing NHANES data have reported a prevalence of MetS of up to 39% of adults (92). Although there are multiple different definitions of MetS, the one most commonly used in clinical medicine comes from the National Cholesterol Education Program’s Adult Treatment Panel III Guidelines (93). MetS represents a constellation of factors, including dyslipidemia, abnormal glucose handling, and high blood pressure.
Excess accumulation of abdominal fat is strongly associated with MetS and is thought to be one of its underlying causes. Several investigators have suggested that increased consumption of fructose-containing sugars may result in an increase in risk factors for MetS. Maersk et al. (26) reported a 6-mo trial of individuals consuming 1 L/d of sucrose-sweetened cola, diet cola, milk, or water. They reported that consumption of sucrose-sweetened cola increased risk factors for MetS. Stanhope et al. (16) compared consumption of fructose with glucose at 25% of calories and reported increases in visceral abdominal fat in the fructose participants. They speculated that this could increase the risk of MetS.
Other investigators have challenged these findings. Sun et al. (94) conducted an analysis of nationally representative data and found no correlation between fructose-containing sugars and prevalence of MetS. RCTs conducted in our research laboratory compared the effects of 8, 18, or 30% of calories from either HFCS or sucrose on body weight and abdominal fat in a cohort of 116 individuals. Although there was an ∼2-pound increase in body mass during the entire cohort, there was no increase in abdominal fat as evaluated by DXA. A subsequent study comparing HFCS at 18% of calories, sucrose at 18% of calories, fructose at 9% of calories, and glucose at 9% of calories in 123 individuals during a 10-wk, free-living period did not show any increase in accumulation of abdominal fat. Moreover, these studies did not show any increase in either systolic or diastolic blood pressure or increase in glucose. A slight decrease in HDL (~1 mg/dL) and increases in TGs (10–14% increase) occurred in these studies, although all values remained within normal limits (67). Thus, any effects of fructose-containing sugars on risk factors for MetS, if present, would appear to be very small.
Does Consumption of Fructose-Containing Sugars Increase the Risk of Fatty Infiltration of the Liver?
Concern about fatty infiltration of the liver and a potential effect of fructose has been evaluated by a number of investigators (95, 96). The basic underlying mechanism of metabolism of fructose in the liver has been suggested by some investigators as a potential reason to be concerned about fat accumulation in the liver (16, 97).
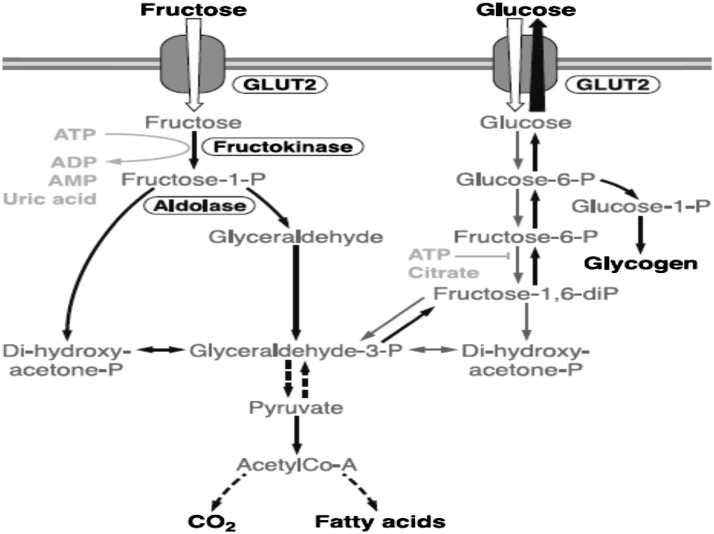
Fructose is metabolized differently than glucose in the liver, as illustrated in Figure 4. More than 90% of absorbed fructose is cleared on first pass by the liver. As shown in this figure, there is an interactive pathway between glucose and fructose metabolism. It has been estimated that >50% of fructose is metabolized into glucose, another 15% into glycogen, 25% into lactate, and a few percent into carbon dioxide (22). In various studies, 1–5% of fructose consumed can be converted into TGs in the process of de novo lipogenesis (DNL). The amount of fat generated through this process in normal human metabolism is on the order of 1% of the amount of fat typically consumed in the human diet (95). Nonetheless, some investigators have suggested that DNL may contribute to increased fat in the liver (96–99) and might ultimately lead to NAFLD, which is the leading cause of chronic liver disease and the need for liver transplantation in the world (27, 28).
FIGURE 4 .
Metabolism of fructose and glucose in the liver. Although there are differences in metabolism, the pathways are interactive, as indicated in the figure. Reproduced from (22) with permission. ADP, adenosine diphosphate; AMP, adenosine monophosphate; ATP, adenosine-5'-triphosphate; CO2, carbon dioxide; GLUT, glucose transporters.
Lê et al. (98) gave descendants of diabetics dosages of fructose of 3.5 mg/kg of lean body mass and found some increased accumulation of liver fat. Stanhope et al. (16) gave individuals 25% of energy as fructose and found that there was increased DNL compared with 25% of energy as glucose. However, other investigators have not found increased liver fat following fructose consumption (100, 101). In a separate study, Lê et al. (101) reported that individuals who consumed 1 mg/kg of fructose of lean body weight did not increase liver fat. Silbernagel et al. (100) reported similar findings in a 4-wk trial. Bravo et al. (69) from our research group reported that individuals who consumed up to 30% of calories from either HFCS or sucrose during a 10-wk, free-living period did not accumulate additional fat in the liver. Chung et al. (102) conducted a systematic review and meta-analysis, which did not find a linkage between fructose consumption and NAFLD. Thus, although there are theoretical models that suggest that an increase in DNL may occur in response to fructose consumption, the literature on liver fat accumulation in response to fructose consumption is mixed. RCTs that have given normally consumed sugars at typical amounts have not demonstrated fat accumulation in the liver (69, 98, 100). Whether fructose consumption can lead to NAFLD is speculative and not supported by recent systematic and meta-analysis (102).
Do Fructose-Containing Sugars Have Different Effects Than Glucose on Neural Pathways?
Some animal experiments have suggested that fructose behaves differently in the brain than does glucose (31, 34). These studies, particularly in rodents, must be treated with great caution given the multiple and important differences between rodent brains and human brains, most prominently the small prefrontal cortex in the rat brain and the large, well-developed one in humans (103, 104). Thus, studies of behavior and cognition in rats often translate poorly to humans (105, 106). Some investigators have compared fructose with glucose utilizing functional MRI in humans (107, 108). It must be emphasized again that fructose and glucose are rarely, if ever, consumed in isolation in the human diet. Moreover, a number of these studies have utilized either boluses of fructose or glucose (108) through abnormal routes (e.g., i.v.) (107) or at large doses of fructose or glucose (e.g., >95% of population consumption levels of fructose) (108).
Purnell et al. (107) explored the neurologic response to 25 g of either fructose or glucose delivered as an i.v. bolus. They reported that although there were no changes in blood flow to the hypothalamus, there were differences between fructose and glucose in blood flow to the cerebral cortex. These investigators speculated that this could potentially result in overeating and ultimately to obesity. It should be noted, however, that the opposite and similar deflections in blood flow to the cortex when comparing fructose with glucose raise the possibility that if the 2 were given together, such as in HFCS or sucrose, these effects would cancel each other out with the net effect similar to the saline control.
Page et al. (108) gave 75 g of either fructose or glucose in oral doses in a random blinded fashion to 20 young, healthy volunteers. They reported that there were differences in hypothalamic blood flow, with glucose suppressing hypothalamic blood flow as measured by arterial spin labeling. They further reported that there were differences between fructose and glucose in brain connectivity.
Although both of these research teams were careful to point out that their studies were designed to explore “proof of concept” and should be taken with considerable caution, these studies were widely misinterpreted by the media and the public to suggest that the data supported the concept of “sugar addiction,” which could lead to overeating.
In addition to the fact that these experiments did not reflect the normal way that sugars are consumed in the human diet, the underlying concept of “sugar addiction” or addiction to any food product has been widely disputed. Ziauddeen et al. (109), Benton (110), and Corwin and Hayes (111) have all written persuasive reviews challenging the concept of food addiction except in very limited settings such as binge-eating disorder.
A pilot study conducted in our research laboratory in collaboration with researchers from Harvard Medical School compared neurologic responses to HFCS given as 18% of calories, sucrose as 18% of calories, fructose as 9% of calories, and glucose as 9% of energy delivered in low-fat milk compared with an unsweetened low-fat milk control condition in 7 participants as a component of a mixed nutrient meal. Preliminary data from this pilot study suggest that all of these sugars appear to behave similarly and not differently from the control condition with regard to hypothalamic blood flow and brain connectivity (112).
Clearly, there is a need for larger, more definitive trials in the area of brain responses to various sugars. It does appear appropriate at the present time to raise concerns about artificial experiments that deliver sugars in ways that they are not normally consumed in the human diet or animal experiments where human neural pathways may be poorly mimicked.
The metabolic and endocrine responses, as well as the health implications of consuming added sugars, particularly in SSBs, continue to cause considerable controversy and debate within the scientific community. Much of the data used to propose differences in metabolism or adverse health consequences from consuming fructose containing sugars have come either from pure fructose vs. pure glucose experiments, epidemiologic studies, or animal data, none of which establish cause and effect in humans.
Although further trials are needed to clarify a number of issues, at the current time, we think that the following conclusions are warranted based on RCTs:
• There is no unique relation between HFCS and obesity.
• There are no metabolic differences between HFCS and sucrose, and both are metabolically different from pure fructose compared with pure glucose.
• There do not appear to be adverse effects on total cholesterol or LDL from consumption of HFCS or sucrose at up to the 90 percentile human fructose consumption level. Small decreases in HDL have occurred and a 10% or greater increase in TGs may occur, particularly at higher doses of these sugars.
• There are no increases in risk factors for diabetes, including insulin, glucose, or insulin resistance, when comparing up with the 90 percentile population of fructose-containing sugars and no differences comparing these sugars with a glucose control.
• Normal population consumption levels of fructose-containing sugars do not appear to increase uric acid or blood pressure.
• Normal consumption amounts of fructose, HFCS, or sucrose do not appear to increase risk factors for MetS.
• Consumption of up to the 90 percentile population consumption level of either HFCS or sucrose does not appear to result in fatty infiltration of the liver.
• There does not appear to be a difference in hypothalamic blood flow when fructose-containing sugars are compared with glucose in the context of mixed nutrient meals, although additional studies will be required to provide more definitive evidence in this area.
These findings suggest we must be very cautious about attributing adverse health consequences to consumption of fructose-containing sugars. RCTs at normal population consumption levels using sugars that are typically consumed in the human diet do not support adverse consequences. Clearly, further RTCs exploring real-world conditions in humans are urgently needed.
Acknowledgments
J.M.R. prepared this manuscript and read and approved the final manuscript.
Footnotes
Abbreviations used: CVD, cardiovascular disease; DGA, Dietary Guidelines for Americans; DNL, de novo lipogenesis; HFCS, high fructose corn syrup; IOM, Institute of Medicine; MetS, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; RCT, randomized controlled trial; SSB, sugar-sweetened beverage.
Literature Cited
- 1.Lustig RH. Fructose: It's “Alcohol Without the Buzz”. Adv Nutr. 2013;4:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White JS. Challenging the fructose hypothesis: new perspectives on fructose consumption and metabolism. Adv Nutr. 2013;4:246–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rippe JM, Angelopoulos TJ. Sucrose, high-fructose corn syrup, and fructose, their metabolism and potential health effects: what do we really know? Adv Nutr. 2013;4:236–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klurfeld DM, Foreyt J, Angelopoulos TJ, Rippe JM. Lack of evidence for high fructose corn syrup as the cause of the obesity epidemic. Int J Obes (Lond). 2013;37:771–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray GA. Fructose: pure, white, and deadly? Fructose, by any other name, is a health hazard. J Diabetes Sci Technol. 2010;4:1003–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rippe JM. The health implications of sucrose, high-fructose corn syrup, and fructose: what do we really know? J Diabetes Sci Technol. 2010;4:1008–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulgoni V. High-fructose corn syrup: everything you wanted to know, but were afraid to ask. Am J Clin Nutr. 2008;88:S1715. [DOI] [PubMed] [Google Scholar]
- 8.Stanhope KL, Havel P. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am J Clin Nutr. 2008;88 Suppl:S1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White JS. Straight talk about high-fructose corn syrup: what it is and what it ain’t. Am J Clin Nutr. 2008;88 Suppl:S1716. [DOI] [PubMed] [Google Scholar]
- 10.White JS. Misconceptions about high-fructose corn syrup: is it uniquely responsible for obesity, reactive dicarbonyl compounds, and advanced glycation end products? J Nutr. 2009;139:S1219–27 [DOI] [PubMed] [Google Scholar]
- 11. American Medical Association. Report 3 of the Council on Science and Public Health. Chicago; American Medical Association; 2008 (A-08)
- 12.American Dietetic Association Use of nutritive and nonnutritive sweeteners. J Am Diet Assoc. 2004;104:255–75 [DOI] [PubMed] [Google Scholar]
- 13.Bray GA. Fructose: should we worry? Int J Obes (Lond). 2008;32:S127–31 [DOI] [PubMed] [Google Scholar]
- 14.Johnson RJ, Gower T, Gollub E. The sugar fix, the high-fructose fallout that is making you fat and sick. New York: Rodale; 2008 [Google Scholar]
- 15.Popkin B. The world is fat: the fads, trends, policies, and products that are fattening the human race. New York: Penguin Group; 2008 [Google Scholar]
- 16.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sánchez-Lozada LG, Le M, Segal M, Johnson RJ. How safe is fructose for persons with or without diabetes? Am J Clin Nutr. 2008;88:1189–90 [DOI] [PubMed] [Google Scholar]
- 18.White J, Foreyt J, Melanson K, Angelopoulos T. High-fructose corn syrup: controversies and common sense. Am J Lifestyle Med. 2010;4:515–20 [Google Scholar]
- 19.Bray GA. Fructose and risk of cardiometabolic disease. Curr Atheroscler Rep. 2012;14:570–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teff KL, Elliott SS, Tschöp M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D’Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–72 [DOI] [PubMed] [Google Scholar]
- 21.Lustig RH. Fat chance: beating the odds against sugar, processed food, obesity, and disease. New York: Hudson Street Press; 2012 [Google Scholar]
- 22.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46 [DOI] [PubMed] [Google Scholar]
- 23.Basu S, Yoffe P, Hills N, Lustig RH. The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data. PLoS ONE. 2013;8:e57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goran MI, Ulijaszek SJ, Ventura EE. High fructose corn syrup and diabetes prevalence: a global perspective. Glob Public Health. 2013;8:55–64 [DOI] [PubMed] [Google Scholar]
- 25.Johnson RJ, Segal M, Sautin Y, Nakagawa T, Feig D, Kang D, Gersch M, Benner S, Sanchez-Lozada L. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906 [DOI] [PubMed] [Google Scholar]
- 26.Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen S, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95:283–9 [DOI] [PubMed] [Google Scholar]
- 27.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40 Suppl 1:S5–10 [DOI] [PubMed] [Google Scholar]
- 28.McCullough AJ. Update on nonalcoholic fatty liver disease. J Clin Gastroenterol. 2002;34:255–62 [DOI] [PubMed] [Google Scholar]
- 29.Stephan BC, Wells JC, Brayne C, Albanese E, Siervo M. Increased fructose intake as a risk factor for dementia. J Gerontol A Biol Sci Med Sci. 2010;65:809–14 [DOI] [PubMed] [Google Scholar]
- 30.Bray GA. Energy and fructose from beverages sweetened with sugar or high-fructose corn syrup pose a health risk for some people. Adv Nutr. 2013;4:220–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funari VA, Herrera VL, Freeman D, Tolan DR. Genes required for fructose metabolism are expressed in Purkinje cells in the cerebellum. Brain Res Mol Brain Res. 2005;142:115–22 [DOI] [PubMed] [Google Scholar]
- 32.Miller CC, Martin RJ, Whitney ML, Edwards GL. Intracerebroventricular injection of fructose stimulates feeding in rats. Nutr Neurosci. 2002;5:359–62 [DOI] [PubMed] [Google Scholar]
- 33.Shu HJ, Isenberg K, Cormier RJ, Benz A, Zorumski CF. Expression of fructose sensitive glucose transporter in the brains of fructose-fed rats. Neuroscience2006;7:140:889–95. [DOI] [PubMed] [Google Scholar]
- 34.Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13:1385–8 [DOI] [PubMed] [Google Scholar]
- 35.Lustig RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc. 2010;110:1307–21 [DOI] [PubMed] [Google Scholar]
- 36.Stanhope KL, Griffen S, Bair B, Swarbrick M, Kelm N, Havel P. Twenty four hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr. 2008;87:1194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox CL, Stanhope K, Schwarz J, Graham J, Hatcher B, Griffen S, Bremer A, Berglund L, McGahan J, Havel P, et al. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr. 2012;66:201–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiser S, Smith JC, Jr, Mertz W, Holbrook J, Scholfield D, Powell A, Canfield W, Canary J. Indices of copper status in humans consuming a typical American diet containing either fructose or starch. Am J Clin Nutr. 1985;42:242–51 [DOI] [PubMed] [Google Scholar]
- 39.Hallfrisch J, Reiser S, Prather E. Blood lipid distribution of hyperinsulinemic men consuming three levels of fructose. Am J Clin Nutr. 1983;37:740–8 [DOI] [PubMed] [Google Scholar]
- 40.Reaven GM. Effects of fructose on lipid metabolism. Am J Clin Nutr. 1982;35:627. [DOI] [PubMed] [Google Scholar]
- 41.Hwang IS, Ho H, Hoffman B, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–6 [DOI] [PubMed] [Google Scholar]
- 42.Health effects of dietary fructose. Am J Clin Nutr. 1993;58 Suppl 5:S721–823 [PubMed] [Google Scholar]
- 43.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–43 [DOI] [PubMed] [Google Scholar]
- 44.Bray GA, Popkin BM. Calorie-sweetened beverages and fructose: what have we learned 10 years later. Pediatr Obes2013;8:242–8. [DOI] [PubMed] [Google Scholar]
- 45.Lustig RH, Schmidt LA, Brindis CD. Public health: the toxic truth about sugar. Nature2012;482:27–9. [DOI] [PubMed] [Google Scholar]
- 46.Yudkin J. Pure, white, and deadly. London: Penguin Books; 1986; [Google Scholar]
- 47.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41 [DOI] [PubMed] [Google Scholar]
- 48.CDC Overweight and obesity: US obesity trends. Atlanta: U.S. Department of Health and Human Services, CDC; 2010 [cited 2013 Jun 17]. Available from: http://www.cdc.gov/obesity/data/adult.html
- 49.WHO Risk factor projects. overweight and obesity; 2005. [cited 2013 Jun 17]. Available from: http://www.who.int/chp/chronic_disease_report/part2_ch1/en/index.html
- 50.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olsen NJ, Heitmann BL. Intake of calorically sweetened beverages and obesity. Obes Rev. 2009;10:68–75 [DOI] [PubMed] [Google Scholar]
- 52.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr. 2012;95:989–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaiser KA, Shikany JM, Keating KD, Allison DB. Will reducing sugar-sweetened beverage consumption reduce obesity? Evidence supporting conjecture is strong, but evidence when testing effect is weak. Obes Rev. 2013;14:620–33.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dolan LC, Potter SM, Burdock GA. Evidence-based review on the effect of normal dietary consumption of fructose on development of hyperlipidemia and obesity in healthy, normal weight individuals. Crit Rev Food Sci Nutr. 2010;50:53–84 [DOI] [PubMed] [Google Scholar]
- 56.Dolan LC, Potter SM, Burdock GA. Evidence-based review on the effect of normal dietary consumption of fructose on blood lipids and body weight of overweight and obese individuals. Crit Rev Food Sci Nutr. 2010;50:889–918 [DOI] [PubMed] [Google Scholar]
- 57.Sievenpiper JL, de Souza RJ, Mirrahimi A, Yu M, Carleton A, Beyene J, Chiavaroli L, Di Buono M, Jenkins A, Leiter L, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med. 2012;156:291–304. [DOI] [PubMed] [Google Scholar]
- 58.USDA/Economic Research Service Food availability (per capita) data system: loss-adjusted food availability; 2012. [cited 2013 Jun 17]. Available from: http://www.ers.usda.gov/data-products/food-availability-%28per-capiat%29-data-system.aspx Calories.xls.
- 59.Lowndes J, Kawiecki D, Angelopoulos T, Rippe J. Fructose containing sugars do not cause changes in weight, body composition or abdominal fat when consumed as part of a eucaloric (weight-stable) diet. Obesity. 2010;18 Suppl 1:S51 [Google Scholar]
- 60.Lowndes J, Kawiecki D, Pardo S, Nguyen V, Melanson KJ. YU Z, Rippe JM. The effects of four hypocaloric diets containing different levels of sucrose or high fructose corn syrup on weight loss and related parameters. Nutr J. 2012;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melanson KJ, Zukley L, Lowndes J, Nguyen V, Angelopoulos T, Rippe J. Effects of high fructose corn syrup and sucrose consumption on circulating glucose, insulin, leptin, and ghrelin and on appetite in normal-weight women. Nutrition2007;23:103–12. [DOI] [PubMed] [Google Scholar]
- 62.Zukley L, Lowndes J, Nguyen V, Brosnahan J, Summers A, Melanson K, Angelopoulos T, Rippe J. Consumption of beverages sweetened with high fructose corn syrup and sucrose produce similar levels of glucose, leptin, insulin and ghrelin in obese females. FASEB. 2007;21:538 [Google Scholar]
- 63.Johnson RK, Appel L, Brands M, Howard B, Lefevre M, Lustig R, Sacks F, Steffen L, Wylie-Rosett J. American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism and the Council on Epidemiology and Prevention. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120:1011–20 [DOI] [PubMed] [Google Scholar]
- 64.Institute of Medicine Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy Press; 2002 [DOI] [PubMed] [Google Scholar]
- 65.USDA, U.S. Department of Health and Human Services Report of the Advisory Committee on the Dietary Guidelines for Americans 2010. 7th ed. Washington, DC: U.S. Government Printing Office; 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lowndes J, Fullerton Z, Rippe J. No changes in plasma uric acid and blood pressure following ten weeks of fructose containing sugar consumption consumption. J Clin Hypertens. 2013;15 Suppl 1:30 [Google Scholar]
- 67.Lowndes J, Kawiecki D, Angelopoulos T, Rippe J. Fructose containing sugars do not result in an atherogenic lipid profile when consumed as part of a eucaloric (weight stable) diet. Circulation. 2010;122:A10906 [Google Scholar]
- 68.Fullerton Z, Lowndes J, Sinnett S, Rippe J. The effects of various consumption levels of high fructose corn syrup and sucrose on circulating glucose, insulin, leptin, active ghrelin and triglycerides. FASEB J2013; 27:858.2. [Google Scholar]
- 69.Bravo S, Lowndes J, Sinnett S, Yu Z, Rippe J. Consumption of sucrose and high fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl Physiol Nutr Metab. 2013;38:681–8 [DOI] [PubMed] [Google Scholar]
- 70. AHA Scientific Statement. Dietary, lifestyle changes can significantly reduce triglycerides. Dallas: AHA; 2011.
- 71.Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr. 2008;88:1419–37 [DOI] [PubMed] [Google Scholar]
- 72.Sievenpiper JL, Wang DD, De Souza RJ, Cozma A, Ha V, Chiavaroli L, Mirrahimi A, Carleton A, Beyene J, Kendall C, et al. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Can J Diabetes. 2012;36:S19. [DOI] [PubMed] [Google Scholar]
- 73.Marckmann P. Dietary treatment of thrombogenic disorders related to the metabolic syndrome. Br J Nutr. 2000;83 Suppl 1:S121–6 [DOI] [PubMed] [Google Scholar]
- 74.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–9 [DOI] [PubMed] [Google Scholar]
- 75.Lowndes J, Sinnett S, Yu Z, Rippe J. Effects of fructose containing sugars on lipids, blood pressure and uric acid when consumed at up to 90th percentile population consumption levels. Circulation. 2012;126:A13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parks EJ, Hellerstein MK. Carbohydrate-induced hypertriacylglycerolemia: historical perspective and review of biological mechanisms. Am J Clin Nutr. 2000;71:412–33 [DOI] [PubMed] [Google Scholar]
- 77.Bantle JP, Laine D, Thomas J. Metabolic effects of dietary fructose and sucrose in types I and II diabetic subjects. JAMA. 1986;256:3241–6 [PubMed] [Google Scholar]
- 78.Gardner C, Wylie-Rosett J, Gidding SS, Steffen L, Johnson R, Reader D, Lichtenstein A. Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2012;126:509–19 [DOI] [PubMed] [Google Scholar]
- 79.Sluijs I, Beulens JW, van der Schouw YT, van der A DL, Buckland G, Kuijsten A, Schulze M, Amiano P, Ardanaz E, Balkau B, et al. Dietary glycemic index, glycemic load, and digestible carbohydrate intake are not associated with risk of type 2 diabetes in eight European countries. J Nutr. 2013;143:93–9 [DOI] [PubMed] [Google Scholar]
- 80.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gallagher D, Kunzia P, Heshka S, Albu J, Heymsfield S, Goodpaster B, Visser M, Harris T. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cozma AI, Sievenpiper J, de Souza R, Chiavaroli L, Ha V, Wang D, Mirrahimi A, Yu M, Carleton A, Di Buono M, et al. Effect of fructose on glycemic control in diabetes: a systematic review and meta-analysis of controlled feeding trials. Diabetes Care. 2012;35:1611–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feig DI, Soletsky B, Johnson R. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension. JAMA. 2008;300:924–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen S, Choi H, Lustig R, Hsu C. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr. 2009;154:807–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van der Schaaf MR, Koomans H, Joles J. Dietary sucrose does not increase twenty-four-hour ambulatory blood pressure in patients with either essential hypertension or polycystic kidney disease. J Hypertens. 1999;17:453–4 [DOI] [PubMed] [Google Scholar]
- 86.Dhingra R, Sullivan L, Jacques P, Wang T, Fox C, Meigs J, D’Agostino R, Gaziano J, Vasan R. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480–8 [DOI] [PubMed] [Google Scholar]
- 87.Sun SZ, Flickinger B, Williamson-Hughes P, Empie M. Lack of association between dietary fructose and hyperuricemia risk in adults. Nutr Metab (Lond). 2010;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lowndes J, Zukley L, Nguyen V, Rippe J. The effect of high fructose corn syrup on uric acid levels in normal weight women. Obesity2008; 16:Suppl 1:S150. [Google Scholar]
- 89.Lowndes J, Zukley L, Nguyen V, Angelopoulos T, Rippe J. The effect of high fructose corn syrup on uric acid in obese women. Obesity. Suppl 2007;15:498-P. [Google Scholar]
- 90.Ha V, Sievenpiper J, de Souza R, Chiavaroli L, Wang D, Cozma A, Mirrahimi A, Yu M, Carleton A, Dibuono M, et al. Effect of fructose on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Hypertension. 2012;59:787–95 [DOI] [PubMed] [Google Scholar]
- 91.Wang DD, Sievenpiper J, de Souza R, Chiavaroli L, Ha V, Cozma A, Mirrahimi A, Yu M, Carleton A, Di Buono M, et al. The effects of fructose intake on uric acid vary among controlled dietary trials. J Nutr. 2012;142:916–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ford ES. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004;27:2444–9 [DOI] [PubMed] [Google Scholar]
- 93.Grundy SM, Brewer H, Cleeman J, Smith S, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8 [DOI] [PubMed] [Google Scholar]
- 94.Sun SZ, Anderson G, Flickinger B, Williamson-Hughes P, Empie M. Fructose and non-fructose sugar intakes in the US population and their associations with indicators of metabolic syndrome. Food Chem Toxicol. 2011;49:2875–82 [DOI] [PubMed] [Google Scholar]
- 95.Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr. 1996;16:523–7 [DOI] [PubMed] [Google Scholar]
- 96.Parks EJ, Skokan L, Timlin M, Dingfelder C. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 2008;138:1039–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lustig RH. Letter to the editor. J Am Diet Assoc. 2011;111:990–3 [Google Scholar]
- 98.Lê K-A, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr. 2006;84:1374–9 [DOI] [PubMed] [Google Scholar]
- 99.McDevitt RM, Bott S, Harding M, McDevitt R, Coward W, Bluck L, Prentice A. De novo lipogenesis during controlled overfeeding with sucrose or glucose in lean and obese women. Am J Clin Nutr. 2001;74:737–46 [DOI] [PubMed] [Google Scholar]
- 100.Silbernagel G, Machann J, Unmuth S, Schick F, Stefan N, Haring H, Fritsche A. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. Br J Nutr. 2011;106:79–86 [DOI] [PubMed] [Google Scholar]
- 101.Lê KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89:1760–5 [DOI] [PubMed] [Google Scholar]
- 102.Chung M, Ma J, Patel K, Berger S, Lichtenstein A. Fructose consumption and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Endocr Rev2013;34:SAT-729. [Google Scholar]
- 103.Uylings HBM, Van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rate and in primates, including humans. 85th vol. Philadelphia: Elsevier; 1991. p. 31–62. [DOI] [PubMed] [Google Scholar]
- 104.Teffer K, Semendeferi K. Human prefrontal cortex: evolution, development, and pathology. Prog Brain Res. 2012;195:191–218 [DOI] [PubMed] [Google Scholar]
- 105.Premack D. Human and animal cognition; continuity and discontinuity. Proc Natl Acad Sci U S A2007;104:13861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sarter M. Animal cognition: defining the issues. Neurosci Biobehav Rev. 2004;28:645–50 [DOI] [PubMed] [Google Scholar]
- 107.Purnell JQ, Klopfenstein BA, Stevens A, Havel P, Adams S, Dunn T, Krisky C, Rooney W. Brain functional magnetic resonance imaging response to glucose and fructose infusions in humans. Diabetes Obes Metab. 2011;13:229–34 [DOI] [PubMed] [Google Scholar]
- 108.Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline G, Naik S, Sinha R, Constable R, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ziauddeen H, Farooqi I, Fletcher P. Obesity and the brain: how convincing is the addiction model? Natl Rev Neurosci. 2012;13:279–86 [DOI] [PubMed] [Google Scholar]
- 110.Benton D. The plausibility of sugar addiction and its role in obesity and eating disorders. Clin Nutr. 2010;29:288–303 [DOI] [PubMed] [Google Scholar]
- 111.Corwin R, Hayes J. Are sugars addictive? In Rippe JM, editor. Fructose, high fructose corn syrup and health. New York: Springer Publishing. In press 2013. [Google Scholar]
- 112.Pena-Gomez C, Alonso-Alonso M, Bravo S, Magerowski G, Sinnett S, Blackburn G, Rippe J. Hypothalamic fMRI responses to different sugars under normal intake conditions: a pilot study. Obesity Society Annual Scientific Meeting; 2013 Nov 11–16; Atlanta, GA.