Abstract
Objectives:
Sleep interruption is often reported by women with hot flashes and night sweats (or vasomotor symptoms, VMS). Although women report that VMS awaken them, polysomnography (PSG) studies have not consistently supported this contention.
Design:
We mimicked menopause using a gonadotropin-releasing hormone agonist (GnRHa) to investigate whether VMS increase awakenings and wake after sleep onset (WASO). VMS, serum estradiol, and at-home PSGs (two pretreatment, two posttreatment) were measured before and after 4 weeks on GnRHa. Regression models were used to determine the effect of increasing VMS frequency on awakenings and WASO, as measured objectively and subjectively.
Participants:
Twenty-nine healthy women (mean 27.3 y).
Setting:
Academic medical center.
Interventions:
Depot GnRHa (leuprolide 3.75-mg).
Results:
Serum estradiol was rapidly and uniformly suppressed on GnRHa. Persistent VMS were reported by 69% of women. The number of nighttime VMS correlated directly with the degree of sleep disturbance. Each additional reported nighttime VMS was associated with a 62% increase from baseline in PSG-measured WASO (P = 0.007), a 3% increase in awakenings (P = 0.05), and 6% increase in %N1 sleep (P = 0.02). Nighttime VMS were also associated with increased perceived WASO (312%; P = 0.02), awakenings (16%; P = 0.007), Insomnia Severity Index (P = 0.03), and Pittsburgh Sleep Quality Index (P = 0.03) scores, and decreased perceived sleep efficiency (P = 0.01). Objectively recorded nighttime VMS correlated with PSG-measured WASO (rs = 0.45, P = 0.02).
Conclusions:
This menopause model demonstrates that nighttime vasomotor symptoms correlate with increased sleep fragmentation. These findings are consistent with a specific contribution of vasomotor symptoms to polysomnography-measured sleep interruption suggesting that nighttime vasomotor symptoms interrupt sleep in the setting of menopause.
Citation:
Joffe H; Crawford S; Economou N; Kim S; Regan S; Hall JE; White D. A gonadotropin-releasing hormone agonist model demonstrates that nocturnal hot flashes interrupt objective sleep. SLEEP 2013;36(12):1977-1985.
Keywords: Menopause, hot flashes, awakenings, wake time after sleep onset, polysomnography
INTRODUCTION
The prevalence of sleep disturbance increases during the menopause transition.1,2 Because repeated awakenings from sleep increase in women undergoing menopause,3 nocturnal hot flashes (or night sweats) are commonly thought to explain the increased prevalence of sleep disturbance associated with meno-pause. Hot flashes and night sweats (collectively called vaso-motor symptoms, VMS) have been linked consistently to chronic insomnia4 and poor subjective sleep quality.1–3,5,6 However, results of studies examining the association of VMS with polysomnography (PSG) measures of sleep have been mixed, with some finding more awakenings in general,7,8 more awakenings only during the first half of the night,9 increased wake after sleep onset (WASO),10 and reduced sleep efficiency,8,10 whereas others report no association.6,11–13 Although a less precise measure of sleep, a small number of actigraphic studies also indicate that women with VMS have more awakenings14 and lower sleep efficiency.15 Although some studies have not shown an association between objective VMS and PSG sleep,11,12 in general, adverse associations with PSG sleep have been detected when VMS were measured objectively,7–10 but not when VMS were measured subjectively.6,13 Data showing that VMS correlate with objective sleep disturbance would validate the commonly experienced perception of sleep disruption in women in midlife with VMS.
Importantly, most studies do not distinguish daytime from nighttime symptoms but rather compare PSG sleep among women with VMS versus those without VMS regardless of the frequency of VMS. In a previous study, we measured changes in sleep continuity using actigraphy, rather than PSG, in a different population of women receiving GnRHa to induce VMS.16 In that study, we found no overall effect of subjectively reported VMS on actigraphic sleep continuity except when the frequency of VMS was taken into account.16 However, as with other studies, we did not distinguish nighttime from daytime VMS, which may have diluted the findings. Although most women have VMS during both day and night, the number and proportion of symptoms experienced at night varies among women. If VMS directly cause sleep interruption, the frequency of nighttime VMS specifically would be expected to be linked to PSG measures of sleep interruption.
VMS are the primary symptom of the menopause transition, affecting up to two-thirds of women.17 Sleep disturbance is a primary reason for women with VMS to pursue medical advice and treatment for their menopause-related symptoms.18 Documenting that perceived nighttime VMS cause sleep interruption would validate the perception of reduced sleep quality that women describe and prioritize treatment of nighttime VMS as a way to improve sleep continuity. It is therefore critical to establish specifically whether hot flashes are causally related to sleep disturbance. However, because all prior studies examining sleep in women with VMS are observational and cross-sectional in design, it is not possible to determine whether VMS precede the onset of observed sleep problems, suggesting a potential etiologic role.
In the current study, we isolated the specific role of nighttime VMS in the generation of PSG-measured and subjectively measured sleep by rapidly inducing VMS with the gonadotropin-releasing hormone agonist (GnRHa) leuprolide in healthy premenopausal volunteers. This GnRHa model is a valuable controlled experimental approach to specifically dissect the effect of nocturnal VMS on sleep because VMS are induced rapidly on GnRHa in two-thirds of women.16,19–23 GnRHa are Food and Drug Administration-approved hormonal agents used to treat endometriosis and fibroids because they suppress production of estradiol, thereby mimicking the hormonal changes of meno-pause. Although all women treated with GnRHa undergo the same hormonal changes,16 the frequency of VMS that develops varies widely and includes some individuals who do not develop any VMS. The current study takes advantage of this known side effect of GnRHa to study the effect of new-onset VMS on sleep.
We examined the effect of VMS on both changes in PSG and subjective sleep quality that occurred after GnRHa was initiated in relation to the number of nighttime, and separately, daytime, subjective VMS that developed after initiation of GnRHa. As evidence of a specific effect of nocturnal VMS on sleep interruption, we hypothesized that more frequent nighttime VMS developing on the GnRHa would result in a greater increase in the amount of WASO and the number of PSG awakenings. In secondary exploratory analyses, we examined whether sleep staging or other measures of sleep continuity were affected by the development of VMS. In addition, nocturnal VMS were recorded objectively to determine the effect of objective VMS on objective and subjective measures of sleep fragmentation.
MATERIALS AND METHODS
Premenopausal volunteers received a single open-label intramuscular injection of the depot GnRHa leuprolide and were then followed to determine whether they reported developing VMS. After excluding women with primary sleep disorders, at-home PSG studies and sleep quality questionnaires were completed prior to and after GnRHa administration. Sleep and VMS diaries were completed daily throughout the study, and nighttime VMS were recorded objectively during the post-GnRHa PSG studies. Serum estradiol was measured serially to confirm suppression of ovarian function.
Study Participants
Twenty-nine healthy premenopausal women 18-45 y old with monthly menstrual cycles and no VMS, sleep disorders, depression, or other psychiatric illnesses were enrolled in the study. Participants were carefully screened to select healthy women with regular menstrual cycles, evidence of ovulation (midluteal serum progesterone > 3 ng/dL), and no VMS (7-day hot flash diary). A routine clinical in-laboratory PSG using American Academy of Sleep Medicine (AASM) scoring procedures24 was conducted to exclude those with previously undiagnosed sleep disordered breathing (SDB, n = 1) and periodic limb movement disorder (PLMD, n = 1). Women with clinically significant depression were excluded using a standardized psychiatric diagnosis instrument (Patient Health Questionnaire, PHQ-9)25 and a score > 16 on the clinician-rated Montgomery-Åsberg Depression Rating Scale (MADRS),26,27 a threshold suggesting clinically relevant depressive symptoms.28 Other psychiatric exclusion criteria included previous diagnosis of major depression, bipolar disorder, psychosis, anorexia nervosa, alcohol and substance-use disorders, or suicide attempts. Additional exclusion criteria were pregnancy, lactation, abnormal laboratory studies (prolactin, thyroid function, liver function, renal function), and use of centrally active medications (e.g., anti-depressants, benzodiazepines, corticosteroids) or hormonal or nonhormonal medications known to suppress hot flashes (e.g., birth control preparations, serotonergic agents, gabapentin).
Study Procedures
After completing the in-laboratory screening PSG and prior to GnRHa administration, participants completed two ambulatory PSG studies, a 7-day sleep diary, and standardized questionnaires measuring perceived sleep quality, insomnia symptoms, and daytime sleepiness. After completing baseline procedures, all participants were given one open-label dose of the depot GnRH agonist leuprolide 3.75 mg during the midluteal phase of the menstrual cycle (approximately 7 days after ovulation, confirmed by urinary luteinizing hormone levels and basal body temperature) to rapidly induce hypoestrogenism and maintain ovarian suppression for the study period.21,22,29–32
Following GnRHa administration, participants were monitored daily with a sleep diary and a VMS diary to determine the development of new-onset VMS. Diaries were completed until both ambulatory posttreatment PSGs studies were obtained 4 w after administration of the GnRHa. Concurrent with both post-treatment PSGs, nighttime VMS were measured objectively using an ambulatory skin conductance monitor.
For safety monitoring purposes, participants were contacted 8 and 12 w after GnRHa administration to establish that menses had resumed and VMS had resolved. All participants provided written informed consent for study procedures, which were approved by the Partners HealthCare Institutional Review Boards.
Study Measures
As stated, a screening PSG was obtained in the laboratory and four study PSGs were conducted in the home. On the screening PSG, all standard AASM-recommended channels were recorded, including respiration (nasal pressure, oxygen saturation [SaO2], and chest-abdominal motion) and bilateral anterior tibialis electromyography (EMG). In the home, objective sleep parameters were obtained using the Safiro (Compu-medics Limited, Charlotte, NC) ambulatory PSG unit. Participants came to our research offices to be hooked up to the unit by our sleep technician. Each pair of PSG studies obtained at baseline and posttreatment were conducted on consecutive nights when logistically possible. Sleep scoring was completed by the Harvard Sleep & EEG Core using standard procedures to define sleep staging, including electroencephalography (EEG; C3-A2, C4-A1, O1-A2, O2-A1), electro-oculography (both eyes), and electromyography (submental). Standard AASM scoring methods were used to define sleep stages N1, N2, N3, and rapid eye movement (REM).24 Awakenings were defined as alpha EEG activity comprising > 15 sec of a 30-sec epoch. PSG scorers were blinded to hot flash status. Lights-out and lights-on times were recorded on an event marker of a time-synched actigraphic watch, with a sleep diary as confirmation, and strong correlation between the two measures (r = 0.93, P < 0.001).
Perceived sleep quality, insomnia symptoms, and daytime sleepiness were assessed using the Pittsburgh Sleep Quality Index (PSQI; range 0-21),33 the Insomnia Severity Index (ISI; range 0-28),34 and the Fatigue Severity Scale (FSS; range 9-63).35 A 7-day sleep diary was completed during the week preceding initiation of GnRHa when baseline PSG studies were obtained and during the final follow-up week when the posttreatment PSG studies were obtained. The diary included questions about the number of awakenings, WASO, and sleep onset latency for each night, enabling sleep efficiency to be calculated.
Subjectively measured VMS were recorded in a daily diary throughout the study. Participants recorded the number of VMS they were experiencing twice each day, once at the end of the day to describe the number of daytime VMS and once in the morning to quantify the number of nocturnal VMS experienced during the previous night.
Objective VMS were measured using the Bahr monitor (Simplex Scientific, Middleton, WI), a small ambulatory monitor worn on the sternum that detects individual VMS events in real-time by measuring transient changes in sternal skin conductance. VMS were identified using the standard Bahr analysis algorithm (Version 1.1.40), as previously described.36 The monitor was time-synched to the PSG so that individual VMS events could be counted and linked temporally to individual awakenings.
Hormone Assays
Serum levels of estradiol, estrone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) were obtained during the follicular phase of the menstrual cycle prior to and 1, 2, and 4 w after GnRHa administration to confirm ovarian suppression. Estradiol and estrone were measured using liquid chromatography, tandem mass spectrometry (Mayo Clinic, Rochester, NY)37,38 in order to achieve accuracy and precision in the low range seen on GnRHa therapy. The interassay coefficient of variation (CV) ranges for estradiol and estrone in the low range studied was 8.6% and 8.7%, respectively.37 Serum LH was measured using a two-site monoclonal nonisotopic system according to the manufacturer's directions (Axsym, Abbott Laboratories, Abbott Park, IL, USA), as previously described.39 LH is expressed in IU/L as equivalents of the Second International Pituitary Standard (80/552 and 92/510). The interassay coefficients of variation are 5.3%, 5.5%, and 7.4% for quality control sera containing 5.6 IU/L, 26.2 IU/L, and 69.0 IU/L, respectively.
Analytical Methods
For descriptive purposes, participants were divided into three VMS frequency groups (persistent and frequent, n = 10; persistent and infrequent, n = 10; no VMS, n = 9). A median split was used to distinguish those with persistent VMS who had more versus less frequent symptoms during the entire follow-up period. The no-VMS group was composed of four women who never reported having symptoms and five who reported < 5 VMS during the entire follow-up period. Because all VMS reported by this latter group occurred exclusively during the daytime and within the first 5 days after receiving leuprolide, weeks before the posttreatment PSGs, they were grouped together with the no-VMS group.
The primary predictor was the number of VMS reported subjectively during the night. For each participant, nighttime VMS frequency was calculated as a daily average of the number of VMS reported on the diary to have occurred during the 1-mo follow-up leading up to the posttreatment PSGs. Parallel analyses were conducted using the number of daytime VMS reported subjectively. Because the daily average of nighttime, and similarly daytime, VMS reported during the follow-up month were strongly correlated with the number of nighttime/daytime VMS reported during the week preceding the post-treatment PSGs (r > 0.91, P < 0.0001, for both comparisons), the average from the month-long follow-up was used for the primary analysis as a more stable estimate of daily VMS. Secondary analyses of nocturnal VMS were conducted using (1) the daily average of nighttime and then daytime VMS calculated from the 7 days preceding the posttreatment PSGs, and (2) using the median number of objectively measured VMS detected on the Bahr monitor during the two posttreatment PSG studies.36
Percent change from pretreatment to posttreatment for each PSG sleep parameter was calculated using the mean of the two pretreatment and posttreatment studies for each participant at each time point. Similar approaches were used for secondary endpoints of N1%, N2%, N3%, REM%, PSG sleep efficiency, and PSG sleep-onset latency, PSQI, ISI, and FSS. The percent change from pretreatment to posttreatment for each sleep diary item (perceived WASO, number of awakenings, sleep efficiency, sleep onset latency) was calculated using the change from the 7-day average at baseline to the 7-day average during the week surrounding the posttreatment PSGs.
Linear regression models were built to examine the effect of nighttime (or daytime) VMS frequency on the primary endpoints of percent change in PSG-measured WASO time and number of awakenings from baseline to study end. Models for daytime VMS were adjusted for nighttime VMS because of confounding by nighttime symptoms. The same approach was used to model the secondary exploratory endpoints (other PSG-measured sleep parameters, sleep diary measures, PSQI, ISI, and FSS scores). Each beta coefficient represents the percent change in each dependent sleep measure (e.g., percent change in WASO time) for each additional VMS reported at night (or in the daytime) in a model adjusting for the baseline value of that sleep parameter (e.g., baseline WASO time). Models for WASO and awakenings were adjusted to take into account intersubject variability in at-home PSG sleep period time (from the event marker).
RESULTS
Study Participants
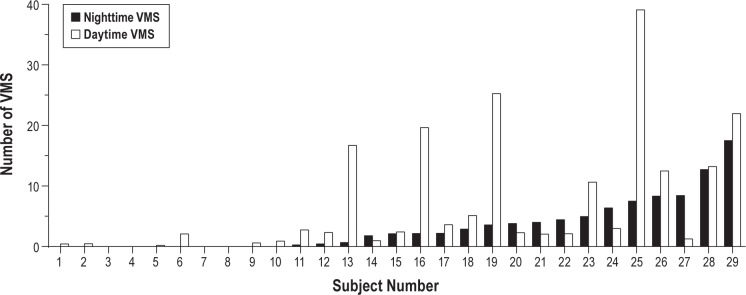
Of 29 study participants, 20 (69%) reported developing VMS that began 11.1 ± 5.5 days after receiving leuprolide and persisted throughout the follow-up period until the posttreatment PSGs. For those reporting persistent VMS (n = 20), the median daily number of VMS reported during the entire follow-up period was 3.7 (interquartile range [IQR] 2.0-7.0) each night and 3.3 (IQR 2.2-15.0) each day. Those with frequent VMS (n = 10) reported a median of 2.3 VMS per night and 2.6 VMS per day, whereas those with infrequent VMS (n = 10) reported a median of 0.4 nighttime and 1.6 daytime VMS (Table 1). During the week leading up to the PSG studies specifically, the median number of VMS reported was 1.6 (IQR 0.4-2.2) each night and 1.9 (IQR 0.9-4.6) each day. Although the number of nighttime and daytime VMS correlated strongly (rs = 0.74, P < 0.001) overall, the number of VMS occurring during the nighttime and daytime varied among study participants, with some women reporting the majority of VMS during the daytime and others experiencing a disproportionate number at night (Figure 1). Of those with daytime VMS, 24% reported having no nighttime VMS; conversely, everyone reporting nighttime VMS also reported daytime VMS.
Table 1.
Subject characteristics according to subsequent development of vasomotor symptoms (VMS)
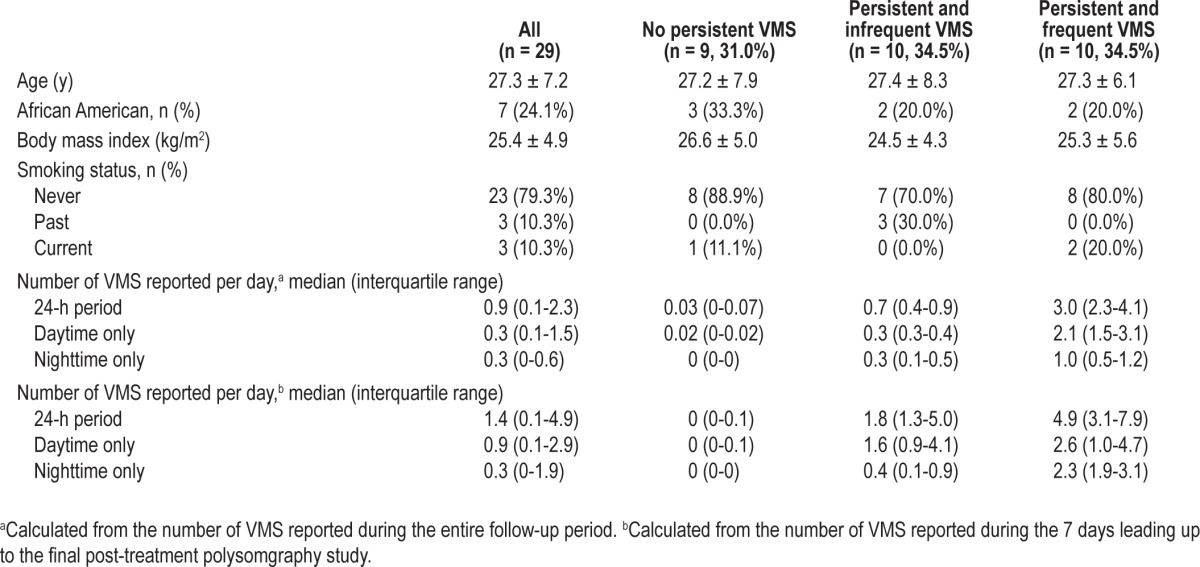
Figure 1.
Distribution of total number of nighttime and daytime vasomotor symptoms (VMS) reported by each individual subject (n = 29) over the 1-mo follow-up, showing interindividual differences in the proportion of VMS that were reported at night. Each pair of black and white vertical bars represents an individual subject.
Participant characteristics are shown in Table 1 for the entire study population and then according to VMS frequency groups. Participants were 27.3 ± 7.2 y old, with a mean body mass index of 25.4 ± 4.9 kg/m2 (range 18.4-34.9); one-fourth were African-American. There were no demographic characteristics associated with the likelihood of VMS developing on GnRHa.
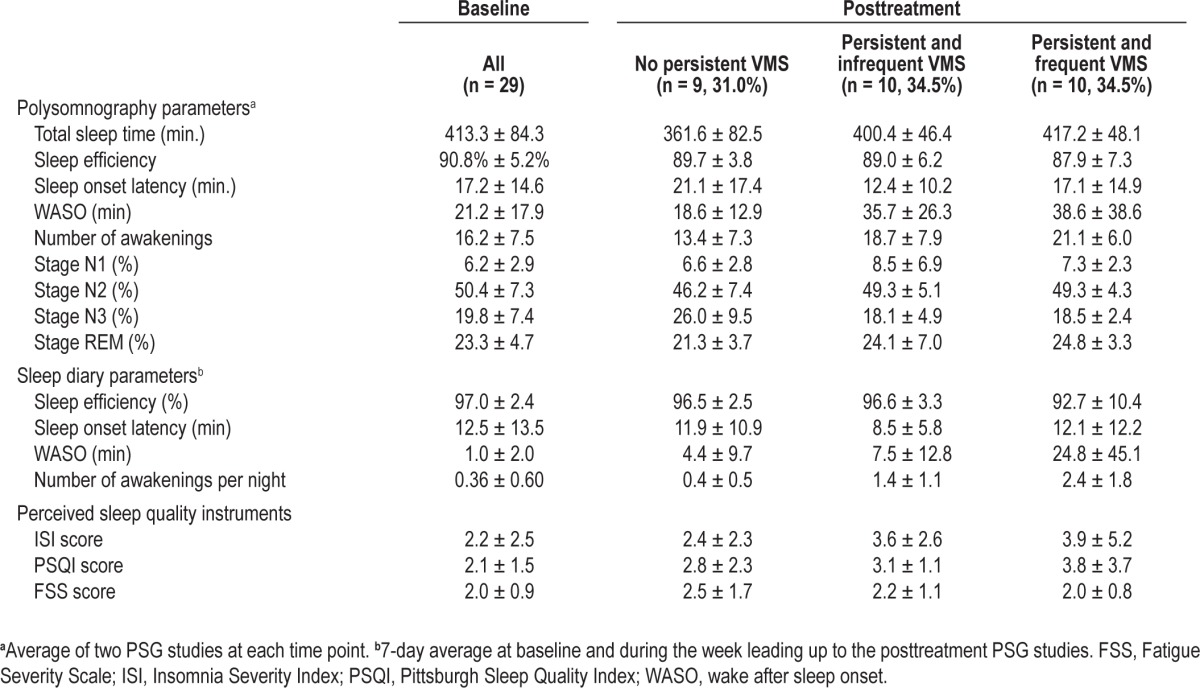
By design, participants were good sleepers without SDB, PLMD, or clinical evidence of insomnia. Ambulatory PSG prior to GnRHa revealed a mean sleep efficiency of 91%, sleep onset latency of 17 min, WASO of 21 min, and 16 awakenings per night (Table 2). Sleep diaries and questionnaires similarly indicated good sleep quality prior to study treatment (Table 2). Baseline sleep characteristics were not associated with the likelihood of developing VMS.
Table 2.
Polysomnography parameters and sleep diary measures for all subjects together (n = 29) prior to and after 1 mo on gonadotropin-releasing hormone agonist therapy by vasomotor symptom frequency group
Changes in Reproductive Hormones
Expected changes in reproductive hormones were observed in all participants. Serum estradiol was suppressed to post-menopausal levels (< 20 pg/mL) in all participants during the follow-up period and the expected decline in LH was observed in all participants (Figure 2A). The rates of decline in estradiol and estrone were not associated with the likelihood of developing VMS regardless of whether VMS were considered by frequency group (Figures 2B and 2C) or using a continuous measure of reported VMS frequency.
Figure 2.
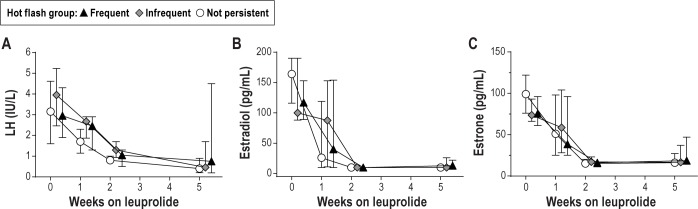
Median and interquartile range for luteinizing hormone (A), serum estradiol (B), and estrone (C) on the day that the gonadotropin-releasing hormone agonist leuprolide was administered in the midluteal phase (week 0) and all subsequent time points. Data are shown by hot flash group (frequent and persistent, n = 10; infrequent and persistent, n = 10; not persistent, n = 9).
Effect of Nighttime VMS
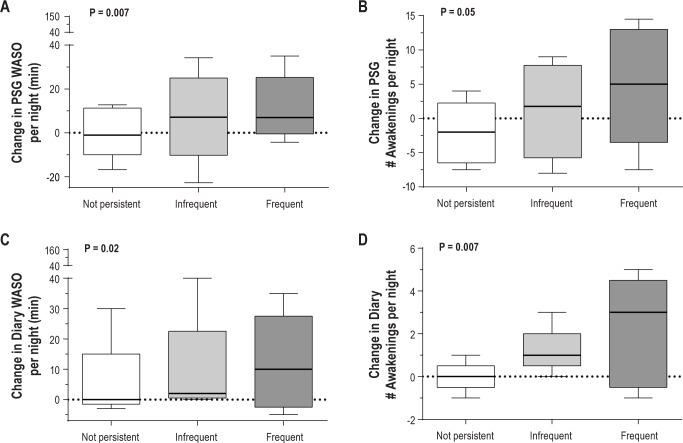
On PSG studies, increasing nighttime VMS frequency resulted in an increase in WASO and in the number of awakenings (Table 3; P = 0.007 and P = 0.05, respectively). On average, there was a 62% increase from baseline in WASO time and a 3% increase in the number of awakenings per night on the PSG for each additional nighttime VMS reported. Within the frequent (n = 10) and infrequent VMS (n = 10), and no-VMS groups (n = 9), the median increase in the WASO time was 14, 6.5, and 0.3 min, respectively (Figure 3A). Similarly, the median increase in the number of awakenings was 6 and 0.8 per night in the frequent and infrequent VMS groups, whereas there was a median decrease of one awakening per night in the no-VMS group (Figure 3b). In addition, the proportion of total sleep time spent in stage N1 increased by 6% in association with increasing nighttime VMS (Table 3; P = 0.02). There was a statistical trend toward a reduction in sleep efficiency in relation to increasing nighttime VMS frequency (P = 0.09), but there was no effect of nighttime VMS frequency on other PSG-measured parameters (Table 3).
Table 3.
Effect of nighttime hot flashes on polysomnographic and subjective measures of sleep
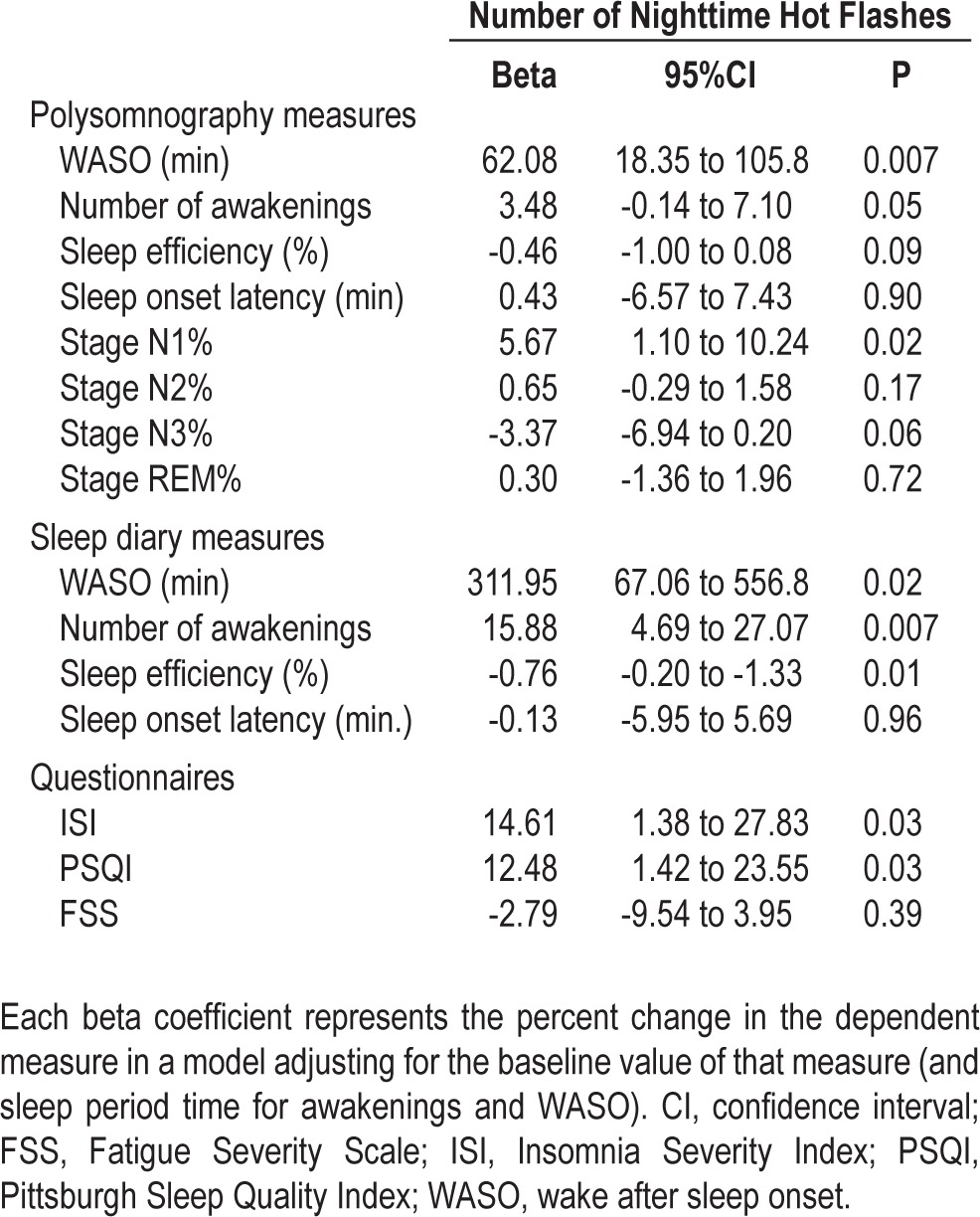
Figure 3.
Effect of nighttime vasomotor symptoms on polysomnography (PSG)-measured (A) wake after sleep onset (WASO); (B) number of awakenings; and subjectively (diary)-measured (C) WASO; (D) number of awakenings. Data are shown by hot flash group (frequent and persistent, n = 10; infrequent and persistent, n = 10; not persistent, n = 9). P values derive from linear regression models of the effect of nighttime VMS frequency on the primary endpoints of percent change in PSG-measured WASO time and number of awakenings adjusting for baseline levels of WASO (or awakenings) and sleep period time.
Consistent with changes in PSG measures, the WASO time and the number of awakenings reported on the sleep diary increased in association with nighttime VMS frequency by 312% and 16%, respectively (Table 3, Figures 3C and 3D; P = 0.02 and P = 0.007, respectively). For the frequent, infrequent, and the no nighttime VMS groups, the median increase in WASO time was 6, 2.5, and 0 min, and the number of awakenings reported per night increased by 2.5, 1, and 0 in each group. Mean duration of wake episodes reported on the diary increased from baseline in relation to the number of nighttime VMS reported (rs = 0.51, P = 0.006). With each additional nighttime VMS reported, self-reported sleep efficiency decreased by 1% (Table 3; P = 0.01), insomnia symptoms increased by 15% (Table 3; P = 0.03), and perceived sleep quality worsened by 13% (Table 3; P = 0.03). There was no effect of nighttime VMS frequency on other sleep diary measures or on FSS scores.
Results were consistent for both objective and subjective sleep parameters when the time frame for quantifying nighttime VMS was restricted to the week leading up the posttreatment PSGs.
Effect of Objectively Recorded Nighttime VMS
Among study participants reporting VMS, we recorded a median of 3.0 (IQR 1.5-4.0) objective VMS during the two posttreatment PSG using a skin conductance monitor. There was a strong correlation between the number of nighttime VMS recorded objectively and the number reported subjectively (rs = 0.57, P = 0.001). The number of nighttime VMS recorded correlated with PSG-measured WASO time (rs = 0.45, P = 0.02), which increased by 6.3 min for each additional objective VMS that was measured (95% confidence interval 0.3-12.8 min). The number of objectively recorded nocturnal VMS also correlated with an increase in the mean duration of PSG-measured wake episodes from baseline (rs = 0.41, P = 0.03). However, objective nighttime VMS did not correlate with the number of awakenings on PSG (rs = 0.27, P = 0.16) or other sleep measures on the PSG, diary, or questionnaires. Sixty-six percent of objectively measured nighttime VMS were associated with an awakening occurring within 5 min of onset of a VMS event.
Effect of Daytime VMS
Prior to accounting for nighttime VMS frequency, the number of PSG-measured awakenings increased by 2% (P = 0.03) and the number of awakenings reported on the diary increased by 7% (P = 0.02) for each additional daytime VMS reported. However, these associations were no longer significant after adjusting for nighttime VMS frequency and there was no independent effect of daytime VMS on any sleep parameter after accounting for nighttime VMS. Results were consistent for both objective and subjective sleep parameters when the time frame for quantifying daytime VMS was restricted to the week leading up the posttreatment PSGs.
DISCUSSION
Using a GnRHa model to induce VMS, we have shown that the number of nighttime VMS correlates directly with the degree of sleep fragmentation. Our results show that the duration of WASO and the number of awakenings, as measured with at-home PSG and sleep diaries, increase in relation to the number of nighttime subjective VMS that develop. WASO time and number of awakenings increased in relation to the number of nighttime VMS reported upon awakening, whereas the number of objectively measured nighttime VMS correlated with WASO time but not awakenings. The effects of nighttime VMS frequency extend to an increase in %N1 sleep, a reduction in diary-rated sleep efficiency, an increase in insomnia symptoms, and a decrease in perceived sleep quality. The number of VMS reported during the daytime was not associated with changes in any sleep parameter after adjusting for nighttime VMS. Our results provide the first experimental evidence that VMS fragment PSG-measured sleep. These findings are consistent with a specific contribution of VMS to PSG-measured sleep interruption, suggesting that nighttime VMS interrupt sleep in the setting of menopause.
Our study shows that nighttime VMS specifically interrupt PSG-measured sleep, but do not prolong sleep onset latency or extensively alter sleep architecture. The increase in WASO duration, awakenings, and %N1 sleep indicate a shift toward greater sleep fragmentation and more light sleep. Sleep onset latency, N2, N3, and REM sleep were not appreciably affected by VMS. These observed changes in objective sleep continuity are consistent with other studies showing that VMS are associated with fragmentation of sleep and reduction of sleep efficiency, as measured by PSG7–10 and actigraphy,14–16 although not all studies have found this association.6,11–13 Previous studies finding no link between VMS and PSG measures of sleep6,11–13 may not have detected an association because women with all levels of VMS frequency— some of whom may have had infrequent nighttime symptoms— were compared categorically against women without VMS. Most studies have used a cross-sectional design contrasting postmenopausal women with VMS versus those without VMS and have not taken into account the specific number of VMS occurring at night. In contrast, in our own previous study of GnRHa-induced VMS, we observed that VMS reduced actigraphically measured sleep efficiency only when the analysis factored in the number of VMS reported.16 Given that VMS occurring at night are the relevant symptom to investigate in sleep studies and that the number of nighttime VMS varies between women, studies similar to the current one provide a more robust quantification by focusing on nighttime VMS frequency.
We observed that the amount of increase in PSG-measured WASO time corresponded to the number of nighttime VMS, regardless of whether VMS were reported in the morning or measured objectively during the night. Analyses of subjectively reported nighttime VMS depend on an individual being awake long enough to remember experiencing a hot flash, which implicitly links subjective VMS at night to awakenings. Individuals who have sufficiently long awakenings to generate memory of the event are more likely to report experiencing sleep disruption and impaired sleep quality, and to have measurable changes of sleep interruption on PSG.40 In contrast, objective recording of VMS at night using a skin conductance measurement is independent of the sleep-wake state. Although not all objective VMS are associated with an awakening, the increase in WASO duration and wake episode duration that we observed in relation to an increasing number of objective nighttime VMS provides further evidence that the physiological changes associated with VMS events are linked to sleep interruption.
In our study, the extent of sleep interruption resulting from nighttime VMS was modest. For example, those who developed frequent nighttime VMS had a median increase from baseline of six awakenings and 14 min of WASO per night on PSG and they reported experiencing a median of 2.5 additional awakenings and 6 additional min of WASO per night relative to baseline. This amount of sleep interruption resulted in a small but statistically significant reduction in self-reported sleep efficiency on the diary and a statistical trend toward a reduction in PSG-measured sleep efficiency. Insomnia symptoms and perceived sleep quality were similarly worsened proportionate to the frequency of nighttime VMS, extending results of studies showing worse sleep quality in women in midlife with VMS versus without VMS17 by demonstrating a “dose” effect of nighttime VMS on sleep quality. Interestingly, our study may have underestimated the deleterious effect of VMS on sleep because, although the number of nighttime VMS reported was similar to that seen in women during the menopause transition,15 we enrolled healthy young premenopausal women who lacked age-related sleep changes, and were good sleepers at baseline. As a result, our study population may have been more resilient than women in midlife to the disruptive effects of nighttime VMS on sleep.
We observed that the increase in PSG awakenings from baseline among those developing new-onset hot flashes exceeds the number of nocturnal VMS. For example, the group with frequent nocturnal VMS had a median of six additional awakenings on PSG after developing VMS, whereas they reported a median of only 3.8 nocturnal VMS subjectively and had 4.0 nocturnal VMS measured objectively. Moreover, we observed that only two-thirds of objectively measured nocturnal VMS were associated with an awakening occurring within 5 min, providing further evidence that a significant proportion of the additional awakenings occurring in those with VMS were not linked with an individual VMS event. These findings suggest that not all awakenings are coupled with an individual hot flash, with some awakenings potentially linked to other related physiologic changes occurring in women with VMS. VMS are triggered by cortical activation,9 and typically follow a transient increase in core body temperature (up to 1°C).41–43 Women with VMS also have underlying changes in core body temperature44 and autonomic nervous system activity,45 independent of individual VMS events. Because increases in core temperature can result in an awakening,46 we hypothesize that the underlying thermal dysregulation in women with VMS may contribute to the number of awakenings. Therefore, the extent of sleep interruption in women developing VMS may reflect the effect of both heat dissipation and sweating, yielding awakenings as well as differential patterns of core temperature and/or autonomic nervous system activity effects on sleep.
The use of an experimental design to study the effect of VMS on sleep provides a strong approach for this research question because changes in sleep from baseline are calculated in response to the onset of VMS. The rigor of study design is balanced by the size of the study sample, which, like other experimental studies,47 is limited by cost considerations and the intensity of study procedures. Inclusion of young premenopausal women eliminates confounding by age-related sleep changes, which may underestimate the magnitude of the effect of nighttime VMS on sleep. PSG sleep patterns observed at baseline in our study participants are consistent with those seen in healthy women of a similar age.48,49
In summary, this study of induced hot flashes provides strong evidence that nocturnal VMS fragment sleep and reduce sleep quality in direct proportion to the increasing number of nighttime VMS, thereby validating the subjective experience of women transitioning through menopause during midlife. By quantifying nighttime VMS, using each study participant as her own control, and isolating VMS effects from those of age-related sleep changes, this study demonstrates an effect of new-onset nighttime VMS on sleep disruption and resolves the apparent paradox in the literature between the effects of VMS on subjective and objective sleep measures.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Joffe has received grant funding from Cephalon/Teva, serves as a consultant for Noven Pharmaceuticals, and serves as an unpaid consultant to Sunovion. Dr. White has served as Chief Medical Officer for Philips Respironics, and currently serves as Chief Scientific Officer for Apnicure Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Drs. Hall and White contributed equally as senior authors. The authors acknowledge Judith Boucher, RPSGT, and Susan Dougherty, RPSGT, for assistance conducting the ambulatory PSG studies, and Daniel Aeschbach, PhD, and Brandon Lockyer, RPSGT, in the Sleep & EEG Core of the Harvard Medical School Division of Sleep Medicine for assistance with sleep stage scoring. The work was performed at Massachusetts General Hospital. Funding source was 5R01MH082922 (to Dr. Joffe). ClinicalTrials.gov Identifier: NCT01116401.
REFERENCES
- 1.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Sleep. 2010;33:539–49. doi: 10.1093/sleep/33.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–90. [PMC free article] [PubMed] [Google Scholar]
- 4.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166:1262–8. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 5.Burleson MH, Todd M, Trevathan WR. Daily vasomotor symptoms, sleep problems, and mood: using daily data to evaluate the domino hypothesis in middle-aged women. Menopause. 2010;17:87–95. doi: 10.1097/gme.0b013e3181b20b2d. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–72. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 7.Erlik Y, Tataryn IV, Meldrum DR, Lomax P, Bajorek JG, Judd HL. Association of waking episodes with menopausal hot flushes. JAMA. 1981;245:1741–4. [PubMed] [Google Scholar]
- 8.Woodward S, Freedman RR. The thermoregulatory effects of menopausal hot flashes on sleep. Sleep. 1994;17:497–501. doi: 10.1093/sleep/17.6.497. [DOI] [PubMed] [Google Scholar]
- 9.Freedman RR, Benton MD, Genik RJ, 2nd, Graydon FX. Cortical activation during menopausal hot flashes. Fertil Steril. 2006;85:674–8. doi: 10.1016/j.fertnstert.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Savard J, Davidson JR, Ivers H, et al. The association between nocturnal hot flashes and sleep in breast cancer survivors. J Pain Symptom Manage. 2004;27:513–22. doi: 10.1016/j.jpainsymman.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Freedman RR, Roehrs TA. Sleep disturbance in menopause. Menopause. 2007;14:826–9. doi: 10.1097/GME.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- 12.Freedman RR, Roehrs TA. Lack of sleep disturbance from menopausal hot flashes. Fertil Steril. 2004;82:138–44. doi: 10.1016/j.fertnstert.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Shaver J, Giblin E, Lentz M, Lee K. Sleep patterns and stability in perimenopausal women. Sleep. 1988;11:556–61. doi: 10.1093/sleep/11.6.556. [DOI] [PubMed] [Google Scholar]
- 14.Ensrud KE, Stone KL, Blackwell TL, et al. Frequency and severity of hot flashes and sleep disturbance in postmenopausal women with hot flashes. Menopause. 2009;16:286–92. doi: 10.1097/gme.0b013e31818c0485. [DOI] [PubMed] [Google Scholar]
- 15.Thurston RC, Santoro N, Matthews KA. Are vasomotor symptoms associated with sleep characteristics among symptomatic midlife women? Comparisons of self-report and objective measures. Menopause. 2012;19:742–8. doi: 10.1097/gme.0b013e3182422973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joffe H, White DP, Crawford SL, et al. Adverse effects of induced hot flashes on objectively recorded and subjectively reported sleep: results of a gonadotropin-releasing hormone agonist experimental protocol. Menopause. 2013;20:905–14. doi: 10.1097/GME.0b013e31828292d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women's health across the nation. Am J Public Health. 2006;96:1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58:348–58. doi: 10.1016/j.maturitas.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi H, Kobori H, Kikuchi I, Sato Y, Mitsuhashi N. A prospective randomized study comparing endocrinological and clinical effects of two types of GnRH agonists in cases of uterine leiomyomas or endometriosis. J Obstet Gynaecol Res. 2000;26:325–31. doi: 10.1111/j.1447-0756.2000.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka T. Effects of herbal medicines on menopausal symptoms induced by gonadotropin-releasing hormone agonist therapy. Clin Exp Obstet Gynecol. 2001;28:20–3. [PubMed] [Google Scholar]
- 21.DeFazio J, Meldrum DR, Laufer L, et al. Induction of hot flashes in premenopausal women treated with a long-acting GnRH agonist. J Clin Endocrinol Metab. 1983;56:445–8. doi: 10.1210/jcem-56-3-445. [DOI] [PubMed] [Google Scholar]
- 22.Blamey RW, Jonat W, Kaufmann M, Bianco AR, Namer M. Goserelin depot in the treatment of premenopausal advanced breast cancer. Eur J Cancer. 1992:810–4. doi: 10.1016/0959-8049(92)90120-q. [DOI] [PubMed] [Google Scholar]
- 23.Fellowes D, Fallowfield LJ, Saunders CM, Houghton J. Tolerability of hormone therapies for breast cancer: how informative are documented symptom profiles in medical notes for ‘well-tolerated’ treatments? Breast Cancer Res Treat. 2001;66:73–81. doi: 10.1023/a:1010684903199. [DOI] [PubMed] [Google Scholar]
- 24.Iber C, Ancoli-Israel S, Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. [Google Scholar]
- 25.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 27.Davidson J, Turnbull CD, Strickland R, Miller R, Graves K. The Montgomery-Asberg Depression Scale: Reliability and validity. Acta Psychiatr Scand. 1986;74:544–8. doi: 10.1111/j.1600-0447.1986.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 28.Rudd MD, Rajab MH. Specificity of the Beck Depression Inventory and the confounding role of comorbid disorders in a clinical sample. Cognitive Therapy Research. 1995;19:51–68. [Google Scholar]
- 29.San Roman GA, Surrey ES, Judd HL, Kerin JF. A prospective randomized comparison of luteal phase versus concurrent follicular phase initiation of gonadotropin-releasing hormone agonist for in vitro fertilization. Fertil Steril. 1992;58:744–9. doi: 10.1016/s0015-0282(16)55322-9. [DOI] [PubMed] [Google Scholar]
- 30.Meldrum DR, Wisot A, Hamilton F, Gutlay AL, Huynh D, Kempton W. Timing of initiation and dose schedule of leuprolide influence the time course of ovarian suppression. Fertil Steril. 1988;50:400–2. doi: 10.1016/s0015-0282(16)60121-8. [DOI] [PubMed] [Google Scholar]
- 31.Gelety TJ, Pearlstone AC, Surrey ES. Short-term endocrine response to gonadotropin-releasing hormone agonist initiated in the early follicular, midluteal, or late luteal phase in normally cycling women. Fertil Steril. 1995;64:1074–80. doi: 10.1016/s0015-0282(16)57963-1. [DOI] [PubMed] [Google Scholar]
- 32.West CP, Baird DT. Suppression of ovarian activity by Zoladex depot (ICI 118630), a long-acting luteinizing hormone releasing hormone agonist analogue. Clin Endocrinol (Oxf) 1987;26:213–20. doi: 10.1111/j.1365-2265.1987.tb00779.x. [DOI] [PubMed] [Google Scholar]
- 33.Buysse DJ, Reynolds CFd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 34.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 35.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 36.Carpenter JS, Newton KM, Sternfeld B, et al. Laboratory and ambulatory evaluation of vasomotor symptom monitors from the Menopause Strategies Finding Lasting Answers for Symptoms and Health network. Menopause. 2012;19:664–71. doi: 10.1097/gme.0b013e31823dbbe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson RE, Grebe SK, DJ OK, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–84. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 38.Siekmann L. Determination of oestradiol-17 beta in human serum by isotope dilution-mass spectrometry. Definitive methods in clinical chemistry, II. J Clin Chem Clin Biochem. 1984;22:551–7. doi: 10.1515/cclm.1984.22.8.551. [DOI] [PubMed] [Google Scholar]
- 39.Welt CK, Pagan YL, Smith PC, Rado KB, Hall JE. Control of follicle-stimulating hormone by estradiol and the inhibins: critical role of estradiol at the hypothalamus during the luteal-follicular transition. J Clin Endocrinol Metab. 2003;88:1766–71. doi: 10.1210/jc.2002-021516. [DOI] [PubMed] [Google Scholar]
- 40.Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355:1064–9. doi: 10.1016/S0140-6736(00)02039-0. [DOI] [PubMed] [Google Scholar]
- 41.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70:332–7. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 42.Freedman RR, Woodward S. Core body temperature during menopausal hot flushes. Fertil Steril. 1996;65:1141–4. [PubMed] [Google Scholar]
- 43.Carpenter JS, Gilchrist JM, Chen K, Gautam S, Freedman RR. Hot flashes, core body temperature, and metabolic parameters in breast cancer survivors. Menopause. 2004;11:375–81. doi: 10.1097/01.gme.0000113848.74835.1a. [DOI] [PubMed] [Google Scholar]
- 44.Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80:2354–8. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 45.Thurston RC, Christie IC, Matthews KA. Hot flashes and cardiac vagal control during women's daily lives. Menopause. 2012;19:406–12. doi: 10.1097/gme.0b013e3182337166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Someren EJ. More than a marker: interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol Int. 2000;17:313–54. doi: 10.1081/cbi-100101050. [DOI] [PubMed] [Google Scholar]
- 47.D'Ambrosio C, Stachenfeld NS, Pisani M, Mohsenin V. Sleep, breathing, and menopause: the effect of fluctuating estrogen and progesterone on sleep and breathing in women. Gend Med. 2005;2:238–45. doi: 10.1016/s1550-8579(05)80053-1. [DOI] [PubMed] [Google Scholar]
- 48.Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30:1283–91. doi: 10.1093/sleep/30.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chuong CJ, Kim SR, Taskin O, Karacan I. Sleep pattern changes in menstrual cycles of women with premenstrual syndrome: a preliminary study. American journal of obstetrics and gynecology. 1997;177:554–8. doi: 10.1016/s0002-9378(97)70145-5. [DOI] [PubMed] [Google Scholar]