Abstract
While leptin is known to increase sympathetic nerve activity (SNA), we tested the hypothesis that leptin also enhances baroreflex control of SNA and HR. Using α-chloralose anesthetized male rats, mean arterial pressure (MAP), HR, lumbar SNA (LSNA), splanchnic SNA (SSNA), and renal SNA (RSNA) were recorded before and for 2 hr after lateral cerebroventricular (LV) leptin or aCSF administration. Baroreflex function was assessed using a four parameter sigmoidal fit of HR and SNA responses to slow ramp (3-5 min) changes in MAP, induced by iv infusion of nitroprusside and phenylephrine. Leptin (3 μg) increased (P<0.05) basal LSNA, SSNA, RSNA, HR and MAP, and the LSNA, SSNA, RSNA, and HR baroreflex maxima. Leptin also increased gain of baroreflex control of LSNA and RSNA, but not of SSNA or HR. The elevations in HR were eliminated by pretreatment with methscopalamine, to block parasympathetic nerve activity; however, after cardiac sympathetic blockade with atenolol, leptin still increased basal HR and MAP and the HR baroreflex maximum and minimum. Leptin (1.5 μg) also increased LSNA and enhanced LSNA baroreflex gain and maximum, but did not alter MAP, HR, or the HR baroreflex. LV aCSF had no effects. Finally, to test if leptin acts in the brainstem, leptin (3 μg) was infused into the 4th ventricle; however, no significant changes were observed. In conclusion, leptin acts in the forebrain to differentially influence baroreflex control of LSNA, RSNA, SSNA and HR, with the latter action mediated via suppression of parasympathetic nerve activity.
Keywords: male rats, RSNA, LSNA, SSNA, methscopolamine, parasympathetic
INTRODUCTION
Clinical and experimental studies have shown that obesity is associated with hypertension, due at least in part to increases in sympathetic nerve activity (SNA).1;2 Considerable effort has been directed toward identifying the underlying mechanisms. As recently reviewed2;3 one potential candidate is leptin, a 16-kDa peptide hormone produced predominantly by white adipocytes. Acute leptin administration increases SNA to multiple organs, including brown adipose tissue, the hindlimb, adrenal gland, and kidney,3 and chronic leptin infusion produces hypertension.2;3 In addition, leptin-deficient obese mice (ob/ob mice) fail to exhibit hypertension, despite massive obesity.2;3
Another deleterious consequence of obesity is impaired sensitivity of the baroreceptor reflex.4-6 Decreased baroreflex function has been identified as a risk factor for subsequent adverse cardiovascular events in humans with type 2 diabetes mellitus.7 However, the mechanism is unknown. While leptin has been considered,8 current information is conflicting. For example, monogenic forms of rodent obesity due to loss of leptin or to mutations of the leptin receptor exhibit decreased baroreflex gain,9-12 suggesting that leptin enhances baroreflex function. Conversely, systemic administration of leptin was reported to be ineffective,13 and microinjection of leptin into the nucleus tractus solitarius (NTS) suppressed baroreflex control of heart rate (HR).14 Therefore, one aim of the present experiments was to further investigate if leptin influences baroreflex function. Given the well-established sympathoexcitatory effects of leptin, we hypothesized that leptin would also enhance the SNA responses to decreases in arterial pressure (i.e. baroreflex gain). A key feature of these experiments is that we compared the effects of leptin on baroreflex control of lumbar SNA (LSNA), renal SNA (RSNA), as well as splanchnic SNA (SSNA). In addition, to test if leptin influences baroreflex control of HR via changes in the activity of the sympathetic nervous system or the parasympathetic nervous system, experiments were performed to determine if the effects of leptin on the HR baroreflex were eliminated by systemic pretreatment with drugs that blocked cholinergic (methscopolamine) or beta-adrenergic (atenolol) receptors.
Leptin has been shown to increase SNA following selective microinjection into several hypothalamic nuclei15-19 and into the NTS.14;20 Therefore, in order to activate these multiple sites concurrently, leptin was infused via a lateral cerebral ventricle (LV). In addition, to begin to identify the sites at which leptin acts to modify baroreflex function, we compared the effects of leptin infusion via the LV, which will activate receptors in both the hypothalamus and NTS, versus the fourth ventricle (4V), to target brainstem leptin receptors.
METHODS
Animals
Forty male (320-450g) Sprague Dawley rats (Charles River Laboratories, Inc) were used for these experiments. All of the rats were acclimated for at least 1 week before experimentation in a room with a 12-hour:12-hour light/dark cycle, with food (LabDiet 5001, Richmond, IN, USA) and water provided ad libitum. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional (Oregon Health & Science University) Animal Care and Use Committee.
Surgery
Under isoflurane anesthesia, a tracheal tube, femoral arterial and venous catheters, and a recording electrode around the lumbar, splanchnic or renal sympathetic nerve were implanted (see Supplement for details). After preparing the rat for icv infusions, a loading dose of α-chloralose (50 mg kg−1; Sigma) was administered intravenously (iv) over 30 min, while isoflurane was slowly withdrawn, and this was followed by a continuous infusion of α-chloralose (25 mg kg−1 h−1) for the duration of the experiment. Throughout the experiment, artificial ventilation with 100% oxygen was maintained, and respiratory rate and tidal volume were adjusted to maintain expired CO2 at 3.5-4.5%. Anesthetic depth was regularly confirmed by the lack of a pressor or withdrawal response to a foot or tail pinch. After completion of surgery and the α-chloralose loading dose, rats were allowed to stabilize for at least 60 min before experimentation.
Baroreflex function
Pulsatile and mean arterial pressures, HR and SNA were continuously recorded, and baroreflex function was assessed as previously described21 (see Supplement for details).Briefly, complete baroreflex curves were constructed by fitting a sigmoidal curve to the changes in HR and SNA induced by slow changes in AP following iv infusion of nitroprusside and phenylephrine. Absolute values of baroreflex gain (BRG) and the mean±SEM of other sigmoidal parameters (baroreflex maxima, minima, and midpoint) are depicted in the figures.
Intracerebroventricular infusions
With a flat skull and using bregma and the dorsal surface of the dura as zero, single-barreled glass pipettes drawn to a small tip were used for both LV and 4V infusions. Coordinates for positioning the LV cannulae were as follows (mm from bregma): 1.0 caudal, 1.4 lateral, and 4.2 dorsal. A pipette was placed into the 4V using the following coordinates (mm): 2.0 caudal to the interaural line, on the midline, and 7.3 ventral to the skull surface. Leptin (R&D Systems) was dissolved in artificial cerebrospinal fluid (aCSF) containing (in mM): 128 NaCl, 2.6 KCl, 1.3 CaCl2, 0.9 MgCl2, 20 NaHCO3, 1.3 Na2HPO4 and 2.0 dextrose, pH 7.4. Correct pipette placement was confirmed at the end of the experiment by infusing ~ 100 nL of 2.5% Alcian blue in 0.5 mM/L of sodium acetate via the same pipette, removing the brain, and verifying the presence of dye in the cerebroventricles.
Experimental Protocols
After stabilization, basal baroreflex function was established by producing at least two baroreflex curves, 30 min apart, with similar gains; the final control curve was used for data analysis. Protocol 1: After reestablishment of basal values, LSNA and HR BRG were measured 1 and 2 hr after LV injection of a dose of leptin that has been shown to inhibit food intake when administered in conscious rats22;23 (3 μg in 3 μL, followed by 5 μg/hr, n=5), a lower leptin dose (1.5 μg in 1.5 μL, followed by 5 μg/hr, n=5) or the aCSF vehicle (0.6 μL/min, n=5). In separate groups of rats, SSNA (n=6) or RSNA (n= 5) and HR BRG were measured after LV injection of the higher leptin dose. The HR and MAP results from the 3 groups of rats receiving the higher leptin dose were combined (n=16). Protocol 2: After establishing basal baroreflex function, either methscopolamine (1 mg/kg, n=4) or atenelol (2mg/kg hr, n=5) was administered iv, at doses previously documented to block cardiac parasympathetic and sympathetic contributions, respectively.24 Thirty min later, baroreflex curves were generated to assess the effects of the blockade alone. Then, leptin was administered icv (3 μg followed by 5 μg/hr), and baroreflex function was reassessed after 1 and 2 hr. Protocol 3: After control measurements, LSNA and HR BRG were measured 1 and 2 hr after leptin (3 μg, followed by 5 μg/hr, n=5) administration into the 4V. In each protocol, basal data were taken as the average of the 30 sec period prior to each BRG measurement.
Statistical Analysis
All data are presented as means ± SEM. Between-group differences were evaluated using one- or two-way ANOVA for repeated measures and the post hoc Newman–Keuls test. P values<0.05 were considered statistically significant.
RESULTS
LV leptin administration increases gain of baroreflex control of LSNA and RSNA, but not of SSNA or HR
LV leptin (3 μg) administration increased basal LSNA and RSNA after 1 and 2 hr; the lower dose of leptin (1.5 μg) increased LSNA only at 2 hr (Figures 1, 2). In contrast, basal SSNA was not significantly elevated until 2 hr after 3 μg leptin (Figure 2). MAP and HR also increased 2 hr following leptin (3 μg) injection; however, 1.5 μg leptin did not significantly alter MAP or HR (Table 1). Basal values were not significantly changed during icv infusion of aCSF (Figure 1 and Table 1).
Figure 1. Effects of LV leptin or aCSF on baseline LSNA.
LV leptin (3 μg, n=5, top) increased basal LSNA at 1 and 2 hr, whereas the lower dose (1.5 μg, n=5) increased basal LSNA only at 2 hr (middle). aCSF (n=5) had no effects on LSNA. *P<0.05 vs CON; †P<0.05 vs aCSF at the same time.
Figure 2. Effects of LV leptin on baseline RSNA and SSNA.
LV leptin (3 μg) increased basal RSNA (n=5, top) at 1 and 2 hr, whereas SSNA (n=6, bottom) was increased only at 2 hr. *P<0.05 vs CON.
Table 1.
Effect of LV leptin and aCSF on basal MAP and HR
| Variable | Control | Leptin 1 hr | Leptin 2 hr |
|---|---|---|---|
| 3 μg leptin (n = 16) ‡ | |||
| MAP (mmHg) | 103 ± 4 | 106 ± 4 | 118 ± 5*† |
| HR (bpm) | 328 ± 14 | 357 ± 16 | 388 ± 19*† |
| 1.5 μg leptin (n = 5) | |||
| MAP (mmHg) | 105 ± 4 | 102 ± 2 | 99 ± 2 |
| HR (bpm) | 345 ± 22 | 342 ± 16 | 355 ± 19 |
| aCSF (n = 5) | |||
| MAP (mmHg) | 102 ± 7 | 98 ± 7 | 98 ± 14 |
| HR (bpm) | 342 ± 9 | 355 ± 9 | 360 ± 5 |
P<0.05 vs Control
P<0.05 vs aCSF, same time.
The MAP and HR results were combined from the separate sets of rats used for studies of LSNA, SSNA, and RSNA. These data were not different between groups (2 way repeated measures ANOVA, insignificant group and interaction).
Baroreflex control of LSNA, SSNA, RSNA, and HR were differentially altered by icv leptin. Leptin increased LSNA (3 and 1.5 μg doses) and RSNA BRG (Figures 3, 4); the LSNA and RSNA reflex maximum and range were also elevated (Figures 3, 4 and Supplement, Tables S1 and S2; see Figure 1S for representative experiment). On the other hand, while the SSNA maximum was elevated 1 and 2 hr after leptin, BRG was not significantly increased (Figure 4 and Supplement, Table S3). Similarly, the higher dose of leptin increased the HR reflex maximum, and BP50 after 2 hr (Supplement, Table S4), without significantly altering HR BRG (Figure 5). Leptin increased the baroreflex minimum of LSNA and HR (Figures 3 and 5; Supplemental Tables S1 and S4), but not of RSNA and SSNA (Figure 4 and Supplemental Tables S2 and S3).
Figure 3. Effects of LV leptin or aCSF on baroreflex control of LSNA.
Both doses of LV leptin (1.5 and 3 μg) increased baroreflex gain, as well as the LSNA baroreflex maximum. In contrast, aCSF (n=5) had no effects on the baroreflex. *P<0.05 vs CON; †P<0.05 vs aCSF at the same time.
Figure 4. Effects of leptin on baroreflex control of RSNA and SSNA.
Leptin (LV, 3 μg) increased the RSNA (n=5; top) baroreflex maximum and gain (insert) after 1 and 2 hr. Leptin also increased the SSNA (n=6; bottom) baroreflex maximum at 1 and 2 hr, and the BP50 at 2 hr (n=6, *P <0.05); however, the SSNA baroreflex gain and minimum were unchanged (top, insert). *P<0.05 vs CON.
Figure 5. Effects of LV leptin or aCSF on baroreflex control of HR.
While neither the lower dose of leptin (n=5) nor aCSF (n=5) influenced the HR baroreflex, the higher dose of leptin (top) increased the HR baroreflex maximum and minimum (n=16) without altering gain. *P<0.05 vs CON. †P<0.05 vs aCSF at the same time.
Methscopolamine blocks the effect of leptin to increase HR and alter baroreflex control of HR
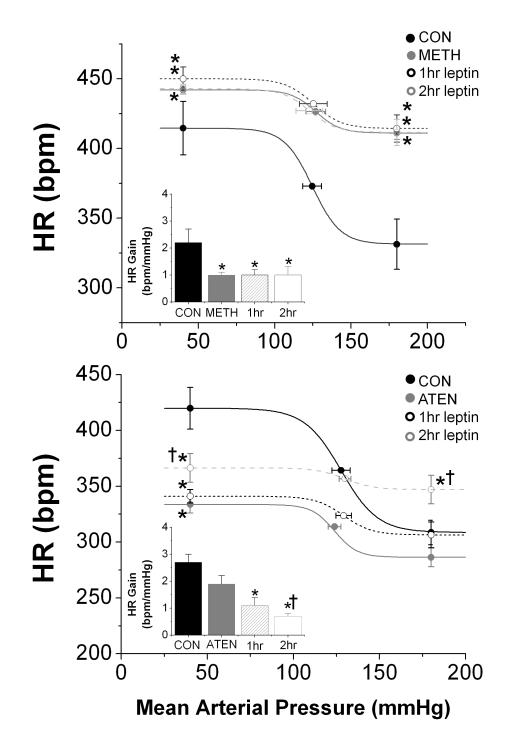
Methscopolamine increased basal HR, without altering MAP; maximum and minimum baroreflex HR were increased and HR BRG was reduced (Table 2 and Figure 6). More importantly, following methscopolamine, leptin failed to alter basal MAP, HR, or influence baroreflex control of HR (Figure 6 and Supplement, Table S5). To determine if the failure of leptin to increase HR was due to a maximal elevation following methscopolamine, the β-agonist, isoproterenol (1 μg/kg) was injected iv after methscopolamine administration and 2 hr of leptin infusion. Notably, isoproterenol increased HR from 409±19 to 495±23 bpm, a value higher than the baroreflex HR maximum (446±2 bpm; P<0.05, n=3).
Table 2.
Effects of methscopolamine and atenolol on MAP and HR, before and after icv leptin (3 μg)
| Variable | Control | Methscopolamine | Leptin 1 hr | Leptin 2 hr |
|---|---|---|---|---|
| MAP (mmHg) | 118 ± 5 | 118 ± 4 | 117 ± 8 | 109 ± 7 |
| HR (bpm) | 355 ± 13 | 403 ± 4* | 409 ± 8* | 411 ± 7* |
| Control | Atenelol | Leptin 1 hr | Leptin 2 hr | |
| MAP (mmHg) | 113 ± 5 | 92 ± 2* | 102 ± 5* | 106 ± 5† |
| HR (bpm) | 351 ± 13 | 318 ± 8* | 326 ± 6* | 348 ± 13† |
P<0.05 vs Control
P<0.05 vs atenolol.
Figure 6. Effects of leptin on the HR baroreflex were blocked by methscopolamine, but not atenolol.
Top. Methscopolamine (1mg/kg, i.v., n=4, solid gray line and circles) elevated the HR baroreflex curve, increased the HR maximum and minimum (P<0.05), but reduced the HR gain (insert, *P<0.05). However, following methscopolamine, leptin did not affect the baroreflex control of HR (dashed and dotted lines). Bottom. Atenolol (2mg/kg·hour, i.v., n=5, solid gray line and circles) depressed the HR baroreflex curve and reduced the HR maximum (P < 0.05). Following atenolol, leptin increased the HR maximum and minimum and reduced the HR gain (insert, P < 0.05). *P<0.05 vs CON; †P<0.05 vs atenolol.
Atenolol reduced basal MAP and HR and decreased the baroreflex HR maximum and the HR range (Figure 6, Table 2, and Supplement, Table S5). Following atenolol, 2 hr of leptin infusion increased basal MAP and HR and elevated the baroreflex HR maximum and minimum (Figure 6 and Supplement, Table S5). Interestingly, HR gain was reduced 2 hr after initiating the leptin infusion (Figure 6) in atenolol-treated rats.
4V leptin does not alter basal or baroreflex control of LSNA, HR and MAP
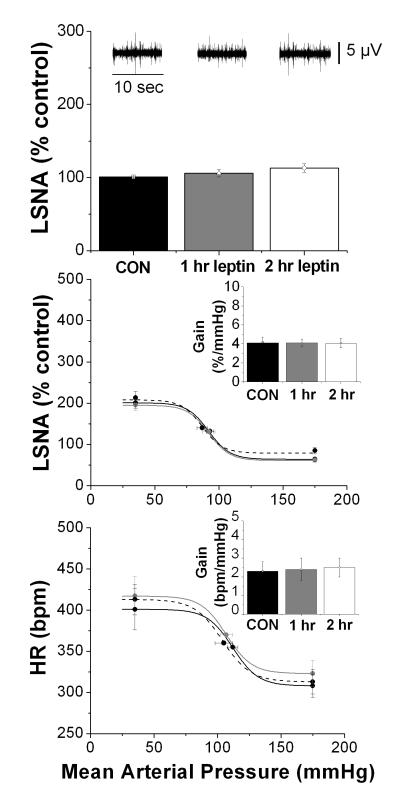
In sharp contrast to the potent effects of leptin administered via the LV, basal MAP, HR and LSNA, as well as BRG and baroreflex parameters, were not altered during 4V leptin infusion (Figure 7 and Supplement, Table S6).
Figure 7. Effects of 4V leptin (3 μg) on basal LSNA and baroreflex control of LSNA and HR.
No significant changes in basal LSNA or in baroreflex control of LSNA and HR were observed following 4V leptin (3μg) infusion.
DISCUSSION
The major goal of this study was to test the hypothesis that leptin acts centrally to enhance baroreflex control of SNA and HR and, if so, to begin to investigate the mechanisms. The important new findings are: 1) leptin increases SSNA (as well as LSNA, RSNA, HR and MAP, as previously reported19); 2) central leptin exerts differential effects on baroreflex control of LSNA, SSNA and HR; 3) the ability of leptin to increase HR and its baroreflex regulation is eliminated by pretreatment with methscopolamine, to block the parasympathetic nervous system, but not by pretreatment with atenolol, to block cardiac sympathetic effects; and 4) these actions of leptin are not observed when leptin is infused via the 4V. Collectively, these data indicate that central leptin acts in the forebrain to influence baroreflex control of SNA and HR, with the latter action mediated via suppression of cardiac parasympathetic tone.
Previous studies have demonstrated that intravenous or central leptin administration increases LSNA, RSNA, HR, and MAP;19;25;26 our results confirm this work. A new finding, however, is that leptin also acts centrally to increase SSNA. Dunbar et al26 reported that icv leptin decreases splanchnic blood flow, which contributes to its pressor effect. The increase in SSNA that we observed suggests that the sympathetic nervous system likely mediates this action. Plasma leptin levels increase after a meal.27 Therefore, the central action of leptin to increase SSNA may help to maintain arterial pressure at a time when the splanchnic circulation is dilating to accept and distribute absorbed nutrients. In addition, in parallel to the actions of insulin,28 meal-induced increases in leptin may contribute to enhanced baroreflex control of LSNA.
Monogenetic impairment of the actions of leptin, due either to loss of leptin or mutations of the leptin receptor, depresses the baroreflex,9-12 suggesting that leptin may support or enhance baroreflex function. However, these rodent models exhibit obesity, and diet-induced obesity also decreases baroreflex control of HR4;5;24 and, in one human study, SNA,29 confounding this suggestion. The present study demonstrates that exogenous leptin administration differentially influences baroreflex function. Increases in baroreflex gain were observed for LSNA and RSNA, but not for SSNA or HR. However, leptin increased the baroreflex maximum and range of LSNA, RSNA, SSNA, and HR, as well as the baroreflex minimum of LSNA and HR. Previously, iv leptin was shown to increase the range and maximum of baroreflex control of RSNA in mice, without enhancing gain.13 Different species or routes of leptin administration may explain these divergent results.
The mechanism by which leptin differentially alters baroreflex control of various autonomic efferents was not identified, but may arise from actions of leptin at different brain sites, or within a site on distinct neurons. In support of this supposition, previous studies have demonstrated that leptin acts differentially to increase SNA at several hypothalamic sites containing leptin receptors.16;18;19 We found that leptin increases the baroreflex minimum for LSNA and HR (but not for SSNA or RSNA; see also13); therefore, leptin can increase LSNA and HR even when baroreflex suppression is maximal at high pressures, suggesting that the neurocircuitry that mediates the increases in LSNA and HR is at least in part independent of, or distal to, brain baroreflex pathways. However, the finding that leptin also increases LSNA baroreflex gain suggests an additional interaction with baroreflex pathways. Thus, the brain neurons that initiate the effects of leptin on baroreflex control of different sympathetic nerves may also project to multiple sites in the brainstem and/or spinal cord.
Obesity mutes leptin’s effect to increase SNA to brown adipose tissue and LSNA, yet leptin-induced increases in RSNA are preserved or enhanced.30;31 Since leptin increases gain of baroreflex control of LSNA, brain resistance to leptin could explain in part the decrease in muscle SNA baroreflex gain reported in obese humans compared to after weight loss.29 On the other hand, because leptin does not influence SSNA baroreflex gain, its loss likely does not likely contribute to the decreased SSNA gain observed in Zucker rats.10 Indeed, Schreihofer et al10 came to the same conclusion, since they found that SSNA baroreflex gain is not impaired in juvenile Zucker rats, but only in older Zucker rats that exhibit considerable obesity.
While the ability of leptin to increase HR has been noted before,14;18;32 the mechanism has not been directly investigated. Nevertheless, previous work has provided indirect evidence that leptin may suppress parasympathetic control of the heart. First, Arnold et al14 reported that NTS microinjection of leptin decreased spontaneous baroreflex sensitivity and the reflex bradycardia following bolus iv injections of phenylephrine, each of which reflect primarily cardiovagal activity. Second, correlations between leptin and indices of cardiac parasympathetic activity have been noted in humans.33 Using a pharmacological approach, we directly tested this hypothesis. Administration of methscopolamine to block the parasympathetic nervous system completely prevented the ability of leptin to elevate HR and right-shift the baroreflex curve. This was not due to a HR ceiling effect, since after methscopolamine and leptin treatment, iv injection of the β-adrenergic agonist, isopreteronol, to mimic cardiac sympathoexcitation, increased HR even higher. Interestingly, methscopolamine also prevented the pressor response to LV leptin. Methscopolamine does not cross the blood-brain barrier, but it can exhibit some cholinergic nicotinic blocking activity.34 Therefore, one explanation for this finding is that methscolpolamine inhibited ganglionic transmission. In support, we have found that iv methscopolamine decreases SNA and arterial pressure when administered after leptin infusion (Li, Shi, and Brooks, unpublished observations). On the other hand, in rats in which cardiac sympathetic activity was blocked with atenolol, leptin increased HR, MAP and right-shifted the baroreflex curve. Collectively, these data suggest that leptin increases HR at any given MAP by suppressing parasympathetic control of the heart. One of the hallmarks of obesity in humans and experimental animals is suppression of cardiac parasympathetic activity4;24;35 in association with elevated leptin levels. Therefore, we speculate that leptin may contribute to impaired vagal activity during obesity. Interestingly, after atenolol, leptin infusion also markedly decreased baroreflex gain. Thus, leptin may also suppress the response of the parasympathetic nervous system to changes in arterial pressure, at least when cardiac sympathetic activity is also reduced. In humans, obesity can reduce sympathetic activity to the heart;1 therefore, leptin may also contribute to the impaired cardiac baroreflex sensitivity that is observed.
Leptin has been shown to increase SNA and HR following microinjection into several hypothalamic nuclei (arcuate nucleus, dorsal medial hypothalamus, lateral hypothalamus, and ventromedial hypothalamus)18;19;36 as well as the NTS.14;20 However, as we previously found with insulin,37 leptin infusion into the 4V, to restrict its actions to the hindbrain, was ineffective. The variance of our study with previous work may be explained by a failure of sufficient leptin to reach its active sites in the NTS, since at least in one report,20 larger doses were used. Regardless of the explanation, our studies show that leptin can act in the forebrain to increase SSNA and influence baroreflex control of LSNA, RSNA, SSNA, and HR. As discussed, in view of the variable effects on baroreflex control of SNA and HR that were observed, more than one site may be involved.
While the results of this study clearly demonstrate a differential effect of leptin on baroreflex function, there are several limitations that must be considered. First, the experiments were performed in anesthetized rats. While we acknowledge that it will be important to repeat these experiments in conscious animals, it is noteworthy that α-chloralose was chosen as the anesthetic, because baroreflex function is relatively preserved. Indeed, the values of LSNA baroreflex gain measured in the present study are essentially identical to values obtained in conscious rats.38;39 Second, the concentrations of leptin achieved in the CSF were certainly supraphysiological. However, high doses are required to establish a significant diffusion gradient, since as a large peptide only limited amounts of leptin penetrate brain from the CSF and only very slowly.40 The fact that the stimulatory effects of leptin on LSNA in the present study were related more to the initial bolus dose than to the total infused amount supports this contention. Importantly, in rats, iv infusion of doses that increase plasma leptin within the physiological-pathophysiological range increase SNA similarly to the present study,25 strongly suggesting that such levels of plasma leptin would also enhance baroreflex control. Third, the short-term effects of leptin that we observed may underestimate the potency of long-term elevations. In support, in agreement with previous work,16;25 we found that the SNA responses to LV leptin are slowly developing and may not have peaked within our two hr study period. Moreover, chronic intracerebroventricular infusion of significantly lower doses of leptin are sufficient to elevate arterial pressure and suppress food intake.41
Perspectives
In both humans and animals, elevations in plasma leptin occur soon after beginning a high fat diet and accruing excess adiposity, in association with activation of the sympathetic nervous system.42-44 Thus, the hypothesis that increased leptin contributes to obesity-induced sympathoexcitation has received considerable attention.2;3 Another hallmark of obesity is depressed basal parasympathetic nerve activity and impaired HR baroreflex function, which also rapidly develops.4-6;24;35;44 The present results suggest that leptin may also contribute to these cardiovascular consequences. On the other hand, in obese humans, baroreflex control of muscle SNA has been reported to be depressed29;45 or preserved.46;47 Therefore, if leptin is involved in SNA baroreflex impairment, then the factor(s) that reduce brain sensitivity to leptin’s action to enhance BRG must vary among individuals. Future experiments are required to identify the factors that modify sensitivity of the brain to leptin and to determine whether and how the impact of these factors varies among obese individuals.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
Leptin acts in the brain to increase SSNA.
Leptin increases LSNA and RSNA baroreflex gain (but not gain of baroreflex control of SSNA or HR) and increases LSNA, SSNA, RSNA, and HR baroreflex maxima and range.
Leptin increases HR via suppression of parasympathetic nerve activity.
Leptin exerts these effects via an action in the forebrain.
What is relevant?
Obesity increases leptin. Therefore, leptin may contribute to the suppression of basal and baroreflex-mediated changes in parasympathetic nerve activity commonly observed in obese individuals and identified as a risk factor for adverse cardiovascular events.
After a meal, like insulin, increases in leptin may contribute to enhanced LSNA baroreflex gain and may counteract splanchnic vasodilation via increases in SSNA.
Summary
Leptin acts in the forebrain to differentially influence baroreflex control of LSNA, SSNA, RSNA, and HR, with the latter action mediated via suppression of parasympathetic nerve activity
Acknowledgments
SOURCES OF FUNDING. This study was supported in part by a National Institutes of Health grant HL088552 and a Grant-in-Aid from the American Heart Association (12GRNT11550018).
Footnotes
DISCLOSURES. None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 2.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahmouni K. Leptin-Induced Sympathetic Nerve Activation: Signaling Mechanisms and Cardiovascular Consequences in Obesity. Curr Hypertens Rev. 2010;6:104–209. doi: 10.2174/157340210791170994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beske SD, Alvarez GE, Ballard TP, Davy KP. Reduced cardiovagal baroreflex gain in visceral obesity: implications for the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2002;282:H630–H635. doi: 10.1152/ajpheart.00642.2001. [DOI] [PubMed] [Google Scholar]
- 5.Straznicky NE, Lambert GW, Lambert EA. Neuroadrenergic dysfunction in obesity: an overview of the effects of weight loss. Curr Opin Lipidol. 2010;21:21–30. doi: 10.1097/MOL.0b013e3283329c62. [DOI] [PubMed] [Google Scholar]
- 6.Aronne LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol. 1995;269:R222–R225. doi: 10.1152/ajpregu.1995.269.1.R222. [DOI] [PubMed] [Google Scholar]
- 7.Okada N, Takahashi N, Yufu K, Murozono Y, Wakisaka O, Shinohara T, Anan F, Nakagawa M, Hara M, Saikawa T, Yoshimatsu H. Baroreflex sensitivity predicts cardiovascular events in patients with type 2 diabetes mellitus without structural heart disease. Circ J. 2010;74:1379–1383. doi: 10.1253/circj.cj-09-0960. [DOI] [PubMed] [Google Scholar]
- 8.Grassi G. Leptin, sympathetic nervous system, and baroreflex function. Curr Hypertens Rep. 2004;6:236–240. doi: 10.1007/s11906-004-0075-8. [DOI] [PubMed] [Google Scholar]
- 9.Goncalves AC, Tank J, Diedrich A, Hilzendeger A, Plehm R, Bader M, Luft FC, Jordan J, Gross V. Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction. Hypertension. 2009;53:387–392. doi: 10.1161/HYPERTENSIONAHA.108.124776. [DOI] [PubMed] [Google Scholar]
- 10.Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2007;293:H2543–H2549. doi: 10.1152/ajpheart.01201.2006. [DOI] [PubMed] [Google Scholar]
- 11.Davis G. Baroreflex and somato-reflex control of blood pressure, heart rate and renal sympathetic nerve activity in the obese Zucker rat. Exp Physiol. 2011;96:623–634. doi: 10.1113/expphysiol.2011.057638. [DOI] [PubMed] [Google Scholar]
- 12.Hilzendeger AM, Goncalves AC, Plehm R, Diedrich A, Gross V, Pesquero JB, Bader M. Autonomic dysregulation in ob/ob mice is improved by inhibition of angiotensin-converting enzyme. J Mol Med (Berl) 2010;88:383–390. doi: 10.1007/s00109-009-0569-6. [DOI] [PubMed] [Google Scholar]
- 13.Hausberg M, Morgan DA, Chapleau MA, Sivitz WI, Mark AL, Haynes WG. Differential modulation of leptin-induced sympathoexcitation by baroreflex activation. J Hypertens. 2002;20:1633–1641. doi: 10.1097/00004872-200208000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Leptin impairs cardiovagal baroreflex function at the level of the solitary tract nucleus. Hypertension. 2009;54:1001–1008. doi: 10.1161/HYPERTENSIONAHA.109.138065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 17.Satoh N, Ogawa Y, Katsuura G, Numata Y, Tsuji T, Hayase M, Ebihara K, Masuzaki H, Hosoda K, Yoshimasa Y, Nakao K. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes. 1999;48:1787–1793. doi: 10.2337/diabetes.48.9.1787. [DOI] [PubMed] [Google Scholar]
- 18.Marsh AJ, Fontes MA, Killinger S, Pawlak DB, Polson JW, Dampney RA. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42:488–493. doi: 10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]
- 19.Montanaro MS, Allen AM, Oldfield BJ. Structural and functional evidence supporting a role for leptin in central neural pathways influencing blood pressure in rats. Exp Physiol. 2005;90:689–696. doi: 10.1113/expphysiol.2005.030775. [DOI] [PubMed] [Google Scholar]
- 20.Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53:375–380. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol. 2011;589:1643–1662. doi: 10.1113/jphysiol.2011.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Cha MJ, Yoo SB, Moon YW, Noh SJ, Jahng JW. Leptin blocks the fasting-induced increase of pERK1/2 in the paraventricular nucleus of rats. Regul Pept. 2010;162:122–128. doi: 10.1016/j.regpep.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 24.McCully BH, Brooks VL, Andresen MC. Diet-induced obesity severely impairs myelinated aortic baroreceptor reflex responses. Am J Physiol Heart Circ Physiol. 2012;302:H2083–H2091. doi: 10.1152/ajpheart.01200.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–2043. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- 27.Lee MJ, Fried SK. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am J Physiol Endocrinol Metab. 2009;296:E1230–E1238. doi: 10.1152/ajpendo.90927.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol. 2010;588:3593–3603. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97:2037–2042. doi: 10.1161/01.cir.97.20.2037. [DOI] [PubMed] [Google Scholar]
- 30.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 31.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55:862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- 32.Ren J. Leptin and hyperleptinemia - from friend to foe for cardiovascular function. J Endocrinol. 2004;181:1–10. doi: 10.1677/joe.0.1810001. [DOI] [PubMed] [Google Scholar]
- 33.Piestrzeniewicz K, Luczak K, Lelonek M, Wranicz JK, Goch JH. Obesity and heart rate variability in men with myocardial infarction. Cardiol J. 2008;15:43–49. [PubMed] [Google Scholar]
- 34.Brown JH, Laiken N. Muscarinic receptor agonists and antagonists. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman’s pharmacological basis of therapeutics. McGraw-Hill; New York: 2011. [Google Scholar]
- 35.Van Vliet BN, Hall JE, Mizelle HL, Montani JP, Smith MJ., Jr Reduced parasympathetic control of heart rate in obese dogs. Am J Physiol. 1995;269:H629–H637. doi: 10.1152/ajpheart.1995.269.2.H629. [DOI] [PubMed] [Google Scholar]
- 36.Enriori PJ, Sinnayah P, Simonds SE, Garcia RC, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pricher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension. 2008;51:514–520. doi: 10.1161/HYPERTENSIONAHA.107.102608. [DOI] [PubMed] [Google Scholar]
- 38.Xu L, Brooks VL. Sodium intake, angiotensin II receptor blockade and baroreflex function in conscious rats. Hypertension. 1997;29:450–457. doi: 10.1161/01.hyp.29.1.450. [DOI] [PubMed] [Google Scholar]
- 39.Xu L, Collister JP, Osborn JW, Brooks VL. Endogenous angiotensin II supports lumbar sympathetic activity in conscious, sodium deprived rats: role of area postrema. Am J Physiol. 1998;275:R46–R55. doi: 10.1152/ajpregu.1998.275.1.R46. [DOI] [PubMed] [Google Scholar]
- 40.Maness LM, Kastin AJ, Farrell CL, Banks WA. Fate of leptin after intracerebroventricular injection into the mouse brain. Endocrinology. 1998;139:4556–4562. doi: 10.1210/endo.139.11.6319. [DOI] [PubMed] [Google Scholar]
- 41.Dubinion JH, da Silva AA, Hall JE. Chronic blood pressure and appetite responses to central leptin infusion in rats fed a high fat diet. J Hypertens. 2011;29:758–762. doi: 10.1097/HJH.0b013e328344280b. [DOI] [PubMed] [Google Scholar]
- 42.Gentile CL, Orr JS, Davy BM, Davy KP. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1834–R1838. doi: 10.1152/ajpregu.00876.2006. [DOI] [PubMed] [Google Scholar]
- 43.Muntzel MS, Al Naimi OA, Barclay A, Ajasin D. Cafeteria diet increases fat mass and chronically elevates lumbar sympathetic nerve activity in rats. Hypertension. 2012;60:1498–1502. doi: 10.1161/HYPERTENSIONAHA.112.194886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, Head GA. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60:163–171. doi: 10.1161/HYPERTENSIONAHA.111.190413. [DOI] [PubMed] [Google Scholar]
- 45.Grassi G, Dell’Oro R, Facchini A, Quarti TF, Bolla GB, Mancia G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens. 2004;22:2363–2369. doi: 10.1097/00004872-200412000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Alvarez GE, Ballard TP, Beske SD, Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol. 2004;287:H414–H418. doi: 10.1152/ajpheart.01046.2003. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.