Abstract
Study Objectives:
Gender differences in the prevalence of sleep apnea/hypopnea syndrome may be mediated via male sex hormones. Our objective was to determine the exact pathway for a testosterone-mediated increased propensity for central sleep apnea via blockade of the 5α-reductase pathway of testosterone conversion by finasteride.
Design:
Randomization to oral finasteride vs. sham, single-center study.
Setting:
Sleep research laboratory.
Participants:
Fourteen healthy young males without sleep apnea
Intervention:
Hypocapnia was induced via brief nasal noninvasive positive pressure ventilation during stable NREM sleep. Cessation of mechanical ventilation resulted in hypocapnic central apnea or hypopnea.
Measurements and Results:
The apnea threshold (AT) was defined as the end-tidal CO2 (PETCO2) that demarcated the central apnea closest to the eupneic PETCO2. The CO2 reserve was defined as the difference in PETCO2 between eupnea and AT. The apneic threshold and CO2 reserve were measured at baseline and repeated after at a minimum of 1 month. Administration of finasteride resulted in decreased serum dihydrotestosterone. In the finasteride group, the eupneic ventilatory parameters were unchanged; however, the AT was decreased (38.9 ± 0.6 mm Hg vs.37.7 ± 0.9 mm Hg, P = 0.02) and the CO2 reserve was increased (-2.5 ± 0.3 mm Hg vs. -3.8 ± 0.5 mm Hg, P = 0.003) at follow-up, with a significantly lower hypocapnic ventilatory response, thus indicating increased breathing stability during sleep. No significant changes were noted in the sham group on follow-up study.
Conclusions:
Inhibition of testosterone action via the 5α-reductase pathway may be effective in alleviating breathing instability during sleep, presenting an opportunity for novel therapy for central sleep apnea in selected populations.
Citation:
Chowdhuri S; Bascom A; Mohan D; Diamond MP; Badr MS. Testosterone conversion blockade increases breathing stability in healthy men during NREM sleep. SLEEP 2013;36(12):1793-1798.
Keywords: Apneic threshold, CO2 reserve, testosterone, finasteride, dihydrotestosterone, 5α-reductase blockade, central sleep apnea, chemoresponsiveness
INTRODUCTION
Gender differences in ventilatory control may contribute to gender differences in the prevalence of sleep apnea/hypopnea syndrome.1,2 Epidemiologic studies demonstrate a paucity of central sleep apnea in premenopausal women.3 Previous work from our laboratory demonstrated that the hypocapnic apneic threshold is lower in women than men.4 Interestingly, the difference in propensity to develop hypocapnic central apnea during sleep may be due to the destabilizing effect of testosterone rather than the stabilizing effect of progesterone. Administration of testosterone to women for 12 days results in diminished CO2 reserve, defined as the degree of hypocapnia required to induce central apnea during sleep.5 Similarly, androgen blockade in men is associated with a decrease in the hypocapnic apneic threshold.6 Thus, male sex hormones appear to play a destabilizing role in ventilatory control during sleep.
The underlying mechanisms of testosterone action on the hypocapnic apneic threshold remain uncertain. Testosterone actions are mediated via one of two pathways.7 The first pathway is the conversion via aromatase to 17β-oestradiol (E2) activity, which exerts its effects by acting on estrogen receptors (ERs).12 There is indirect evidence suggesting that the respiratory effects of testosterone are mediated through the aromatase pathway conversion to 17β-oestradiol. For example, aging and gonadectomy in male rats prevented the development of respiratory long-term facilitation (LTF), an excitatory phenomenon evoked by repetitive hypoxia.8 Testosterone replacement restored LTF in gonadectomized male rats; however, this effect required the conversion of testosterone to estrogen by aromatase.9 Similarly, we have found that hormone replacement therapy with estrogen and progesterone decreases the apneic threshold during sleep in postmenopausal women.10
The second testosterone pathway is the conversion of testosterone by 5α-reductase to the biologically active 5α-dihydrotestosterone (DHT), exerting its effects by acting on androgen receptors, which are abundant in the CNS.7,11 While sexual dimorphisms have been attributed to this pathway, whether this pathway is implicated in the male predominance of sleep apnea is not known. Specific investigations into 5α-reductase inhibition on ventilatory control during sleep would allow us to clarify the mechanism of testosterone mediated destabilization of ventilation during sleep.13 Thus, the purpose of this study was to determine the role of the 5α-reductase pathway on the hypopcapnic apneic threshold during sleep. We hypothesized that blockade of the alpha reductase pathway with finasteride would result in increased CO2 reserve and decreased hypocapnic apneic threshold.
METHODS
Participants
The Human Investigation Committees of Wayne State University School of Medicine and Detroit Veterans Affairs Medical Center approved the experimental protocols. Informed written consent was obtained from 14 healthy participants free of daytime sleepiness and medical disorders. Screening polysomnography (PSG) confirmed the absence of sleep apnea (apnea-hypopnea index < 5/h). The subjects were randomized to receive finasteride (finasteride group) or undergo sham study (sham group).
Breathing Circuit
The following methodology has been previously described by our group.4,5,14 Each participant was connected to the breathing circuit via a nasal mask. An appropriate-sized, airtight silicone nasal mask (Respironics, Murrysville, PA) was glued to the face to prevent mask leaks. The mask was connected to a Plateau Exhalation Valve (Respironics, Inc, Pittsburgh, PA), via a heated pneumotachometer. The valve, which provides a continuous leak path in the breathing circuit and serves as an exhaust vent, was connected to the inspiratory line. Participants were restricted to nasal breathing by placing tape over the mouth. During the Mechanical Ventilation (MV) Protocol (see below), hyperventilation was achieved using a pressure support ventilator (Quantum PSV, Healthdyne Technologies, Marietta, GA) or a bilevel positive airway pressure (PAP) machine (Resmed Sullivan VPAP II ST-A) with a minimum achievable EPAP of 2-4 cm H2O.
Measurements
Electroencephalogram (EEG), electrooculogram (EOG), and chin electromyogram (EMG) were recorded using the International 10-20 system of electrode placement (EEG: C3-A2 and C4-A1; EOG, O-A2). Inspiratory airflow was measured by a heated pneumotachometer (Model 3700A, Hans Rudolph, Kansas City, MO) that was attached to a pressure transducer (Validyne, Northridge, CA). The tidal volume (VT) was obtained from the electronic integration of the flow signal (model FV156 Integrator, Validyne, Northridge, CA). To confirm the central etiology of apnea and to ascertain upper airway mechanics, supraglottic pressure (PSG) was measured using a pressure transducer tipped catheter (Model TC-500XG, Millar Instruments, Houston, TX), with the tip positioned in the hypopharynx. The hypopharyngeal position was obtained by advancing the catheter tip 2 cm after it disappeared behind the tongue. PETCO2 readings were obtained continuously by an infrared analyzer (Model CD-3A, AEI Technologies, Pittsburgh, PA) from tubing placed in the nares via a port in nasal mask. Arterial oxygen saturation (SaO2) was measured by a pulse oximeter (Biox 3700, Ohmeda). The signals were displayed on a polygraph recorder (Grass model 15, Astro-Med, Inc., West Warwick, RI) and recorded using Powerlab data acquisition software (AD Instruments, Colorado Springs, CO) for detailed analysis.
Experimental Protocol
Overview
Seven healthy male subjects without sleep disordered breathing received treatment with a 5α-reductase inhibitor, finasteride, at a 5 mg dose, orally every day for a minimum of 1 month, until a repeat study was conducted. The participants in the finasteride group underwent the experimental protocol at baseline without the drug and then on subsequent nights at intervals of one month for the duration of the study. For comparison, a sham group of 7 healthy males was studied at baseline and on a subsequent night after one month. The experimental protocol was conducted during normal nocturnal sleep. Study participants were instructed to limit total sleep time to a maximum of 4-5 h on the night preceding the study.
Mechanical Ventilation (MV) Protocol
Participants assumed a supine position for the entire experimental protocol conducted during stable stage 2 or stage 3 sleep. We used noninvasive positive pressure mechanical ventilation (MV) to determine the apneic threshold as described previously.4,5,14 Mechanical ventilation was applied for 3 min, in the spontaneous-timed mode during stable NREM sleep. In brief, to achieve this, the inspiratory positive airway pressure was increased gradually in 1 to 2 cm H2O increments starting from 2-4 cm H2O at the beginning of each MV trial, while keeping EPAP fixed at the minimum level allowed by the machine. Mechanical ventilation was terminated after 3 min during expiration by returning the inspiratory positive airway pressure to the baseline expiratory positive airway pressure. The ensuing hypocapnia resulted in either a hypopnea or central apnea. Central apnea was defined as an expiratory time ≥ 5 s. If an apnea was not induced, additional hyperventilation trials at intervals of 5 minutes were completed until an apnea was evident. The MV protocol was repeated in both the finasteride and sham groups as noted above. A blood sample was drawn at the end of each protocol night and tested for sex hormone levels, including, dihydrotestosterone, testosterone and estradiol levels for the finasteride group. Finasteride is a 5-α reductase inhibitor that blocks the conversion of testosterone to dihydrotestosterone (DHT). Thus, low hormone levels of testosterone and high levels of DHT allow confirmation that the participant had ingested the drug.16 DHT is the active component that mediates testosterone activity at the sex-hormone receptor site.
Data Analysis
Sleep staging and scoring of arousals were completed using standard criteria, analyzing trials with stable NREM sleep.17 We analyzed MV trials accompanied by a stable stage N2 or N3 sleep state for all study nights under experimental drug or sham conditions. During the control period of the study, 5 breaths recorded immediately prior to the onset of MV were averaged. Likewise, during the MV period, the last 5 mechanically ventilated breaths prior to the return to baseline expiratory positive airway pressure were averaged. The nadir breath immediately following MV was recorded. The data analysis methodology has been previously described.4,5,14,15 The apnea threshold (AT) was defined as the PETCO2 that demarcated the central apnea closest to the eupneic PETCO2. The CO2 reserve was defined as the difference in PETCO2 between eupneic PETCO2 (control) and AT PETCO2 (i.e., Δ PETCO2). The “hypocapnic ventilatory response” was calculated for each trial as the ratio of change in V̇I between control and apnea to the Δ PETCO2 – AT, (Δ V̇I / Δ PETCO2), i.e., this is the slope of the ventilatory response.14,15 The “hypocapnic ventilatory response” values were normalized to baseline values. The inspiratory upper airway resistance, RUA, was measured on the linear portion of the pressure-flow loop during inspiration. RUA was calculated for the control period on the baseline study night and subsequently on the follow-up study night.
The CO2 production, to determine if the observed physiological changes were related to change in metabolic rate, was also assessed on baseline and subsequent study nights. CO2 production was calculated using the equation: VCO2 = PETCO2 × (VA/0.863) where VA (alveolar ventilation) = V̇I – VD and VD (dead space in mL) = subject weight in pounds, and the estimated respiratory quotient is 0.863.18 The results are expressed as mL/min per kilogram of body weight.
Statistical Analysis
A commercially available computer statistical package was used to analyze the data (Sigma Stat 3.11.0, SPSS). The level of statistical significance was set at P ≤ 0.05. Data from the final night was compared with that at baseline in the finasteride group, as this was most representative of the drug effect. Sham nights were compared at baseline and on the subsequent night. For normally distributed data, paired t-tests were performed to compare eupneic minute ventilation, eupneic PETCO2, apneic threshold, CO2 reserve and the hypocapnic ventilatory response, recorded during the mechanical ventilation trials completed at baseline and on the subsequent night with finasteride. Similarly t-tests were performed for the sham group, except for nonparametric data (hypocapnic ventilatory response), where a signed rank test was performed. The results are presented as mean ± standard error of mean (SEM) unless specified otherwise.
RESULTS
All 14 male participants were young and healthy: finasteride group (n = 7): age: 24.4 ± 6.8 (mean ± standard deviation [SD]) years, BMI: 25.2 ± 4.7 kg/m2; sham group (n = 7): age: 25.2 ± 4.7years, BMI: 24.1 ± 3.8 kg/m2. There were no significant differences between the 2 groups in age or BMI. There were no adverse effects reported by the participants in the finasteride group.
Effect of Finasteride on Ventilation and Upper Airway Mechanics
The administration of finasteride resulted in decreased serum dihydrotestosterone (DHT) from 44.0 ± 16.7 ng/dL at baseline to 11.8 ± 5.5 ng/dL (P = 0.01) after 1-2 months, indicating adherence to finasteride prescription. The total serum testosterone levels in the finasteride group at baseline vs. follow-up nights were 462.2 ± 111.9 ng/dL vs. 535.3 ± 139.3 ng/ dL (mean ± SD) (P = ns), respectively. There was no change in the eupneic V̇I or PETCO2 (Figures 1A, B) on the follow-up night from the baseline study night. The CO2 production was also unchanged between the baseline and the follow-up study nights (Table 1).
Figure 1.
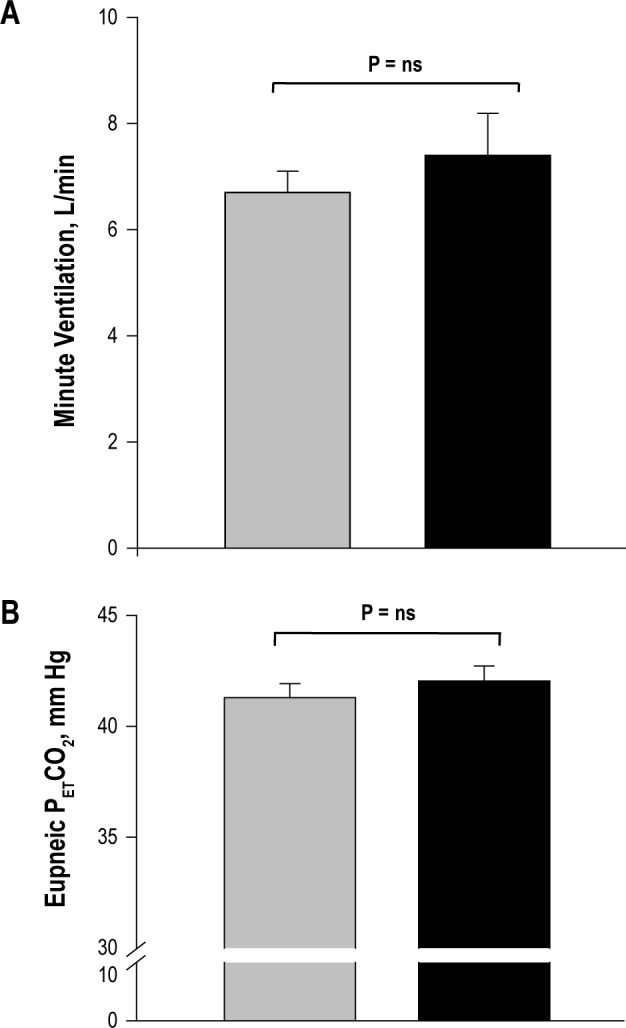
Grouped data comparing the eupneic minute ventilation (A) and end-tidal CO2 (B) at baseline and follow-up experimental nights in the finasteride group. The bars represent averaged data for baseline (gray) and follow-up experimental (black) nights, respectively. No significant changes were noted (P = ns).
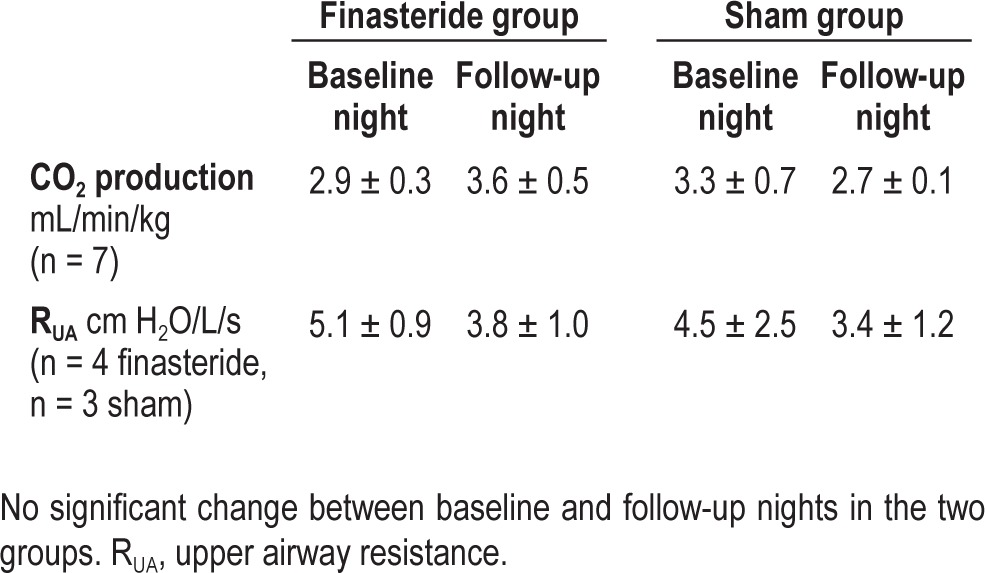
Table 1.
CO2 production and upper airway resistance in the two groups
Effect of Finasteride on the Hypocapnic Apneic Threshold
The administration of finasteride was associated with changes in chemoresponsiveness manifested by significantly decreased hypocapnic ventilatory response (Figure 4). Subsequently, the PETCO2 demarcating the apneic threshold decreased (38.9 ± 0.6 mm Hg vs. 37.7 ± 0.9 mm Hg, P = 0.02) and the CO2 reserve increased (-2.5 ± 0.3 mm Hg vs. -3.8 ± 0.5 mm Hg, P = 0.003) following treatment with finasteride compared to the baseline night study (Figures 2 and 3, respectively). The CO2 production was unchanged at baseline vs. follow-up nights (Table 1).
Figure 4.
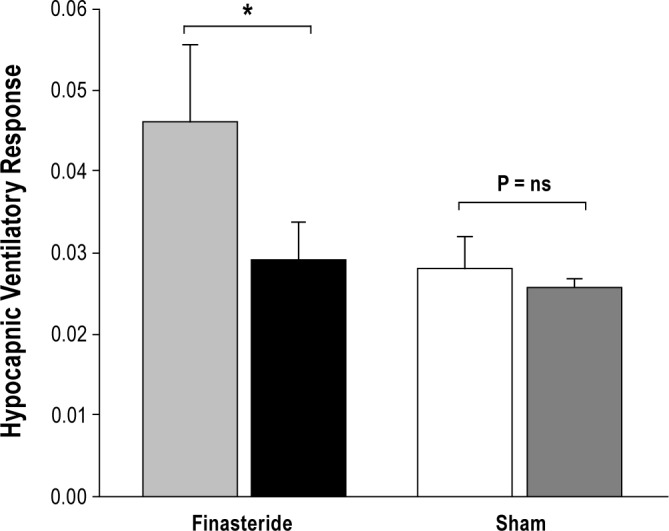
Grouped data comparing the normalized hypocapnic ventilatory response in the two groups, finasteride and sham. The bars represent averaged data for baseline and follow-up experimental nights (first 2 bars: light gray and black bars, respectively) in the finasteride group, and the baseline and follow-up nights (next 2 bars: white and dark gray, respectively) in the sham group. There was a significant decline in the hypocapnic ventilatory response in the finasteride group (*P = 0.047) but no significant change was observed in the sham group.
Figure 2.
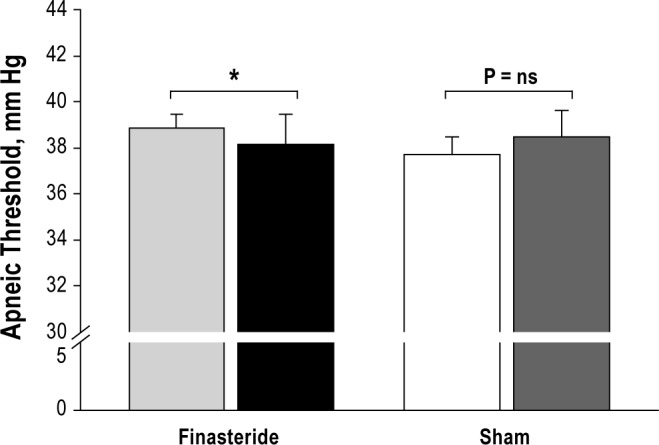
Grouped data comparing the apneic threshold in the finasteride and sham groups. The bars represent averaged data for baseline and follow-up experimental nights (first 2 bars: light gray and black bars, respectively) in the finasteride group, and the baseline and follow-up nights (next 2 bars: white and dark gray, respectively) in the sham group. There was a significant reduction in the apneic threshold in the finasteride group, *P = 0.02, with no significant change noted in the sham group.
Figure 3.
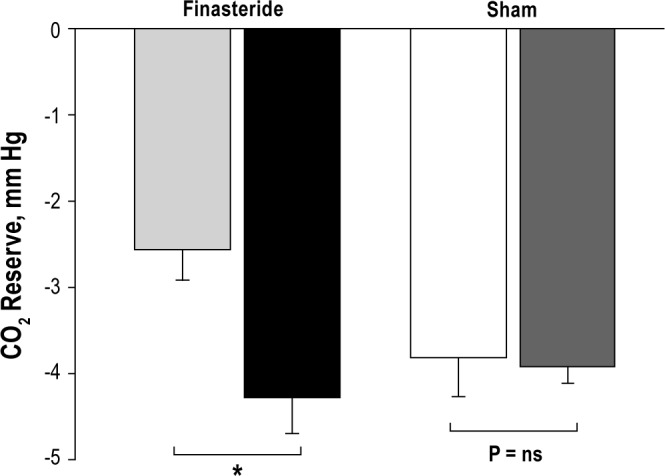
Grouped data comparing the CO2 reserve in the finasteride and sham groups. The bars represent averaged data for baseline and follow-up experimental nights (first 2 bars: light gray and black bars, respectively) in the finasteride group, and the baseline and follow-up nights (next 2 bars: white and dark gray, respectively) in the sham group. There was a significant increase in the CO2 reserve, *P = 0.003 in the finasteride group with no significant change in the sham group.
In addition to above, the baseline control period inspiratory RUA values were not different on the follow-up night in 4 participants in whom the data were available. In the 4 participants in whom RUA data were available, baseline vs. follow-up night values for the eupneic CO2 (41.3 ± 1.1 vs. 42.4 ± 1.2, P = 0.1) and eupneic V̇I (6.8 ± 0.5 vs. 6.9 ± 0.5 L/min, P = 0.9) were unchanged after ingestion of finasteride. In addition, in these 4 participants, the direction of change after ingestion of finasteride, for AT (38.8 ± 1.1 vs. 37.7 ± 1.4 mm Hg, P = 0.1, baseline vs. finasteride), CO2 reserve (-2.9 ± 0.4 vs. -4.4 ± 0.5 mm Hg, P = 0.02), and the hypocapnic ventilatory response (0.04 ± 0.0 vs. 0.02 ± 0.0%/ mm Hg, P = 0.06) was maintained. The CO2 production was also unchanged in this subgroup: 3.0 ± 0.4 mL/min/kg and 4.0 ± 0.8 mL/min/kg (P = ns) at baseline and follow-up nights, respectively.
Sham Study
Likewise, the hypocapnic ventilatory response, the CO2 reserve, and the PETCO2demarcating the apneic threshold did not change in the sham group between the baseline study and the follow-up study nights (Figures 2, 3, and 4). No significant changes in the eupneic PETCO2, (42.3 ± 0.9 vs. 42.0 ± 1.0 mm Hg, P = ns) or eupneic minute ventilation (7.7 ± 0.9 L/min vs. 7.1 ± 0.5 L/min, P = ns) were noted on the baseline study night vs. the follow-up study night. The CO2 production was also unchanged (Table 1). In the sham group, acceptable inspiratory RUA data were available in only 3 individuals (inadequate supraglottic pressure signals in others) (Table 1).
DISCUSSION
Summary of Findings
Our study demonstrated that blockade of 5α-reductase with finasteride resulted in: (1) decreased DHT without change in total serum testosterone levels, (2) decreased hypocapnic ventilatory chemoresponsiveness, (3) increased CO2 reserve associated with decreased PETCO2 that demarcates the apneic threshold, thus, indicating increased breathing stability during sleep with finasteride.
Mechanisms of Testosterone Influence on the Apneic Threshold
Ventilatory control during sleep operates as a negative feedback closed-loop cycle, often described by using the engineering concept of “loop gain” as a framework for breathing instability.19 The propensity to hypocapnic central apnea during NREM sleep is determined by the interplay between two physiological processes. The first process is the effectiveness of the lung/respiratory system in lowering the PETCO2 in response to hyperventilation (plant gain). The lack of change in baseline V̇I or PETCO2 indicates that there was no change in plant gain following administration of finasteride. The second process is the chemoreflex gain (controller gain), which represents the effect of a change in end-tidal CO2 on ventilation.19 Our study revealed that the ventilatory response to hypocapnia decreased after finasteride administration, resulting in a widened CO2 reserve. Thus, finasteride was associated with decreased hypocapnic chemoreflex sensitivity. Our study demonstrated that administration of finasteride altered the apneic threshold in a manner similar to decreased serum testosterone by leuprolide acetate. These changes were not related to alterations in the metabolic rate. Altogether, we interpret these findings as an indication that DHT mediates the effect of testosterone on the apneic threshold. It is unclear whether blockade of the aromatase activity would yield similar results to blockade of the reductase. This needs to be studied further.
Site of Finasteride Effect on the Apneic Threshold
The alteration of the apneic threshold following finasteride administration indicates activity on the central medullary chemoreceptors. This inference is supported by studies showing that cells with large numbers of androgen receptors have been identified in the central nervous system of several species, including birds,20 amphibians,21 rats,22 and rhesus monkeys.23,24 Interestingly, the distribution of androgen concentrating cells is most extensive in the primate model; androgens are taken up by cells in the midbrain, pons, cerebellum, and medulla. Whether androgen receptors are present in human brain and whether they contribute to ventilatory control is not certain.
Alteration of the apneic threshold with testosterone manipulation could also be due to hypocapnia at the peripheral chemoreceptors; hypocapnic disfacilitation of the carotid bodies would decrease ventilatory motor output and hence facilitate the development of central apnea with sustained hyperventilation. Evidence in the literature suggests that testosterone may influence the function of peripheral chemoreceptors. Tatsumi et al. measured the ventilatory and carotid sinus response to hypoxia in neutered male cats after testosterone or placebo administration.25 Testosterone increased the carotid sinus nerve response to hypoxia, suggesting that peripheral chemoresponsiveness was altered by testosterone. However, decreased hypoxic chemo-responsiveness after unilateral central nervous system section raised the possibility that central influences may also play a role in the hypoxic ventilatory response. Our protocol allowed testing for whether testosterone alters chemoresponsiveness.
Testosterone and 5α-dihydrotestosterone (DHT) mediate their effects through binding and activating an intracellular androgen receptor (AR), followed by gene transcription and protein synthesis.26,27 The human AR is among the nuclear receptor super-family, which acts as a ligand-inducible transcription factor through a genomic pathway modulating transcription of target genes, requiring hours or days to produce their actions through protein synthesis. However, some of the rapid androgen effects cannot be explained by a genomic pathway and are mediated through rapid non-genomic effects. For example, testosterone elicits rapid release of prolactin within five minutes in adult male rats. Although our findings are consistent with a genomic effect, our findings do not allow us to ascertain the relative contribution of non-genomic pathways.
Methodological Considerations
The present study utilized noninvasive ventilation as a model to evaluate propensity to hypocapnic central apnea; our laboratory has used and validated this intervention in multiple studies. Several considerations may influence the interpretation of the findings. First, the validity of the CO2 reserve in our experimental model is predicated on the assumption that finasteride was does not alter cerebral blood flow response to hypocapnia and does not alter central to end-tidal CO2 difference. Second, our design does not allow us to determine a dose response or a time response. Third, our study group participants were healthy non-obese and non-apneic volunteers; the effect of finasteride on patients with sleep apnea/hypopnea syndrome, especially those with low serum testosterone, cannot be inferred from our data. Fourth, the design and duration of the study precluded a crossover design. While baseline hypocapnic ventilatory response values were higher compared to the sham group, the response to finasteride was consistent. The baseline CO2 reserve and hypocapnic ventilatory response values were greater in the sham group, likely, due to individual variability. Furthermore, the change in CO2 reserve in our study following finasteride was 1.25 ± 0.7 mm Hg (mean ± SD) with a reduction in AT of 1.15 ± 0.9 mm Hg (mean ± SD). Our sample size was sufficient to detect the changes with a power of 80%. Thus, our study possessed adequate power to detect changes in the CO2 reserve and the AT. Fifth, our analysis included only trials with stable NREM sleep state to ensure that sleep state changes did not influence the apneic threshold. We are unable to determine the effect of finasteride on ventilatory control during REM sleep. Finally, our data do not allow us to ascertain the effect of finasteride on peripheral-central chemoreceptor interdependence during NREM sleep.28
Implications for the Pathogenesis and Management of Central Apnea
Pharmacologic therapy for sleep apnea is virtually nonexistent.29,30 This study identifies a potential novel therapeutic pathway for treating central sleep apnea in adults based on the rationale of testosterone inhibition to stabilize breathing during sleep. While this study was completed in healthy young men, the effect of the drug in individuals with central sleep apnea should be studied further. Clinical trials to study the effect of 5α-reductase inhibition for the treatment of sleep apnea in subgroups of individuals are needed.
A number of 5α-reductase inhibitor drugs have been marketed for the treatment of benign prostatic hypertrophy and male pattern of hair loss; however, since the conclusion of this study protocol, a new warning regarding the risk for prostate cancer with these agents was issued by the FDA, even though the risk is low.31,32 The new safety information demonstrated an overall reduction in prostate cancer diagnoses with finasteride 5 mg treatment due to a decreased incidence of lower risk forms of prostate cancer. However, there was an increased incidence of high-grade prostate cancer with finasteride treatment. The FDA noted that that the 5α-reductase inhibitor drugs “remain safe and effective for their approved indications” and health professionals should discuss the risks and benefits prior to use.31,32 Thus, the therapy may be applicable only in specific populations where the benefits outweigh the risks. These populations may include older adults with central sleep apnea due to heart failure, and elderly men and postmenopausal women with central sleep apnea.
In summary, we have identified that inhibition of testosterone action via the 5α-reductase pathway may be effective in alleviating breathing instability during sleep, presenting an opportunity for novel pharmacologic therapy for the treatment of central sleep apnea in selected populations.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the Veterans Affairs VHA Research Service and the National Heart, Lung, and Blood Institute. Dr. Chowdhuri is a recipient of a VHA Career Development Award-2 from the Department of Veterans Affairs. Dr. Diamond is a stockholder and serves on the board of directors for Advanced Reproductive Care Inc. He is also a stockholder in DS Biotech. He serves as a consultant for Actamax, Auxogyn, ZSX Medical, Halt Medical, and Neomed. He has served as a clinical investigator for Boehringer-Ingelheim, Abbott, and BioSante and has received research funding from Ferring Pharmaceuticals, and EMD Serono. The other authors have indicated no financial conflicts of interest. Work was performed at the John D. Dingell Veterans Affairs Medical Center, Detroit, MI.
ACKNOWLEDGMENTS
The authors acknowledge R. Gill for providing technical assistance with the studies.
REFERENCES
- 1.Jensen D, Wolfe LA, O'Donnell DE, Davies GA. Chemoreflex control of breathing during wakefulness in healthy men and women. J Appl Physiol. 2005;98:822–8. doi: 10.1152/japplphysiol.01208.2003. [DOI] [PubMed] [Google Scholar]
- 2.Saaresranta T, Polo O. Hormones and breathing. Chest. 2002;122:2165–72. doi: 10.1378/chest.122.6.2165. [DOI] [PubMed] [Google Scholar]
- 3.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 4.Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol. 2000;89:192–9. doi: 10.1152/jappl.2000.89.1.192. [DOI] [PubMed] [Google Scholar]
- 5.Zhou XS, Rowley JA, Demirovic F, Diamond MP, Badr MS. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol. 2003;94:101–7. doi: 10.1152/japplphysiol.00264.2002. [DOI] [PubMed] [Google Scholar]
- 6.Mateika JH, Omran Q, Rowley JA, Zhou XS, Diamond MP, Badr MS. Treatment with leuprolide acetate decreases the threshold of the ventilatory response to carbon dioxide in healthy males. J Physiol. 2004;561:637–46. doi: 10.1113/jphysiol.2004.071811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celotti F, Melcangi RC, Negri-Cesi P, Poletti A. Testosterone metabolism in brain cells and membranes. J Steroid Biochem Mol Biol. 1991;40:673–8. doi: 10.1016/0960-0760(91)90289-h. [DOI] [PubMed] [Google Scholar]
- 8.Zabka AG, Mitchell GS, Behan M. Ageing and gonadectomy have similar effects on hypoglossal long-term facilitation in male Fischer rats. J Physiol. 2005;563:557–68. doi: 10.1113/jphysiol.2004.077511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson NR, Bird IM, Behan M. Testosterone restores respiratory long term facilitation in old male rats by an aromatase-dependent mechanism. J Physiol. 2011;589:409–21. doi: 10.1113/jphysiol.2010.198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep. 2006;29:95–103. doi: 10.1093/sleep/29.1.95. [DOI] [PubMed] [Google Scholar]
- 11.Celotti F, Melcangi RC, Martini L. The 5 alpha-reductase in the brain: molecular aspects and relation to brain function. Front Neuroendocrinol. 1992;13:163–215. [PubMed] [Google Scholar]
- 12.Zabka AG, Mitchell GS, Behan M. Conversion from testosterone to oestradiol is required to modulate respiratory long-term facilitation in male rats. J Physiol. 2006;576:903–12. doi: 10.1113/jphysiol.2006.114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Negri-Cesi P, Colciago A, Celotti F, Motta M. Sexual differentiation of the brain: role of testosterone and its active metabolites. J Endocrinol Invest. 2004;27:120–7. [PubMed] [Google Scholar]
- 14.Chowdhuri S, Sinha P, Pranathiageswaran S, Badr MS. Sustained hyperoxia stabilizes breathing in healthy individuals during NREM sleep. J Appl Physiol. 2010;109:1378–83. doi: 10.1152/japplphysiol.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol. 2010;108:369–77. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amory JK, Wang C, Swerdloff RS, et al. The effect of 5 alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab. 2007;92:1659–65. doi: 10.1210/jc.2006-2203. [DOI] [PubMed] [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 18.West JB, editor. Respiratory physiology- the essentials. Baltimore: Williams & Wilkins; 1974. [Google Scholar]
- 19.Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2005;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 20.Lea RW, Clark JA, Tsutsui K. Changes in central steroid receptor expression, steroid synthesis, and dopaminergic activity related to the reproductive cycle of the ring dove. Microsc Res Tech. 2001;55:12–26. doi: 10.1002/jemt.1152. [DOI] [PubMed] [Google Scholar]
- 21.Kelley DB. Locations of androgen-concentrating cells in the brain of Xenopus laevis: autoadiography with 3H-dihydrotestosterone. J Comp Neurol. 1981;199:221–31. doi: 10.1002/cne.901990206. [DOI] [PubMed] [Google Scholar]
- 22.Stumpf WE, Sar M. Steroid hormone target cells in the extrahypothalamic brain stem and cervical spinal cord: neuroendocrine significance. J Steroid Biochem. 1979;11:801–7. doi: 10.1016/0022-4731(79)90015-3. [DOI] [PubMed] [Google Scholar]
- 23.Rees HD, Michael RP. Brain cells of the male rhesus monkey accumulate 3H-testosterone or its metabolites. J Comp Neurol. 1982;206:273–7. doi: 10.1002/cne.902060307. [DOI] [PubMed] [Google Scholar]
- 24.Sheridan PJ, Weaker FJ. Androgen receptor systems in the brain stem of the primate. Brain Res. 1982;235:225–32. doi: 10.1016/0006-8993(82)91002-2. [DOI] [PubMed] [Google Scholar]
- 25.Tatsumi K, Hannhart B, Pickett CK, Weil JV, Moore LG. Effects of testosterone on hypoxic ventilatory and carotid body neural responsiveness. Am J Respir Crit Care Med. 1994;149:1248–53. doi: 10.1164/ajrccm.149.5.8173766. [DOI] [PubMed] [Google Scholar]
- 26.Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266:510–8. [PubMed] [Google Scholar]
- 27.Losel RM, Falkenstein E, Feuring M, et al. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- 28.Dempsey JA, Smith CA, Blain GM, Xie A, Gong Y, Teodorescu M. Role of central/peripheral chemoreceptors and their interdependence in the pathophysiology of sleep apnea. Adv Exp Med Biol. 2012;758:343–9. doi: 10.1007/978-94-007-4584-1_46. [DOI] [PubMed] [Google Scholar]
- 29.Veasey SC, Guilleminault C, Strohl KP, Sanders MH, Ballard RD, Magalang UJ. Medical therapy for obstructive sleep apnea: a review by the Medical Therapy for Obstructive Sleep Apnea Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 2006;29:1036–44. doi: 10.1093/sleep/29.8.1036. [DOI] [PubMed] [Google Scholar]
- 30.Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35:17–40. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. Department of Health and Human Services. U.S. Food and Drug Administration. FDA Drug Safety Communication: 5-alpha reductase inhibitors (5-ARIs) may increase the risk of a more serious form of prostate cancer. [Accessed Dec 1, 2012]. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm258314.htm.
- 32.U.S. Department of Health and Human Services. U.S. Food and Drug Administration. Questions and Answers: 5-alpha reductase inhibitors (5-ARIs) may increase the risk of a more serious form of prostate cancer. [Accessed Dec 1, 2012]. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm258358.htm.


