Abstract
Streptococcus (S.) pneumoniae is a common causative pathogen in pneumonia. Serine protease orthologs expressed by a variety of bacteria have been found of importance for virulence. Previous studies have identified two serine proteases in S. pneumoniae, HtrA (high-temperature requirement A) and PrtA (cell wall-associated serine protease A), that contributed to virulence in models of pneumonia and intraperitoneal infection respectively. We here sought to identify additional S. pneumoniae serine proteases and determine their role in virulence. The S. pneumoniae D39 genome contains five putative serine proteases, of which HtrA, Subtilase Family Protein (SFP) and PrtA were selected for insertional mutagenesis because they are predicted to be secreted and surface exposed. Mutant D39 strains lacking serine proteases were constructed by in-frame insertion deletion mutagenesis. Pneumonia was induced by intranasal infection of mice with wild-type or mutant D39. After high dose infection, only D39ΔhtrA showed reduced virulence, as reflected by strongly reduced bacterial loads, diminished dissemination and decreased lung inflammation. D39ΔprtA induced significantly less lung inflammation together with smaller infiltrated lung surface, but without influencing bacterial loads. After low dose infection, D39ΔhtrA again showed strongly reduced bacterial loads; notably, pneumococcal burdens were also modestly lower in lungs after infection with D39Δsfp. These data confirm the important role for HtrA in S. pneumoniae virulence. PrtA contributes to lung damage in high dose pneumonia; it does not however contribute to bacterial outgrowth in pneumococcal pneumonia. SFP may facilitate S. pneumoniae growth after low dose infection.
Introduction
The bacterium Streptococcus (S.) pneumoniae is a major global cause of human disease [1]. S. pneumoniae is the most frequent cause of community-acquired pneumonia and a common pathogen in sepsis, the incidence being greatest at the extremes of age and in immune compromised individuals [2]. Although the discovery of antibiotics and the development of vaccines have reduced the health burden associated with pneumococcal infections, S. pneumoniae still causes over 2 million deaths annually [1] and increased bacterial resistance against currently available antibiotics could make pneumococcal infections an even larger health threat in the future [3]. Consequently, additional knowledge about this bacterium and its virulence factors is of importance.
In 1991, Courtney [4] was the first to describe a role for serine proteases as virulence factors for S. pneumoniae. Since then, serine protease orthologs have been found in many bacteria and numerous roles in virulence and pathogenesis have been described [5]–[16]. One of these serine proteases is HtrA (high-temperature requirement A), which has been identified as a virulence factor in several bacterial species. In S. pneumoniae, HtrA is important for bacterial stress response and protein quality control, and has a role in competence [11], [14], [17]. As a posttranslational regulator, HtrA is involved in bacteriocin activity and cell division [8], [15], [16]. HtrA has been identified as an important virulence factor for S. pneumoniae, i.e. HtrA deficient pneumococci demonstrated a dramatically reduced virulence in models of pneumonia and bacteremia [11]. Another investigation identified PrtA (cell wall-associated serine protease A) as a pneumococcal serine protease important for virulence after intraperitoneal infection [5].
In this study, we searched for additional serine proteases as virulence factors of S. pneumoniae. By reannotating the S. pneumoniae D39 genome using a subsystems approach [18] and by screening all proteins for the presence of serine protease associated domains using Interproscan [19], we identified SFP (subtilase family protein) as an additional surface-exposed pneumococcal serine protease besides HrtA and PrtA, and generated directed gene knockout mutants of htrA (SPD_2068), sfp (SPD_1753) and prtA (SPD_0558) in S. pneumoniae strain D39. We tested their virulence in an in vivo pneumonia model by inoculating mice with viable wild-type (WT) and mutant S. pneumoniae via the airways, and compared several outcome parameters 48h after infection.
Materials and Methods
Serine protease search
All proteins encoded in the genome of S. pneumoniae D39 where screened for the presence of serine protease associated domains using Interproscan [19]. Domains IPR009003 and IPR001940 identified HtrA, domain IPR008357 identified SFP, while domains IPR000209 and IPR015500 identified both SFP and PrtA as predicted to encode serine proteinases. An additional search for proteins predicted to have serine protease activity was performed by examining membership of GO category 0008236, resulting in the identification of SPD_1765 and SPD_1920. Blast analysis of the proteome of S. pneumoniae D39 with HtrA, SFP and PrtA with an E-value cut-off of 0.1 showed limited protein sequence similarity of SFP with PrtA, but no other putative serine proteases were identified. Furthermore the S. pneumoniae D39 genome was re-annotated using the +6RAST subsystems approach [18] and the resulting annotations where searched for protease encoding proteins. No other obvious serine proteases could be detected using these methods. Subcellular localization prediction of the putative serine proteases was performed with SignalP [20].
Construction of directed deletion mutants
Directed-deletion mutants of S. pneumoniae D39 lacking HtrA (D39ΔhtrA), SFP (D39Δsfp) or PrtA (D39ΔprtA) were generated by allelic exchange of the target gene with a spectinomycin resistance marker essentially as described previously [21]. Briefly, an extension PCR was performed to join 400–500 bp 5’ and 3’ flanking sequences of the target gene with the spectinomycin resistance cassette (obtained from pR412T7). The resulting PCR products were introduced by competent stimulating peptide (CSP-1)-induced transformation into D39. Transformants were selected on the basis of spectinomycin resistance and were checked by PCR for recombination at the desired location on the chromosome. Subsequently, the D39 WT strain was transformed with 1 μg chromosomal DNA isolated from the mutants to prevent the accumulation of inadvertent mutations elsewhere on the chromosome. At the same time, D39 was mock-transformed to obtain a coupled WT strain. All primers used in this study are shown in Table 1.
Table 1. Oligonucleotide primers used in this study.
| Primer name | Target a, b | Sequence (5'- 3') |
| Primers for generation of directed mutants | ||
| PBpR412_L | SpecR cassette | GCCGCTCTAGAACTAGTGG |
| PBpR412_R | SpecR cassette | GATACCCCTCGAATTGACGC |
| PBTnMr9 | SpecR cassette control primer | CAATGGTTCAGATACGACGAC |
| SPD_2068_L1 | htrA left flanking region | TTCCCTTCAATGGCTAACAC |
| SPD_2068_L2 | htrA left flanking region | CCACTAGTTCTAGAGCGGCAAACTACCCAAGGCTCCAC |
| SPD_2068_R1 | htrA right flanking region | GACTTGCCCATCTTATCTGC |
| SPD_2068_R2 | htrA right flanking region | GCGTCAATTCGAGGGGTATCACCATTCTATCGGAGACACC |
| SPD_2068_C | htrA gene control primer | TCGCTGAGAATCTGTGTCAG |
| SPD_1753_L1 | sfp left flanking region | CCTCTTGGATTAGAGACAATC |
| SPD_1753_L2 | sfp left flanking region | CCACTAGTTCTAGAGCGGCTTTCGCTAGTCTGAGTGTG |
| SPD_1753_R1 | sfp right flanking region | TAGTTGTGGTAACCTGTTTGC |
| SPD_1753_R2 | sfp right flanking region | GCGTCAATTCGAGGGGTATCATTACAAGTCAGTTTAGTTG |
| SPD_1753_C | sfp gene control primer | CTTTCGCCTCCACCAGTAAC |
| SPD_0558_L1 | prtA left flanking region | TTCAAACCACGTCAACGTCG |
| SPD_0558_L2 | prtA left flanking region | CCACTAGTTCTAGAGCGGCTGCAGCTGTAGTTAGTGAC |
| SPD_0558_R1-2 | prtA right flanking region | TAACCGTCCAATAGACTTCG |
| SPD_0558_R2 | prtA right flanking region | GCGTCAATTCGAGGGGTATCAGCCCAGACACTATTAGCTG |
| SPD_0558_C | prtA gene control primer | GTGTCTGCTAAGACTACCTC |
In vitro growth assay
Mid-log growing mutant or WT S. pneumoniae were diluted to an optical density (OD) of 0.1 (620 nm wavelength) in Todd Hewitt broth (Oxoid microbiology products, Thermo Scientific, Hampshire, UK) with 0.5% Yeast extract (THY). Cultures were incubated at 37°C in a 5.0% CO2 incubator. OD was measured every hour for the next 5 hours.
Animals
Specific pathogen-free C57BL/6 male and female mice were purchased from Harlan Sprague-Dawley (Horst, the Netherlands). Experimental groups were age- and sex matched, and housed in the Animal Research Institute Amsterdam under standard care. All experiments were conducted with mice between 10 and 12 weeks of age.
Ethics statement
This study was carried out in concordance with the ‘Wet op de Dierproeven’ in the Netherlands. The Institutional Animal Care and Use Committee of the Academic Medical Center approved all experiments. All efforts were made to minimize suffering. Induction of pneumonia happened under isoflurane anaesthesia.
Experimental study design
Pneumonia was induced by intranasal inoculation with S. pneumoniae D39, D39ΔhtrA, D39Δsfp or D39ΔprtA (serotype 2; 5×105 or 5×104 colony forming units (CFU) in 50 µL isotonic saline) using previously described methods [22]–[24]. Mice were euthanized 48 hours after induction of pneumonia (N = 8 mice per group). Blood was obtained from the inferior vena cava and diluted 4:1 with citrate. Bronchoalveolar lavage fluid (BALF), lung, spleen and liver were harvested as described [22]–[24] and organs were homogenised in five volumes of sterile isotonic saline. The left lung lobe was fixed in 10% buffered formalin and embedded in paraffin. Total cell numbers in BALF were determined by an automated cell counter (Coulter Counter, Coulter Electronics, Hialeah, FL, USA). Differential cell counts were performed on cytospin preparations stained with a modified Giemsa stain (Diff-Quick; Dade Behring AG, Düdingen, Switzerland). For bacterial quantification blood, BALF, and organ homogenates were serially diluted by 10-fold in sterile isotonic saline and plated onto sheep-blood agar plates. Following 16 hours of incubation at 37°C CFU were counted. For further measurements, homogenates were diluted 1:1 with lysis buffer (300 mM NaCl, 30 mM Tris, 2 mM MgCl2, 2 mM CaCl2, 1% (v/v) Triton X-100, pH 7.4) with protease inhibitor mix and incubated for 30 minutes on ice, followed by centrifugation at 680 g for 10 minutes. Supernatants were stored at –20°C until analysis.
Histopathology
Four-micrometer sections of the left lung lobe were stained with hematoxylin and eosin (H&E). Slides were coded and scored by a pathologist blinded for group identity for the following parameters: interstitial inflammation, endothelialitis, bronchitis, oedema, pleuritis and presence of thrombi. All parameters were rated separately from 0 (condition absent) to 4 (most severe condition). The total histopathological score was expressed as the sum of the scores of the individual parameters, with a maximum of 24. In addition, pulmonary infiltrate was scored as a percentage of total lung surface occupied by confluent infiltrates.
Assays
Interleukin (IL)-6, tumor necrosis factor alpha (TNF-α), keratinocyte-derived cytokine (KC) and IL-1β were measured using commercially available ELISA kits (R&D Systems, Abingdon, UK). Myeloperoxidase (MPO; Hycult, Uden, the Netherlands) was measured by ELISA according to manufacturers’ instructions.
Statistical analysis
Data are expressed as box and whisker plots showing the smallest observation, lower quartile, median, upper quartile and largest observation, or as medians with interquartile ranges. Comparisons between groups were first performed using Kruskal-Wallis one-way analysis of variance test; in case of significant differences, differences between groups were tested using the Mann-Whitney U test. All analyses were done using GraphPad Prism version 5.01 (GraphPad Software, San Diego, CA). P-values < 0.05 were considered statistically significant.
Results
S. pneumoniae D39 genome contains three putative serine proteases
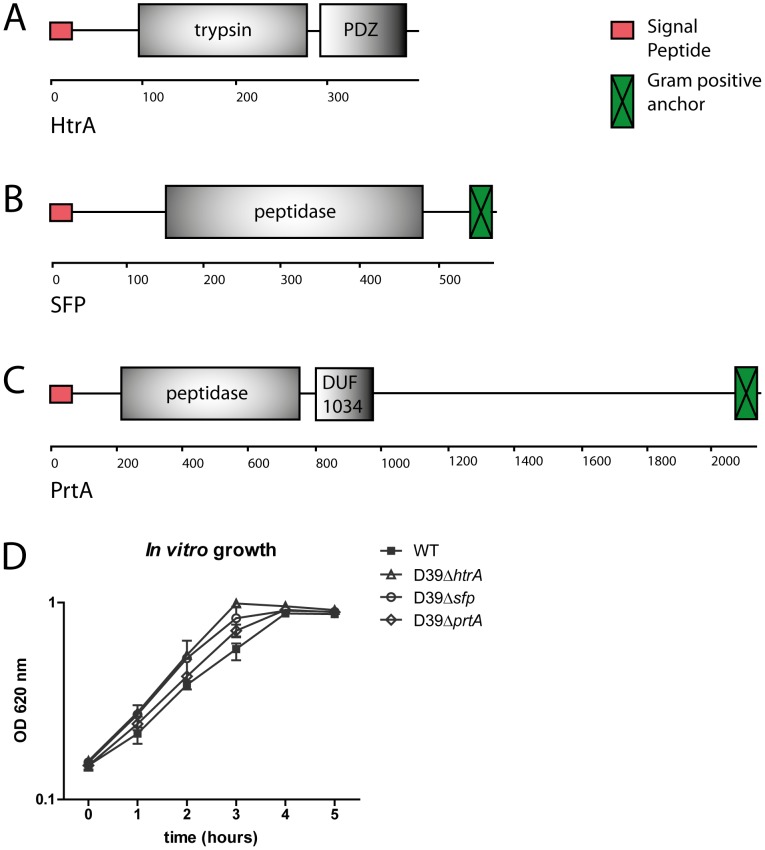
To identify all serine protease proteins encoded in the genome of S. pneumoniae D39, we screened the D39 proteome for the presence of serine protease domains using Interproscan as described in the Experimental procedures. Furthermore, the D39 genome was re-annotated to identify additional proteases. This led to the identification of several proteases, of which only HtrA (SPD_2068), PrtA (SPD_558) and SFP (SPD_1753) were predicted to have both serine protease activity (figure 1A-C) and be secreted and surface-exposed based on the presence of a signal sequence [20]. The predicted PDZ (Post synaptic density protein, Drosophila disc large tumor suppressor, and Zonula occludens-1 protein) domain in HtrA could be involved in protein-protein interactions. The DUF (Domain of Unknown Function) 1034 domain in PrtA is predicted to have serine-type endopeptidase activity. Two additional putative serine proteases were identified by their membership of GO category 0008236 (SPD_1765 and SPD_1920), but they were excluded from further analysis because they did not contain a signal sequence or a cell surface exposed serine protease domain. BLAST analysis of the D39 proteome with HtrA, PrtA and SFP did not reveal any additional putative serine protease encoding genes.
Figure 1. HtrA, SFP and PrtA: structure and in vitro growth of mutant S. pneumoniae.
Structure of serine protease HtrA (A), SFP (B) and PrtA (C), predicted by SMART PROTEIN online sequence analysis (http://smart.embl-heidelberg.de/). Number of amino acids is indicated underneath each enzyme. The predicted PDZ (Post synaptic density protein, Drosophila disc large tumor suppressor, and Zonula occludens-1 protein) domain in HtrA could be involved in protein-protein interactions. The DUF (Domain of Unknown Function) 1034 domain in PrtA is predicted to have serine-type endopeptidase activity. (D) Mutant S. pneumoniae strains did not show reduced growth compared to WT S. pneumoniae in vitro.
S. pneumoniae D39ΔhtrA, but not D39Δsfp or D39ΔprtA, displays diminished growth and dissemination in vivo
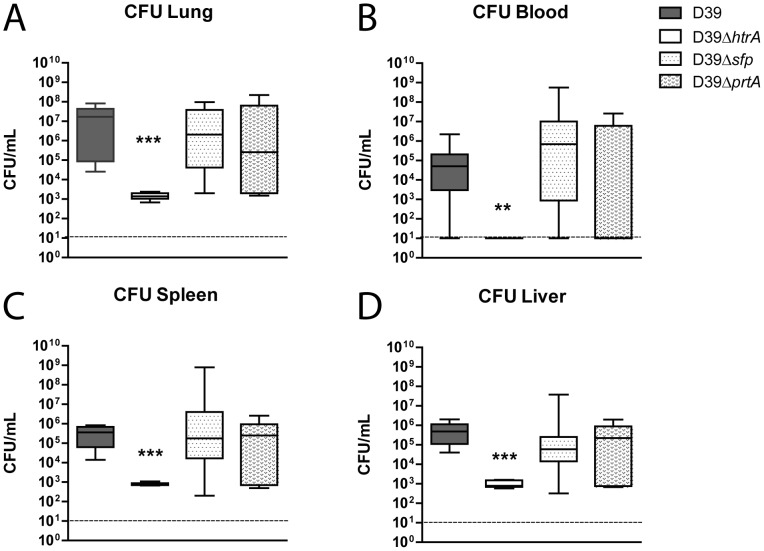
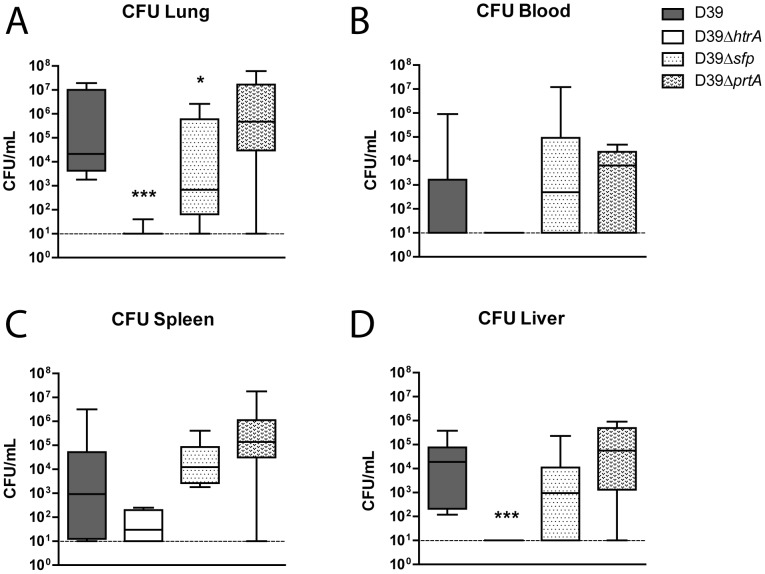
Mutant S. pneumoniae strains did not show reduced growth compared to WT S. pneumoniae in THY at 37 °C and 5.0% CO2 (figure 1D). To study the role of the three serine proteases in S. pneumoniae virulence, we infected mice with 5×105 CFU of either WT D39, D39ΔhtrA, D39Δsfp or D39ΔprtA via the airways and quantified bacterial loads at the primary site of infection (lungs) and distant body sites (blood, spleen and liver) 48 hours later (figure 2). WT S. pneumoniae D39, D39Δsfp and D39ΔprtA had multiplied in the lungs, accompanied by dissemination to spleen and liver; the counts of these three pneumococcal strains were similar in all body sites examined. In contrast, D39ΔhtrA counts were markedly lower in the lungs and in all distant body sites when compared with the other three strains (P < 0.0005). In addition, no bacteria could be detected in the blood of D39ΔhtrA infected animals (P < 0.005).
Figure 2. S. pneumoniae D39ΔhtrA, but not D39Δsfp or D39ΔprtA, displays diminished growth and dissemination in vivo.
Mice were infected with WT or mutant S. pneumoniae (5×105 CFU) via the intranasal route and euthanized 48 hours later. Bacterial counts were determined in lung (A), blood (B), spleen (C) and liver (D). Data are expressed as box- and whisker plots depicting the smallest observation, lower quartile, median, upper quartile and largest observation. N = 8 mice per group at each time point. *** P < 0.005 versus WT S. pneumoniae.
Reduced lung inflammation during pneumonia caused by S. pneumoniae D39ΔhtrA or D39ΔprtA
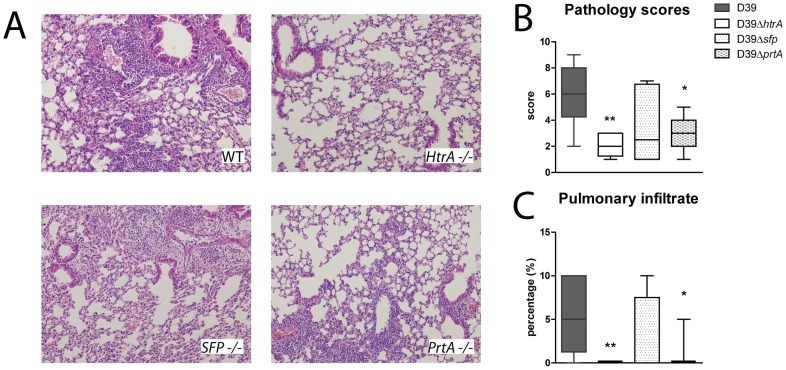
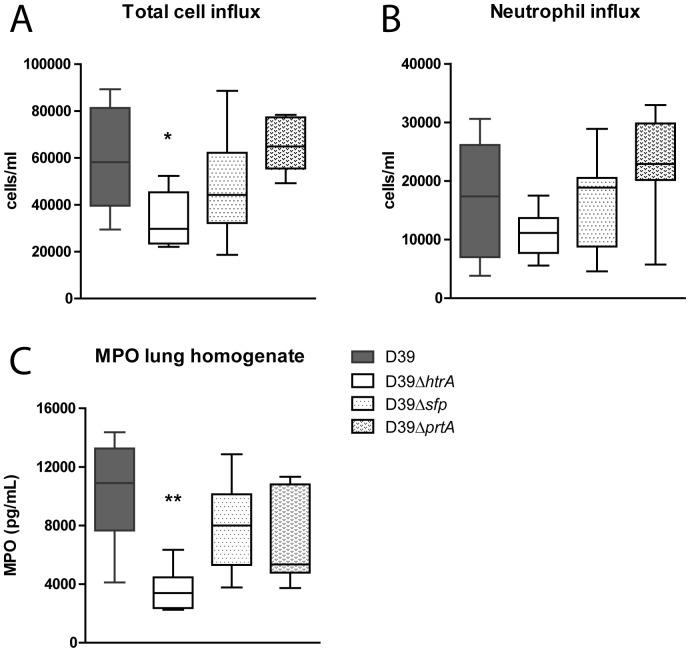
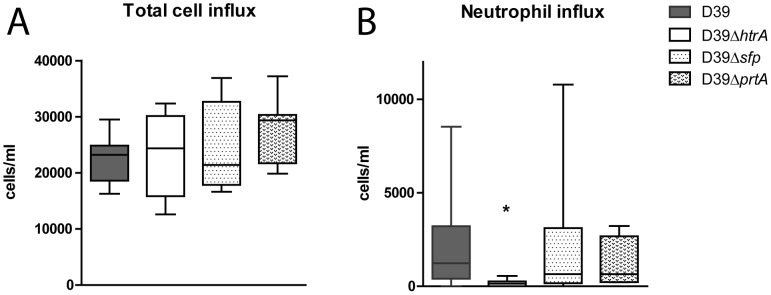
This model of pneumococcal pneumonia is associated with histological features in the lung characteristic for lower respiratory tract infection, including interstitial inflammation, endothelialitis, edema, inflammatory infiltrates and pleuritis [25]. To determine the impact of the three S. pneumoniae serine proteases on the induction of these inflammatory alterations, we semi-quantitatively scored lung histology slides after pneumonia caused by WT D39, D39ΔhtrA, D39Δsfp or D39ΔprtA (figure 3). The lungs of D39ΔhtrA infected mice displayed significantly less inflammation and a smaller infiltrated lung surface (P < 0.01). Remarkably, in spite of similar bacterial loads, the lungs of D39ΔprtA infected mice also displayed lower histopathology scores when compared with lungs of WT D39 infected mice, together with a smaller infiltrated lung surface (P < 0.05). Considering that neutrophils play a key role in the inflammatory response during respiratory tract infection by S. pneumoniae [1], [26], we next determined total cell and neutrophil counts in BALF after infection with WT D39, D39ΔhtrA, D39Δsfp or D39ΔprtA (figure 4). We additionally measured MPO concentration in whole lung homogenates, as a marker of neutrophil content of lung tissue. Total cell counts were lower in BALF obtained from D39ΔhtrA infected mice (P < 0.05); a similar trend was observed for neutrophil influx. In accordance, whole lung MPO concentrations were lower after infection with D39ΔhtrA (P < 0.05). The deletion of either sfp or prtA did not influence cell recruitment. Cytokines and chemokines play an eminent role in the regulation of inflammation during pneumonia [1], [26]. Therefore, we measured cytokines (TNFα, IL-1β, IL-6) and a chemokine (KC) in whole lung homogenates as an additional readout for pulmonary inflammation. Lung IL-1β, IL-6 and KC levels were lower in D39ΔhtrA when compared with the other three S. pneumoniae strains (table 2).
Figure 3. Reduced lung inflammation during pneumonia caused by S. pneumoniae D39ΔhtrA and D39ΔprtA.
Mice were infected with WT or mutant S. pneumoniae (5×105 CFU) via the intranasal route and euthanized 48 hours later. (A) Representative microphotographs of H&E stained lung sections of WT or mutant S. pneumoniae infected mice (10 times original magnification). (B) Total lung histopathology scores expressed as box- and whisker plots depicting the smallest observation, lower quartile, median, upper quartile and largest observation. (C) Pulmonary infiltrate as percentage of total lung surface. No infiltrates were observed in the D39ΔhtrA infected group; only one mouse with an infiltrate was observed in the D39ΔprtA infected group. N = 8 mice per group at each time point. * P < 0.05, ** P < 0.01 versus WT S. pneumoniae.
Figure 4. Diminished cell and neutrophil influx during pneumonia caused by S. pneumoniae D39ΔhtrA.
Mice were infected with WT or mutant S. pneumoniae (5×105 CFU) via the intranasal route and euthanized 48 hours later. Cell (A) and neutrophil (B) influx were determined on BALF cytospin preparations. As a marker of neutrophil influx in lung tissue, MPO was measured in whole lung homogenates (C). Data are expressed as box- and whisker plots depicting the smallest observation, lower quartile, median, upper quartile and largest observation. N = 8 mice per group at each time point. * P < 0.05 versus WT S. pneumoniae.
Table 2. Lung cytokine and chemokine levels.
| S. pneumoniae | D39 | D39ΔhtrA | D39Δsfp | D39ΔprtA | |
| Lung homogenate, t = 48h (pg/mL) | |||||
| IL-6 | 1689 (257 – 2864) | 174 (136 – 220) ** | 1076 (220 – 3045) | 260 (174 – 3717) | |
| TNF-α | 498 (216 – 857) | 220 (189 – 277) | 592 (229 – 1290) | 285 (171 – 805) | |
| KC | 7164 (747 – 8697) | 362 (322 – 444) ** | 3218 (659 – 7962) | 482 (300 – 8355) | |
| IL-1β | 724 (116 – 1117) | 81 (73 – 97) ** | 363 (129 – 859) | 106 (79 – 1047) | |
Mice were infected with the S. pneumoniae strain indicated (5×105 CFU) via the intranasal route and euthanized 48 hours later. Data are expressed as medians and interquartile range of 7 or 8 mice per group. ** P < 0.01 versus WT S. pneumoniae.
Modest role for SFP after low dose infection
The data presented above confirm that HtrA plays a significant role in S. pneumoniae virulence [11]. We argued that the contribution of PrtA and SFP to S. pneumoniae virulence could be more subtle and as such not noticed in our model with a relatively high bacterial dose. Accordingly, we repeated our infection experiments with a 10-fold lower inoculum (5×104 CFU). These studies again revealed the reduced virulence of D39ΔhtrA, as reflected by lower bacterial loads in lungs (P < 0.005) and liver (P < 0.005) at 48 hours post infection (figure 5). Of interest, pneumococcal burdens were also modestly but significantly lower in lungs after infection with D39Δsfp (P < 0.05); this difference with WT D39 was not observed in blood or distant organs. Cell influx in low dose pneumonia was modest (Figure 6), with less than half of the total cell counts in BALF compared to high dose infection. No difference in total cell numbers was observed between WT or mutant S. pneumoniae infected mice (figure 6A). Neutrophil influx determined by cell differentiation on cytospin slides however was diminished in D39ΔhtrA infected animals (figure 6B) (P < 0.05).
Figure 5. HtrA strongly contributes to virulence after low dose infection with a more modest role for SFP.
Mice were infected with a 10-fold lower inoculum of WT or mutant S. pneumoniae (relative to the infectious dose used in the experiments shown in figures 2–4; 5×104 CFU) via the intranasal route and euthanized 48 hours later. Bacterial counts were determined in lung (A), blood (B), spleen (C) and liver (D). Data are expressed as box- and whisker plots depicting the smallest observation, lower quartile, median, upper quartile and largest observation. N = 8 mice per group at each time point. * P <0.05, ** P < 0.01, *** P < 0.005 versus WT S. pneumoniae.
Figure 6. Diminished cell and neutrophil influx during pneumonia caused by low dose S. pneumoniae D39ΔhtrA.
Mice were infected with a 10-fold lower inoculum of WT or mutant S. pneumoniae (relative to the infectious dose used in the experiments shown in figures 2–4; 5×104 CFU) via the intranasal route and euthanized 48 hours later. Cell (A) and neutrophil (B) influx were determined on BALF cytospin preparations. Data are expressed as box- and whisker plots depicting the smallest observation, lower quartile, median, upper quartile and largest observation. N = 8 mice per group at each time point. * P < 0.05 versus WT S. pneumoniae.
Discussion
Serine protease orthologs have been found in many bacteria, contributing to their virulence to a significant extent [4]-[14]. Previous research identified the serine protease HtrA as a major virulence factor in S. pneumoniae during experimentally induced pneumonia [11], [14]. We here sought for additional S. pneumoniae serine proteases and determined their role in virulence during respiratory tract infection in vivo. Our main findings are that the S. pneumoniae D39 genome expresses three putative secreted, surface-exposed serine proteases: HtrA, SFP and PrtA. We confirmed reduced virulence after high and low dose infection of D39ΔhtrA [11], [14], as reflected by strongly reduced bacterial loads, diminished systemic dissemination and decreased lung inflammation. After high dose infection D39ΔprtA induced significantly less lung inflammation without influencing bacterial loads. Pneumococcal burdens were also modestly but significantly lower in lungs after low dose infection with D39Δsfp. These data reveal two additional pneumococcal serine proteases that can modify the host response during pneumonia, albeit clearly to a more modest extent than HtrA.
Structure and function of HtrA have been widely studied. S. pneumoniae HtrA has been identified to help bacteria survive environmental pressures such as elevated temperature, oxidative stress, and osmotic stress [11]. Another study revealed an important role for HtrA during S. pneumoniae cell division [15]. HtrA additionally has a distinct role in bacteriocin activity by reducing pneumocin expression [8], [16]. Pneumocins mediate intra- and interspecies competition in vitro and have been shown to provide a competitive advantage in vivo [16]. In the present study, we confirmed the strongly reduced virulence of D39ΔhtrA in pneumonia [11]. D39ΔhtrA infected animals displayed more than 3 logs lower bacterial counts in their lungs at 48 hours after infection; in addition, their lungs showed minimal signs of lower respiratory tract infection upon histopathological examination. There was significantly less dissemination to distant organs in D39ΔhtrA infected mice. Notably, D39ΔhtrA could be detected in spleen and liver, although blood cultures were sterile in all experiments. Ibrahim et al [11] concluded that D39ΔhtrA S. pneumoniae did not disseminate from the lungs based on negative blood cultures; our data, however, indicate that D39ΔhtrA is able to spread to distant body sites.
It is known that apolactoferrin can kill many species of bacteria, including Streptococcus pneumoniae. Lactoferricin, an N-terminal peptide of apolactoferrin, and fragments of it are even more bactericidal than apolactoferrin. PrtA cleaves apolactoferrin, greatly enhancing the killing activity of apolactoferrin and its cleavage products [13]. Thus, in theory, PrtA deficiency might cause increased rather than decreased virulence due to a diminished capacity of apolactoferrin to kill S. pneumoniae. Nonetheless, the only in vivo study performed thus far with PrtA deficient S. pneumoniae showed decreased virulence compared to WT S. pneumoniae when injected intraperitoneally in mice [5]. Our pneumonia model is more relevant to determine the role of PrtA in S. pneumoniae virulence, as pneumonia is the primary illness caused by pneumococci. In both high and low dose infection, bacterial outgrowth, pulmonary cell influx and cytokine release were similar after induction of pneumonia with D39ΔprtA or WT S. pneumoniae. Interestingly, histopathology scores of lungs and the percentage of infiltrated lung surface were significantly lower in D39ΔprtA infected animals than in mice inoculated with WT S. pneumoniae, suggesting that PrtA has a modest role in the induction of pulmonary inflammation during pneumococcal pneumonia without influencing cellular influx or cytokine release in the lungs, and bacterial multiplication and dissemination.
To our knowledge, the role of SFP in pneumococcal virulence has not been studied before. SFP has homology with S. agalactiae CspA, a serine protease capable of inactivating chemokines in vitro [7]. While after high dose infection the growth of D39Δsfp was indistinguishable from that of WT S. pneumoniae, this mutant strain demonstrated slightly reduced growth in the lungs in low dose pneumonia. This modest role of SFP mediated virulence should be confirmed in additional studies using different S. pneumoniae strains.
We identified two additional hypothetical proteins, SPD_1765 and SPD_1920, predicted to have serine protease activity. These were not examined further here as their serine protease domain was likely not exposed to the outer cell surface. It may be of interest to examine the roles of intracellular serine protease activity for SPD_1765 and SPD_1920 in S. pneumoniae virulence in future research.
The current investigation addressed the role of three serine proteases expressed by S. pneumoniae (HtrA, SFP and PrtA) in virulence in vivo by comparing bacterial growth and dissemination and the accompanying inflammatory response in the lung after 48 hours of airway infection by newly generated deletion mutants. This time point was selected since our main endpoint of interest was bacterial growth; the 48-hour time point reflects late stage pneumonia shortly before mice are expected to die. Our data confirm the previously reported important role for HtrA in S. pneumoniae virulence [11]. In this previous paper, reconstitution of HtrA into D39ΔhtrA reverted D39ΔhtrA to its full virulence [11]. We here created a new HtrA deletion mutant and our investigation is limited by the fact that we did not reconstitute HtrA in our independently generated D39ΔhtrA strain. Of note, however, our main objective was to identify new pneumococcal serine proteases as virulence factors in pneumonia. Since our results only reveal a significant role for the already established HrtA [11], we think that the absence of a HrtA reconstitution experiment does not jeopardize our main conclusion (i.e., that the other proteases identified do not significantly contribute to the virulence of S. pneumoniae). In contrast to its reported role in intraperitoneal infection [5] we here show that PrtA does not influence bacterial multiplication and dissemination in pneumococcal pneumonia, however, PrtA had a modest role in the induction of pulmonary inflammation. Finally, in the first studies reported to date, we provide evidence that SFP may facilitate S. pneumoniae growth after low dose infection of the lower respiratory tract, although clearly these data need to be confirmed in independent experiments involving more time points. Together these data firmly establish that virulence of S. pneumoniae is dominated by HtrA.
Acknowledgments
The authors would like to thank J.B. Daalhuisen and M. ten Brink for their technical support.
Funding Statement
This work was supported by an AMC PhD Scholarship to S.F. de Stoppelaar. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. van der Poll T, Opal SM (2009) Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 374: 1543–1556. [DOI] [PubMed] [Google Scholar]
- 2. Dockrell DH, Whyte MK, Mitchell TJ (2012) Pneumococcal pneumonia: mechanisms of infection and resolution. Chest 142: 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garau J (2002) Treatment of drug-resistant pneumococcal pneumonia. Lancet InfectDis 2: 404–415. [DOI] [PubMed] [Google Scholar]
- 4. Courtney HS (1991) Degradation of connective tissue proteins by serine proteases from Streptococcus pneumoniae. Biochem Biophys Res Commun 175: 1023–1028. [DOI] [PubMed] [Google Scholar]
- 5. Bethe G, Nau R, Wellmer A, Hakenbeck R, Reinert RR, et al. (2001) The cell wall-associated serine protease PrtA: a highly conserved virulence factor of Streptococcus pneumoniae. FEMS Microbiol Lett 205: 99–104. [DOI] [PubMed] [Google Scholar]
- 6. Boehm M, Hoy B, Rohde M, Tegtmeyer N, Baek KT, et al. (2012) Rapid paracellular transmigration of Campylobacter jejuni across polarized epithelial cells without affecting TER: role of proteolytic-active HtrA cleaving E-cadherin but not fibronectin. Gut Pathog 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bryan JD, Shelver DW (2009) Streptococcus agalactiae CspA is a serine protease that inactivates chemokines. J Bacteriol 191: 1847–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dawid S, Sebert ME, Weiser JN (2009) Bacteriocin activity of Streptococcus pneumoniae is controlled by the serine protease HtrA via posttranscriptional regulation. J Bacteriol 191: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edwards RJ, Taylor GW, Ferguson M, Murray S, Rendell N, et al. (2005) Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J Infect Dis 192: 783–790. [DOI] [PubMed] [Google Scholar]
- 10. Hoy B, Geppert T, Boehm M, Reisen F, Plattner P, et al. (2012) Distinct roles of secreted HtrA proteases from gram-negative pathogens in cleaving the junctional protein and tumor suppressor E-cadherin. J Biol Chem 287: 10115–10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ibrahim YM, Kerr AR, McCluskey J, Mitchell TJ (2004) Role of HtrA in the virulence and competence of Streptococcus pneumoniae. Infect Immun 72: 3584–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyon WR, Caparon MG (2004) Role for serine protease HtrA (DegP) of Streptococcus pyogenes in the biogenesis of virulence factors SpeB and the hemolysin streptolysin S. Infect Immun 72: 1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mirza S, Wilson L, Benjamin WH Jr, Novak J, Barnes S, et al. (2011) Serine protease PrtA from Streptococcus pneumoniae plays a role in the killing of S. pneumoniae by apolactoferrin. Infect Immun 79: 2440–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sebert ME, Palmer LM, Rosenberg M, Weiser JN (2002) Microarray-based identification of htrA, a Streptococcus pneumoniae gene that is regulated by the CiaRH two-component system and contributes to nasopharyngeal colonization. Infect Immun 70: 4059–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsui HC, Keen SK, Sham LT, Wayne KJ, Winkler ME (2011) Dynamic distribution of the SecA and SecY translocase subunits and septal localization of the HtrA surface chaperone/protease during Streptococcus pneumoniae D39 cell division. MBio 2. [DOI] [PMC free article] [PubMed]
- 16.Kochan TJ, Dawid S (2013) The HtrA protease of Streptococcus pneumoniae controls density dependent stimulation of the bacteriocin blp locus via disruption of pheromone secretion. J Bacteriol. [DOI] [PMC free article] [PubMed]
- 17. Cassone M, Gagne AL, Spruce LA, Seeholzer SH, Sebert ME (2012) The HtrA protease from Streptococcus pneumoniae digests both denatured proteins and the competence-stimulating peptide. J Biol Chem 287: 38449–38459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, et al. (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zdobnov EM, Apweiler R (2001) InterProScan--an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17: 847–848. [DOI] [PubMed] [Google Scholar]
- 20. Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8: 785–786. [DOI] [PubMed] [Google Scholar]
- 21. Burghout P, Bootsma HJ, Kloosterman TG, Bijlsma JJ, de Jongh CE, et al. (2007) Search for genes essential for pneumococcal transformation: the RADA DNA repair protein plays a role in genomic recombination of donor DNA. J Bacteriol 189: 6540–6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rijneveld AW, Florquin S, Bresser P, Levi M, De WV, et al. (2003) Plasminogen activator inhibitor type-1 deficiency does not influence the outcome of murine pneumococcal pneumonia. Blood 102: 934–939. [DOI] [PubMed] [Google Scholar]
- 23. Rijneveld AW, Weijer S, Bresser P, Florquin S, Vlasuk GP, et al. (2006) Local activation of the tissue factor-factor VIIa pathway in patients with pneumonia and the effect of inhibition of this pathway in murine pneumococcal pneumonia. Crit Care Med 34: 1725–1730. [DOI] [PubMed] [Google Scholar]
- 24. Van Den Boogaard FE, Brands X, Schultz MJ, Levi M, Roelofs JJ, et al. (2011) Recombinant human tissue factor pathway inhibitor exerts anticoagulant, anti-inflammatory and antimicrobial effects in murine pneumococcal pneumonia. J ThrombHaemost 9: 122–132. [DOI] [PubMed] [Google Scholar]
- 25. Dessing MC, Florquin S, Paton JC, van der Poll T (2008) Toll-like receptor 2 contributes to antibacterial defence against pneumolysin-deficient pneumococci. Cell Microbiol 10: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paterson GK, Orihuela CJ (2010) Pneumococci: immunology of the innate host response. Respirology 15: 1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]