Abstract
We identified a novel human homologue of the rat FE65 gene, hFE65L, by screening the cytoplasmic domain of beta-amyloid precursor protein (beta PP) with the "interaction trap." The cytoplasmic domains of the beta PP homologues, APLP1 and APLP2 (amyloid precursor-like proteins), were also tested for interaction with hFE65L. APLP2, but not APLP1, was found to interact with hFE65L. We confirmed these interactions in vivo by successfully coimmunoprecipatating endogenous beta PP and APLP2 from mammalian cells overexpressing a hemagglutinin-tagged fusion of the C-terminal region of hFE65L. We report the existence of a human FE65 gene family and evidence supporting specific interactions between members of the beta PP and FE65 protein families. Sequence analysis of the FE65 human gene family reveals the presence of two phosphotyrosine interaction (PI) domains. Our data show that a single PI domain is sufficient for binding of hFE65L to the cytoplasmic domain of beta PP and APLP2. The PI domain of the protein, Shc, is known to interact with the NPXYp motif found in the cytoplasmic domain of a number of different growth factor receptors. Thus, it is likely that the PI domains present in the C-terminal moiety of the hFE65L protein bind the NPXY motif located in the cytoplasmic domain of beta PP and APLP2.
Full text
PDF


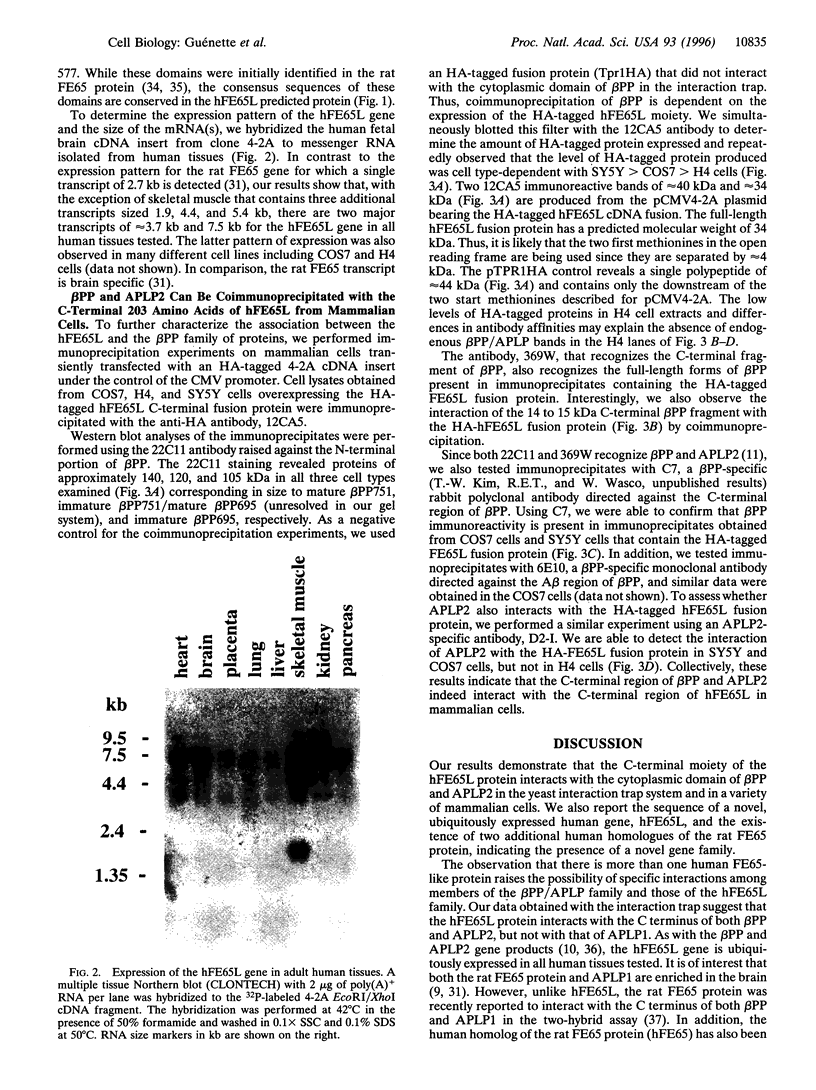
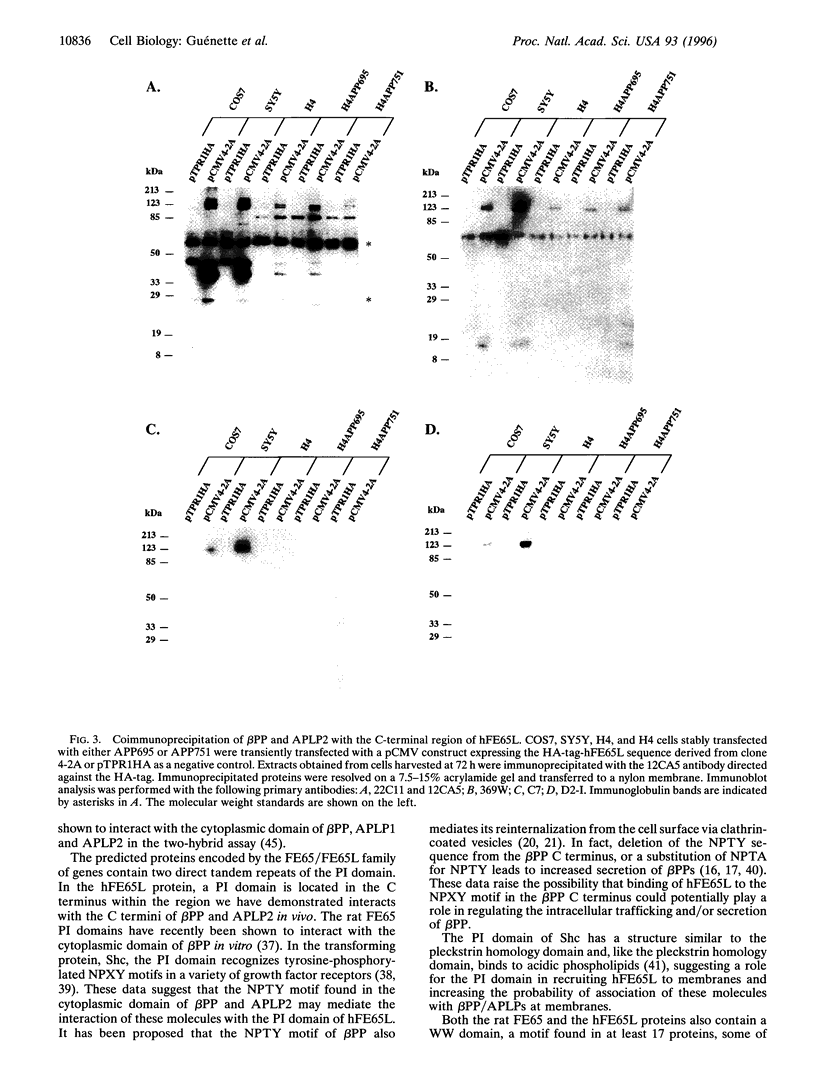

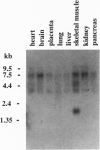
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaikie P., Immanuel D., Wu J., Li N., Yajnik V., Margolis B. A region in Shc distinct from the SH2 domain can bind tyrosine-phosphorylated growth factor receptors. J Biol Chem. 1994 Dec 23;269(51):32031–32034. [PubMed] [Google Scholar]
- Bork P., Margolis B. A phosphotyrosine interaction domain. Cell. 1995 Mar 10;80(5):693–694. doi: 10.1016/0092-8674(95)90347-x. [DOI] [PubMed] [Google Scholar]
- Bork P., Sudol M. The WW domain: a signalling site in dystrophin? Trends Biochem Sci. 1994 Dec;19(12):531–533. doi: 10.1016/0968-0004(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Buxbaum J. D., Gandy S. E., Cicchetti P., Ehrlich M. E., Czernik A. J., Fracasso R. P., Ramabhadran T. V., Unterbeck A. J., Greengard P. Processing of Alzheimer beta/A4 amyloid precursor protein: modulation by agents that regulate protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):6003–6006. doi: 10.1073/pnas.87.15.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Chen H. I., Sudol M. The WW domain of Yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7819–7823. doi: 10.1073/pnas.92.17.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Umans L., Van Leuven F., Van Den Berghe H. Study of the synthesis and secretion of normal and artificial mutants of murine amyloid precursor protein (APP): cleavage of APP occurs in a late compartment of the default secretion pathway. J Cell Biol. 1993 Apr;121(2):295–304. doi: 10.1083/jcb.121.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duilio A., Zambrano N., Mogavero A. R., Ammendola R., Cimino F., Russo T. A rat brain mRNA encoding a transcriptional activator homologous to the DNA binding domain of retroviral integrases. Nucleic Acids Res. 1991 Oct 11;19(19):5269–5274. doi: 10.1093/nar/19.19.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estus S., Golde T. E., Kunishita T., Blades D., Lowery D., Eisen M., Usiak M., Qu X. M., Tabira T., Greenberg B. D. Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science. 1992 Feb 7;255(5045):726–728. doi: 10.1126/science.1738846. [DOI] [PubMed] [Google Scholar]
- Faraonio R., Minopoli G., Porcellini A., Costanzo F., Cimino F., Russo T. The DNA sequence encompassing the transcription start site of a TATA-less promoter contains enough information to drive neuron-specific transcription. Nucleic Acids Res. 1994 Nov 25;22(23):4876–4883. doi: 10.1093/nar/22.23.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein K. M., Hunihan L. W., Roberts S. B. Altered cleavage and secretion of a recombinant beta-APP bearing the Swedish familial Alzheimer's disease mutation. Nat Genet. 1994 Mar;6(3):251–255. doi: 10.1038/ng0394-251. [DOI] [PubMed] [Google Scholar]
- Fiore F., Zambrano N., Minopoli G., Donini V., Duilio A., Russo T. The regions of the Fe65 protein homologous to the phosphotyrosine interaction/phosphotyrosine binding domain of Shc bind the intracellular domain of the Alzheimer's amyloid precursor protein. J Biol Chem. 1995 Dec 29;270(52):30853–30856. doi: 10.1074/jbc.270.52.30853. [DOI] [PubMed] [Google Scholar]
- Fukuchi K., Kamino K., Deeb S. S., Smith A. C., Dang T., Martin G. M. Overexpression of amyloid precursor protein alters its normal processing and is associated with neurotoxicity. Biochem Biophys Res Commun. 1992 Jan 15;182(1):165–173. doi: 10.1016/s0006-291x(05)80126-3. [DOI] [PubMed] [Google Scholar]
- Gustafson T. A., He W., Craparo A., Schaub C. D., O'Neill T. J. Phosphotyrosine-dependent interaction of SHC and insulin receptor substrate 1 with the NPEY motif of the insulin receptor via a novel non-SH2 domain. Mol Cell Biol. 1995 May;15(5):2500–2508. doi: 10.1128/mcb.15.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyuris J., Golemis E., Chertkov H., Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993 Nov 19;75(4):791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Haass C., Koo E. H., Mellon A., Hung A. Y., Selkoe D. J. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992 Jun 11;357(6378):500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- Haass C., Koo E. H., Mellon A., Hung A. Y., Selkoe D. J. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992 Jun 11;357(6378):500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Haass C., Selkoe D. J. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993 Dec 17;75(6):1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. S., Spruyt M. A., Brown A. M., Sahasrabudhe S. R., Blume A. J., Vitek M. P., Muenkel H. A., Sonnenberg-Reines J. The release of Alzheimer's disease beta amyloid peptide is reduced by phorbol treatment. J Biol Chem. 1994 Mar 18;269(11):8376–8382. [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Squazzo S. L. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994 Jul 1;269(26):17386–17389. [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A., Sisodia S. S., Trowbridge I. S. Characterization of sorting signals in the beta-amyloid precursor protein cytoplasmic domain. J Biol Chem. 1995 Feb 24;270(8):3565–3573. [PubMed] [Google Scholar]
- Niman H. L., Houghten R. A., Walker L. E., Reisfeld R. A., Wilson I. A., Hogle J. M., Lerner R. A. Generation of protein-reactive antibodies by short peptides is an event of high frequency: implications for the structural basis of immune recognition. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4949–4953. doi: 10.1073/pnas.80.16.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstedt C., Caporaso G. L., Thyberg J., Gandy S. E., Greengard P. Identification of the Alzheimer beta/A4 amyloid precursor protein in clathrin-coated vesicles purified from PC12 cells. J Biol Chem. 1993 Jan 5;268(1):608–612. [PubMed] [Google Scholar]
- Podlisny M. B., Tolan D. R., Selkoe D. J. Homology of the amyloid beta protein precursor in monkey and human supports a primate model for beta amyloidosis in Alzheimer's disease. Am J Pathol. 1991 Jun;138(6):1423–1435. [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996 Aug;2(8):864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989 Dec;16(5-6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Slunt H. H., Thinakaran G., Von Koch C., Lo A. C., Tanzi R. E., Sisodia S. S. Expression of a ubiquitous, cross-reactive homologue of the mouse beta-amyloid precursor protein (APP). J Biol Chem. 1994 Jan 28;269(4):2637–2644. [PubMed] [Google Scholar]
- Sudol M., Bork P., Einbond A., Kastury K., Druck T., Negrini M., Huebner K., Lehman D. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J Biol Chem. 1995 Jun 16;270(24):14733–14741. doi: 10.1074/jbc.270.24.14733. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Cheung T. T., Cai X. D., Odaka A., Otvos L., Jr, Eckman C., Golde T. E., Younkin S. G. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994 May 27;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Thinakaran G., Sisodia S. S. Amyloid precursor-like protein 2 (APLP2) is modified by the addition of chondroitin sulfate glycosaminoglycan at a single site. J Biol Chem. 1994 Sep 2;269(35):22099–22104. [PubMed] [Google Scholar]
- Wasco W., Bupp K., Magendantz M., Gusella J. F., Tanzi R. E., Solomon F. Identification of a mouse brain cDNA that encodes a protein related to the Alzheimer disease-associated amyloid beta protein precursor. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10758–10762. doi: 10.1073/pnas.89.22.10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasco W., Gurubhagavatula S., Paradis M. D., Romano D. M., Sisodia S. S., Hyman B. T., Neve R. L., Tanzi R. E. Isolation and characterization of APLP2 encoding a homologue of the Alzheimer's associated amyloid beta protein precursor. Nat Genet. 1993 Sep;5(1):95–100. doi: 10.1038/ng0993-95. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Dawes L. R., Fisher S., Villa-Komaroff L., Oster-Granite M. L., Neve R. L. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989 Jul 28;245(4916):417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Aizawa T., Hayashi Y. Degeneration in vitro of post-mitotic neurons overexpressing the Alzheimer amyloid protein precursor. Nature. 1992 Sep 3;359(6390):64–67. doi: 10.1038/359064a0. [DOI] [PubMed] [Google Scholar]
- Zhou M. M., Ravichandran K. S., Olejniczak E. F., Petros A. M., Meadows R. P., Sattler M., Harlan J. E., Wade W. S., Burakoff S. J., Fesik S. W. Structure and ligand recognition of the phosphotyrosine binding domain of Shc. Nature. 1995 Dec 7;378(6557):584–592. doi: 10.1038/378584a0. [DOI] [PubMed] [Google Scholar]