Abstract
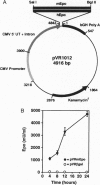
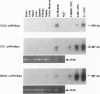
Erythropoietin (Epo)-responsive anemia is a common and debilitating complication of chronic renal failure and human immunodeficiency virus infection. Current therapy for this condition involves repeated intravenous or subcutaneous injections of recombinant Epo. In this report, we describe the development of a novel muscle-based gene transfer approach that produces long-term expression of physiologically significant levels of Epo in the systemic circulation of mice. We have constructed a plasmid expression vector, pVRmEpo, that contains the murine Epo cDNA under the transcriptional control of the cytomegalovirus immediate early (CMV-IE) promoter, the CMV-IE 5' untranslated region, and intron A. A single intramuscular (i.m.) injection of as little as 10 micrograms of this plasmid into immunocompetent adult mice produced physiologically significant elevations in serum Epo levels and increased hematocrits from preinjection levels of 48 +/- 0.4% to levels of 64 +/- 3.3% 45 days after injection. Hematocrits in these animals remained elevated at greater than 60% for at least 90 days after a single i.m. injection of 10 micrograms of pVRmEpo. We observed a dose-response relationship between the amount of plasmid DNA injected and subsequent elevations in hematocrits. Mice injected once with 300 micrograms of pVRmEpo displayed 5-fold increased serum Epo levels and elevated hematocrits of 79 +/- 3.3% at 45 days after injection. The i.m. injected plasmid DNA remained localized to the site of injection as assayed by the PCR. We conclude that i.m. injection of plasmid DNA represents a viable nonviral gene transfer method for the treatment of acquired and inherited serum protein deficiencies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr D., Tubb J., Ferguson D., Scaria A., Lieber A., Wilson C., Perkins J., Kay M. A. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995 Mar;2(2):151–155. [PubMed] [Google Scholar]
- Dai Y., Roman M., Naviaux R. K., Verma I. M. Gene therapy via primary myoblasts: long-term expression of factor IX protein following transplantation in vivo. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10892–10895. doi: 10.1073/pnas.89.22.10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Schwarz E. M., Gu D., Zhang W. W., Sarvetnick N., Verma I. M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan J., Pan L. C., Pavlath G. K., Travis M. A., Lanctot A. M., Blau H. M. Systemic delivery of human growth hormone by injection of genetically engineered myoblasts. Science. 1991 Dec 6;254(5037):1509–1512. doi: 10.1126/science.1962213. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Litzky L., Wilson J. M. Prolonged transgene expression in cotton rat lung with recombinant adenoviruses defective in E2a. Hum Gene Ther. 1994 Oct;5(10):1217–1229. doi: 10.1089/hum.1994.5.10-1217. [DOI] [PubMed] [Google Scholar]
- Goldwasser E., Sherwood J. B. Radioimmunoassay of erythropoietin. Br J Haematol. 1981 Jul;48(3):359–363. doi: 10.1111/j.1365-2141.1981.tb02726.x. [DOI] [PubMed] [Google Scholar]
- Hamamori Y., Samal B., Tian J., Kedes L. Myoblast transfer of human erythropoietin gene in a mouse model of renal failure. J Clin Invest. 1995 Apr;95(4):1808–1813. doi: 10.1172/JCI117859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamori Y., Samal B., Tian J., Kedes L. Persistent erythropoiesis by myoblast transfer of erythropoietin cDNA. Hum Gene Ther. 1994 Nov;5(11):1349–1356. doi: 10.1089/hum.1994.5.11-1349. [DOI] [PubMed] [Google Scholar]
- Hartikka J., Sawdey M., Cornefert-Jensen F., Margalith M., Barnhart K., Nolasco M., Vahlsing H. L., Meek J., Marquet M., Hobart P. An improved plasmid DNA expression vector for direct injection into skeletal muscle. Hum Gene Ther. 1996 Jun 20;7(10):1205–1217. doi: 10.1089/hum.1996.7.10-1205. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Landen C. N., Rothenberg S. R., Taylor L. A., Leland F., Wiehle S., Fang B., Bellinger D., Finegold M., Thompson A. R. In vivo hepatic gene therapy: complete albeit transient correction of factor IX deficiency in hemophilia B dogs. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2353–2357. doi: 10.1073/pnas.91.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarchand P., Jaffe H. A., Danel C., Cid M. C., Kleinman H. K., Stratford-Perricaudet L. D., Perricaudet M., Pavirani A., Lecocq J. P., Crystal R. G. Adenovirus-mediated transfer of a recombinant human alpha 1-antitrypsin cDNA to human endothelial cells. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6482–6486. doi: 10.1073/pnas.89.14.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M., Cornefert-Jensen F., Hartikka J., Felgner J., Rundell A., Margalith M., Dwarki V. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum Gene Ther. 1993 Aug;4(4):419–431. doi: 10.1089/hum.1993.4.4-419. [DOI] [PubMed] [Google Scholar]
- Mendell J. R., Kissel J. T., Amato A. A., King W., Signore L., Prior T. W., Sahenk Z., Benson S., McAndrew P. E., Rice R. Myoblast transfer in the treatment of Duchenne's muscular dystrophy. N Engl J Med. 1995 Sep 28;333(13):832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- Morgan J. E. Cell and gene therapy in Duchenne muscular dystrophy. Hum Gene Ther. 1994 Feb;5(2):165–173. doi: 10.1089/hum.1994.5.2-165. [DOI] [PubMed] [Google Scholar]
- Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989 Jan 12;337(6203):176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Siegfried W., Yoshimura K., Yoneyama K., Fukayama M., Stier L. E., Päkkö P. K., Gilardi P., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991 Apr 19;252(5004):431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Setoguchi Y., Danel C., Crystal R. G. Stimulation of erythropoiesis by in vivo gene therapy: physiologic consequences of transfer of the human erythropoietin gene to experimental animals using an adenovirus vector. Blood. 1994 Nov 1;84(9):2946–2953. [PubMed] [Google Scholar]
- Tripathy S. K., Black H. B., Goldwasser E., Leiden J. M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996 May;2(5):545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- Tripathy S. K., Goldwasser E., Lu M. M., Barr E., Leiden J. M. Stable delivery of physiologic levels of recombinant erythropoietin to the systemic circulation by intramuscular injection of replication-defective adenovirus. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11557–11561. doi: 10.1073/pnas.91.24.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent N., Ragot T., Gilgenkrantz H., Couton D., Chafey P., Grégoire A., Briand P., Kaplan J. C., Kahn A., Perricaudet M. Long-term correction of mouse dystrophic degeneration by adenovirus-mediated transfer of a minidystrophin gene. Nat Genet. 1993 Oct;5(2):130–134. doi: 10.1038/ng1093-130. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Ludtke J. J., Acsadi G., Williams P., Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992 Sep;1(6):363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Yang Y., Ertl H. C., Wilson J. M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994 Aug;1(5):433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Yang Y., Li Q., Ertl H. C., Wilson J. M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995 Apr;69(4):2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S. N., Kurachi K. Expression of human factor IX in mice after injection of genetically modified myoblasts. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3357–3361. doi: 10.1073/pnas.89.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]