Abstract
Traumatic brain injury (TBI), particularly explosive blast-induced TBI (bTBI), has become the most prevalent injury among military personnel. The disruption of cognitive function is one of the most serious consequences of bTBI because its long-lasting effects prevent survivors fulfilling their active duty and resuming normal civilian life. However, the mechanisms are poorly understood and there is no treatment available. This study investigated the effects of adenosine A2A receptor (A2AR) on bTBI-induced cognitive deficit, and explored the underlying mechanisms. After being subjected to moderate whole-body blast injury, mice lacking the A2AR (A2AR knockout (KO)) showed less severity and shorter duration of impaired spatial reference memory and working memory than wild-type mice did. In addition, bTBI-induced cortical and hippocampal lesions, as well as proinflammatory cytokine expression, glutamate release, edema, cell loss, and gliosis in both early and prolonged phases of the injury, were significantly attenuated in A2AR KO mice. The results suggest that early injury and chronic neuropathological damages are important mechanisms of bTBI-induced cognitive impairment, and that the impairment can be attenuated by preventing A2AR activation. These findings suggest that A2AR antagonism is a potential therapeutic strategy for mild-to-moderate bTBI and consequent cognitive impairment.
Keywords: adenosine, brain trauma, cognitive impairment, experimental, neuroprotection
Introduction
In the recent conflicts in Iraq and Afghanistan, traumatic brain injury (TBI), especially blast-induced TBI (bTBI) caused by explosive devices, has become the most prevalent military injury and is described as the ‘signature injury' of the current military operations.1 Many of those having persistent neuropsychiatric symptoms caused by bTBI may fail to receive medical attention because the diagnosis is nonspecific and symptoms may be attributed to other psychiatric disorders such as posttraumatic stress disorder.2 Cognitive dysfunction is one of the most frequent neuropsychiatric disorders after blast exposure. Indeed, there is a growing consensus that blast can produce subtle injuries in the brain and that the psychiatric symptoms exhibited by blast victims have a biologic basis.3 Yet, little is understood about the mechanisms and diagnosis of bTBI, and still less is known about what treatments are most beneficial for acute neurotrauma and its long-term sequelae.
The adenosine A2A receptor (A2AR) is one of the four adenosine receptors (A1, A2A, A2B, and A3), all of which are G-protein coupled. The activity of A2AR regulates the release of several neurotransmitters, modulates neuronal excitability and synaptic plasticity, and glia function, consequently affecting various behavioral functions including cognition.4 The A2AR is present in both neurons and glial cells, where it is involved in gliosis and inflammation. Therefore, A2AR is thought to play important roles in modulating various brain insults.5 Our previous studies have shown that severe whole-body blast exposure causes notable brain injury, including inflammation and gliosis.6 We have also shown that A2AR inactivation attenuates cerebral injury in a mouse TBI model.7 We therefore speculated that A2AR deficiency may protect against bTBI-induced neuropathogenesis including inflammation, gliosis, and cognitive impairment.
In this study, we used an A2AR knockout (KO) mouse model to evaluate the effect of A2AR on memory deficit and its neuropathological and molecular correlates in both early and prolonged stages after blast injury. The data we present here provide direct evidence that genetic inactivation of A2AR can ameliorate cognitive impairment and attenuate neuropathological damage induced by blast injury in mice.
Materials and methods
Animals
The mice used in this study were from Dr Chen.5 The A2AR homozygous KO mice and their wild-type (WT) littermates were generated and identified as previously described.5, 8 Briefly, congenic global A2AR KO mice on a C57BL/6 background were generated by backcrossing global A2AR KO on mixed (129-Steel × C57BL/6) genetic background to C57BL/6 mice for 13–15 generations. Male mice 2 to 3 months old (weighing 22 to 26 g) were used. The mice were housed and maintained with free access to food and water in a temperature- and humidity-controlled room under a 12-hour light/dark cycle in the Experimental Center of Medical Animal of the Daping Hospital/Research Institute of Surgery, the Third Military Medical University (Chongqing, China). All animal procedures were approved by the Administration of Affairs Concerning Experimental Animals Guidelines of Third Military Medical University. All efforts were made to minimize the number used and animal suffering, in accordance with the ARRIVE guidelines.
Blast Injury Model
The bio-shock tube (BST-I) apparatus was used to produce blast injury as previously described9, 10 with minor modifications. Unanesthetized mice were placed into individual cages (1 mouse per cage) that were further secured on a metal shelf to restrict movement of their body during a rapid blast impact and to prevent subsequent secondary/tertiary blast injuries. All the cages were positioned at the same vertical plane inside the BST-I bio-shock tube apparatus to ensure equal pressure exposure. The peak positive pressure was 423.3±3.91 kPa and lasted 55.49±1.99 ms. Uninjured A2AR KO and WT mice were used as controls. After being subjected to blast injury, all mice (except those tested for learning and memory function) were killed at different time points (24 hours, and 1, 4, and 8 weeks) to collect tissue samples for further assays.
Morris Water Maze (MWM) Test
At 1, 4, and 8 weeks after blast injury, mice (n=12 for each WT group and n=10 for each KO group per test period) were tested for learning and memory performance using MWM paradigms as described previously.11, 12 The test consisted of three parts: visible cue task, reference memory task, and working memory task. The same cohorts of mice were tested for both reference memory and working memory in each test period (1, 4, or 8 weeks). To avoid repeated testing of the same subjects, separate cohorts of mice were subjected to MWM test at different test period after blast exposure. The schematic representation of method and process for MWM is shown in Figure 1.
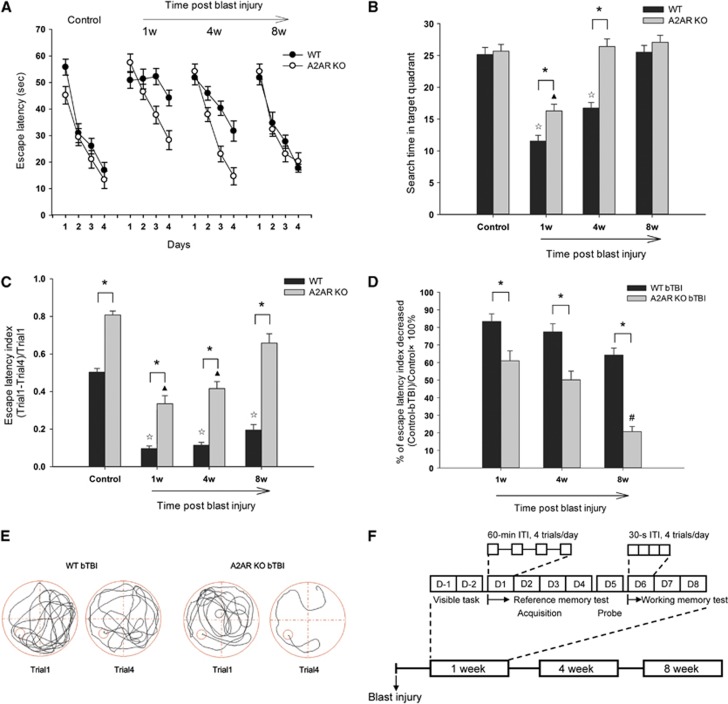
Figure 1.
Spatial reference memory (A, B) and working memory (C) impairment were ameliorated in adenosine A2A receptor (A2AR) knockout (KO) mice after blast-induced traumatic brain injury (bTBI). There was no significant difference between wild-type (WT)-control and KO-control groups in the (A) acquisition phase (F(2, 345)=0.587, P=0.624, two-way analysis of variance (ANOVA)) and in the (B) probe phase (P=0.74). (A) There were significant ‘genotype × day interaction' effects on the escape latency between WT-bTBI and KO-bTBI groups at 1 week (F(2, 348)=5.344, P=0.001, two-way ANOVA) and 4 weeks (F(2, 348)=5.67, P<0.001, two-way ANOVA) after blast injury. At 4 weeks after bTBI, escape latency in KO-bTBI mice recovered to the level of KO-control (F(2, 318)=0.789, P=0.501, two-way ANOVA). (B) When the platform was removed, search time in the former target quadrant was enhanced in KO-bTBI mice at 1 and 4 weeks after bTBI, compared with the WT-bTBI mice (P<0.01, t-test). Compared with the WT-bTBI group, the KO-bTBI mice exhibited significantly higher escape latency index (C), ‘genotype × time interaction' effect (F(2, 255)=4.061, P=0.008, two-way ANOVA), and better recovery amplitude (D) at all three time phases examined, indicating attenuated working memory impairment in KO-bTBI mice. *P<0.001 between the two groups; ΔP<0.01 compared with WT control; ▴P<0.01 compared with KO control; #P<0.01, compared with KO-bTBI at 1 week. (E) Representative swimming tracks of injured mice in the Morris water maze (MWM). (F) Schematic representation of the method and process for MWM (n=12 for each WT group and n=10 for each KO group per test period).
At the beginning of each test period, a 2-day visible cue task (days −2 and −1) was used to pretrain mice to escape from the water by locating the ‘visible' platform.12 The platform was made ‘visible' by a local cue place directly above the escape platform and was varied between the 2 days. This served to familiarize the mice with the apparatus and procedure, to allow them to acquire the necessary escape response, and to screen out those mice that had trouble in swimming or escape behavior.
The spatial reference memory tests consisted of an acquisition phase (days 1–4) and a probe/retention phase (day 5) as previously described,11, 13 with minor modifications. (A) During the acquisition phase, the platform was placed in a constant position (in the center of one of the four quadrants). Mice were tested four times per day for four consecutive days. Time spent in finding the hidden platform was recorded as escape latency. Mice failing to find the platform within 60 seconds were guided by the experimenter to the platform, on which it remained for 20 seconds, and was recorded as a latency of 60 seconds. In each trial, each mouse was started at a randomly selected starting point. The intertrial interval was ∼60 minutes. (B) During the probe phase, the platform was removed. Mice were allowed to swim for 60 seconds. The time the mice spent in searching for the former platform quadrant was recorded to assess retention and retrieval of spatial reference memory.
From days 6 to 8, an adaptation of a four-trial ‘repeated acquisition protocol'11 was used to test working memory. The platform was moved to a randomly chosen new position every day, but was kept in the same position for all trials on the same day. The mice were placed at the same starting point in all the four consecutive trials of 1 day and were permitted to swim for 60 seconds or until they located the platform. Working memory was measured by escape latency index ((trial 1−trial 4)/trial 1), i.e., the reduction of escape latency from trial 1 (when the platform was unknown) to trial 4. The interval between trials was 30 seconds.
Neurologic Deficit Scoring
At preblast injury and 1, 3, and 7, days after blast injury, mice were scored for neurologic deficits as described previously:5, 7 (0) no observable neurologic deficits (normal); (1) failure to extend one of the forepaws (mild); (2) circling to the right or left side of the body (moderate); and (3) loss of walking or righting reflex (severe).
Assay of Glutamate Levels in the Cerebrospinal Fluid
At 24 hours, and 1, 4, and 8 weeks after blast injury, cerebrospinal fluid (CSF) of mice (n=4–6 per group) was collected for determination of glutamate levels by high-performance liquid chromatography using ortho-phthaldialdehyde precolumn derivatization and fluorescence detection as previously reported.14, 15
Determination of Inflammatory Cytokine mRNA Levels
Quantitative PCR was used to analyze the mRNA expression of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in temporal cortex and hippocampus of both sides of the brain at 24 hours and 1, 4, and 8 weeks after blast injury (n=6–8 per group).7, 15 The relative abundance of the target gene was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the data were expressed as ratios relative to the control. The primers were as follows: TNF-α, forward 5′-AATGGCCTCCCTCTCATCAG-3′ and reverse 5′-CCACTTGGTGGTTTG CTACG-3′ IL-1β, forward 5′-GTGTGACGTTCCCATTAGAC-3′ and reverse 5′-CATTGAGGTGGAGAGCTTTC-3′ GAPDH, forward 5′-AGGTTGTCTCCTGCGACTTCA-3′ and reverse 5′-TGGTCCAGGGTTTCTTACTCC-3′.
Histopathological Evaluation
At 24 hours and 1, 4, and 8 weeks after blast injury, anesthetized mice (n=3–5 per group) were killed by transcardial injection with saline followed by 4% paraformaldehyde. For immunohistochemistry, coronal paraffin brain sections of 4 μmol/L thickness were incubated with antiglial fibrillary acidic protein (GFAP, 1:1500), anti-aquaporin-4 (AQP4) (1:750), and anti-neuronal nuclei (NeuN, 1:600) diluted in phosphate-buffered saline for detection of GFAP, AQP4, and NeuN, respectively. Sections were then visualized using 3,3′-diaminobenzidine. Image Pro Plus 4.5 (Media Cybernetics, Rockville, MD, USA) was used to analyze the results as described previously.15, 16 The average value was calculated from three to five different mice per group. For hippocampal analysis, three slices per mouse with one field per slice were measured; for cortex analysis, two slices per mouse with four fields per slice were measured. All primary antibodies were purchased from Abcam, Cambridge, MA, USA. All histologic assessments were performed by an observer masked to the grouping.
Western Blot Assays
To evaluate the protein levels of TNF-α, IL-1β, and AQP4 and to investigate the activation of A2AR, western blot analysis was performed (n=3 to 4) using antibodies against TNF-α, IL-1β, AQP4 (Abcam), adenyl cyclase (AC), and phospholipase C (PLC) (Santa Cruz Biotechnology, Dallas, TX, USA), respectively. GAPDH (Santa Cruz Biotechnology) served as the endogenous control. Image Pro Plus 4.5 was used to analyze the results. The relative quantity of the target protein was normalized to GAPDH.
Statistical Analysis
All data are expressed as mean±s.e.m. The effects of genotype, day, and genotype × day interaction in the MWM were determined by two-way analysis of variance (ANOVA). The comparisons of neurologic deficit scores among groups were analyzed by Mann–Whitney U-test. Specific differences between the two genotypes were analyzed using Student's t-test, and differences between two sessions of the same test using paired t-test. All other statistical comparisons between groups were performed by factorial ANOVA followed by Bonferroni's post hoc test. A value of P<0.05 was considered statistically significant.
Results
The A2A Receptor Deficiency Attenuated Memory Impairment Induced by Blast Injury
All mice survived during the period of study. On an initial task of MWM for spatial reference memory, both WT-control and KO-control mice showed rapid decreases in swimming distance (Supplementary Figure 3A) and escape latency (Figure 1A) over the 4 days of training and nearly identical search time in the probe phase (Figure 1B), indicating that deficiency of A2AR had little effect on basal reference memory. At 1 week after bTBI, WT mice showed no decrease in escape latency during acquisition phase and search time in the target quadrant in the probe phase that was decreased by 50% compared with control mice, indicating severely compromised reference memory. In contrast, reference memory was relatively preserved in A2AR KO-bTBI mice: they showed a smaller, but still significant, decrease in escape latency compared with KO-control mice. In addition, search time in the target quadrant was significantly longer in KO-bTBI mice than for WT-bTBI mice (Figures 1A and 1B). By 4 weeks after bTBI, both parameters of reference memory had returned to normal in KO-bTBI mice, whereas full recovery was seen in WT-bTBI mice until 8 weeks after bTBI (Figures 1A and 1B).
In our spatial working memory paradigm, the escape latency index ((T1−T4)/T1) of the WT-bTBI and KO-bTBI groups was significantly lower than their control groups respectively, for at least 8 weeks, indicating a long-lasting effect of blast injury on working memory. Moreover, the KO mice exhibited significantly higher escape latency index at baseline and at all points after bTBI (Figure 1C) and a smaller decrease in escape latency index after bTBI (Figure 1D) compared with WT mice. Thus, inactivation of A2AR attenuated both extent and duration of bTBI-induced working memory impairment. Although there appeared to be a difference in escape latency index at baseline between WT-control and KO-control mice, two-way ANOVA analysis was used to exclude the effect of this difference on postinjury comparison. It confirmed the greater recovery in KO-bTBI mice than WT-bTBI mice for working memory deficiency. Moreover, indistinguishable swimming speed over the different days in the MWM test (Supplementary Figure 3C) indicated no difference in spontaneous activity between groups and excluded the effect of motor activity variance on the results of memory test.
Genetic Inactivation of A2A Receptor Attenuated Blast-Induced Cerebral Cortical and Hippocampal Lesions
Mild-to-moderate neurologic deficit was observed in some of the injured mice in the first 3 days after bTBI, but disappeared by 1 week after blast injury (data not shown). At 24 hours after blast injury, KO-bTBI mice exhibited significantly lower neurologic deficit scores than WT-bTBI mice (Figure 2A). Glutamate levels in CSF were elevated in both WT and A2AR KO mice at 24 hours after bTBI, declining to baseline by 1 week after blast injury; however, the amplitude of the increase was significantly lower in KO-bTBI mice than WT-bTBI mice (Figure 2B).
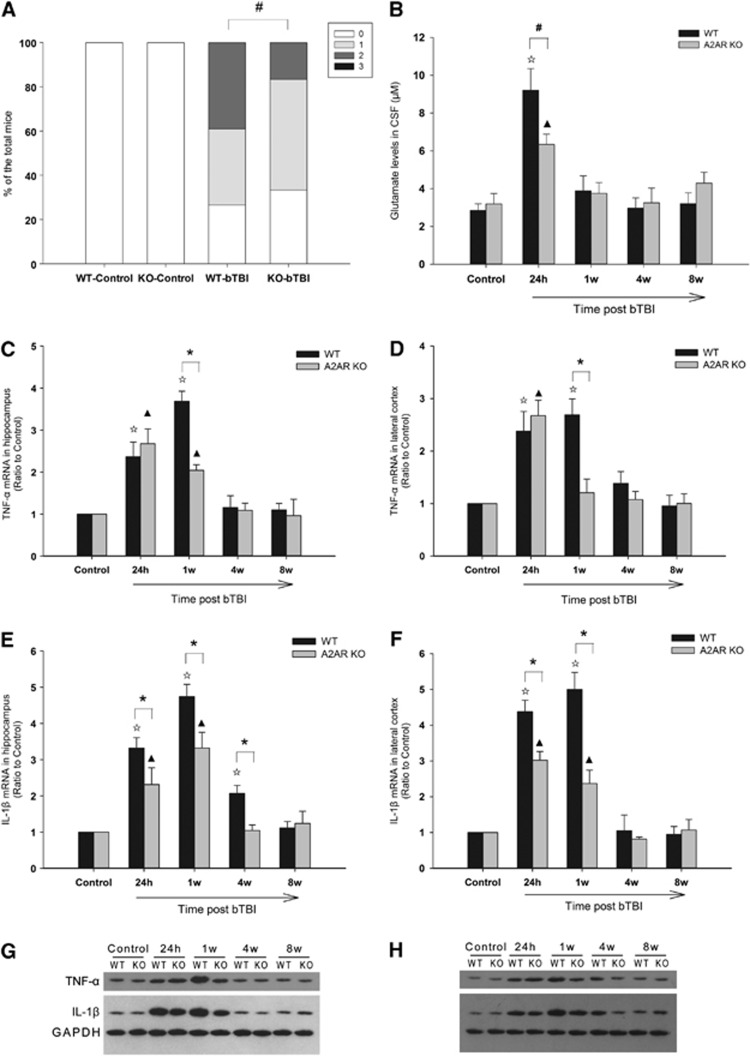
Figure 2.
Genetic inactivation of adenosine A2A receptor (A2AR) attenuated neurologic deficit, glutamate level in cerebrospinal fluid (CSF), and proinflammatory cytokine expression in brain tissue after blast-induced traumatic brain injury (bTBI). (A) The neurologic deficit of the knockout (KO)-bTBI group was significantly alleviated compared with the wild-type (WT)-bTBI group at 24 hours after blast exposure (Mann–Whitney U-test; n=64 mice in WT group and n=60 mice in KO group.) (B) Glutamate levels in KO-bTBI mice were significantly reduced when compared with WT-bTBI mice at 24 hours after blast exposure (n=6 mice in WT group and n=5 mice in KO group per time point). (C–H) The A2AR deficiency attenuated and shortened the inflammatory cytokine tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) expression at both mRNA and protein levels. Compared with WT-bTBI mice, increasing of TNF-α at 1 week after bTBI (C, D, G, H) and IL-1β at 24 hours and 1 week after bTBI (E–H) in both hippocampus and cortex were attenuated in KO-bTBI mice (n=6–8 per group). *P<0.01 between the two groups; ΔP<0.01 compared with WT control; ▴P<0.01 compared with KO control; #P<0.05 between the two groups.
Levels of proinflammatory cytokines were measured as an indicator of neuroinflammation. In WT-bTBI mice, the TNF-α and IL-1β mRNA production were elevated in hippocampus and lateral cortex by 24 hours after bTBI, remained at equal or higher levels at 1 week after injury, and returned to baseline by 4 weeks (except for IL-1β mRNA in the hippocampus, which remained elevated at 4 weeks, returning to baseline by 8 weeks after bTBI). In KO-bTBI mice at 24 hours after bTBI, TNF-α and IL-1β mRNA levels in the hippocampus (Figures 2C and 2E) and lateral cortex (Figures 2D and 2F) were elevated to levels similar to those in WT mice. However, levels of both cytokine mRNAs returned to baseline significantly more rapidly in KO-bTBI mice than in WT-bTBI mice (Figures 2C–2F). These results indicate that genetic inactivation of A2AR attenuated neurologic deficit and excitotoxicity during the early phase of bTBI and decreased the level and the duration of neuroinflammation. Protein levels of these two inflammatory cytokines assessed by western blot further confirmed the results from mRNA assay.
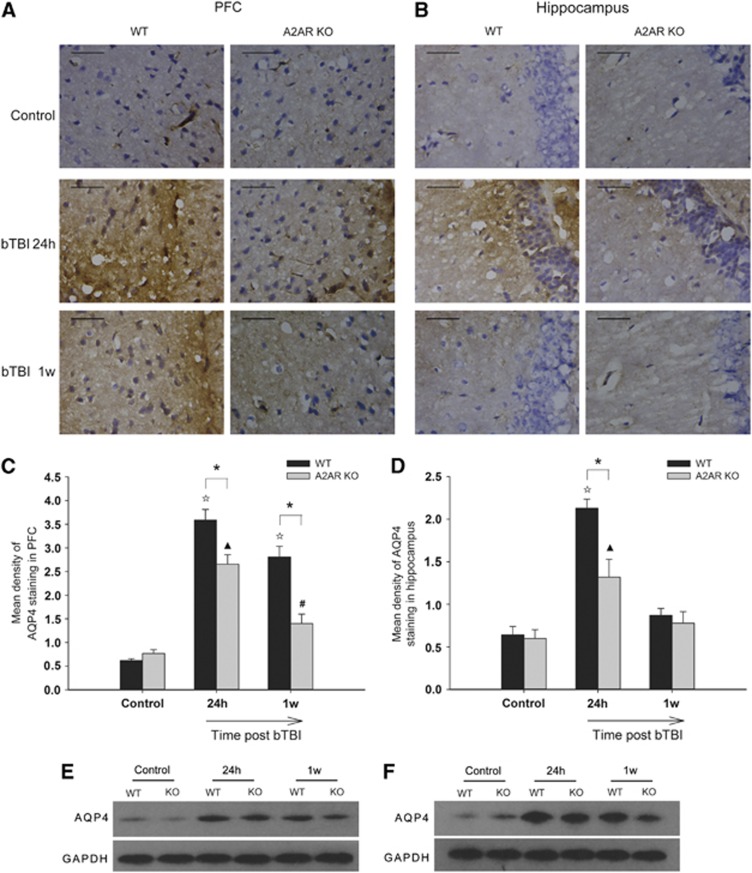
Brain water content was measured before and after blast injury by the wet–dry method as we reported previously,14 but no significant differences within groups were observed. We then examined AQP4 protein expression by immunohistochemical and western blot analysis. The results showed that AQP4 expressions in the prefrontal cortex (PFC) and the hippocampal dentate gyrus sector were significantly reduced in KO-bTBI mice compared with WT-bTBI mice at both 24 hours and 1 week after blast injury (Figure 3), suggesting that A2AR deficiency reduced cerebral edema at both early and late stages.
Figure 3.
Genetic inactivation of adenosine A2A receptor (A2AR) reduced the aquaporin-4 (AQP4) expression after blast injury. Representative photomicrographs of AQP4 immunostaining in (A) prefrontal cortex (PFC) and (B) hippocampal dentate gyrus of wild-type (WT) and A2AR knockout (KO) mice at 24 hours and 1 week after blast-induced traumatic brain injury (bTBI). After bTBI, AQP4 immunoreactivity increased in both regions in WT and A2AR KO mice, with a smaller increase in the A2AR KO. We quantified this result using mean density on express levels of AQP4 immunostaining in PFC (C) and hippocampus (D). (E) and (F) represent AQP4 protein levels by western blot. N=6 per group. Scale bars represent 50 μm. *P<0.01 between the two groups; ΔP<0.01 compared with WT control; ▴P<0.01 compared with KO control; #P<0.05 compared with KO control.
To explore the effect of A2AR deletion on blast-induced neuropathology, we examined two well-established neuropathological markers at various time points after blast injury.
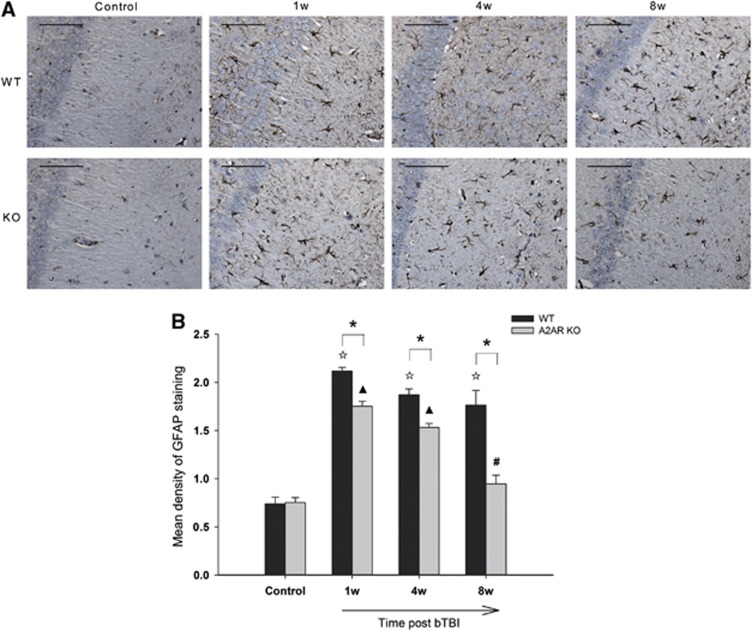
We used GFAP staining as an indicator of reactive astrocytosis after blast injury. Blast injury induced a strong increase in density of GFAP-immunostained astrocytes and also induced profuse and hypertrophic cytoplasmic extensions in the hippocampal CA1 regions of both WT and A2AR KO mice (Figure 4A). However, there was significantly less GFAP staining in KO-bTBI mice than in WT-bTBI mice at both 1 and 4 weeks after bTBI, and GFAP staining returned to baseline in A2AR KO mice, although still present in WT mice at 8 weeks after blast injury (Figure 4B). These results indicate that A2AR deficiency reduced the intensity and duration of gliosis after blast injury.
Figure 4.
Genetic inactivation of adenosine A2A receptor (A2AR) reduced astrocytosis after blast injury. Glial fibrillary acidic protein (GFAP) immunostaining (A) followed by semiquantitative analysis (B) revealed that the densities of astrocytes in the hippocampal CA1 regions of both the A2AR knockout (KO) and wild-type (WT) groups increased markedly after blast injury compared with the control groups. However, compared with the WT mice, fewer activated astrocytes were observed in A2AR KO mice, indicating that A2AR deficiency reduced chronic astrocytosis caused by blast injury (n=4 to 6 per group). Scale bars represent 50 μm. *P<0.01 between the two groups; ΔP<0.01 compared with WT control; ▴P<0.01 compared with KO control; #P<0.05 compared with KO control.
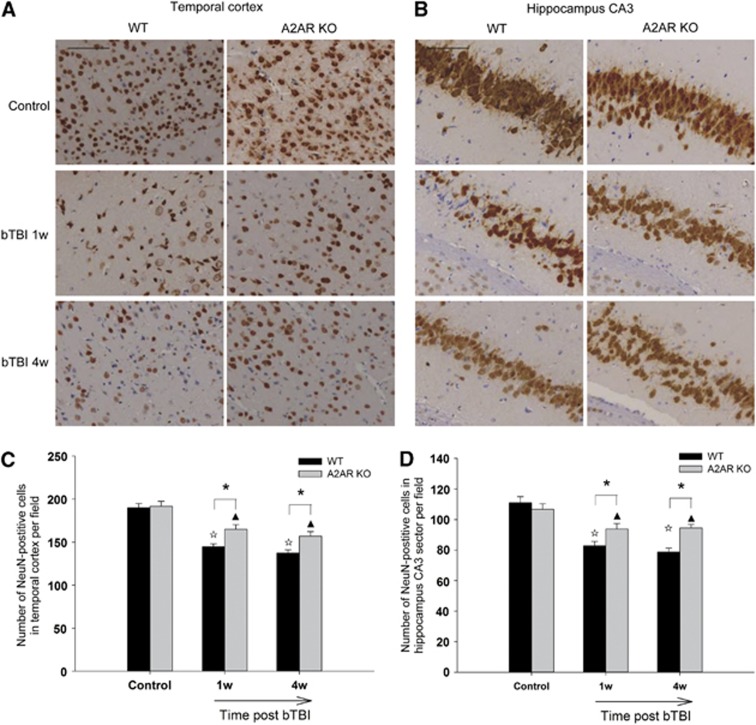
To examine neuronal damage and loss, we looked at NeuN staining in several brain regions. The results showed that the number of NeuN-positive neurons in the temporal cortex and hippocampus CA3 sector reduced significantly in WT-bTBI mice than in KO-bTBI mice at 1 and 4 weeks after blast injury (Figure 5). Blast injury also resulted in distortion of apical dendrites and shrinkage of neurons in especially the WT-bTBI mice. Moreover, NeuN staining decrease was also observed in hippocampal CA1 region, white matter, and PFC in some of the mice, whereas there were no significant differences between WT and KO mice in these regions. In addition, NeuN staining did not change significantly in thalamus and striatum when comparing bTBI mice with control mice.
Figure 5.
Genetic inactivation of adenosine A2A receptor (A2AR) attenuated neuronal loss and degeneration after blast injury. The number of neuronal nuclei (NeuN)-positive neurons in temporal cortex (A, C) and CA3 sector of the hippocampus (B, D) was reduced more significantly in wild-type (WT)-blast-induced traumatic brain injury (bTBI) mice when compared with that in knockout (KO)-bTBI mice at 1 week and 4 weeks after blast exposure. Pyramidal neurons in control groups showing apical dendrites are in parallel arrays. The WT-bTBI mice showing distorted projecting dendrites and condensed and shrunken neurons that were more serious than that in KO-bTBI mice (n=4–6 per group). Scale bars represent 50 μm. *P<0.05 between the two groups; ΔP<0.01 compared with WT control; ▴P<0.01 compared with KO control.
Distinct Signaling Pathways Associated with A2A Receptor Were Activated at Different Time Points at Different Brain Regions after Blast-Induced Traumatic Brain Injury
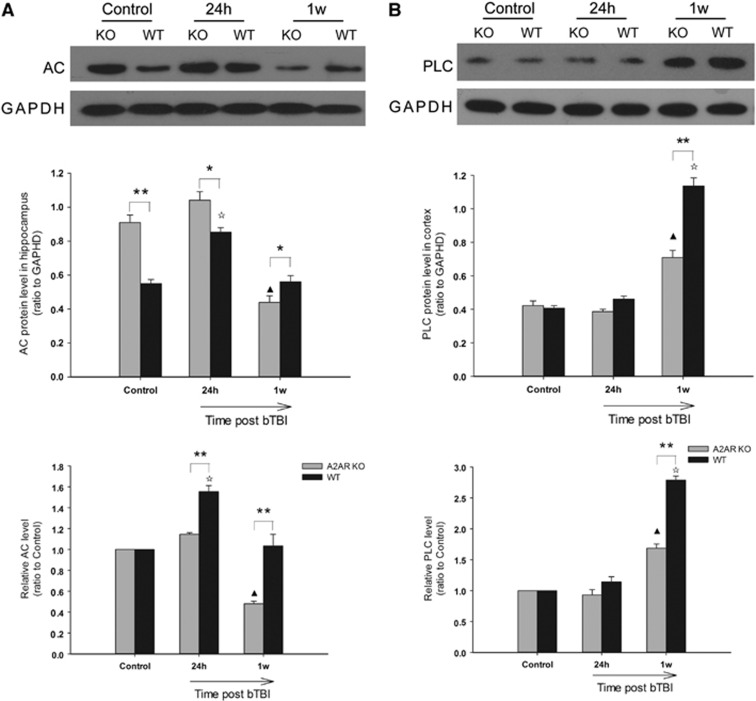
To determine whether A2AR was actually activated in the blast TBI model, we investigated two predominant signaling molecules downstream of A2AR, namely AC and PLC. Western blots analysis showed that AC protein production in the hippocampus at 24 hours after bTBI (Figure 6A) and PLC production in the cortex at 1 week after bTBI in WT-bTBI mice increased more significantly than in KO-bTBI mice, indicating that A2AR was indeed activated at these time points and that different signaling pathways were involved in the effect of A2AR in different brain regions at different time points of bTBI.
Figure 6.
Signaling of adenosine A2A receptor (A2AR) activation after blast-induced traumatic brain injury (bTBI). Western blot after semiquantitative analysis showed that adenyl cyclase (AC) level in hippocampus increased more significantly at 24 hours after bTBI (A) and that phospholipase C (PLC) level in cortex increased more significantly at 1 week after bTBI (B) in wild-type (WT) mice than that in knockout (KO) mice, respectively. It revealed that A2AR was stimulated to trigger different signaling pathways at different time points after bTBI in different brain regions (n=3 to 4 per group). *P<0.05 between the two groups; **P<0.01 between the two groups; ΔP<0.01 compared with WT control; ▴P<0.01 compared with KO control.
Discussion
Mild-to-Moderate Blast Injury Leads to Impairment of Learning and Memory Functions and Neuropathological Changes
Various animal and human studies have suggested that even mild blast exposure may cause not only structural damage but also functional changes in brains.3, 17 Here we show that mild-to-moderate whole-body blast injury that does not cause serious immediate visible neurologic deficits leads to long-term cognitive deficits, including impairment of reference and working memory. Notably, impairment of working memory persisted for at least 8 weeks. The long-lasting cognitive consequence of blast injury assessed in this study was longer than that in previous works.3
Moreover, we found evidence of neuroinflammation, brain edema, increased CSF glutamate, neuronal degeneration and loss, and astrogliosis in the cortex and hippocampus in mice exposed to blast, consistent with the results of previous studies showing that blast injury-induced neurotrauma mimics many clinically relevant features of TBI.18 Our results support the hypothesis suggested by Cernak et al.19, 20 that behavioral and psychological changes after cortical injury are the result of afferent hyperexcitability and increased release of neurotransmitters. However, distinct from previous studies using controlled cortical impact injury where injury was localized near the impact site,21 these results show that blast-induced brain injuries are diffuse, occurring in multiple regions such as temporal cortical, prefrontal cortical and subcortical regions, cerebella, and hippocampus. Both ipsilateral and contralateral sides of the brain are vulnerable. The wide distribution of tissue insult, especially the cortex and hippocampus, may be one reason that the external neurologic motor deficits were relatively minor and transient, whereas the internal neuropsychiatric effects were persistent. Thus, this study offers further support for a correlation between memory dysfunction after bTBI and the appearance of molecular and cellular abnormalities throughout the brain.
Inactivation of A2A Receptor Preserves Cognitive Function and Reduces Secondary Brain Injury in Both Early and Late Stages of Blast-Induced Traumatic Brain Injury
In this study, both the memory impairment and the neuropathogenesis seen in WT mice at short and longer time points after blast exposure were alleviated in A2AR KO mice, suggesting that A2AR deficiency plays a neuroprotective role in both early and prolonged stages of bTBI, in accordance with the results of our previous studies in a TBI model,7, 22 and other studies in models of acute brain insult and neurodegenerative diseases.23, 24
Both glutamate excitotoxicity and neuroinflammation have been proposed as possible mechanisms underlying the secondary injury that occurs in TBI. They can interact to trigger a cascade reaction that results in neuronal injury and amplification of brain damage. In the central nervous system, glutamate can be released from presynaptic glutamatergic terminals. The A2AR located at these terminals can directly regulate the amount and frequency of glutamate release and outflow.25 Our results suggest that the suppression of glutamate level may be responsible for the neuroprotective effect provided by A2AR deletion against bTBI in the acute stage, as has been described in other models of brain injury.26
Failure to clear the synaptic cleft of glutamate can overstimulate postsynaptic glutamate receptors, promoting neuronal death.27 In this study, NeuN immunostaining showed that neuronal loss and shrinkage in KO-bTBI mice were significantly reduced at 1 week after blast exposure. This may result from suppression of glutamate release and other toxic reaction and could explain the relative protection of cognitive function in the KO animals.
Neuroinflammation contributes directly to neuronal injury and is targeted as a causative mediator of cognitive dysfunction in acute brain insult and neurodegenerative disorders. Interleukin-1β in particular has been associated with reduced hippocampal plasticity and long-term contextual and spatial memory impairment.28 Our previous study showed that lack of A2AR significantly suppressed inflammation and neuropathological changes in the acute phase in a mouse TBI model.7 We also determined that a high local glutamate level switched the effect of A2AR from anti-inflammatory to proinflammatory and aggravated brain injury at 24 hours after TBI in mice.14 In this study, A2AR deficiency decreased both the amplitude and duration of the increase in IL-1β and TNF-α expression after blast injury. The reduced neuroinflammation may explain why A2AR deficiency not only exerts neuroprotective effects in the early phase after bTBI, but also reduces the progression of neurodegeneration in the late phase, as revealed by NeuN and GFAP staining in cortex and hippocampus.
Sustained neuroinflammation is closely correlated with cognitive dysfunction and neurodegeneration. Reactive astrogliosis is considered a hallmark of neuroinflammation29 and of many neurodegenerative conditions.30 During the late phase of bTBI, we detected significantly elevated expression of GFAP in astrocytes, combined with characteristic profuse and hypertrophic cytoplasmic extensions on astrocytes in the hippocampal CA1 region. These characteristics of astrocytosis were reduced significantly in KO-bTBI mice, consistent with previous findings showing that A2AR deficiency reduces astrocytosis in primary astrocytes31 in vitro and in a sciatic nerve injury model.29, 32
Normal astrocytes provide essential services for brain homeostasis and neuronal function, including metabolic support for neurons, glutamate uptake and conversion, and regulating water balance by altering the level of AQP4 expression. Aquaporin-4 is the most abundant and the main water channel mediating water movement in the brain. Abnormal astrocytes contribute to the formation of edema and make neurons vulnerable to neurotoxins including proinflammatory cytokines, resulting in further injury to neurons, thus accelerating neurodegeneration and cognitive dysfunction. We found in this study that AQP4 expression in the PFC was significantly attenuated in KO-bTBI mice than in WT-bTBI mice, indicating that disturbed water balance and edema is regulated by A2AR. We also found that pyramidal neurons in the hippocampal CA3 sector were noticeably shrunken and condensed, and there was a significant decrease in number of cortical neurons, suggesting compromised neuronal function. This apparent neuronal loss and damage may translate into memory deficit. Interestingly, this neuronal damage was significantly decreased by inactivation of A2AR. We speculate that both the inhibition of the secondary injury in the early phase and astroglial response in prolong stage of bTBI may lead to the improved behavioral outcomes including retention of learning and memory functions in A2AR KO mice.
In addition, cerebrovascular insults are considered an important contributor to blast-induced brain injuries. Also, adenosine A2AR plays significant roles in regulating cerebral blood flow (CBF) by producing vasodilatation and increasing CBF.33, 34 Given that bTBI can induce hypotension and ischemia, activation of A2AR may exert protective effect by increasing CBF. On the contrary, because increased CBP may promote intracranial hypertension, inactivation of A2AR may also exert protective effect by inhibiting CBP. This is particularly too compounded to explain in this study but is needed to be elucidated in our future work.
Moreover, although only mild-to-moderate brain injury was observed in this model, peripheral organ (such as lung) injury was evident. Because blast-induced neurotrauma may be affected by local and cerebral as well as systemic responses including peripheral organ damage and blood surge,35, 36 systemic responses could not be excluded when discussing the impact factors of bTBI. The effects and the underlying mechanisms also need further investigations.
Inactivation of A2A Receptor Alleviated Blast-Induced Traumatic Brain Injury-Induced Impairment of Spatial Reference and Working Memory
Our results showed that bTBI significantly impaired both reference and working memory; however, the effect on working memory was much more persistent. Performance on the reference memory task returned to normal in WT mice by 4 weeks, whereas working memory was still impaired at 8 weeks after bTBI, the longest time point we examined. The A2AR deficiency was protective against bTBI-induced effects on both types of memory, decreasing both the degree and duration of memory impairment. Our results are consistent with previous pharmacological and genetic studies suggesting that A2AR activity modifies the spatial memory process in models of injury and disease in rodents.37, 38
Deficits in memory functions are at the core of pathophysiology for many neuropsychiatric disorders.39 The development and consolidation of long-term reference memory is mainly dependent on the hippocampus, whereas the failure of working memory performance is a major factor in cognitive malfunction related to disturbed network activity in the PFC and integrity of other regions such as the temporal cortex, hippocampus, basal ganglia, and thalamus. In this study, blast exposure resulted in diffuse multiregion damages to brain tissue. This may explain the long-lasting deficit in working memory and the long-term effect of A2AR deficiency on working memory rather than reference memory.
Interestingly, we found that A2AR inactivation still exerted a beneficial effect on working memory at 8 weeks after bTBI, even though there were no obvious neuroinflammatory or neuropathological changes at this time point. This suggests that there may be some unknown mechanisms involved in the protective effect of A2AR inactivation during late stages of bTBI. In a previous study using a TBI model, we found that inactivation of A2AR signaling is compensated by upregulation of A1R expression that may result in endogenous adenosine acting predominately at A1R to result in neuroprotection.7 In blast model in the present study, we investigated the possible signaling pathways (AC and PLC) associated with A2AR in vivo. The results suggested that different signaling pathways were involved in the effect of A2AR in different brain regions at different time points of bTBI. Additional studies are needed to elucidate the underlying signaling transduction mechanism involved in the bTBI model.
Furthermore, caffeine, the most widely consumed stimulant all over the world, is a nonselective adenosine receptor antagonist. Previous experimental and clinical studies have suggested that chronic caffeine treatment exerts neuroprotective effects in TBI by antagonizing A2AR15 and that higher caffeine levels in CSF predicting a better clinical outcome in TBI patients.40 In summary, these data suggest that treatment with an A2AR antagonist (such as caffeine) is a promising therapeutic and prophylactic strategy for mild-to-moderate bTBI and consequent cognitive impairment. Our results also suggest that inhibiting or reversing brain insult as early as possible may be an effective way to improve the long-term neuropathological and behavioral outcomes of bTBI.
Acknowledgments
The authors thank Professor Zhi-Yong Yin and senior engineers Xiao-Yan Li and Xin Ning for their technical support on the BST-I bio-shock tube. They also thank Dr Susan E Lewis and Dr Hai-Ying Shen for critically reading and editing the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by grants from the National Natural Science Foundation of China (No. 31171022 and No. 81201461) and Key Project of Medicine and Health of PLA (No. 08G098).
Supplementary Material
References
- Sayer NA. Traumatic brain injury and its neuropsychiatric sequelae in war veterans. Annu Rev Med. 2012;63:405–419. doi: 10.1146/annurev-med-061610-154046. [DOI] [PubMed] [Google Scholar]
- Sayer NA, Nelson D, Nugent S. Evaluation of the Veterans Health Administration traumatic brain injury screening program in the upper Midwest. J Head Trauma Rehabil. 2011;26:454–467. doi: 10.1097/HTR.0b013e3181ff393c. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra160. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Ferre S, Vaugeois JM, Chen JF. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des. 2008;14:1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Zhao Y, Chen XY, Xiong RP, Lu JL, Chen JF, et al. Differential alteration of heat shock protein 90 in mice modifies glucocorticoid receptor function and susceptibility to trauma. J Neurotrauma. 2010;27:373–381. doi: 10.1089/neu.2009.0926. [DOI] [PubMed] [Google Scholar]
- Li W, Dai S, An J, Xiong R, Li P, Chen X, et al. Genetic inactivation of adenosine A2A receptors attenuates acute traumatic brain injury in the mouse cortical impact model. Exp Neurol. 2009;215:69–76. doi: 10.1016/j.expneurol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Yu L, Huang Z, Mariani J, Wang Y, Moskowitz M, Chen JF. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10:1081–1087. doi: 10.1038/nm1103. [DOI] [PubMed] [Google Scholar]
- Wang ZG. [An experimental study of blast injury] Zhonghua Yi Xue Za Zhi. 1989;69:7–12. [PubMed] [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X, Savic J. Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain Inj. 2001;15:593–612. doi: 10.1080/02699050010009559. [DOI] [PubMed] [Google Scholar]
- Zhou SJ, Zhu ME, Shu D, Du XP, Song XH, Wang XT, et al. Preferential enhancement of working memory in mice lacking adenosine A(2A) receptors. Brain Res. 2009;1303:74–83. doi: 10.1016/j.brainres.2009.09.082. [DOI] [PubMed] [Google Scholar]
- Wei CJ, Singer P, Coelho J, Boison D, Feldon J, Yee BK, et al. Selective inactivation of adenosine A(2A) receptors in striatal neurons enhances working memory and reversal learning. Learn Mem. 2011;18:459–474. doi: 10.1101/lm.2136011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Dai SS, Zhou YG, Li W, An JH, Li P, Yang N, et al. Local glutamate level dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury. J Neurosci. 2010;30:5802–5810. doi: 10.1523/JNEUROSCI.0268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Dai S, An J, Li P, Chen X, Xiong R, et al. Chronic but not acute treatment with caffeine attenuates traumatic brain injury in the mouse cortical impact model. Neuroscience. 2008;151:1198–1207. doi: 10.1016/j.neuroscience.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Dai SS, Wang H, Yang N, An JH, Li W, Ning YL, et al. Plasma glutamate-modulated interaction of A2AR and mGluR5 on BMDCs aggravates traumatic brain injury-induced acute lung injury. J Exp Med. 2013;210:839–851. doi: 10.1084/jem.20122196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Merkle AC, Koliatsos VE, Bilik JM, Luong QT, Mahota TM, et al. The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol Dis. 2011;41:538–551. doi: 10.1016/j.nbd.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab. 2010;30:255–266. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Savic J, Zunic G, Pejnovic N, Jovanikic O, Stepic V. Recognizing, scoring, and predicting blast injuries. World J Surg. 1999;23:44–53. doi: 10.1007/s002689900563. [DOI] [PubMed] [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X, Savic J. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J Trauma. 2001;50:695–706. doi: 10.1097/00005373-200104000-00017. [DOI] [PubMed] [Google Scholar]
- Harting MT, Jimenez F, Adams SD, Mercer DW, Cox CS., Jr. Acute, regional inflammatory response after traumatic brain injury: implications for cellular therapy. Surgery. 2008;144:803–813. doi: 10.1016/j.surg.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SS, Li W, An JH, Wang H, Yang N, Chen XY, et al. Adenosine A2A receptors in both bone marrow cells and non-bone marrow cells contribute to traumatic brain injury. J Neurochem. 2010;113:1536–1544. doi: 10.1111/j.1471-4159.2010.06716.x. [DOI] [PubMed] [Google Scholar]
- Popoli P, Pintor A, Domenici MR, Frank C, Tebano MT, Pezzola A, et al. Blockade of striatal adenosine A2A receptor reduces, through a presynaptic mechanism, quinolinic acid-induced excitotoxicity: possible relevance to neuroprotective interventions in neurodegenerative diseases of the striatum. J Neurosci. 2002;22:1967–1975. doi: 10.1523/JNEUROSCI.22-05-01967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui L, Duan W, Tian H, Li C, Zhu J, Chen JF, et al. Adenosine A 2A receptor deficiency reduces striatal glutamate outflow and attenuates brain injury induced by transient focal cerebral ischemia in mice. Brain Res. 2009;1297:185–193. doi: 10.1016/j.brainres.2009.08.050. [DOI] [PubMed] [Google Scholar]
- Popoli P, Frank C, Tebano MT, Potenza RL, Pintor A, Domenici MR, et al. Modulation of glutamate release and excitotoxicity by adenosine A2A receptors. Neurology. 2003;61:S69–S71. doi: 10.1212/01.wnl.0000095216.89483.a2. [DOI] [PubMed] [Google Scholar]
- Chen JF, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, et al. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol. 2007;83:310–331. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Scott HA, Gebhardt FM, Mitrovic AD, Vandenberg RJ, Dodd PR. Glutamate transporter variants reduce glutamate uptake in Alzheimer's disease. Neurobiol Aging. 2011;32:e551–511. doi: 10.1016/j.neurobiolaging.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, et al. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bura SA, Nadal X, Ledent C, Maldonado R, Valverde O. A 2A adenosine receptor regulates glia proliferation and pain after peripheral nerve injury. Pain. 2008;140:95–103. doi: 10.1016/j.pain.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Liem RK, Messing A. Dysfunctions of neuronal and glial intermediate filaments in disease. J Clin Invest. 2009;119:1814–1824. doi: 10.1172/JCI38003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Cottini L, Fumagalli M, Ceruti S, Abbracchio MP. Blockade of A2A adenosine receptors prevents basic fibroblast growth factor-induced reactive astrogliosis in rat striatal primary astrocytes. Glia. 2003;43:190–194. doi: 10.1002/glia.10243. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Greco A, Potenza RL, Pezzola A, Blum D, Bantubungi K, et al. Effects of the adenosine A2A receptor antagonist SCH 58621 on cyclooxygenase-2 expression, glial activation, and brain-derived neurotrophic factor availability in a rat model of striatal neurodegeneration. J Neuropathol Exp Neurol. 2007;66:363–371. doi: 10.1097/nen.0b013e3180517477. [DOI] [PubMed] [Google Scholar]
- Kusano Y, Echeverry G, Miekisiak G, Kulik TB, Aronhime SN, Chen JF, et al. Role of adenosine A2 receptors in regulation of cerebral blood flow during induced hypotension. J Cereb Blood Flow Metab. 2010;30:808–815. doi: 10.1038/jcbfm.2009.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek PM, Hendrich KS, Jackson EK, Wisniewski SR, Melick JA, Shore PM, et al. Characterization of the effects of adenosine receptor agonists on cerebral blood flow in uninjured and traumatically injured rat brain using continuous arterial spin-labeled magnetic resonance imaging. J Cereb Blood Flow Metab. 2005;25:1596–1612. doi: 10.1038/sj.jcbfm.9600154. [DOI] [PubMed] [Google Scholar]
- Cernak I. The importance of systemic response in the pathobiology of blast-induced neurotrauma. Front Neurol. 2010;1:151. doi: 10.3389/fneur.2010.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Savic J, Malicevic Z, Zunic G, Radosevic P, Ivanovic I, et al. Involvement of the central nervous system in the general response to pulmonary blast injury. J Trauma. 1996;40:S100–S104. doi: 10.1097/00005373-199603001-00023. [DOI] [PubMed] [Google Scholar]
- Cunha GM, Canas PM, Melo CS, Hockemeyer J, Muller CE, Oliveira CR, et al. Adenosine A2A receptor blockade prevents memory dysfunction caused by beta-amyloid peptides but not by scopolamine or MK-801. Exp Neurol. 2008;210:776–781. doi: 10.1016/j.expneurol.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Pamplona FA, Prediger RD. Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Front Biosci. 2008;13:2614–2632. doi: 10.2741/2870. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Shearer DK, Locascio JJ, Growdon JH, Corkin S. Working memory in mild Alzheimer's disease and early Parkinson's disease. Neuropsychology. 2003;17:230–239. doi: 10.1037/0894-4105.17.2.230. [DOI] [PubMed] [Google Scholar]
- Sachse KT, Jackson EK, Wisniewski SR, Gillespie DG, Puccio AM, Clark RS, et al. Increases in cerebrospinal fluid caffeine concentration are associated with favorable outcome after severe traumatic brain injury in humans. J Cereb Blood Flow Metab. 2008;28:395–401. doi: 10.1038/sj.jcbfm.9600539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.