Abstract
Study Objectives:
Identifying the neurochemistry and neural circuitry of sleep regulation is critical for understanding sleep and various sleep disorders. Fruit flies display sleep-like behavior, sharing essential features with sleep of vertebrate. In the fruit fly's central brain, the mushroom body (MB) has been highlighted as a sleep center; however, its neurochemical nature remains unclear, and whether it promotes sleep or wake is still a topic of controversy.
Design:
We used a video recording system to accurately monitor the locomotor activity and sleep status. Gene expression was temporally and regionally manipulated by heat induction and the Gal4/UAS system.
Measurements and Results:
We found that expressing pertussis toxin (PTX) in the MB by c309-Gal4 to block Go activity led to unique sleep defects as dramatic sleep increase in daytime and fragmented sleep in nighttime. We narrowed down the c309-Gal4 expressing brain regions to the MB α/β core neurons that are responsible for the Go-mediated sleep effects. Using genetic tools of neurotransmitter-specific Gal80 and RNA interference approach to suppress acetylcholine signal, we demonstrated that these MB α/β core neurons were cholinergic and sleep-promoting neurons, supporting that Go mediates an inhibitory signal. Interestingly, we found that adjacent MB α/β neurons were also cholinergic but wake-promoting neurons, in which Go signal was also required.
Conclusion:
Our findings in fruit flies characterized a group of sleep-promoting neurons surrounded by a group of wake-promoting neurons. The two groups of neurons are both cholinergic and use Go inhibitory signal to regulate sleep.
Citation:
Yi W; Zhang Y; Tian Y; Guo J; Li Y; Guo A. A subset of cholinergic mushroom body neurons requires go signaling to regulate sleep in Drosophila. SLEEP 2013;36(12):1809-1821.
Keywords: Mushroom body, Go signaling, cholinergic neurons, sleep
INTRODUCTION
People suffer from various sleep disorders such as insomnia and hypersomnia,1 leading to a clear need to identify the neurophysiology and neurochemistry of sleep and wakefulness. Drosophila has emerged as a new animal model for sleep research, because its rest behavior shares cardinal features with mammalian sleep characteristics such as prolonged reversible immobility, increased arousal thresholds,2,3 altered brain electrical activity,4,5 and homeostatic influence.6 The neurochemistry basis in fruit fly sleep has been suggested to be functionally conserved.1,7 As in mammals, dopamine (DA) and octopamine (OA, the insect equivalent of norepinephrine) exert wake-promoting function, whereas serotonin (5-HT) and γ–aminobutyric acid (GABA) mainly promote sleep in fruit flies (flies for short).1,8–13 The convenient genetic tools in fruit flies have enabled in-depth studies of the neural circuits related with dopamine, octopamine, and 5-HT systems in sleep regulation, providing further insights into the understanding of sleep neurophysiology.14–16 Nevertheless, the function of the cholinergic system in sleep has not been investigated in fruit flies.
The mushroom body (MB), a paired symmetrical neuropil in the brain, has been highlighted as a sleep control center in fruit flies,17,18 and mediates many of the effects of DA and 5-HT on sleep.11,19,20 Two G protein pathways have been reported to function in the MB in sleep regulation: stimulating the excitatory Gs pathway promotes wakefulness,17,18 while activating Go, belonging to the inhibitory Gi/o subfamily, promotes sleep,21 suggesting that the MB plays a sleep-inhibiting role. However, it has also been reported that blocking the MB output inhibits sleep.18 The role of the MB in sleep control is thus complex and remains controversial. In addition, although the MB has been found to be modulated by various types of neurons, the neurochemistry nature of the MB neurons themselves has been poorly understood.22
Here, we show that inhibiting Go activity in a restricted subtype of MB neurons, the MB α/β core neurons, leads to increased sleep. We further demonstrate that these MB neurons are cholinergic and their acetylcholine (Ach) output is required for sleep regulation. Finally, we find that the adjacent MB neurons play opposite roles in sleep regulation and also require Go signaling and ACh output for their function.
METHODS
Drosophila Strains
Fruit fly strains were kindly gifted as follows: UAS-GoGTP by Dr. Andrew Tomlinson (Columbia University); UAS-PTX by Dr. Gregg Roman (University of Houston); UAS-mc* by Dr. Yuh-Nung Jan (University of California at San Francisco); MB-Gal80 by Dr. Scott Waddell (University of Massachusetts Medical School); Cha3.3kb-Gal80 by Dr. Yi Rao (Peking University); GAD1-Gal80 by Dr. Takaomi Sakai (Tokyo Metropolitan University); c309-Gal4 by Dr. Amita Sehgal (University of Pennsylvania); D52H-Gal4 by Dr. Yi Zhong (Tsinghua University). UAS-vAChTRNAi (#40918) was obtained from the Vienna Drosophila RNAi Center; UAS-ChATRNAi (12345R-4), sca109–68-Gal4 and NP lines from the Drosophila Genetic Resource Center. UAS-mCD8::GFP (#5137), R15E01-Gal4, and tub-Gal80ts (#7018 and #7019) from the Bloomington Stock Center. Flies were raised on standard cornmeal, sugar, yeast, and agar medium, under 12 h/12 h light/dark (LD) conditions. Flies were maintained at 24°C, except those used for heat-induction experiments that were maintained at 18°C.
Sleep and Circadian Rhythm Assays
Sleep analysis was performed as described previously.21 Virgin female 4- to 6- day-old flies were placed individually in 65 mm × 5 mm monitor tubes with food containing 5% sucrose in 2% agar and entrained for 24-36 h before sleep recording. Lights on was at ZT 0 (circadian time 08:00) and lights off at ZT 12 (circadian time 20:00). Locomotor activity was monitored in the LD cycle using a video system.23,24 Infrared LED lights were used to give constant illumination for video recording. Web cameras with a 640 × 480 resolution were pretreated to allow reception of only infrared light. Images were acquired every 5 sec and further processed by Pysolo24 to determine the location of the flies. Sleep was defined as no detectable movement for 5 min or longer.3 Sleep parameters were calculated using Matlab (MathWorks, Natick, MA). Waking activity was calculated as the total walking distance divided by the total time awake.
For experiments without heat induction, sleep was assayed for three LD cycles at 24°C. In heat induction experiments, sleep was monitored in two LD cycles at 18°C as a preheat control to measure the baseline sleep, and flies were then exposed to a 12-h 31°C heat shock from ZT 12 to ZT 0. After a 12-h recovery period, sleep data from the next two LD cycles were collected at 18°C as the postheat sleep level.
For free-running circadian rhythm and sleep assay, flies were treated and recorded in the same condition as mentioned previously, except that the experiments were performed in constant darkness. Locomotor activity was measured after the recovery for 5 days and calculated in Clocklab (Actimetrics, Wilmette, IL) to obtain the parameters of the circadian rhythm. The power of the rhythm was calculated by chi-square method at a confidence level of 0.01.
Data Analysis
All statistical analyses were conducted using SPSS (SPSS Inc., Chicago IL). The Wilks-Shapiro test was used to determine the normality of the data. Preheat and postheat records of individual flies were compared with two-tailed paired Student t-tests. The least significant difference t test (LSD) was used as a post hoc test after one-way analysis of variance. Data were presented as mean behavioral responses and error bars represented the standard error of the mean (SEM). Differences between groups were considered significant if the probability of error was less than 0.05 (P < 0.05).
Immunohistochemistry
The expression pattern of Gal4 lines was visualized by the membrane targeted green fluorescent protein (mCD8::GFP, mGFP) expression. In all morphological experiments except those in Supplemental Figure S4, mGFP expression was induced at 31°C for 3 days in flies 7-9 days posteclosion. In Figure S4, mGFP expression was continually induced or suppressed after egg-laying (Figure S4A, S4C), or induced for 3 days before eclosion (Figure S4B). The brains of female adult fruit flies were dissected in cold phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde in PBS at room temperature (RT) for 40 min. After three 20-min washes in phosphate buffered saline Triton X-100 (PBST, 0.3% Triton X-100 in PBS), samples were blocked with blocking buffer (5% normal goat serum in PBST) for 1 h at RT. Samples were then incubated with primary antibodies (mouse anti-FasII at 1:100 from Developmental Studies Hybridoma Bank, Iowa, and rabbit anti-GFP immunoglobulin G (IgG) at 1:800 from Invitrogen, Carlsbad, CA) in blocking buffer at 4°C overnight. After three 20-min washes in PBST, brains were incubated at 4°C overnight with secondary antibodies Cy3 goat anti-mouse IgG (1:500; Invitrogen) and Alexa Fluor 488 goat anti-rabbit IgG (1:1000; Invitrogen) in blocking buffer. After three 20-min washes in PBST, brains were mounted using Vectashield mounting medium (Vector Labs, California) and imaged with a Leica SP5II confocal microscope. Z stack images were scanned at 1-μm section intervals with a resolution of 1,024 × 1,024 pixels. Confocal stacks were processed using ImageJ (NIH, Bethesda, MA) to create the projection view.
Inverse Polymerase Chain Reaction
Genomic DNA of c309-Gal4 or sca109–68-Gal4 flies was extracted and digested with HpaII (New England Biolabs Inc., Ipswich). DNA fragments were then self-ligated overnight at 16°C using T4 DNA ligase (Invitrogen). Ligated fragments containing the 5' end of P{GawB} and flanking genomic DNA were amplified by polymerase chain reaction (PCR) with the primer pair, 5'-CTCCACAATTCCGTTGGATT-3' and 5'-CTATCGACGGGACCACCTTA-3'. The PCR product was sequenced and a BLAST search of the flanking DNA sequence against the Drosophila genome sequence (Flybase.org) was performed to localize the insertion site in the fly genome.
RESULTS
Blocking Go Activity by c309-Gal4 Elevates Sleep in Flies
For sleep research in Drosophila, a video recording system has been newly developed, which could detect subtle movements as small as 0.38 mm, approximately 1/10 of the fly body length.23 Thus, we set up this system in our laboratory and manually confirmed that it could faithfully read the fly position (Figure S1). To prevent potential developmental effects, we adopted the temporal and regional gene expression targeting (TARGET) system. In this system, a conventional Gal4/UAS system drives tissue-specific expression of an effecter gene in a Gal4-dependent manner. The Gal4 transcriptional activity can be inhibited by a ubiquitously expressed and temperature-sensitive Gal80 repressor, Gal80ts, at 18°C, whereas the repression of Gal4 is relieved by shifting the temperature to 31°C, resulting in expression of this effecter gene.25 The effecter gene expression was only induced by a 12-h heat-shock in 7- to 9-day-old adult flies in all our experiments except otherwise stated (Figure 1A). Thus, the effect of temporal gene expression on sleep could be determined by comparing sleep profiles between the preheat and post-heat recording period (Figure 1A).
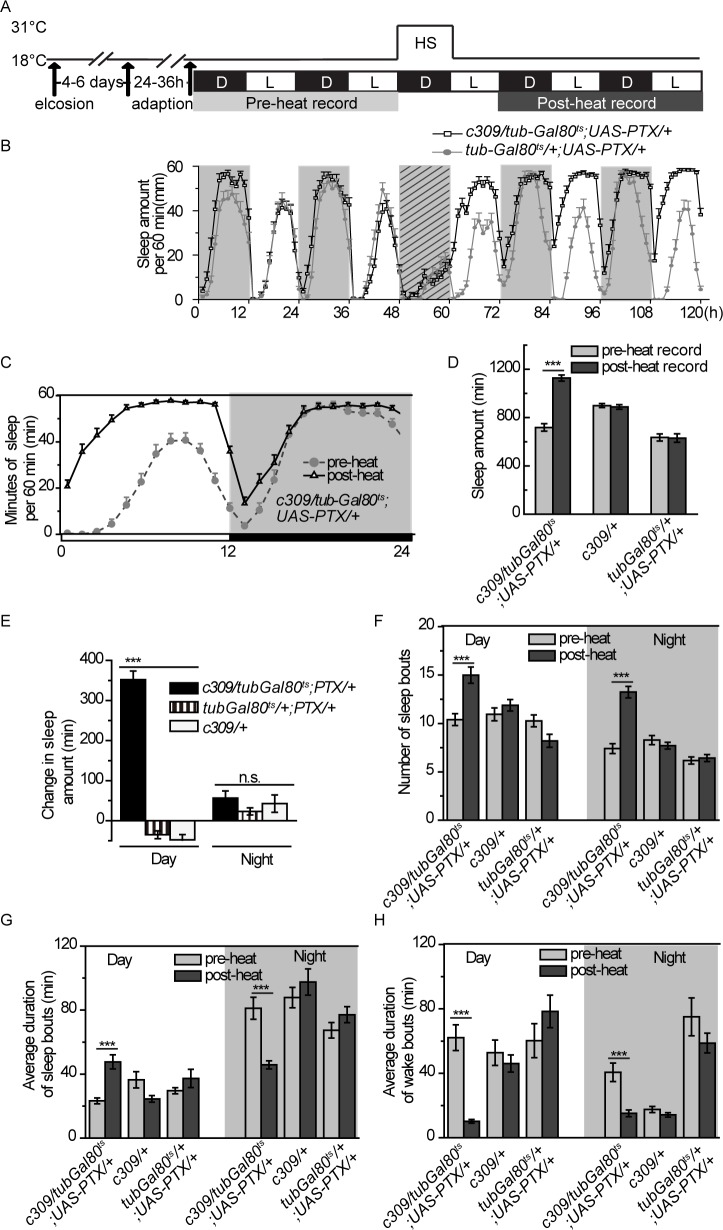
Figure 1.
Blocking Go signaling in c309 neurons leads to sleep increase. (A) Procedure for sleep recording and heat induction. White and black bars show light/dark (L/D) periods. Fly sleep was monitored for 2 days before (light gray) and after (dark gray) heat shock induction. (B) Sleep profiles for c309-Gal4/ tub-Gal80ts;UAS-PTX/+ flies and their parental flies tub-Gal80ts/+;UAS-PTX/+ during the sleep recording procedure. Diagonal crossing lines represent heat shock period. (C) Sleep profiles for c309-Gal4/tub-Gal80ts;UAS-PTX/+ are averaged for the preheat or the post-heat recording days. (D-H) Sleep parameters are analyzed and averaged in the preheat or the post-heat recording days for c309-Gal4/tub-Gal80ts;UAS-PTX/+ flies and their parental controls c309-Gal4/+ and tub-Gal80ts/+;UAS-PTX/+flies: daily sleep amount (D), change in sleep amount calculated by subtracting sleep before heat induction from sleep after heat induction (E), number of sleep bouts (F), average duration of sleep bout during the day or night (G), and average duration of wake bouts during the day or night (H). n = 30-36 in each group. Data were analyzed by one-way analysis of variance in (E) and paired Student t-test in other panels. Data are presented as the mean ± standard error of the mean. ***P < 0.001; n.s, no significant difference, P > 0.05.
We previously reported that inhibiting Go activity by PTX (a specific inhibitor of Go activity in flies26,27) in the MB led to decreased sleep levels in flies.21 Surprisingly, temporally expressing PTX with c309-Gal4, another MB-expressing Gal4, led to dramatic increases in sleep amount (Figures 1B through 1D). We observed temporally reduced sleep amount during the heat shock treatment in both c309 > PTX and control flies (Figure 1B). To exclude the possibility that the sleep increase after heat shock-induced PTX expression is due to sleep rebound to heat-induced sleep loss, we performed the heat shock induction during the daytime, during which the sleep amount was unaffected (Figure S2A). We also observed a remarkable increase in sleep amount after expressing PTX in c309 neurons (Figure S2B). Thus, these results suggest that expression of PTX in c309 neurons results in sleep increase, and indicate that this increase is not a result of heat-induced sleep loss.
Analyzing the sleep profile in details, we noticed that the excessive sleep occurred predominantly during the daytime, whereas the amount of nighttime sleep was unchanged (Figure 1E). The increased daytime sleep was attributable to both prolonged sleep bout duration and more sleep bouts (Figure 1F and 1G), suggesting the flies suffered from excessive daytime sleepiness. Meanwhile, the wake bout duration during the daytime was significantly decreased (Figure 1H), suggesting that c309 > PTX flies had difficulty maintaining long periods of wakefulness when flies are supposed to be mainly wakeful. In contrast, nighttime sleep was fragmented upon PTX expression, indicated by a significantly increased number of sleep bouts, as well as a decreased average duration of individual sleep bouts (Figure 1F and 1G). Wake bout duration at night was also shortened (Figure 1H), reflecting more frequent sleep/wake switches and instability of both the sleep and wake states at night.
The fragmented sleep/wake state implied a weakened circadian rhythm, and we thus tested this possibility under constant dark (DD) condition. Our results showed that temporally expressing PTX in c309-Gal4 expressing neurons (termed c309 neurons) did not substantially influence period length or rhythmicity, but only decreased the relative rhythmic power (Table S1). We further measured the sleep status of the flies in DD condition and found that temporally blocking Go activity in c309 neurons also induced dramatic sleep increment and increased sleep/wake bout number, similar to that in LD condition (Figure S3). These results suggested that the sleep defects in c309 > PTX flies were not resulted from circadian rhythm problem. We noticed that heat shock also induces moderate increase in sleep bout number in tub-Gal80ts/+;UAS-PTX/+ control flies (Figure S3C). We suspect that the insertions of tub-Gal80ts and/or UAS-PTX transgenes affect the fly sensitivity to heat to some, albeit subtle, extent, which may only be observed under DD conditions.
Elevating Go Activity by c309-Gal4 Decreases Sleep Amount
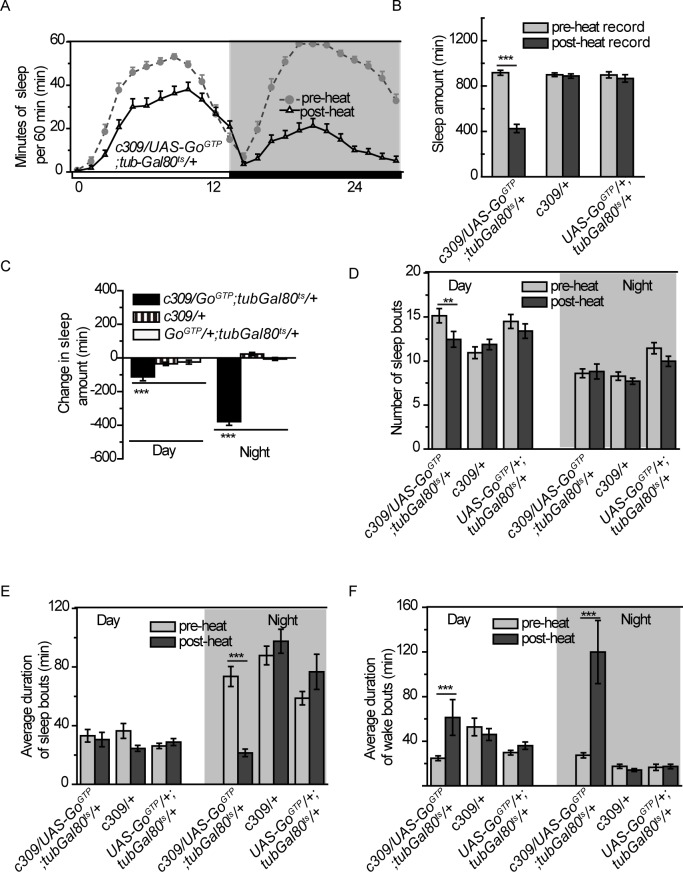
To further understand the function of Go activity in sleep, we adopted GoGTP, a constitutively active form of one Go subunit, to temporally elevate Go activity in c309 neurons in the adult stage (Figure 1A). In contrast to hypersomnia resulting from blocking Go activity, elevating Go activity in c309 neurons led to a significant decrease in sleep amount (Figures 2A and 2B). Quantification of sleep parameters revealed that elevating Go activity in c309 neurons induced sleep decrease during both the daytime and nighttime (Figure 2C). During the daytime, sleep bout number decreased, but sleep duration was comparable to control flies (Figures 2D and 2E). On the contrary, sleep bout number during the nighttime was unchanged, whereas sleep duration was dramatically decreased (Figures 2D and 2E). Wake bout duration was increased during both daytime and nighttime, indicating more consolidated wakefulness (Figure 2F). Taken together with the results from c309 > PTX flies, we suggest that Go functions in c309 neurons to reinforce wakefulness, and that the signals mediated by Go may represent wake-promoting signals.
Figure 2.
Elevating Go signaling in c309 neurons decreases sleep. (A) Sleep profiles before or after temporally expressing GoGTP using c309-Gal4 with tub-Gal80ts. (B-F) Sleep parameters before (light gray bar) or after heat induction (dark gray bar) were analyzed for c309-Gal4/UAS-GoGTP;tub-Gal80ts/+ flies and their parental controls c309-Gal4/+ and UAS-GoGTP/+;tub-Gal80ts/+flies: daily sleep amount (B), change in sleep amount during daytime and nighttime (C), number of sleep bouts (D), average duration of sleep bouts during daytime and nighttime (E), and average duration of wake bouts during daytime and nighttime (F). n = 30-36 in each group. Data were analyzed by one-way analysis of variance in (C) and with paired Student t-test in other panels. Data are presented as means ± standard error of the mean. ***P < 0.001; n.s, no significant difference, P > 0.05.
Go Functions in c309-labeled MB α/β Core Neurons to Regulate Sleep
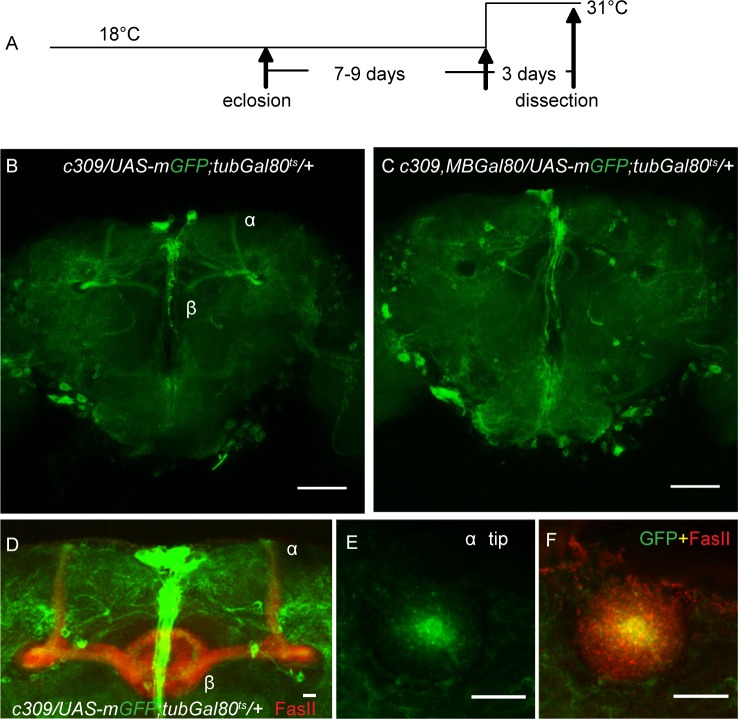
We next examined which of the neurons labeled by c309-Gal4 were responsible for Go signal-mediated sleep effects. When mCD8::GFP (mGFP) was continuously expressed by c309-Gal4, all of the MB α/β and γ neurons and some neurons scattered through the central brain were strongly labeled (Figure S4A), consistent with a previous report.28 However, when we induced GFP expression for 3 days in 7- to 9-day-old flies by heat shock (Figure 3A), we observed far fewer expression regions that were driven by c309-Gal4 (Figure 3B). It should be noted that the age of these flies used corresponds to the age of flies for which heat shock was administered in our behavioral studies (Figures 1A and 3A). In particular, expression in the MB was restricted to the α/β core neurons, whose axons run exclusively into the core area of the α/β lobes22 (Figures 3D through 3F). When heat induction was performed for 3 days before eclosion, stronger expression in all α/β and γ subtypes was found (Figure S4B). Thus, these results indicate that the differences in the c309-Gal4 expression pattern are due to different developmental stages.
Figure 3.
c309-Gal4 is expressed in MB α/β core neurons in adult stage flies. (A) Procedure of heat induction of mCD8::GFP for examining the expression pattern. (B-C) In the MB region, c309-Gal4 has restricted expression, which could be eliminated by MB-Gal80. (D-F) Projection view of the MB lobe area (D) and a single horizontal section of the tip of the α lobe (E-F). FasII signal (red) shows the neuropils of MB neurons. GFP signal (green), representing c309-Gal4 expressing neurons, is only observed in the core area of MB α/β lobe. Scale bars represent 50 μm in B and C, and 10 μm in D-F.
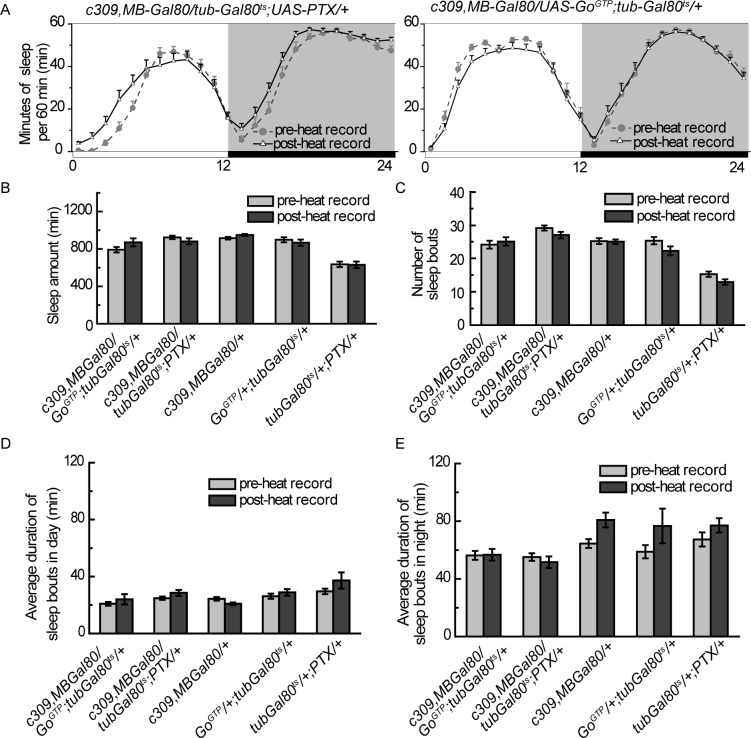
To determine whether these restricted MB neurons or the scattered neurons outside the MB (termed as non-MB neurons) were responsible for the sleep defects, we introduced MB-Gal80 to specifically inhibit Gal4 activity in the MB neurons29 (Figure 3C). Combining MB-Gal80 with c309-Gal4 completely blocked the PTX expression induced sleep increment and eliminated the GoGTP expression induced sleep reduction (Figures 4A and 4B). In addition, the number of sleep/wake bouts and sleep bout duration in both daytime and nighttime were unchanged in both PTX and GoGTP experiments (Figures 4C through 4E). These results demonstrated that manipulating Go activity in c309-labeled non-MB neurons had no effect on either total sleep amount or sleep structure, suggesting that the c309-MB neurons are responsible for the sleep defects in c309 > PTX and c309 > GoGTP flies.
Figure 4.
MB-Gal80 eliminates PTX- and GoGTP- induced sleep effects in c309-Gal4. (A) Sleep profiles are comparable before and after heat induction when MB-Gal80 is introduced. (B-E) Temporally expression of PTX or GoGTP with c309-Gal4,MB-Gal80 leads to no significant change in sleep amount (B), number of sleep bouts (C), sleep bout duration in daytime (D) or sleep bout duration in nighttime (E). Light gray bars and dark gray bars indicate sleep parameters for 2 light-dark (LD) days before and after heat induction, respectively. Data were analyzed by paired Student t-tests; n = 30-36 in each group. Data are presented as means ± standard error of the mean. ***P < 0.001, paired Student t-test.
We also examined another G protein pathway, Gs pathway, with the same heat induction paradigm. Cyclic-AMP-dependent protein kinase (PKA) is a downstream molecule of the Gs pathway, and continually expressing mc*, a constitutively active mouse PKA catalytic subunit, by c309-Gal4 suppresses sleep.17 As expected, we observed severely reduced and fragmented sleep when mc* was temporally expressed in c309 neurons, however, sleep was still severely reduced and fragmented when c309-Gal4 was combined with MB-Gal80 (Figure S5). We therefore suggest that Gs may play a role in sleep regulation in both MB and non-MB neurons, whereas the Go signal regulates sleep only in MB neurons within the c309 neurons.
It has been reported that Go signaling is involved in loco-motor activity control.30 Although we noticed decreased waking activity (the walking distance divided by waking time) in both c309 > PTX and c309 > GoGTP flies (Figure S6), the total sleep amount increased in c309 > PTX but decreased in c309 > GoGTP flies (Figures 1C and 2C). Moreover, c309,MBGal80 > PTX flies also exhibited greatly reduced waking activity (Figure S6), whereas these flies exhibited normal sleep amount (Figure 4B). These results suggest that manipulating Go activity in c309 non-MB neurons may influence locomotor activity; however, our video recording and analysis system can reliably detect different sleep statuses even though flies have locomotor activity defects. The sleep defects we observed should therefore not result from deficient locomotor activity of flies. We also used FasII staining to check the gross morphology of the MB α/β and γ lobe. We found that expression of PTX or GoGTP over a 12-h period did not alter the global structures of the MBs when examined at both the end of the post-heat recording (Figure S7), and 20 days after expression (Figure S8). Thus, we suggest that the behavioral changes are not due to potential long-term structural change of the MBs.
Go is Required in Different MB Subgroups That Have Opposite Functions in Sleep Regulation
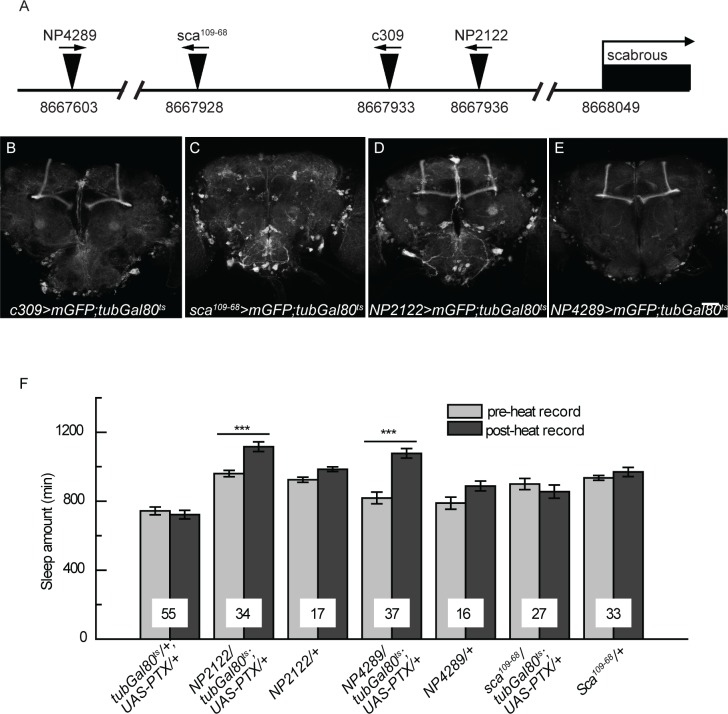
Enhancer trap-based Gal4 lines with adjacent insertion sites in the genome may share similar expression patterns. We mapped the insertion site of c309-Gal4 to 8,667,933 on the second chromosome, upstream of the gene scabrous. We checked the expression patterns of several adjacent Gal4 lines (Figure 5A) using the heat-induction procedure in adult flies (Figure 3A), and unexpectedly their expression patterns were different. Compared with c309-Gal4, sca109–68-Gal4 showed a very weak signal in the MB but similar expression outside the MB (Figures 5B and 5C), whereas NP2122-Gal4 and NP4289-Gal4 showed strong expression in MB α/β core neurons and much less expression outside the MB (Figures 5D and 5E). These Gal4 lines allow us to further examine the locus of Go function in sleep regulation. Blocking Go activity by temporally expressing PTX with NP2122-Gal4 or NP4289-Gal4, but not sca109–68-Gal4, led to a significant increase in sleep amount, as was the case in c309 > PTX flies (Figure 5F). These results further support our hypothesis that Go activity in the MB α/β core neurons is responsible for sleep defects in c309 > PTX flies.
Figure 5.
Inhibiting Go signal in c309-related Gal4 lines regulates sleep in different ways. (A) Schematic diagram of the P-element insertion sites of several Gal4 lines in the scabrous promoter region. Arrows above the triangles indicate insertion orientation. The numbers below the line represent insertion sites on the second chromosome. (B-D) Heat-induced expression patterns are shown for Gal4 lines with the existence of tub-Gal80ts. c309 (B), NP2122-Gal4 (D) and NP4289-Gal4 (E) drive strong expression in the MB α/β core area, whereas Sca109–68-Gal4 (C) drives weak expression in the MB. Scale bar represents 50 μm. (F) Temporal expression of PTX with NP2122- or NP4289- Gal4, but not with Sca109–68-Gal4, leads to increases in sleep amount. Numbers on bars indicate the number of flies in each group. Data were analyzed by paired Student t-test. ***P < 0.001.
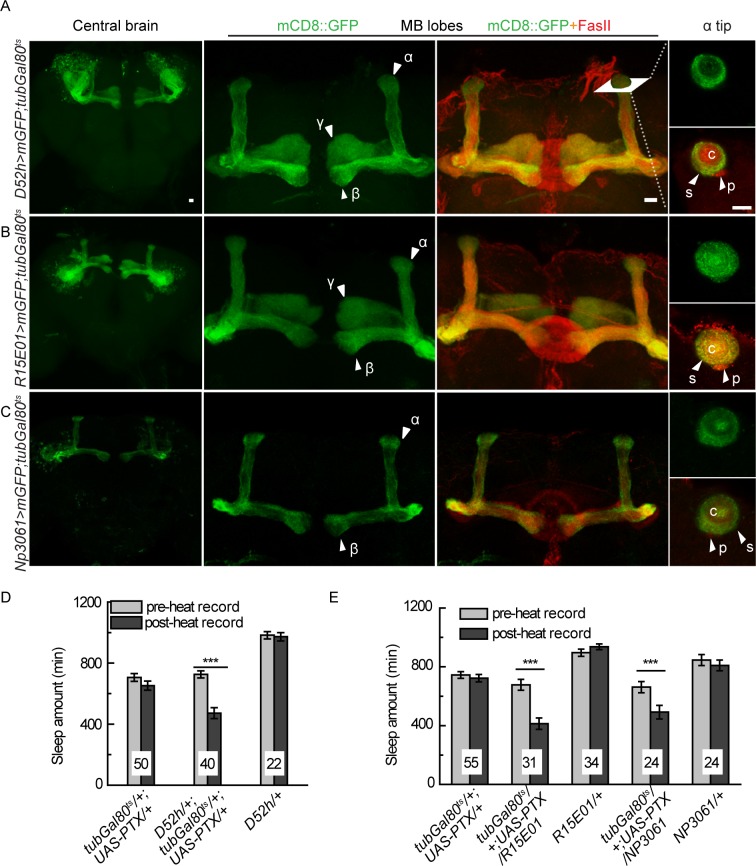
We previously reported that blocking Go activity by PTX in the MB reduced sleep amount,21 and thus we wondered which group of MB neurons was responsible for the opposite sleep regulation. We used three MB Gal4 lines, which showed restricted expression in the MB and rare signal in other brain regions when induced by heat shock in adult flies (Figure 3A). D52H-Gal4 showed strong expression in α/β surface neurons, moderate expression in posterior neurons, but no expression in the core area, whereas R15E01-Gal4 and NP3061-Gal4 labeled all α/β core, surface, and posterior subtypes of neurons. D52H-Gal4 and R15E01-Gal4 also drove expression in the γ neurons (Figures 6A through 6C).
Figure 6.
Inhibition of the endogenous Go signal in multiple MB-Gal4 lines decreases sleep. (A-C) Heat-induced expression patterns are shown for respective Gal4 lines with tub-Gal80ts. From left to right are projection views of the central brain, the MB lobes, the MB lobes co-stained with FasII and a single horizontal section at the tip of α lobe. (A) D52H-Gal4 drives strong expression in MB α/β surface and γ area, moderate expression in the MB α/β posterior and weak expression in the α/β core areas. (B) R15E01-Gal4 drives strong expression in MB α/β surface, posterior, core, and γ areas. (C) NP3061-Gal4 drives strong expression in MB α/β surface, and core area and moderate expression in MB α/β posterior area. c, core; p, posterior; s, surface. Scale bar represents 10 μm. (D and E) Temporal expression of PTX with these Gal4s leads to significantly decreased sleep amount. The numbers on bars indicate the number of flies in each group. Data were analyzed using paired Student t-tests. ***P < 0.001.
Temporally inducing PTX expression by D52H-Gal4 led to significantly reduced sleep amount (Figure 6D). When introducing MB-Gal80 to abolish D52H-Gal4 activity in the MB expression, sleep amount was statistically indistinguishable before and after heat shock induction, suggesting that Go activity in the α/β surface/posterior and γ neurons is required to promote sleep (Figures S9A and S9D). Interestingly, blocking Go activity in R15E01-Gal4 or NP3061-Gal4 labeled neurons also significantly decreased sleep (Figure 6E). Meanwhile, these sleep effects could be abolished by the presence of MB-Gal80, further supporting that their MB neuron expression was required (Figures S9B through S9D). The expression patterns of these three Gal4 lines overlap in the α/β surface/ posterior neurons. Therefore, our results demonstrate that in addition to the α/β core neurons, the Go signal is also required in other subtypes of MB neurons, such as the α/β surface/posterior neurons, and their effects on sleep regulation are opposite to the α/β core neurons.
MB α/β Core Neurons are Cholinergic and Down-regulating ACh Signal Inhibits Sleep
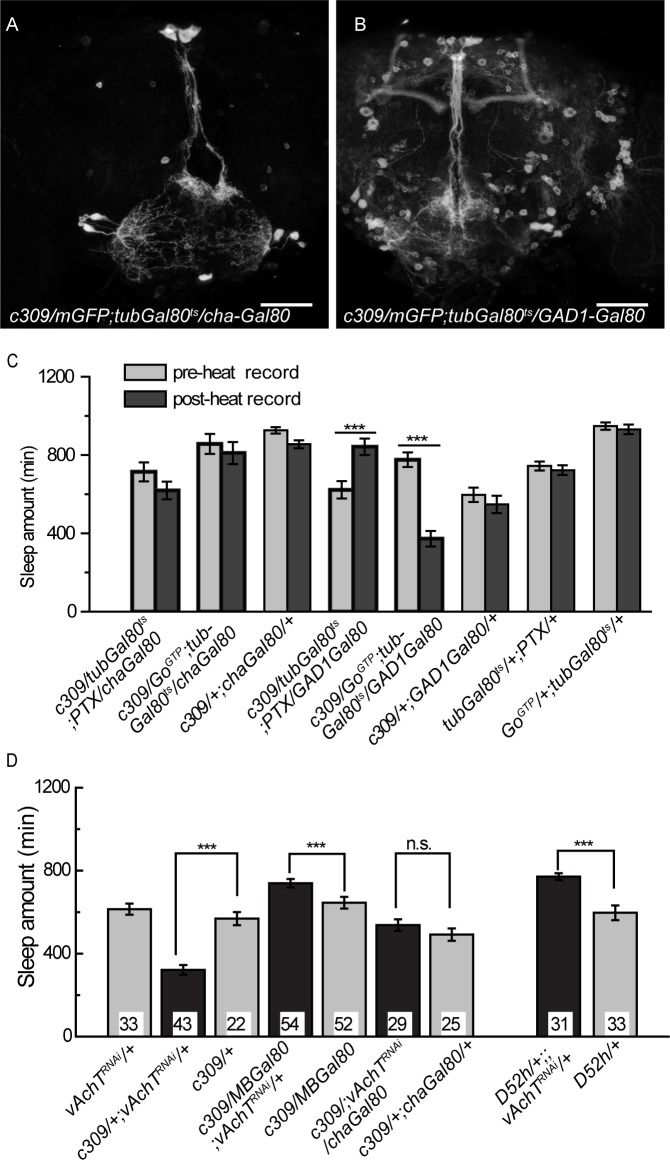
To examine which neurotransmitter(s) are used in these MB α/β core neurons, we used Gal80 tools to inhibit expression in specific neural subtypes. Cha3.3kb-Gal80 and GAD1-Gal80 are reported to suppress Gal4 activity in cholinergic neurons31 and GABAergic neurons,32 respectively. We found that c309-Gal4 expression in the MB was not affected by the presence of GAD1-Gal80, but was completely eliminated by Cha-Gal80 (Figures 7A and 7B), suggesting that c309-MB neurons are cholinergic but not GABAergic. We then examined the sleep status of these flies and found that combining Cha-Gal80, but not GAD1-Gal80, with c309-Gal4 could block sleep changes induced by temporally inhibiting (with PTX) or elevating (with GoGTP) Go activity (Figure 7C). These results imply that c309-MB neurons that function in sleep control are cholinergic.
Figure 7.
c309-MB neurons are cholinergic, and reducing the ACh signal in these neurons affects sleep. (A-B) c309 expression in the MB is blocked by Cha-Gal80 but not GAD1-Gal80 in adult stage flies. Scale bar represents 50 μm. (C) Cha-Gal80, but not GAD1-Gal80, eliminates sleep defects induced by temporally expression of PTX or GoGTP mediated by c309-Gal4. n = 25-39 in each group. (D) Continuously expressing vAChTRNAi by c309-Gal4 leads to reduced sleep, which is abolished by MB-Gal80 or Cha-Gal80. Sleep amount is increased in D52H > vAChTRNAi flies. Numbers on the bars indicate the number of flies in each group. ***P < 0.001 in paired Student t-tests (C) or one-way analysis of variance (D). n.s., no significant difference.
Vesicular acetylcholine transporter (vAChT) is required for acetylcholine transport and release.33 Sleep was not affected when we temporally knocked down vAChT by RNAi approach in the c309 neurons at the adult stage for up to 5 days (data not shown), possibly due to insufficient knockdown or residue vAChT protein from early stages. When vAChT was continuously knocked down in c309 neurons, flies showed significantly reduced amounts of sleep compared with their parental controls (Figure 7D). Combining Cha-Gal80 or MB-Gal80 with c309-Gal4 completely blocked this sleep reduction (Figure 7D). We also noticed that the presence of MB-Gal80 not only eliminated this sleep reduction, but also caused an increase of sleep amount (Figure 7D). We suggest that some c309 neurons outside the MBs regulated sleep through ACh output, whereas Go signaling was not acquired in these neurons for sleep regulation. Choline acetyltransferase (ChAT) is expressed only in cholinergic neurons and is critical for the synthesis of acetylcholine.34 By knocking down ChAT with c309-Gal4, we observed similar sleep amount decrease, which could also be eliminated by MB-Gal80 (Figure S10). Continuous knocking down of vAChT or ChAT in the MBs may affect MB development, thus causing the sleep defects. We then checked the morphology of the MBs in these adult flies by FasII staining. The mushroom bodies were morphologically normal in all flies examined (Figures S11A through S11E). Thus, these observations suggest that c309-MB neurons are cholinergic, and that the ACh signal in these neurons plays an essential role in sleep regulation.
Since under continuous induction, c309-Gal4 also drives expression in the α/β surface/posterior and γ neurons, in addition to MB α/β core neurons (Figure S4A). Thus we wondered which group of the MB neurons contributes to the sleep decrease in c309 > vAChTRNAi flies. We observed that when D52H-Gal4 was allowed to continually drive mGFP expression, the mGFP reporter signal was hardly detected in the MB α/β core neurons, whereas strong expression was observed in the MB α/β surface/posterior and γ neurons, which is identical to heat induction at adult stage only (Figures S12A through S12C). Introducing cha-Gal80 could eliminate the expression in the MB γ neurons and strongly reduced the signal in the α/β surface/posterior neurons (Figures S12D through S12F), implying that the α/β surface/posterior and γ neurons could also be largely cholinergic. We thus used D52H-Gal4 and continuously expressed vAChTRNAi to reduce ACh signal in the surface/posterior and γ neurons. Continuous knocking down of vAChT in D52h neurons did not cause long-term structural defects in the MB (Figure S11F). In contrast with the sleep reduction in c309 > vAChTRNAi flies, these D52H > vAChTRNAi flies showed increased sleep compared to their parental controls (Figure 7D), which could be eliminated by the presence of MB-Gal80 or Cha-Gal80 (Figure S12G). Therefore, the sleep-suppressing effect in c309 > vAChTRNAi flies should not be attributed to the surface/ posterior and γ neurons. These results suggest that the ACh signal is required in the MB α/β core neurons to promote sleep, and in surface/posterior and γ neurons to promote wakefulness.
Notably, reducing the ACh signal in MB neurons resulted in similar sleep defects to those in c309 > GoGTP flies, including decreased total sleep amount, fewer sleep bouts during the daytime, and comparable sleep bout numbers and shorter sleep bout duration during the nighttime (Figure S13 and Figures 2D and 2E). These findings suggest that in these cholinergic MB neurons, Go functions as an inhibitory signal in terms of ACh output.
DISCUSSION
Using a video system, we performed an in-depth investigation on sleep in flies and obtained a detailed picture of MB function in sleep regulation. We found that two adjacent subtypes of MB cholinergic neurons require both Go signaling and Ach output to modulate sleep. Furthermore, these two subtypes of MB neurons play opposite roles in sleep regulation.
Functionally Antagonistic Layers of MB α/β Lobe
Among MB neurons, the latest born neurons form the core layer of the α/β lobe, thus are called α/β core neurons.35 They are morphologically similar to adjacent α/β surface neurons, but could be labeled by different enhancer-trap based Gal4 lines as shown in previous studies22,28,36 and in this study (Figures 3B, 5D, and 5E), suggesting different gene expression profiles in different subtypes. Recently, the MB α/β core neurons have been highlighted to play a special permissive role for long-term memory consolidation in Drosophila.37 In the sleep research, although the MB is reported mainly as a wake-promoting brain region, it has also been suggested that MB α/β core neurons have sleep-promoting function by manipulating PKA activity within the Gs signaling.17 However, the Gal4 lines are also expressed in non-MB regions, such as the pars intercerebras (PI), in which PKA signal is also required to regulate sleep.16 Thus, it remains unclear whether the MB contains both sleep-and wake-promoting neurons, and how they are organized. In this study using the TARGET system, c309 non-MB neurons also contributed greatly to the PKA-mediated sleep regulation (Figure S5); however, by combining with MB-Gal80 and comparing with Gal4 lines having close insertion sites (sca109–68, NP2122 and NP4289), we demonstrated that the sleep defects when manipulating Go activity in c309 neurons are attributed to the MB α/β core neurons. Taken together with the opposite sleep effects of blocking Go and knocking-down ACh signal in various Gal4s labeling nonoverlapping MB subtypes, we demonstrate that the MB α/β core neurons are responsible for sleep-promoting effects, whereas the adjacent subtypes of MB neurons exert wake-promoting function.
It is notable that these functional antagonistic MB neurons are spatially very close, forming the inner layer and outer layer of the MB α/β lobe, respectively, raising the possibility that these neurons may have direct connections. In the behavior experiments, when blocking the inhibitory Go signaling in both subtypes, the sleep profile was more like that when Go was blocked only in the surface neurons (Figure 6E). We thus suspect that there may have been functional interaction between these two MB layers during sleep regulation. Mutual inhibitory interactions between the sleep-promoting and wake-promoting circuits have been proposed to control sleep/wake switches in the “flip-flop” way,38 whereas the molecular pathway has not been uncovered. The requirement of inhibitory Go signaling in both sleep- and wake- promoting neurons suggest that Go may play an essential role in mediating the inhibitory signals between the antagonistic neuropils.
Cholinergic Nature of Subsets of MB Neurons
Although the MB has been highlighted for playing important functions in sleep as well as learning and memory, its neurochemistry has not been well characterized. Immunostaining of ChAT detects signals in the MB area,39 however, it is not conclusive enough to determine whether MB neurons express ChAT or merely receive projections from cholinergic neurons.40 The 3.3 Kb sequence upstream of ChAT gene directs expression largely similar to the distribution of endogenous ChAT protein.41 Cha3.3kb-Gal80 in which Gal80 expression was directed by this flanking sequence, could eliminate the Gal4 expression in the MB neurons in previous work42 and this study (Figure 7A), supporting that MB neurons are cholinergic. Notably, we show that knocking down vAChT and ChAT with c309-Gal4 results in the sleep decrement, which can be eliminated by Cha3.3kb-Gal80 or MB-Gal80 (Figure 7D and Figure S10). These results provide the first functional evidence that at least a portion of MB neurons are cholinergic and their ACh signal is required for sleep regulation in flies.
In mammals, pharmacological and electrophysiological studies suggest the cholinergic nuclei in the brainstem promote rapid eye movement sleep or wake, whereas in the forebrain they correlate with wake only.43,44 Direct lesions of different putative cholinergic nuclei showed different effects in rapid eye movement sleep, suggesting complicated functions of ACh system in sleep.45 In our study of flies, reducing ACh output in different subsets of cholinergic neurons affected sleep in different ways (Figure 7D), suggesting there are also different cholinergic neural clusters in flies exerting distinct effects on sleep regulation. Interestingly, the functionally opposite cholinergic neuron subtypes are adjacent to each other. The relative simple neural circuits of flies will allow us to further study the cholinergic system in sleep control.
Go Signaling and Cholinergic Neurons in Sleep Disorder
Of significant interest, blocking Go activity in the MB α/β core neurons led to special sleep phenomena. These flies displayed greatly increased daytime sleep when they should be wakeful (Figure 1E), resembling hypersomnia in mammals. Moreover, they also showed fragmented nighttime sleep and frequent sleep/wake switches (Figures 1F through 1H). These phenomena are always found in narcolepsy, one of the most common hypersomnia sleep disorders, but not in other types of hypersomnia such as idiopathic hypersomnia.46 Narcolepsy is closely correlated with deficiency in orexin system. Orexin is reported to activate Gα11 and the inhibitory PTX-sensitive Gi/o protein in vitro and in vivo in mesopontine neurons, including the cholinergic nuclei.47,48 Furthermore, increased ACh output has been observed in narcolepsy mouse models,49 whereas whether and how the cholinergic system is involved in the pathology of narcolepsy are unclear. Our work with fruit flies showed that blocking the inhibitory Go activity in specific cholinergic neurons led to several narcolepsy-like sleep defects, suggesting insufficient inhibition of special cholinergic cluster may be directly related to this sleep disorder. Future studies on the function of the Go and ACh in narcolepsy in particular neural circuits may provide new understanding for the pathogenesis of narcolepsy.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by grants from the “973” Projects (Grant 2011CBA00400 to Dr. Aike Guo), the National Science Foundation of China (Grant 31130027 to Dr. Aike Guo, 31070956 and 91132709 to Dr. Yan Li), One Hundred Talent Project of the Chinese Academy of Sciences (Grant KSCX2-YW-R-156 to Dr. Yan Li), and the “Strategic Priority Research Program” of the Chinese Academy of Sciences (Grant XDB02040100).
ACKNOWLEDGMENTS
The authors thank Y. N. Jan, A. Sehgal, A. Tomlinson, S. Waddell, G. Roman, T. Sakai, Y. Rao, and Y. Zhong, as well as the Bloomington Stock center, the Vienna Drosophila RNAi center and the Drosophila Genetic Resource center for fly stocks. We are grateful to X. D. Fu for help in writing the software, to Z. P. Zhang, Z. H. Wu, and H. M. Lu for help with manuscript preparing, to J. W. Hou for technical assistance, and to members of the Guo Lab for critical discussions.
ABBREVIATIONS
- ACh
acetylcholine
- ChAT
Choline acetyltransferase
- DA
dopamine
- DAMS
Drosophila activity monitor system
- DD
constant dark condition
- LD
light-dark condition
- MB
Mushroom body
- mGFP
membrane-targeted-GFP
- OA
octopamine
- PIs
pars intercerebralis
- PKA
protein kinase A
- PTX
pertussis toxin
- REM
rapid eye movement sleep
- TARGET
the temporal and regional gene expression targeting system.
- vAChT
vesicular acetylcholine transporter
- ZT
zeitgeber time
SUPPLEMENTAL MATERIAL
Free-running circadian rhythm parameters for flies after 12-h heat shock treatment and 12-h recovery.
The video recording system for sleep analysis in flies. (A) Cartoon schematic diagram of an individual fly in a tube under video recording. (B) A representative picture captured by the video system. Coordinates of each fly are marked with an X. Movements of flies can be recognized at any position in the tube, including on the food (as in tube 1), close to the food (tube 8), in the middle (tube 3), and close to the cotton (tube 2). By manually validating the activities of these four flies, the rate of false negatives (active but taken as inactive) and false positives (inactive but taken as active) during different time zones is listed.
Temporal expression of pertussis toxin (PTX) in c309 neurons leads to sleep increment. (A) Sleep profiles of c309-Gal4/tub-Gal80ts;UAS-PTX/+ flies (black hollow squares, n = 29) and its parental control flies of tub-Gal80ts/+;UAS-PTX/+ (gray solid circles, n = 25). White and gray background colors show light/dark periods. (B) Sleep amount was significantly increased in c309-Gal4/tub-Gal80ts;UASPTX/+ flies after expressing PTX for 12 h of heat shock induction during the daytime. Data are presented as the mean ± standard error of the mean.
Blocking Go signaling in c309 neurons leads to hypersomnia during constant dark (DD) condition. (A) Sleep profiles of c309-Gal4/ tub-Gal80ts;UAS-pertussis toxin (PTX)/+ flies (black hollow squares, n = 15) and the parental control flies of tub-Gal80ts/+;UAS-PTX/+ (gray solid circles, n = 8). Light gray and dark gray background colors show subjective light/dark periods. (B and C) Sleep amount (B) and bout number (C) is significantly increased after temporally inducing PTX expression in c309 neurons. Data are analyzed by paired Student t-test. ***P < 0.001; *P < 0.05. Data are presented as the mean ± standard error of the mean. Numbers on the bars represent group number.
c309-Gal4 expression pattern in different stages. The fly genotype is c309-Gal4/UAS-mCD8::GFP;tub-Gal80ts/+. (A) Flies were maintained continually at the restrictive temperature of tub-Gal80ts (31°C) to allow Gal4 activity until being dissected 7 days after eclosion. The GFP signal shows strong expression in the entire mushroom body (MB) including the α/β and γ lobes. (B) Flies were kept at 18°C and switched to 31°C 3 days before eclosion and were dissected right after eclosion. GFP expression is also strong in MB lobe area, including the α/β and γ lobes. (C) Flies were kept continually in permissive temperature of tub-Gal80ts (18°C) to block Gal4 activity until being dissected 7 days after eclosion. There is rare GFP signal in the brain. Scale bars represent 50 μm.
Temporal expression of mc* in either all c309 neurons or c309 non-mushroom body (MB) neurons decreases sleep. (A) Change in sleep amount (calculated by subtracting sleep before heat induction from sleep after heat-induction) in c309-Gal4/UAS-mc*;tub-Gal80ts/+ flies or in c309-Gal4,MB-Gal80/UAS-mc*; tub-Gal80ts/+ flies are statistically indistinguishable, but significantly decreased from that in their parental controls c309-Gal4/+ flies, c309-Gal4,MB-Gal80/+ flies or UAS-mc*/+; tub-Gal80ts/+ flies. Data are analyzed by one-way analysis of variance. ***P < 0.001; n.s., no significant difference, P > 0.05. (B) Temporal expression of mc* in either all c309 neurons or c309 non-MB neurons decreases sleep bout duration. Paired Student t-tests; ***P < 0.001. Data are presented as means ± standard error of the mean. n = 30-36 in each group.
Modulating Go activity in c309 neurons decreases the waking activity of flies. Temporal expression of pertussis toxin (PTX) or GoGTP with c309-Gal4 or c309-Gal4,MB-Gal80 induces significant decreases in waking activity (the total walking distance divided by the total waking time). Light gray bars and dark gray bars indicate waking activity for 2 light-dark days before or after heat induction, respectively. Data are analyzed by paired Student t-tests. ***P < 0.001; n = 30-36 in each group. Data are presented as means ± standard error of the mean.
Temporal expression of pertussis toxin (PTX) or GoGTP did not affect the global morphology of mushroom bodies (MBs) at the end of the postheat recording. (A) Procedure for heat induction and dissection for B through E. Expression of PTX or GoGTP was induced for 12 h and the fly brains were dissected at about 60 h after heat shock. (B-E) Representative projection view of the fly brains for each genotype. Gray signal indicates the FasII staining signal. (F) Procedure for dissection for G and H. The flies were kept in 18°C to block PTX or GoGTP expression before dissection. (G and H) Representative projection view of the fly brains for each genotype. Gray signal indicates the FasII staining signal. n = 10-15 for each genotype. Scale bar represents 50 μm.
Temporal expression of pertussis toxin (PTX) or GoGTP did not affect the general morphology of mushroom bodies (MBs) at the 20th day after heat induction. (A) Procedure for heat induction and dissection for B through F. PTX or GoGTP was induced to express for 12 h and the fly brains were dissected 20 days after heat shock. (B-F) Representative projection view of the fly brains for each genotype. Gray signal indicates the FasII staining signal. n = 10-15 for each genotype. Scale bar represents 50 μm.
Mushroom body (MB)-Gal80 eliminates pertussis toxin (PTX)-induced sleep effects in multiple MB lines. (A-C) Gal4 activity in the MB neuropil was blocked by MB-Gal80. (D) In the presence of MB-Gal80, temporal expression of PTX with NP3061, R15E01 or D52h lead to no significant change in sleep amount. Light gray bars and dark gray bars indicate sleep parameters for 2 light-dark days before and after heat induction, respectively. Data were analyzed by paired Student's t-tests; n = 30-36 in each group. Data are presented as means ± standard error of the mean. n.s., no significant difference by paired Student t-test. Scale bar represents 50 mm.
Knocking-down chat in c309-MB neurons decreases sleep. Continuously expressing chatRNAi by c309-Gal4 leads to reduced sleep amount, which is abolished by MB-Gal80. The numbers on bars indicate the number of flies in each group. ***P < 0.001 in one-way analysis of variance. Data are presented as means ± standard error of the mean.
Continuous expression of vAchTRNAi or chat-RNAi in c309 or D52h neurons did not affect the general morphology of MBs. (A-F) Representative projection view of the fly brains for each genotype. Gray signal indicates the FasII staining signal. n = 10-15 for each genotype. Scale bar represents 50 μm.
MB-Gal80 and Cha-Gal80 blocked the sleep increment by continual expression of vAChTRNAi in d52h neurons. (A-C) Continuous expressing GFP shows strong signal in D52h MB γ and α/β surface and posterior area. (D-F) Cha-Gal80 inhibited 52h-Gal4 activity in MB γ area and reduced Gal4 activity in the MB α/β area. Projection view of the MB lobe area (left) and a single horizontal section of the tip of the α lobe (right). FasII signal (red) shows the neuropils of MB neurons. Scale bar represents 20 μm. (G) In the presence of MB-Gal80 or cha-Gal80, sleep amount was not significantly increased by expressing vAChTRNAi. n = 20-33 for each genotype. n.s, no significant difference by one-way analysis of variance.
Continual expression of vAChTRNAi in c309-MB neurons affects daytime and night-time sleep differently. (A) Number of daytime sleep bouts is decreased in c309 > vAChTRNAi flies but not in c309,MB-Gal80 > vAChTRNAi flies (left), whereas it is not affected in either fly during the nighttime (right). (B) Duration of sleep bouts is slightly (daytime) or severely (nighttime) decreased in c309 > vAChTRNAi flies. This effect was eliminated by combining with MB-Gal80. n = 16-17 for each genotype. *P < 0.05, ***P < 0.001, n.s., no significant difference by one-way analysis of variance.
REFERENCES
- 1.Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendricks JC, Finn SM, Panckeri KA, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–38. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 3.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–7. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 4.Nitz DA, van Swinderen B, Tononi G, Greenspan RJ. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr Biol. 2002;12:1934–40. doi: 10.1016/s0960-9822(02)01300-3. [DOI] [PubMed] [Google Scholar]
- 5.van Swinderen B, Nitz DA, Greenspan RJ. Uncoupling of brain activity from movement defines arousal states in Drosophila. Curr Biol. 2004;14:81–7. [PubMed] [Google Scholar]
- 6.Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G, Cirelli C. Sleep homeostasis in Drosophila melanogaster. Sleep. 2004;27:628–39. doi: 10.1093/sleep/27.4.628. [DOI] [PubMed] [Google Scholar]
- 7.Allada R, Siegel JM. Unearthing the phylogenetic roots of sleep. Curr Biol. 2008;18:R670–9. doi: 10.1016/j.cub.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–84. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–75. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Crocker A, Sehgal A. Octopamine regulates sleep in drosophila through protein kinase A-dependent mechanisms. J Neurosci. 2008;28:9377–85. doi: 10.1523/JNEUROSCI.3072-08a.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–62. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci. 2008;11:354–9. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parisky KM, Agosto J, Pulver SR, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–82. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueno T, Tomita J, Tanimoto H, et al. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat Neurosci. 2012;15:1516–23. doi: 10.1038/nn.3238. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Liu S, Kodama L, Driscoll MR, Wu MN. Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr Biol. 2012;22:2114–23. doi: 10.1016/j.cub.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crocker A, Shahidullah M, Levitan IB, Sehgal A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron. 2010;65:670–81. doi: 10.1016/j.neuron.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–60. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 18.Pitman JL, McGill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–6. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- 19.Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol. 2008;18:1110–7. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebestky T, Chang JS, Dankert H, et al. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64:522–36. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo F, Yi W, Zhou M, Guo A. Go signaling in mushroom bodies regulates sleep in Drosophila. Sleep. 2011;34:273–81. doi: 10.1093/sleep/34.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–55. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman JE, Raizen DM, Maycock MH, Maislin G, Pack AI. A video method to study Drosophila sleep. Sleep. 2008;31:1587–98. doi: 10.1093/sleep/31.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilestro GF, Cirelli C. pySolo: a complete suite for sleep analysis in Drosophila. Bioinformatics. 2009;25:1466–7. doi: 10.1093/bioinformatics/btp237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–8. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 26.Thambi NC, Quan F, Wolfgang WJ, Spiegel A, Forte M. Immunological and molecular characterization of Go alpha-like proteins in the Drosophila central nervous system. J Biol Chem. 1989;264:18552–60. [PubMed] [Google Scholar]
- 27.Madalan A, Yang X, Ferris J, Zhang S, Roman G. G(o) activation is required for both appetitive and aversive memory acquisition in Drosophila. Learn Mem. 2012;19:26–34. doi: 10.1101/lm.024802.111. [DOI] [PubMed] [Google Scholar]
- 28.Aso Y, Grübel K, Busch S, Friedrich AB, Siwanowicz I, Tanimoto H. The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet. 2009;23:156–72. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- 29.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron. 2007;53:103–15. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang M, Gold MS, Boulay G, et al. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc Natl Acad Sci USA. 1998;95:3269–74. doi: 10.1073/pnas.95.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitamoto T. Conditional disruption of synaptic transmission induces male-male courtship behavior in Drosophila. Proc Natl Acad Sci USA. 2002;99:13232–7. doi: 10.1073/pnas.202489099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakai T, Kasuya J, Kitamoto T, Aigaki T. The Drosophila TRPA channel, Painless, regulates sexual receptivity in virgin females. Genes Brain Behav. 2009;8:546–57. doi: 10.1111/j.1601-183X.2009.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitamoto T, Xie X, Wu C-F, Salvaterra PM. Isolation and characterization of mutants for the vesicular acetylcholine transporter gene in Drosophila melanogaster. J Neurobiol. 2000;42:161–71. [PubMed] [Google Scholar]
- 34.Kitamoto T, Ikeda K, Salvaterra PM. Analysis of cis-regulatory elements in the 5′ flanking region of the Drosophila melanogaster choline acetyltransferase gene. J Neurosci. 1992;12:1628–39. doi: 10.1523/JNEUROSCI.12-05-01628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurusu M, Awasaki T, Masuda-Nakagawa LM, Kawauchi H, Ito K, Furukubo-Tokunaga K. Embryonic and larval development of the Drosophila mushroom bodies: concentric layer subdivisions and the role of fasciclin II. Development. 2002;129:409–19. doi: 10.1242/dev.129.2.409. [DOI] [PubMed] [Google Scholar]
- 36.Takamatsu Y, Nakagoshi H, Rachidi M, Lopes C, Nishida Y, Ohsako S. Characterization of the dCaMKII-GAL4 driver line whose expression is controlled by the Drosophila Ca2+/calmodulin-dependent protein kinase II promoter. Cell Tissue Res. 2002;310:237–52. doi: 10.1007/s00441-002-0631-y. [DOI] [PubMed] [Google Scholar]
- 37.Huang C, Zheng X, Zhao H, et al. A permissive role of mushroom body alpha/beta core neurons in long-term memory consolidation in Drosophila. Curr Biol. 2012;22:1981–9. doi: 10.1016/j.cub.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 38.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorczyca MG, Hall JC. Immunohistochemical localization of choline acetyltransferase during development and in Chats mutants of Drosophila melanogaster. J Neurosci. 1987;7:1361–9. doi: 10.1523/JNEUROSCI.07-05-01361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johard HA, Enell LE, Gustafsson E, Trifilieff P, Veenstra JA, Nassel DR. Intrinsic neurons of Drosophila mushroom bodies express short neuropeptide F: relations to extrinsic neurons expressing different neurotransmitters. J Comp Neurol. 2008;507:1479–96. doi: 10.1002/cne.21636. [DOI] [PubMed] [Google Scholar]
- 41.Sejourne J, Placais P-Y, Aso Y, et al. Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nat Neurosci. 2011;14:903–10. doi: 10.1038/nn.2846. [DOI] [PubMed] [Google Scholar]
- 42.Wu C-L, Xia S, Fu T-F, et al. Specific requirement of NMDA receptors for long-term memory consolidation in Drosophila ellipsoid body. Nat Neurosci. 2007;10:1578–86. doi: 10.1038/nn2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz JR, Roth T. Neurophysiology of sleep and wakefulness: basic science and clinical implications. Curr Neuropharmacol. 2008;6:367–78. doi: 10.2174/157015908787386050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiromani PJ, Gillin JC, Henriksen SJ. Acetylcholine and the regulation of REM sleep: basic mechanisms and clinical implications for affective illness and narcolepsy. Annu Rev Pharmacol Toxicol. 1987;27:137–56. doi: 10.1146/annurev.pa.27.040187.001033. [DOI] [PubMed] [Google Scholar]
- 45.Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res. 1988;458:285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- 46.Chesson AL. Classification of sleep disorders. In: Lee-Chiong TL, editor. Sleep: a comprehensive handbook. New Jersey: John Wiley & Sons, Inc; 2005. pp. 83–9. [Google Scholar]
- 47.Moreno-Balandrán E, Garzón M, Bódalo C, Reinoso-Suárez F, De Andrés I. Sleep-wakefulness effects after microinjections of hypocretin 1 (orexin A) in cholinoceptive areas of the cat oral pontine tegmentum. Eur J Neurosci. 2008;28:331–41. doi: 10.1111/j.1460-9568.2008.06334.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Y, Miwa Y, Yamanaka A, et al. Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci. 2003;92:259–66. doi: 10.1254/jphs.92.259. [DOI] [PubMed] [Google Scholar]
- 49.Kalogiannis M, Grupke SL, Potter PE, et al. Narcoleptic orexin receptor knockout mice express enhanced cholinergic properties in laterodorsal tegmental neurons. Eur J Neurosci. 2010;32:130–42. doi: 10.1111/j.1460-9568.2010.07259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Free-running circadian rhythm parameters for flies after 12-h heat shock treatment and 12-h recovery.
The video recording system for sleep analysis in flies. (A) Cartoon schematic diagram of an individual fly in a tube under video recording. (B) A representative picture captured by the video system. Coordinates of each fly are marked with an X. Movements of flies can be recognized at any position in the tube, including on the food (as in tube 1), close to the food (tube 8), in the middle (tube 3), and close to the cotton (tube 2). By manually validating the activities of these four flies, the rate of false negatives (active but taken as inactive) and false positives (inactive but taken as active) during different time zones is listed.
Temporal expression of pertussis toxin (PTX) in c309 neurons leads to sleep increment. (A) Sleep profiles of c309-Gal4/tub-Gal80ts;UAS-PTX/+ flies (black hollow squares, n = 29) and its parental control flies of tub-Gal80ts/+;UAS-PTX/+ (gray solid circles, n = 25). White and gray background colors show light/dark periods. (B) Sleep amount was significantly increased in c309-Gal4/tub-Gal80ts;UASPTX/+ flies after expressing PTX for 12 h of heat shock induction during the daytime. Data are presented as the mean ± standard error of the mean.
Blocking Go signaling in c309 neurons leads to hypersomnia during constant dark (DD) condition. (A) Sleep profiles of c309-Gal4/ tub-Gal80ts;UAS-pertussis toxin (PTX)/+ flies (black hollow squares, n = 15) and the parental control flies of tub-Gal80ts/+;UAS-PTX/+ (gray solid circles, n = 8). Light gray and dark gray background colors show subjective light/dark periods. (B and C) Sleep amount (B) and bout number (C) is significantly increased after temporally inducing PTX expression in c309 neurons. Data are analyzed by paired Student t-test. ***P < 0.001; *P < 0.05. Data are presented as the mean ± standard error of the mean. Numbers on the bars represent group number.
c309-Gal4 expression pattern in different stages. The fly genotype is c309-Gal4/UAS-mCD8::GFP;tub-Gal80ts/+. (A) Flies were maintained continually at the restrictive temperature of tub-Gal80ts (31°C) to allow Gal4 activity until being dissected 7 days after eclosion. The GFP signal shows strong expression in the entire mushroom body (MB) including the α/β and γ lobes. (B) Flies were kept at 18°C and switched to 31°C 3 days before eclosion and were dissected right after eclosion. GFP expression is also strong in MB lobe area, including the α/β and γ lobes. (C) Flies were kept continually in permissive temperature of tub-Gal80ts (18°C) to block Gal4 activity until being dissected 7 days after eclosion. There is rare GFP signal in the brain. Scale bars represent 50 μm.
Temporal expression of mc* in either all c309 neurons or c309 non-mushroom body (MB) neurons decreases sleep. (A) Change in sleep amount (calculated by subtracting sleep before heat induction from sleep after heat-induction) in c309-Gal4/UAS-mc*;tub-Gal80ts/+ flies or in c309-Gal4,MB-Gal80/UAS-mc*; tub-Gal80ts/+ flies are statistically indistinguishable, but significantly decreased from that in their parental controls c309-Gal4/+ flies, c309-Gal4,MB-Gal80/+ flies or UAS-mc*/+; tub-Gal80ts/+ flies. Data are analyzed by one-way analysis of variance. ***P < 0.001; n.s., no significant difference, P > 0.05. (B) Temporal expression of mc* in either all c309 neurons or c309 non-MB neurons decreases sleep bout duration. Paired Student t-tests; ***P < 0.001. Data are presented as means ± standard error of the mean. n = 30-36 in each group.
Modulating Go activity in c309 neurons decreases the waking activity of flies. Temporal expression of pertussis toxin (PTX) or GoGTP with c309-Gal4 or c309-Gal4,MB-Gal80 induces significant decreases in waking activity (the total walking distance divided by the total waking time). Light gray bars and dark gray bars indicate waking activity for 2 light-dark days before or after heat induction, respectively. Data are analyzed by paired Student t-tests. ***P < 0.001; n = 30-36 in each group. Data are presented as means ± standard error of the mean.
Temporal expression of pertussis toxin (PTX) or GoGTP did not affect the global morphology of mushroom bodies (MBs) at the end of the postheat recording. (A) Procedure for heat induction and dissection for B through E. Expression of PTX or GoGTP was induced for 12 h and the fly brains were dissected at about 60 h after heat shock. (B-E) Representative projection view of the fly brains for each genotype. Gray signal indicates the FasII staining signal. (F) Procedure for dissection for G and H. The flies were kept in 18°C to block PTX or GoGTP expression before dissection. (G and H) Representative projection view of the fly brains for each genotype. Gray signal indicates the FasII staining signal. n = 10-15 for each genotype. Scale bar represents 50 μm.
Temporal expression of pertussis toxin (PTX) or GoGTP did not affect the general morphology of mushroom bodies (MBs) at the 20th day after heat induction. (A) Procedure for heat induction and dissection for B through F. PTX or GoGTP was induced to express for 12 h and the fly brains were dissected 20 days after heat shock. (B-F) Representative projection view of the fly brains for each genotype. Gray signal indicates the FasII staining signal. n = 10-15 for each genotype. Scale bar represents 50 μm.
Mushroom body (MB)-Gal80 eliminates pertussis toxin (PTX)-induced sleep effects in multiple MB lines. (A-C) Gal4 activity in the MB neuropil was blocked by MB-Gal80. (D) In the presence of MB-Gal80, temporal expression of PTX with NP3061, R15E01 or D52h lead to no significant change in sleep amount. Light gray bars and dark gray bars indicate sleep parameters for 2 light-dark days before and after heat induction, respectively. Data were analyzed by paired Student's t-tests; n = 30-36 in each group. Data are presented as means ± standard error of the mean. n.s., no significant difference by paired Student t-test. Scale bar represents 50 mm.
Knocking-down chat in c309-MB neurons decreases sleep. Continuously expressing chatRNAi by c309-Gal4 leads to reduced sleep amount, which is abolished by MB-Gal80. The numbers on bars indicate the number of flies in each group. ***P < 0.001 in one-way analysis of variance. Data are presented as means ± standard error of the mean.
Continuous expression of vAchTRNAi or chat-RNAi in c309 or D52h neurons did not affect the general morphology of MBs. (A-F) Representative projection view of the fly brains for each genotype. Gray signal indicates the FasII staining signal. n = 10-15 for each genotype. Scale bar represents 50 μm.
MB-Gal80 and Cha-Gal80 blocked the sleep increment by continual expression of vAChTRNAi in d52h neurons. (A-C) Continuous expressing GFP shows strong signal in D52h MB γ and α/β surface and posterior area. (D-F) Cha-Gal80 inhibited 52h-Gal4 activity in MB γ area and reduced Gal4 activity in the MB α/β area. Projection view of the MB lobe area (left) and a single horizontal section of the tip of the α lobe (right). FasII signal (red) shows the neuropils of MB neurons. Scale bar represents 20 μm. (G) In the presence of MB-Gal80 or cha-Gal80, sleep amount was not significantly increased by expressing vAChTRNAi. n = 20-33 for each genotype. n.s, no significant difference by one-way analysis of variance.
Continual expression of vAChTRNAi in c309-MB neurons affects daytime and night-time sleep differently. (A) Number of daytime sleep bouts is decreased in c309 > vAChTRNAi flies but not in c309,MB-Gal80 > vAChTRNAi flies (left), whereas it is not affected in either fly during the nighttime (right). (B) Duration of sleep bouts is slightly (daytime) or severely (nighttime) decreased in c309 > vAChTRNAi flies. This effect was eliminated by combining with MB-Gal80. n = 16-17 for each genotype. *P < 0.05, ***P < 0.001, n.s., no significant difference by one-way analysis of variance.