Abstract
Study Objectives:
Nocturnal cardiovascular events are more frequent at the beginning and end of the night. It was proposed that this pattern reflects the nocturnal distribution of sleep and sleep stages. Using heart rate variability (HRV), we recently showed an interaction between the circadian system and vigilance states on the regulation of cardiac rhythmicity. Here, we further investigate this interaction in order to clarify the specific effects of sleep stages on the regulation of the heart.
Design:
Participants underwent a 72-h ultradian sleep-wake cycle procedure in time isolation consisting of alternating 60-min wake episodes in dim light and 60-min nap opportunities in total darkness.
Setting:
Time isolation suite.
Patients or participants:
Fifteen healthy young participants; two were subsequently excluded.
Interventions:
N/A.
Measurements and Results:
The current study revealed that sleep onset and progression to deeper sleep stages was associated with a shift toward greater parasympathetic modulation, whereas rapid eye movement (REM) sleep was associated with a shift toward greater sympathetic modulation. We found a circadian rhythm of heart rate (HR) and high-frequency power during wakefulness and all non-REM sleep stages. A significant circadian rhythm of HR and sympathovagal balance of the heart was also observed during REM sleep. During slow wave sleep, maximal parasympathetic modulation was observed at ∼02:00, whereas during REM sleep, maximal sympathetic modulation occurred in the early morning.
Conclusion:
The circadian and sleep stage-specific effects on heart rate variability are clinically relevant and contribute to the understanding of the degree of cardiovascular vulnerability during sleep.
Citation:
Boudreau P; Yeh WH; Dumont GA; Boivin DB. Circadian variation of heart rate variability across sleep stages. SLEEP 2013;36(12):1919-1928.
Keywords: Autonomic nervous system, circadian rhythms, heart rate variability, sleep
INTRODUCTION
Adverse cardiovascular events exhibit a clear circadian pattern, with maximal occurrence during morning hours.1 Indeed, there is a ∼40% higher incidence in acute myocardial infarction, sudden cardiac death, atrioventricular block, ventricular fibrillation, ventricular tachycardia, and ischemic events between 06:00-12:00 relative to the rest of the day.2 We and others have recently provided evidence that an interaction between circadian and sleep-wake dependent processes regulates heart rate variability (HRV), an accepted method to quantify autonomic nervous system (ANS) cardiac modulation. These observations could contribute to peak cardiovascular vulnerability observed in the morning.3,4 Although nighttime is assumed to be cardioprotective, a meta-analysis revealed a bimodal distribution of myocardial infarctions, sudden cardiac death, and implanted cardioverter-defibrillator at night with peak cardiovascular risk at the beginning and end of the night.5
Sleep stages alternate throughout a normal sleep period and correlate with changes in HRV. During nonrapid eye movement (NREM) sleep, the cardiovascular system is stable and parasympathetic cardiac modulation is stronger.6–11 During rapid eye movement (REM) sleep, the cardiovascular system is unstable and greatly influenced by surges in sympathetic activity.9–11 The increased circadian propensity to REM sleep in the early morning could, in part, explain the coincidental increased cardiac risk. Indeed, REM sleep could precipitate numerous adverse cardiac events such as arrhythmia,12 acute myocardial infarction,5 and sudden cardiac death,2 whereas NREM sleep could increase the risk of ischemic events in susceptible patients.13 Sleep disruption associated with a variety of sleep disorders such as sleep disordered breathing,14 periodic limb movements (PLMs),15 insomnia,16 and other medical conditions including nocturia17 and depression18 has also been associated with cardiovascular diseases. Prior studies have shown that misalignment between the sleep schedule and the endogenous circadian clock as observed in night shift workers may lead to elevated risk of adverse cardiovascular events.19
Based on this evidence, we believe it is important to investigate the interaction between sleep stages and circadian phase on HRV. Our aim is to determine how different sleep stages occurring at conventional or unusual circadian phases can affect HRV and increase cardiovascular risk in patients with cardiovascular diseases, sleep disruption, or in populations with shifted sleep schedules. Here, we used a 72-h ultradian sleep-wake cycle (USW) procedure in constant posture, consisting of 60-min wake episodes in dim light (< 10 lux) alternating with 60-min nap episodes in total darkness. To our knowledge, we are the first to report sleep stage specific effects and HRV across a complete circadian cycle in humans.
METHODS
Participants
Fifteen healthy participants (12 men, 3 women; mean age ± standard deviation [SD]: 24.6 ± 4.5 y) with normal body mass index (body mass index [BMI], mean ± SD: 22.5 ± 2.1 kg/ m2) provided informed consent prior to their participation in this study. Demographic data and screening procedures were reported in a prior publication.3 Prior to laboratory entry, participants maintained a regular 8-h sleep period for ≥ 2 w based on their habitual sleep-wake schedule. Compliance was verified by daily phone calls to the laboratory at bedtimes and wake times, sleep-wake log, and actigraphic recordings during the week preceding laboratory admission (AW-64, Mini Mitter-Respirotronics, Bend, OR, USA).
Procedures
Participants were studied individually in a time isolation suite in the laboratory for 5 consecutive days. On the first night, a baseline 8-h sleep episode was planned according to the participant's habitual sleep schedule. Upon awakening, participants began a 72-h USW procedure consisting of 60-min wake episodes in dim light (< 10 lux) alternating with 60-min nap episode in total darkness (< 0.3 lux), and therefore was composed of 36 wake and 36 nap episodes in constant conditions.3 All experimental procedures were approved by the Douglas Mental Health University Institute Research Ethics Board and are within the ethical standards of the Declaration of Helsinki.
Measures and Data Processing
Core body temperature (CBT) was monitored (every 15 sec) using a thermistor (Steri-Probe, Cincinnati Sub-Zero Prod ucts Inc., Cincinnati, OH, USA) inserted 10 cm into the rectum and connected to an in-house data acquisition system. A dual-harmonic regression model without serial correlated noise3 was used to assess the minimum of individual CBT curves with a circadian period between the imposed limit of 23.91-24.41 h.
Electrocardiogram (EKG) was continuously recorded using a vest with built-in electrodes (LifeShirt, Vivometrics, Ventura, CA, USA; n = 9, 200 Hz) or using the EKG channel of our polysomnographic (PSG) recording system (EKG channel; 512 Hz; n = 6; high- and low-pass filtered: 1 Hz and 35 Hz, respectively). No significant group difference in HRV levels were found between these two recording methods so results were combined. In the six participants recorded with our PSG system, an automatic blood pressure measurement cuff was inflated once per nap episode. When a blood pressure measurement was associated with PSG-confirmed arousal (i.e., at least one 30-sec epoch scored as wake following blood pressure measurement), the corresponding EKG segment was removed from the analysis. This occurred 15 times in total and lasted ≤ 2.5 min in all cases. R-peaks were extracted from the EKG signal using a validated automatic detection software (VivoLogic, Vivometrics, Ventura, CA, USA).20 R-R intervals (RRI) were visually inspected for ectopic beats and artifacts, and were manually corrected by linear interpolation. Spectral power of RRI was calculated by discrete wavelet transform (DWT) using an ad hoc Matlab program (Matlab 7.4, The Math-Works, Natick, MA, USA) and the Wavelab toolbox.3,21 HRV was described as the spectral power in two standard frequency bands (i.e., high-frequency (HF) power: 0.15-0.30 Hz; low-frequency (LF) power: 0.0375-0.15 Hz).22 Prior to statistical analysis, HRV data were collapsed into 30-sec bins and normalized as percent deviation from individual's mean (i.e., mean calculated for the entire USW procedure).
PSG sleep recordings were performed across the USW procedure on a computerized system (Harmonie, Natus Medical Inc., Montreal, Qc, Canada). PSG recordings of all sleep periods and naps included a central and occipital electroencephalogram (EEG), electrooculogram (EOG), and submental electromyogram (EMG). All of these channels were sampled at 250 Hz (n = 9) or 512 Hz (n = 6). High- and low-pass filters were applied on EEG (0.3 Hz and 35 Hz), EMG (5 Hz and 35 Hz), and EOG (0.1 Hz and 15 Hz). PSG sleep recordings were visually scored according to standard criteria using 30-sec epochs.23 The following parameters were calculated for each nap. Sleep onset latency (SOL) was defined as the time interval between lights out and the first occurrence of at least two consecutive epochs of stage 1 (S1) sleep or any occurrence of deeper sleep stages. SOL was given a value of 60 min if no sleep occurred during a nap episode. REM sleep onset latency was the time interval between sleep onset and the first occurrence of an epoch of REM sleep. REM sleep onset latency was given a value of 60 min if no REM sleep occurred during a nap episode. NREM sleep was the amount of time spent in stage 1-4 (S1-S4) sleep, and slow wave sleep (SWS) was the amount of time spent in S3 and S4 sleep. Total sleep time (TST) was defined as the time spent in S1 to S4 plus REM sleep. Sleep efficiency (SE) was the percentage of the 60-min nap spent asleep (i.e., TST (min) / 60 (min) × 100%). S0 was classified as wake before or after sleep onset (WBSO and WASO, respectively). PLMs and sleep apnea/hypopnea were ruled out during the first nocturnal sleep period. For PLMs, EMG of the left and right anterior tibialis was recorded. Leg movements occurring at intervals of 4.0-90.0 sec and clustered in groups of four or greater were considered PLMs, in accordance with Coleman's criteria.24 Respiratory parameters were monitored with bucconasal thermistance and airflow pressure transducer. AASM-recommended criteria25 for defining apnea (≥ 90% reduction in airflow for ≥ 10 sec) and hypopnea (≥ 30% reduction in airflow for ≥ 10 sec) were used. Two participants were excluded (one with PLMs index = 13.9/h and one with apnea-hypopnea index = 5.85/h), leaving 13 participants with an apnea-hypopnea and PLMs index < 5/h.
Data and Statistical Analyses
Circadian Variation of Sleep Parameters
Each 30-sec sleep epoch during the USW procedure was assigned a circadian phase between 0° to 359.9° relative to the individual CBT minimum (set at 0°). Data were then collapsed into 30° circadian bins and folded every 360° (24 h) to obtain averaged individual's curves illustrating a complete circadian cycle of each sleep parameter for each subject. Similarly, SOL and REM SOL of each nap were attributed a circadian phase based on the time of lights out. The circadian variation of sleep parameters was statistically confirmed with a nonlinear mixed model using the nlmixed SAS procedure (SAS Institute Inc, Cary, NC, USA) as described in Eq. 1 without the stage effect.
HRV Regulation by Vigilance States
HRV data collected during the USW procedure were binned according to their corresponding sleep stages. A one-way repeated-measures analysis of variance was used to compare HRV among wake episodes and different vigilance states observed during naps (i.e., WBSO, WASO, S1, S2, SWS, and REM sleep). Significant main effects were further investigated using Tukey HSD pairwise comparisons. Linear mixed-model analysis (mixed procedure, SAS Institute Inc, Cary, NC, USA) was used to investigate the effect of SE and SWS on HRV parameters measured during each nap.
Circadian Variation of HRV During Each Vigilance State
HRV measurements were assigned a vigilance state (i.e., S0, S1, S2, SWS, REM sleep) and a circadian phase between 0° to 359.9° relative to the individual's CBT minimum (set at 0°). For each vigilance state, data were collapsed into 5° circadian bins. This bin size was chosen to conserve temporal localization of each sleep stage. A nonlinear mixed model was applied to HRV values using the nlmixed SAS procedure (SAS Institute Inc, Cary, NC, USA) in order to compare circadian phase and amplitude of each HRV parameter between sleep stages. The model is described as follows:
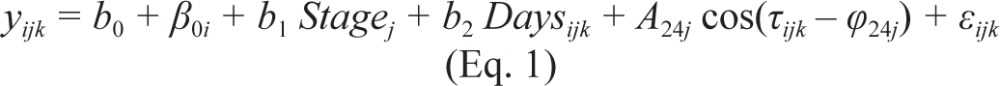 |
where yijk denotes the kth value for a given HRV parameter in the jth sleep stages for the ith participant at circadian degree τijk. The Y-intercept is described by b0 and β0i (fixed and random effects, respectively). The vigilance state effect is described by b1 (fixed effects). The time since the start of the experiment effect is described by b2 (fixed effect, % change/d). The amplitude of the 24-h rhythm in each state is described by A24j. Corresponding phase of peak activity (or acrophase) of this rhythm is described by φ24j. The residual error is assumed to be normally distributed εijk ∼ N(0,σ2) and independent. There was no significant improvement in adding another harmonic to our model when using Akaike Information Criterion (AIC), so we used a single harmonic. Student t test statistic was used to test whether fixed effect values were different from zero. Rhythms were considered significant if their amplitude was significantly different from zero. Differences in amplitude and phase of the 24-h component between vigilance states were tested using the F statistic. Each parameter was tested for significance at the level of P ≤ 0.05 and values are expressed as mean ± standard error of the mean (SEM).
RESULTS
The beginning of the USW procedure was scheduled based on each participant's habitual wake time (average ± SEM; 08:15 ± 00:12), and only data collected during the USW procedure was used for analysis. CBT minimum occurred on average (± SEM) at 05:23 ± 00:17. Sleep and HRV measurements were given a circadian phase between 0° and 359° based on the time relative to individual CBT minimum.
Circadian Variation of Sleep Parameters
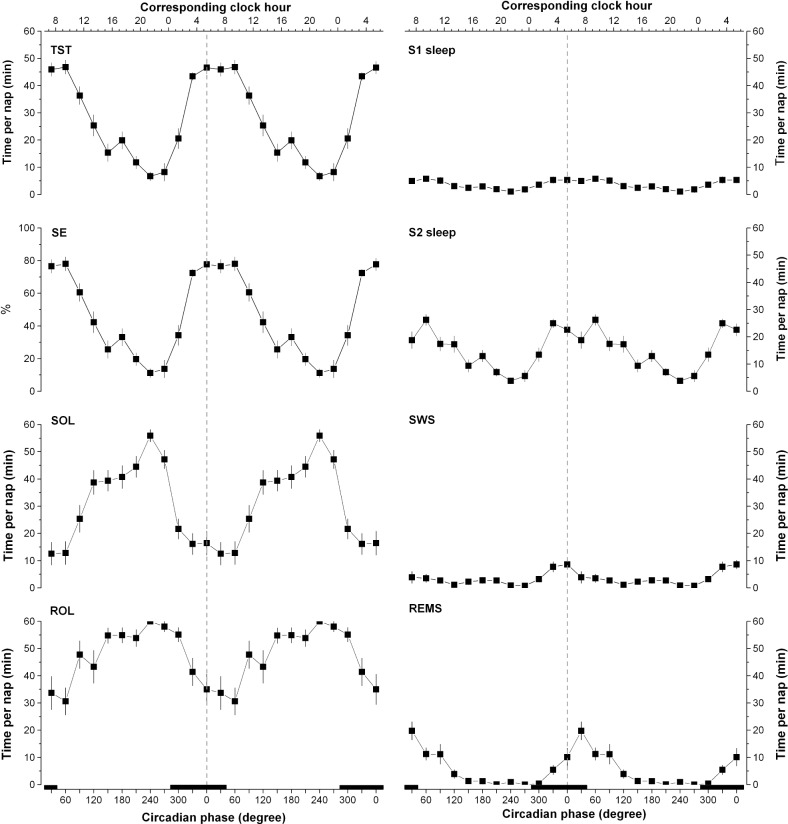
On average, participants slept for 27.3 ± 1.0 min/nap across each day of the 72-h USW procedure (day 1: 27.0 min/nap; day 2: 27.2 min/nap; day 3: 27.3 min/nap; no significant difference between days); 43.5 ± 1.4 min/nap during the four naps spanning the habitual nocturnal sleep episode, and 19.2 ± 1.1 min/ nap during the eight naps spanning the habitual wake period. A significant circadian rhythm of each sleep parameter was observed (P ≤ 0.0001; Figure 1). TST, SE, and S2 sleep measured during each nap episodes were maximal during the time spanning the habitual sleep period, whereas SOL was minimal at that time. S1 and SWS demonstrated a similar pattern, although it was of much smaller circadian amplitude than that of S2 and REM sleep. REM sleep showed a sharp peak in the morning at the end of the habitual sleep period with minimal values the rest of the day. The circadian variation of REM sleep onset latency followed the inverse pattern.
Figure 1.
Circadian variation of polysomnographic (PSG) sleep measures obtained throughout the ultradian sleep-wake cycle procedure. TST, total sleep time; SE, sleep efficiency; SOL, sleep onset latency; REMS, rapid-eye movement sleep; ROL, REM onset latency; S1, stage 1 sleep; S2, stage 2 sleep; SWS, slow wave sleep. Results were folded every 360° and repeated over two circadian cycles for illustrative purposes. A nonlinear mixed model was applied to individual subjects' 30°-binned data using nlmixed SAS procedure. All PSG measures had a significant circadian amplitude (amplitude significantly different from 0, P < 0.05). The vertical dotted line corresponds to the core body temperature (CBT) minimum. Bottom X axes represent circadian phase and top X axes represent the corresponding clock time (CBT minimum at 0° = 05:23). Black bars along the X axes represent the time of projected habitual nocturnal sleep episodes. All values are means ± standard error of the mean.
HRV Regulation by Vigilance States
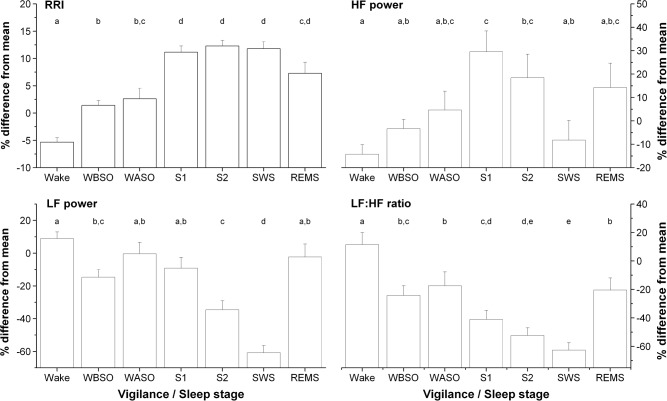
For each participant, HRV data were binned according to their corresponding sleep stage. A significant main effect of sleep stages was found on RRI and every HRV parameter (P ≤ 0.0002; Figure 2). Pairwise comparisons revealed that mean RRI was significantly lower during wake episodes compared to all sleep states measured during nap episodes (P ≤ 0.006). During nap episodes, the mean RRI measured during S1, S2, and SWS was significantly greater than that observed during WBSO (P < 0.0001) and WASO (P ≤ 0.0002). There was no significant difference in RRI between S1, S2, and SWS. REM sleep was associated with increased RRI compared to that of wake episodes and WBSO (P = 0.02), but similar to that of WASO (P = 0.13).
Figure 2.
Heart rate variability (HRV) measures obtained during each vigilance state (i.e., Wake: wake episodes; WBSO, wake before sleep onset; WASO, wake after sleep onset; S1, stage 1 sleep; S2, stage 2 sleep; SWS, slow wave sleep; REMS, rapid eye movement sleep). HRV measures were binned according their associated vigilance state, regardless of the nap number or circadian phase. The Y axis of HRV measures represents the % deviation from the subject's mean across the ultradian sleep-wake cycle procedure. Thus, a value of 0% represents individuals' mean. For each HRV component, letters above each column are used to indicate significant differences between vigilance/sleep stages, i.e., when two vigilance/sleep stages have no common letter, there are significantly different (P < 0.05). All values are mean ± standard error of the mean.
HF power observed during wakefulness (i.e., wake episodes, WBSO, or WASO) was comparable to that observed during SWS and REM sleep. HF power was maximal during S1 sleep, significantly increased compared to wake episodes (P < 0.001), WBSO (P = 0.02), and SWS (P = 0.005). HF power was significantly higher during S2 sleep compared to wake episodes only. The LF power level was higher during wake episodes, WASO, S1, and REM sleep, declined during S2 (P ≤ 0.02), and reached its lowest value during SWS (P ≤ 0.01). During REM sleep, the LF power was similar to that observed during wakefulness and S1. The LF:HF ratio was highest during wake episodes, progressively declined with deeper sleep stages, and reached its lowest value during SWS. This value was significantly reduced during SWS compared to all other stages (P ≤ 0.04), except that of S2 sleep. The LF:HF ratio of S2 was comparable to that of S1. During REM sleep, the LF:HF ratio was comparable to that of WBSO and WASO (P ≥ 0.99), significantly reduced compared to wake episodes (P = 0.0004), and significantly increased compared to that of all NREM sleep stages (P ≤ 0.001).
Results of the linear mixed model looking at the effect of SE and SWS on HRV indicate that the level of sleep disruption in naps significantly affected HRV (P ≤ 0.014). More specifically, we found that a 10% reduction in SE was associated with a mean (± SEM) reduction of 1.40 ± 0.13% and 3.12 ± 0.86% in RRI and HF power, respectively, as well as an increase of 1.61 ± 0.56% and 3.78 ± 0.54% in LF power and LF:HF ratio, respectively. In addition, a 10% increase in the amount of SWS within naps was associated with a 3.82 ± 0.72% increase in mean RRI, as well as a reduction of 17.30 ± 2.78% and 16.54 ± 1.53% in LF power and LF:HF ratio, respectively. No significant effect of SWS duration was observed on HF power (P = 0.47).
Circadian Regulation of HRV During Vigilance States
Each 30-sec sleep epoch and corresponding HRV data collected throughout the USW procedure was assigned a circadian phase. We have previously reported a significant circadian rhythm of RRI and other HRV parameters during wake episodes.3 These curves are reproduced in the current manuscript for comparative purposes (gray lines, Figure 3).
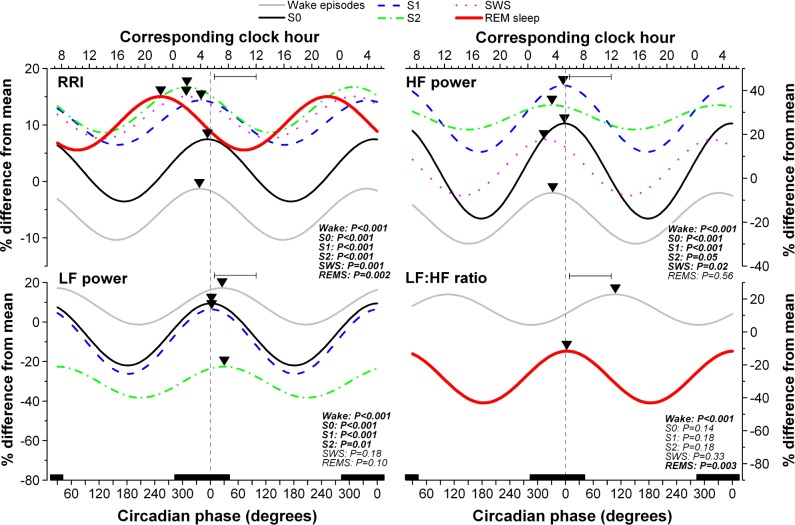
Figure 3.
Circadian variation of consecutive R-R intervals (RRIs, top left panel), high-frequency (HF) power (top right panel), low frequency (LF) power (lower left panel) and the LF:HF ratio (lower right panel) during wake episodes and vigilance states of nap episodes (i.e., S0, wake during nap episode; S1, stage 1 sleep; S2, stage 2 sleep; SWS, slow wave sleep; REMS, rapid eye movement sleep). The Y axis of each HRV component represents the % deviation from the subject's mean. Results were folded every 360° and repeated over two circadian cycles for illustrative purposes. A nonlinear mixed model was applied to individual subjects' data using nlmixed SAS procedure based on equation S1. Only the significant fitted cosine functions are shown for each state. P values are reported for each state. The vertical dotted line corresponds to the minimum core body temperature (CBT). Bottom X axes represent circadian phase and top X axes represent the corresponding clock time (CBT minimum at 0° = 05:23). Black bars along the X axes represent the time of projected habitual nocturnal sleep episodes. Inverted triangles illustrate the acrophase of each significant rhythm. Peak time of adverse cardiovascular events (based on information in Muller1) are illustrated by the horizontal bar within each panel.
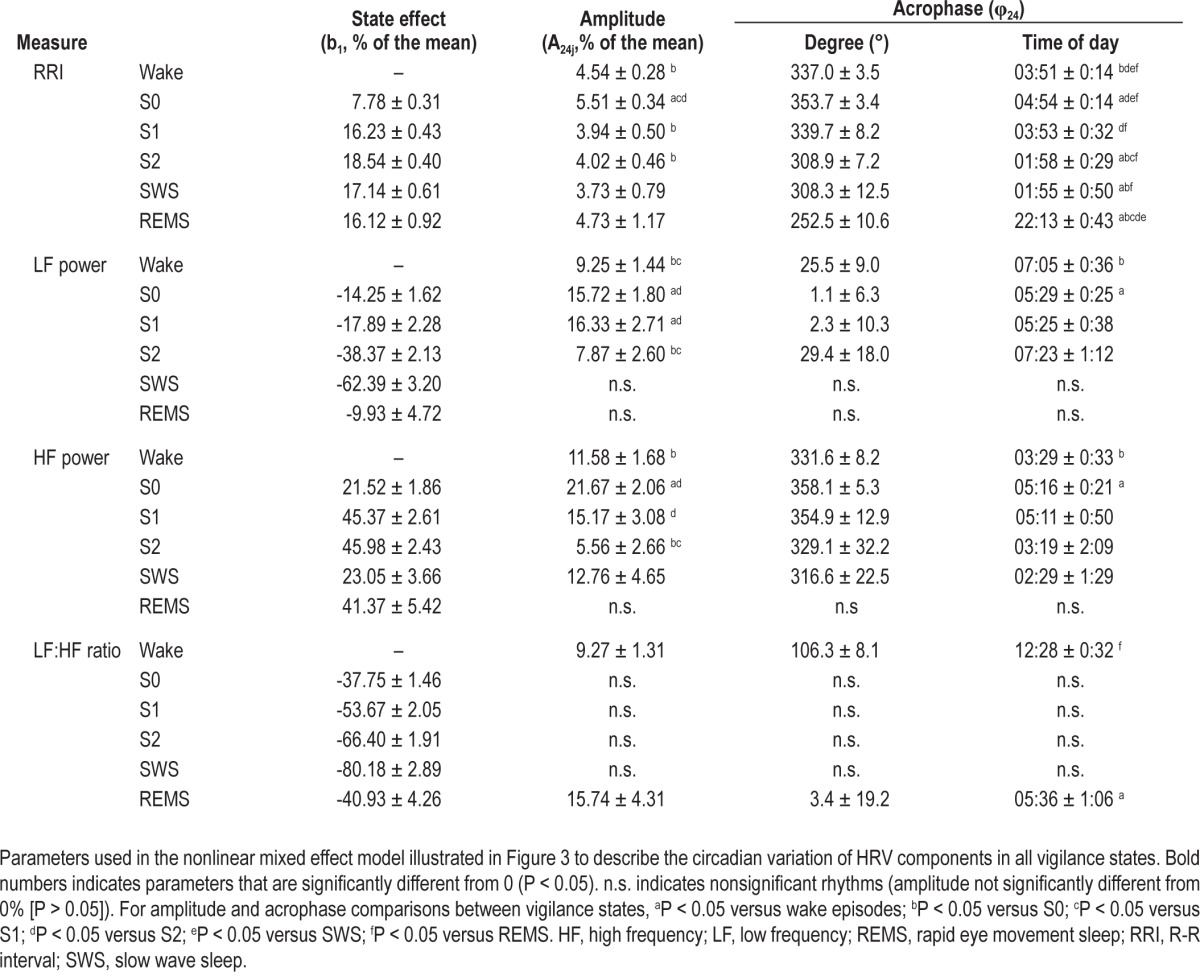
Details of the circadian variation of HRV during each sleep stages are presented in Figure 3 and Table 1. A significant circadian rhythm was observed for RRI during each vigilance state (P ≤ 0.002); for HF power during all sleep stages (P ≤ 0.05) except REM sleep; for LF power during all sleep stages (P ≤ 0.02) except SWS and REM sleep; and for the LF:HF ratio only during wake episodes and REM sleep (P ≤ 0.003). Circadian amplitude and acrophase of each HRV parameter varied between sleep stages. In summary, during S2 and SWS, RRI peaked at the beginning of the night and was advanced compared to the CBT minimum (P ≤ 0.03). During REM sleep, the acrophase of RRI occurred in the evening (252.5° ± 10.6° or 22:13 ± 00:43), significantly advanced relative to that of other vigilance states (P ≤ 0.005). During REM sleep, RRI was lower, thus HR maximal, around the time of habitual awakening (72.5° ± 10.6° or 10:13 ± 00:42). The acrophase of HF and LF power rhythms measured during sleep was aligned with CBT minimum, such that HF and LF power peaked at the end of the night when CBT was at its nadir. There was a trend for the HF power acrophase during SWS to be advanced relative to the CBT minimum (P = 0.07). The acrophase of the LF:HF ratio during REM sleep occurred in the early morning, at a time coincidental with the CBT minimum, significantly advanced compared to that of wake episodes (P < 0.001).
Table 1.
Parameters defining the circadian variation of HRV during each sleep stage
DISCUSSION
HRV Regulation by Vigilance States
The initial RRI increase associated with turning the lights out3 was followed by an additional increase after sleep onset, consistent with prior findings.26 We observed a progressive shift toward greater parasympathetic modulation (lower LF:HF ratio) with increasing sleep depth. These ANS changes are consistent with prior HRV studies,6–10 direct sympathetic nerve recordings,11 and plasma catecholamine levels27 measured in humans during sleep. During REM sleep, our results suggest an increased shift toward greater sympathetic modulation at a level comparable to that of wakefulness, in line with reported surges in sympathetic nerve activity measured by microneurography11 or HRV.9,10
We showed that parasympathetic cardiac modulation (absolute HF power) is elevated around sleep onset, during S1, S2, and REM sleep, but reduced during SWS compared to S1. This apparent reduction in parasympathetic modulation of the heart during SWS is consistent with studies reporting the absolute HF power during sleep,7,26 but not with others using the normalized HF power (nHF = HF/(LF + HF))8,10,26 showing maximal values during SWS. Using our USW data, we were indeed able to replicate these results where nHF is maximal during SWS (data not shown). Early studies linked the parasympathetic modulation of the heart with the absolute HF power28 and were validated by animal and human experiments using vagal denervation,29 vagus nerve excitation, blockage,30 and recordings.31 We consider that the nHF power emphasizes the balance of the two branches of the ANS rather than the parasympathetic component.22,32 Another study used pharmaceutical agents to show that although HF is essentially linearly related to parasympathetic activation within physiological RRI ranges (600-1300 ms), HF may decrease with high parasympathetic activity at greater RRI (> ∼1300 ms).33 Although we cannot exclude that this ceiling effect might explain the lower HF observed during SWS, it is unlikely as mean RRI during SWS (± SEM) was 1101.3 ± 12.51 ms, below the limit observed by Goldberger et al.33 Thus, we believe that activity of both ANS branches goes down with sleep depth, but that this reduction is less pronounced for the parasympathetic compared to the sympathetic modulation of the heart, leading to a reduced LF:HF ratio during SWS.
A recent publication by Kuo et al.34 showed an initial increase of HF power prior to sleep onset in rats, followed by a decline within 5 min of quiet sleep. Our increased HF power during S1 sleep compared to deeper sleep stages may result from a similar phenomenon. Increased HF power and LF:HF ratio followed quiet-to-paradoxical sleep transitions in that experiment.34 In line with our increased HF power and LF:HF ratio during REM sleep, it indicates that an increase in sympathetic modulation of the heart, and not a withdrawal in parasympathetic activity, contributes to the shift toward greater LF:HF ratio and sympathetic modulation observed during transitions from NREM to REM sleep.
WASO and reduced SE were associated with shifts toward sympathetic cardiac modulation (i.e., increased LF, LF:HF; reduced RRI). These findings are consistent with an increased HR and sympathetic modulation during spontaneous35 or evoked36 arousals in healthy or sleep disordered patients.37 These evidences suggest that sleep fragmentation is associated with changes in HRV which may have clinical implications.
Circadian Regulation of HRV During Each Vigilance State
The circadian variation of all sleep measures is consistent with findings of prior studies using an USW procedure38,39 or a forced desynchrony protocol.40 These analyses, combined with our previous results on hormonal and body temperature rhythm,3,41 confirm the validity the USW procedure to investigate the circadian variation of physiological parameters.
Although the endogenous nature of HR rhythm during wakefulness has been well established,3,42 to our knowledge, this is the first time a significant circadian rhythmicity of HR is described during each sleep stage in humans, apart from our recent publication on cardiorespiratory coherence.43 Hu et al.42 reported a circadian rhythm of RRI during sleep opportunities, a result that we recently corroborated using our USW procedure.3 In our study, the circadian variation of HF power observed during wakefulness persisted during NREM sleep, whereas that of the LF power was lost with deeper NREM sleep stages. Interestingly, the circadian rhythmicity of the LF:HF ratio was nonsignificant during all NREM sleep stages and present during REM sleep. Prior studies conducted throughout a 7-h nighttime sleep period (without circadian phase assessment) report inconsistent results about sympathovagal balance on the heart rate night.26,44 The USW procedure assesses circadian phase while minimizing the confounding effects of time spent asleep or awake, and thus constitutes an advantageous experimental approach for investigating the interaction of circadian and sleep stage-specific processes on HRV.
Our results show that the circadian acrophase of each HRV index differed across sleep stages, thus strongly supporting an interaction between these processes on the ANS cardiac modulation. The circadian regulation of sleep-specific changes resulted in more pronounced RRI and HF increases during S2 and SWS when these sleep stages occurred in the first part of the night (acrophase ± SEM, RRI-S2: 01:58 ± 00:29, RRI-SWS: 01:55 ± 00:50, HF-S2: 03:19 ± 02:09 HF-SWS: 02:29 ± 01:29) compared to the afternoon. This time is coincidental with highest occurrence of SWS in subjects living on a day-oriented schedule. Similarly, REM sleep was associated with higher HR and LF:HF ratio when it occurred in the morning (nadir RRI-REM sleep: 10:13 ± 0:43, acrophase LF:HF-REM sleep: 05:36 ± 1:06) compared to the evening, a time coincidental with peak REM sleep propensity. Thus, the sleep stage-specific variation of RRI and HRV parameters across circadian phases is such that it enhances the effect of SWS and REM sleep on ANS cardiac modulation at peak circadian propensity of these sleep stages. These observations add support to the hypothesis that early morning is a high vulnerability period for adverse cardiovascular events. As the acrophase of the LF:HF ratio is delayed during wake episodes compared to REM sleep (Figure 3), waking up in the morning could contribute to extend this vulnerability period throughout the morning.1 Moreover, this interaction suggests that sleeping at night is associated with a greater increase in RRI and HF power than sleeping at adverse circadian phases. Going to bed in the afternoon would lead to the fastest HR during NREM and REM sleep. This would be the case of a night shift worker who would wait a few hours after his shift to go to bed.
Neuronal pathways linking the ANS, circadian, and sleep systems exist and could explain our observations. Some brain regions inactive during REM sleep (REM-off), including the dorsal raphe nucleus, the locus coeruleus, and the ventrolateral part of the periaqueductal gray matter,45 are also involved in the ANS control of the heart.46 The suprachiasmatic nucleus (SCN) projects to the paraventricular nucleus of the hypothalamus, which is one of the most important hypothalamic regions involved in central ANS control.47 The presence of multisynaptic autonomic projections from SCN neurons to the heart was shown in rats.48 Compared to REM sleep and wakefulness, NREM sleep was also shown to alter SCN neuronal activity in rats49 which could in turn influence HRV. The SCN receives cholinergic projections from the pedunculopontine (REM-on) and laterodorsal tegmental nuclei (REM-on), serotoninergic projections from the dorsal raphe nucleus (REM-off), and noradrenergic projections from the locus coeruleus (REM-off), all implicated in generation of REM sleep.45,49 In relation to our results, it is possible that reduced and elevated SCN firing rate during SWS and REM sleep, respectively, leads to variable strength in the SCN output, which in turn modulates the circadian amplitude in ANS modulation of the heart.
Clinical Implications
Reduced HRV in different medical conditions or in healthy subjects is associated with increased cardiovascular risk.22,50,51 The prognostic value of HRV after acute myocardial infarction and in other diseases is recognized in prior literature. In follow-up studies looking at postmyocardial infarction patients, nonsurvivors had on average a reduction of ∼30-40% in LF power and ∼45-50% in HF power compared to survivors.50,52,53 Similarly, Galinier et al. showed a ∼32-50% higher HF and LF power level at baseline between those who survived chronic heart failure compared to those who did not in a 3-y follow-up study.51 The peak-to-trough amplitude of the circadian variation that we observed is between ∼16-32% for the LF power and between ∼11-43% for the HF power. When combined with the important sleep stage-specific effect (e.g. -62% in LF power during SWS), we think that the circadian and sleep stage-specific regulation of the heart is of major clinical importance in diseased patients.
Awakenings after sleep onset increase HR, blood pressure, and sympathetic modulation of the heart.11,36,37 Medical conditions17,18 and sleep disorders14–16 causing frequent awakenings during sleep have been associated with higher cardiovascular risk. Our results show that reduced SE, greater number of WASO, and increased REM sleep duration (during which spontaneous awakenings also increase) are associated with a shift toward greater sympathetic modulation. How these changes in HRV translate to increased cardiovascular risk will require further research.
Few studies investigated the role of NREM and REM sleep on the pathogenesis of cardiac events. REM sleep is associated with surges in sympathetic nerve activity, HR, and blood pressure, as well as reduced baroreceptor sensitivity,11 challenging the cardiovascular system. NREM sleep is considered cardio-protective and increased HF power during NREM sleep is a good indicator of cardiac health.8 However, bradycardia and hypotension during SWS could also lead to reduced coronary perfusion, which in turn could lead to asystolic, ischemic, or thrombolic events in cardiac patients. According to our results and the nocturnal sleep stages distribution, it is tempting to hypothesize that acute cardiac events related to the parasym-pathetic or sympathetic systems would be more prevalent in the beginning or in the end of the night, respectively. Evidence supports such biphasic prevalence in adverse cardiac events at night,5 though the relation between a specific cardiac events, a corresponding ANS division, and a sleep state is difficult to establish. For instance, myocardial ischemia could be explained by reduced coronary blood flow during NREM sleep, or increased metabolic demand during REM sleep. Increased sympathetic modulation of the heart during REM sleep is consistent with reports of increased morning incidence in acute myocardial infarction, sudden cardiac death, atrioventricular block, ventricular fibrillation, and ventricular tachycardia.2 An increase in myocardial infarction, sudden cardiac death, paroxysmal atrial fibrillation onset, and automatic implantable cardioverter-defibrillator discharges has been reported in the first part of the night during increased SWS prevalence,5 and NREM sleep was shown to precipitate multiple myocardial ischemic events in pigs in the presence of a flow-limiting coronary stenosis.13 This indicates that SWS could lead to myocardial ischemia in patients suffering from coronary artery diseases.
A majority of night shift workers live in a state of chronic desynchronization between their circadian system and their imposed sleep-wake cycle.54 A recent meta-analysis suggests a possible association between ischemic heart diseases and shift work.19 Our data indicate that sleeping at unconventional circadian phases could result in higher HR and reduced parasym-pathetic modulation. Thus, a recommendation would be that night shift workers plan their sleep periods in the morning to maximize the shift toward greater parasympathetic modulation during SWS. Even if morning hours are also associated with maximal REM sleep sympathetic modulation, this maximal sympathetic level is still lower than that observed during wakefulness. Conversely, shift workers susceptible to ischemic or bradycardia events that could be worsened by elevated para-sympathetic levels should plan their main sleep period in the afternoon/evening before a night shift.13 Initiating sleep in the afternoon just prior to a nightshift reduces the homeostatic sleep pressure for the following night shift but can also substantially impair social and family life.55 Recommendations of optimal sleep schedule for night shift workers should thus be considered according to which sleep state could be more beneficial/challenging based on preexisting medical conditions. Shift work can substantially modulate sleep organization and efficiency, and lead to acute and chronic sleep deprivation. In any case, cardiac patients should avoid sleep deprivation because it leads to SWS or REM sleep rebound the following night.
Limitations
As a result of the circadian distribution of sleep stages, REM sleep or SWS periods were short or missing at certain circadian phases (Figure 1). Missing data combined with elevated inter-individual variability may have reduced our statistical power to detect significant HRV circadian amplitude in SWS or REM sleep. Our statistical analyses included random effect parameters to account for interindividual variability. We designed our 60/60-min USW procedure to minimize the amount of sleep restriction compared to the classic 60-30 min USW procedure.56 However, an effect of sleep deprivation was still observed in our study. As a correction factor, we included a time-in-experiment effect in our equation.3 Although the HF power is mostly recognized as an index of parasympathetic modulation of the heart, there is debate about the physiological significance of the LF:HF ratio as a quantitative tool to evaluate the sympathovagal balance of the heart because of inconsistent results in certain situations and a lack of formal definition.57 Furthermore, we did not correct for breathing frequency. Nevertheless, HRV is generally accepted as an index of cardiovascular health and results of the current manuscript are in line with those that we recently reported using cardiorespiratory coherence (i.e., adjusting for the breathing frequency).43
CONCLUSION
The current study provides evidence that circadian and sleep stage-specific processes interact to influence the ANS modulation of the heart. These observations strongly suggest that the regulation of the ANS modulates the diurnal variation of cardiovascular vulnerability by enhancing sleep stage-specific effects at specific times of day. Sympathetic modulation during REM sleep was increased in the morning. Although probably protective in healthy individuals, the circadian variation of HR and HF power during NREM sleep may also accentuate morbidity and mortality in cardiac disease patients. Better knowledge of the physiological mechanisms responsible for the circadian regulation of the heart during sleep could lead to the development of pharmacological tools targeting specific sleep stages or circadian phases for the benefit of cardiac patients.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by an operating grant from the Canadian Institute of Health Research; and salary support from the Fonds de la recherche du Québec-Santé (to Dr. Boivin); the Standard Life Foundation (to Dr. Boivin); and the Institut de Recherche Robert-Sauvé en Santé et en Sécurité du Travail (to Mr. Boudreau). Dr. Boivin is CEO and Scientific Director of Alpha Logik Consultants, Inc., and has received travel support from Servier and Merck Canada. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the research participants, staff and students of the Centre for Study and Treatment of Circadian Rhythms for their contributions to this investigation. We also thank Dr. Sylvie Rhéaume, Dr. Alain Solignac, and Abdelmadjid Azzoug, RN, for medical supervision; Dr. Claire-Dominique Walker for salivary cortisol assays; Francine Duquette for dietary advices; Manon Robert for her assistance on the sleep recordings; and Véronique Pagé for statistical advices; and Dr. Ari Shechter for manuscript revision.
REFERENCES
- 1.Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999;12:35S–42S. doi: 10.1016/s0895-7061(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 2.Portaluppi F, Tiseo R, Smolensky MH, Hermida RC, Ayala DE, Fabbian F. Circadian rhythms and cardiovascular health. Sleep Med Rev. 2012;16:151–66. doi: 10.1016/j.smrv.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Boudreau P, Yeh WH, Dumont GA, Boivin DB. A circadian rhythm in heart rate variability contributes to the increased cardiac sympathovagal response to awakening in the morning. Chronobiol Int. 2012;29:757–68. doi: 10.3109/07420528.2012.674592. [DOI] [PubMed] [Google Scholar]
- 4.Scheer FA, Hu K, Evoniuk H, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A. 2010;107:20541–6. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavery CE, Mittleman MA, Cohen MC, Muller JE, Verrier RL. Nonuniform nighttime distribution of acute cardiac events: a possible effect of sleep states. Circulation. 1997;96:3321–7. doi: 10.1161/01.cir.96.10.3321. [DOI] [PubMed] [Google Scholar]
- 6.Burgess HJ, Penev PD, Schneider R, Van Cauter E. Estimating cardiac autonomic activity during sleep: impedance cardiography, spectral analysis, and Poincare plots. Clin Neurophysiol. 2004;115:19–28. doi: 10.1016/s1388-2457(03)00312-2. [DOI] [PubMed] [Google Scholar]
- 7.Busek P, Vankova J, Opavsky J, Salinger J, Nevsimalova S. Spectral analysis of the heart rate variability in sleep. Physiol Res. 2005;54:369–76. [PubMed] [Google Scholar]
- 8.Vanoli E, Adamson PB, Ba L, Pinna GD, Lazzara R, Orr WC. Heart rate variability during specific sleep stages. A comparison of healthy subjects with patients after myocardial infarction. Circulation. 1995;91:1918–22. doi: 10.1161/01.cir.91.7.1918. [DOI] [PubMed] [Google Scholar]
- 9.Gronfier C, Simon C, Piquard F, Ehrhart J, Brandenberger G. Neuroendocrine processes underlying ultradian sleep regulation in man. J Clin Endocrinol Metab. 1999;84:2686–90. doi: 10.1210/jcem.84.8.5893. [DOI] [PubMed] [Google Scholar]
- 10.Cabiddu R, Cerutti S, Viardot G, Werner S, Bianchi AM. Modulation of the Sympatho-Vagal Balance during Sleep: Frequency Domain Study of Heart Rate Variability and Respiration. Front Physiol. 2012;3:45. doi: 10.3389/fphys.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–7. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 12.Holty JE, Guilleminault C. REM-related bradyarrhythmia syndrome. Sleep Med Rev. 2011;15:143–51. doi: 10.1016/j.smrv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Kuklov A, Kehoe RF, Crystal GJ. Sleep-induced hypotension precipitates severe myocardial ischemia. Sleep. 2008;31:1215–20. [PMC free article] [PubMed] [Google Scholar]
- 14.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo BB, Blackwell T, Ancoli-Israel S, Stone KL, Stefanick ML, Redline S. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) Study. Circulation. 2011;124:1223–31. doi: 10.1161/CIRCULATIONAHA.111.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chien KL, Chen PC, Hsu HC, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–84. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kupelian V, Rosen RC, Link CL, et al. Association of urological symptoms and chronic illness in men and women: contributions of symptom severity and duration--results from the BACH Survey. J Urol. 2009;181:694–700. doi: 10.1016/j.juro.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mezick EJ, Hall M, Matthews KA. Are sleep and depression independent or overlapping risk factors for cardiometabolic disease? Sleep Med Rev. 2011;15:51–63. doi: 10.1016/j.smrv.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost P, Kolstad HA, Bonde JP. Shift work and the risk of ischemic heart disease - a systematic review of the epidemiologic evidence. Scand J Work Environ Health. 2009;35:163–79. doi: 10.5271/sjweh.1319. [DOI] [PubMed] [Google Scholar]
- 20.Heilman KJ, Porges SW. Accuracy of the LifeShirt (Vivometrics) in the detection of cardiac rhythms. Biol Psychol. 2007;75:300–5. doi: 10.1016/j.biopsycho.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckheit JB, Donoho DL. WaveLab and reproducible research. 1995. [cited; Technical report]. Available from: http://www-stat.stanford.edu/∼wavelab/Wavelab_850/wavelab.pdf.
- 22.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–81. [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. Los Angeles: Brain Information Service, Brain Research Institute, UCLA; 1968. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. [Google Scholar]
- 24.Coleman RM. Periodic movements in sleep (nocturnal myoclonus) and restless legs syndrome. In: Guilleminault C, editor. Sleeping and waking disorders: indications and techniques. Menlo-Park: Addison-Wesley; 1982. pp. 265–95. [Google Scholar]
- 25.Iber C, Ancoli-Israel S, Chesson A, Quan SF. Westcherster, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 26.Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10:253–64. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 27.Rasch B, Dodt C, Molle M, Born J. Sleep-stage-specific regulation of plasma catecholamine concentration. Psychoneuroendocrinology. 2007;32:884–91. doi: 10.1016/j.psyneuen.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–3. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 29.Chiou CW, Zipes DP. Selective vagal denervation of the atria eliminates heart rate variability and baroreflex sensitivity while preserving ventricular innervation. Circulation. 1998;98:360–8. doi: 10.1161/01.cir.98.4.360. [DOI] [PubMed] [Google Scholar]
- 30.Medigue C, Girard A, Laude D, Monti A, Wargon M, Elghozi JL. Relationship between pulse interval and respiratory sinus arrhythmia: a time- and frequency-domain analysis of the effects of atropine. Eur J Physiol. 2001;441:650–5. doi: 10.1007/s004240000486. [DOI] [PubMed] [Google Scholar]
- 31.Kuo TB, Lai CJ, Huang YT, Yang CC. Regression analysis between heart rate variability and baroreflex-related vagus nerve activity in rats. J Cardiovasc Electrophysiol. 2005;16:864–9. doi: 10.1111/j.1540-8167.2005.40656.x. [DOI] [PubMed] [Google Scholar]
- 32.Burr RL. Interpretation of normalized spectral heart rate variability indices in sleep research: a critical review. Sleep. 2007;30:913–9. doi: 10.1093/sleep/30.7.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberger JJ, Challapalli S, Tung R, Parker MA, Kadish AH. Relationship of heart rate variability to parasympathetic effect. Circulation. 2001;103:1977–83. doi: 10.1161/01.cir.103.15.1977. [DOI] [PubMed] [Google Scholar]
- 34.Kuo TB, Shaw FZ, Lai CJ, Yang CC. Asymmetry in sympathetic and vagal activities during sleep-wake transitions. Sleep. 2008;31:311–20. doi: 10.1093/sleep/31.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sforza E, Chapotot F, Lavoie S, Roche F, Pigeau R, Buguet A. Heart rate activation during spontaneous arousals from sleep: effect of sleep deprivation. Clin Neurophysiol. 2004;115:2442–51. doi: 10.1016/j.clinph.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Blasi A, Jo J, Valladares E, Morgan BJ, Skatrud JB, Khoo MC. Cardiovascular variability after arousal from sleep: time-varying spectral analysis. J Appl Physiol. 2003;95:1394–404. doi: 10.1152/japplphysiol.01095.2002. [DOI] [PubMed] [Google Scholar]
- 37.Sforza E, Pichot V, Cervena K, Barthelemy JC, Roche F. Cardiac variability and heart-rate increment as a marker of sleep fragmentation in patients with a sleep disorder: a preliminary study. Sleep. 2007;30:43–51. doi: 10.1093/sleep/30.1.43. [DOI] [PubMed] [Google Scholar]
- 38.Shechter A, Varin F, Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33:647–56. doi: 10.1093/sleep/33.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carskadon MA, Dement WC. Sleep studies on a 90-minute day. Electroencephalogr Clin Neurophysiol. 1975;39:145–55. doi: 10.1016/0013-4694(75)90004-8. [DOI] [PubMed] [Google Scholar]
- 40.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shechter A, Boudreau P, Varin F, Boivin DB. Predominance of distal skin temperature changes at sleep onset across menstrual and circadian phases. J Biol Rhythms. 2011;26:260–70. doi: 10.1177/0748730411404677. [DOI] [PubMed] [Google Scholar]
- 42.Hu K, Ivanov P, Hilton MF, et al. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci U S A. 2004;101:18223–7. doi: 10.1073/pnas.0408243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boudreau P, Brouse CJ, Dumont GA, Boivin DB. Sleep-wake and circadian-dependent variation of cardiorespiratory coherence. Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE; 2012 Aug 28 2012-Sept 1 2012, 2012; pp. 3817–20. [DOI] [PubMed] [Google Scholar]
- 44.Scholz UJ, Bianchi AM, Cerutti S, Kubicki S. Vegetative background of sleep: spectral analysis of the heart rate variability. Physiol Behav. 1997;62:1037–43. doi: 10.1016/s0031-9384(97)00234-5. [DOI] [PubMed] [Google Scholar]
- 45.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 46.Iversen S, Iversen I, Saper CB. The autonomic nervous system and the hypothalamus. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. 4th edition. New York: McGraw-Hill; 2000. pp. 960–81. [Google Scholar]
- 47.Buijs RM, la Fleur SE, Wortel J, et al. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol. 2003;464:36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- 48.Scheer FA, Ter Horst GJ, van Der Vliet J, Buijs RM. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am J Physiol Heart Circ Physiol. 2001;280:H1391–9. doi: 10.1152/ajpheart.2001.280.3.H1391. [DOI] [PubMed] [Google Scholar]
- 49.Deboer T, Vansteensel MJ, Detari L, Meijer JH. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat Neurosci. 2003;6:1086–90. doi: 10.1038/nn1122. [DOI] [PubMed] [Google Scholar]
- 50.Lanza GA, Guido V, Galeazzi MM, et al. Prognostic role of heart rate variability in patients with a recent acute myocardial infarction. Am J Cardiol. 1998;82:1323–8. doi: 10.1016/s0002-9149(98)00635-3. [DOI] [PubMed] [Google Scholar]
- 51.Galinier M, Pathak A, Fourcade J, et al. Depressed low frequency power of heart rate variability as an independent predictor of sudden death in chronic heart failure. Eur Heart J. 2000;21:475–82. doi: 10.1053/euhj.1999.1875. [DOI] [PubMed] [Google Scholar]
- 52.Perkiomaki JS, Jokinen V, Tapanainen J, Airaksinen KE, Huikuri HV. Autonomic markers as predictors of nonfatal acute coronary events after myocardial infarction. Ann Noninvasive Electrocardiol. 2008;13:120–9. doi: 10.1111/j.1542-474X.2008.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balanescu S, Corlan AD, Dorobantu M, Gherasim L. Prognostic value of heart rate variability after acute myocardial infarction. Med Sci Monit. 2004;10:CR307–15. [PubMed] [Google Scholar]
- 54.Boivin DB, Tremblay GM, James FO. Working on atypical schedules. Sleep Med. 2007;8:578–89. doi: 10.1016/j.sleep.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Akerstedt T. Is there an optimal sleep-wake pattern in shift work? Scand J Work Environ Health. 1998;24:18–27. [PubMed] [Google Scholar]
- 56.Carskadon MA, Dement WC. Distribution of REM sleep on a 90 minute sleep-wake schedule. Sleep. 1980;2:309–17. [PubMed] [Google Scholar]
- 57.Prakash ES. ‘Sympathovagal balance from heart rate variability: an obituary’, but what is sympathovagal balance? Exp Physiol. 2012;97:1140. doi: 10.1113/expphysiol.2012.067322. [DOI] [PubMed] [Google Scholar]