Abstract
Objectives and Methods
Data from the memory training arm (n = 629) of the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial were examined to characterize change in memory performance through five years of follow-up as a function of memory training, booster training, adherence to training, and other characteristics.
Results
Latent growth model analyses revealed that memory training was associated with improved memory performance through year five, but that neither booster training nor training adherence significantly influenced this effect. Baseline age was associated with change in memory performance attributable to the passage of time alone (i.e., to aging). Higher education and better self-rated health were associated with greater change in memory performance after training.
Discussion
These findings confirm that memory training can aid in maintaining long-term improvements in memory performance. Booster training and adherence to training do not appear to attenuate rates of normal age-related memory decline.
Keywords: ACTIVE trial, memory training, training adherence, cognitive training, aging, memory decline
The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study is a multi-site, longitudinal trial of the effect of three types of cognitive training interventions (memory, inductive reasoning, and speed of processing) on cognitive and functional abilities in older adults (K. Ball et al., 2002; Jobe et al., 2001; Unverzagt et al., 2009). Initial (K. Ball et al., 2002) and long-term (S. L. Willis et al., 2006) follow-up studies from ACTIVE demonstrate the potential for improvement and maintenance of training-related cognitive performance in older adults. Findings from previous cognitive training studies show immediate, positive effects specific to the cognitive abilities that are the focus of training (McDougall et al., 2010; A. Neely & Bäckman, 1993b; G. Rebok & Balcerak, 1989; Schmidt, Dijkstra, Berg, & Deelman, 1999; S. Willis & Schaie, 1986). Studies with a long-term follow-up period report training benefits lasting up to five years (K. Ball et al., 2002; Borella, Carretti, Riboldi, & De Beni, 2010; Hastings & West, 2009; A. S. Neely & Backman, 1993a; O'Hara et al., 2007; Stigsdotter & Backman, 1989; S. Willis & Nesselroade, 1990; S. L. Willis et al., 2006). However, the findings are inconsistent (Anschutz, Camp, Markley, & Kramer, 1987; O'Hara et al., 2007; Scogin & Bienias, 1988).
Despite advances in cognitive training research, the impact of demographic factors such as age, sex, education, cognitive functioning, and health status on training responsiveness remains understudied. Some evidence suggests that individuals who are younger (Brooks, Friedman, Pearman, Gray, & Yesavage, 1999; Verhaeghen, Marcoen, & Goossens, 1992; Yesavage, Sheikh, Friedman, & Tanke, 1990) and those with initially higher cognitive functioning (R. Hill, Yesavage, Sheikh, & Friedman, 1989; Langbaum, Rebok, Bandeen-Roche, & Carlson, 2009; McKitrick et al., 1999; Verhaeghen & Marcoen, 1996; Yesavage et al., 1990) benefit more from training. However, not all studies replicate these findings (e.g., (Dorfman & Ager, 1989; Rasmusson, Rebok, Bylsma, & Brandt, 1999; Schmidt et al., 1999; Wolters, Theunissen, Bemelmans, Does, & Spinhoven, 1996)). Likewise, the impact of sex, education, and baseline health status on training-related cognitive improvement is not well understood. Of three studies we are aware of that investigated the association between education and memory training gains, none reported significant associations (Dorfman & Ager, 1989; Rasmusson et al., 1999; Wolters et al., 1996). Although it is possible that individuals with the most education may benefit most from memory training, longitudinal studies are needed to determine whether education affects the maintenance of training gains (Langbaum et al., 2009). With respect to health status, Rasmusson and colleagues (1999) reported that participants with improved cognitive performance after memory training took fewer total medications and reported a history of heart disease less often than those whose performance did not improve.
Providing “booster training” at some point after participants have completed initial training has been found to improve the maintenance of training (K. Ball et al., 2002; Kliegl, Smith, & Baltes, 1990; Schaie, 1996). Willis et al. (2006) reported significant booster effects at the five-year follow-up of the ACTIVE study for the reasoning and speed of processing interventions, but not for the memory intervention. Although this was true for the entire memory-trained group, certain subgroups defined by demographic characteristics may have benefited from booster training more than other subgroups. Thus, there is a need to investigate whether booster training is more effective among particular subgroups of individuals and to further investigate effects of booster training on trajectories of memory performance.
In addition, researchers have not thoroughly examined how adherence to training may impact outcomes. Although studies have examined the effectiveness of memory training among older adults (see (G. W. Rebok, Carlson, & Langbaum, 2007; Verhaeghen et al., 1992); for reviews), little attention has focused on the degree of training adherence, defined here by the number of training sessions attended, needed to produce meaningful gains in memory performance. There is great variability in the number of training sessions across studies. Instruction in mnemonic techniques over the course of only one or two sessions can lead to gains in selected aspects of memory (e.g., (G. Rebok & Balcerak, 1989)), although most interventions are typically between 5 and 15 sessions (60 to 75 minutes per session) for group-based memory training (G. W. Rebok et al., 2007). The wide variability in the number of training sessions across studies speaks to the need to better understand the dose of training needed to yield sustainable benefits. Variability in adherence (attendance) in ACTIVE allows us to get a sense of how many sessions are required to produce positive results by examining the association between dose and response.
The present study used data from the memory training arm of the ACTIVE trial to investigate the effects of participant characteristics (age, sex, education, cognitive functioning, and health status), booster training, and adherence to memory training, on training responsiveness. We hypothesized that individuals who are younger and are in better physical and cognitive health at baseline would demonstrate greater benefits of training on memory performance. We also anticipated that booster training and adherence to training would be associated with maintenance of training gains in memory performance.
Methods
Participants
Participants were older adults from the memory-trained group of the ACTIVE trial recruited from six university-based field centers. Participants were excluded from the trial if they showed evidence of substantial cognitive impairment (Mini-Mental State Examination score of less than 23 (MMSE; (Folstein, Folstein, & McHugh, 1975)) or a self-reported diagnosis of dementia), visual impairment (self-report of excessive difficulty reading newspaper print or tested visual acuity worse than 20/50), functional decline (difficulty with activities of daily living (ADLs)), or a history of certain medical conditions that would predispose them to functional decline or mortality, such as cancer or stroke (Jobe et al., 2001). The study protocol was approved by the institutional review boards at all sites, and the trial was monitored by a data safety monitoring board. Written informed consent was obtained from all participants.
A total of 2,802 participants were randomized to a no-contact control condition (n = 698) or one of three training conditions: memory training (n = 703), inductive reasoning training (n = 699), or speed of processing training (n = 702). All participants did not receive training at the same time; training was administered over six waves, or replicates. The administration of the word-list learning tasks for the first replicate of data collection differed from that in later replicates; thus, we excluded from analyses data from memory-trained individuals who received training during the first replicate (n = 74). The present investigation uses participants randomized to the memory-training condition after the first replicate and followed up immediately after training and at one, two, three, and five years after initial training (n = 629). The ACTIVE control group is not included in the present analysis because the focus is on training and participant factors that contribute to training benefits.
Procedures
Participants completed a battery of cognitive, sensory, and functional tests at the baseline visit (prior to randomization), and again immediately after training, and at one, two, three, and five years after training.
Memory training involved the following: (a) ten 60- to 75-minute sessions; (b) small-group settings with individual and group exercises led by certified trainers; (c) focus on strategies for learning and remembering; (d) demonstration of strategy use; (e) practice with exemplar problems; (f) feedback on performance; (g) fostering self-efficacy regarding performance; (h) applying learned strategies to real-world tasks; (i) individualized training experiences; and (j) social interaction (Jobe et al., 2001). The first session was dedicated to educating participants about how memory works and how to get the most out of memory training. During sessions two through five, participants received instruction on memory strategies, including categorization methods, visualization strategies, method of loci, and organizational strategies for text recall (i.e., story mnemonics, sentence mnemonics). During the five remaining sessions, participants practiced each of the memory strategies, but no new strategies were introduced.
Prior to the first and third annual assessments, individuals who completed at least 8 of the 10 initial training sessions were randomized to either receive or not to receive additional memory training sessions (i.e., booster training). Booster training consisted of four 75-minute refresher classes designed to reinforce strategies already taught during the initial training sessions to help participants maintain performance gains.
Measures
At each assessment participants completed several cognitive tests, including three memory measures: modified versions of the Hopkins Verbal Learning Test (HVLT), Rey-Auditory Verbal Learning Test (AVLT), and the Rivermead Behavioral Memory Test's (RBMT) Paragraph Recall task. During the HVLT (Brandt, 1991), respondents are read a 12-item list of semantically related words (four words from each of three semantic categories) for three learning trials. In each trial, participants were presented with the same list of words by audiotape, which they were then asked to recall in any order. During the AVLT (Rey, 1941), participants are presented with a 15-item list of unrelated words, which they are asked to write down immediately over five repeated trials. As with the HVLT, participants were given the same list of words in each trial by audiotape, and were asked to write down the words in any order. However, after the fifth trial, participants were read a different list (interference trial) followed by the original list again (short-delay retention trial). The RBMT-Paragraph Recall task (Wilson, Cockburn, & Baddeley, 1985) measures prose memory. Participants are read a four-to five-sentence paragraph containing 21 distinct propositions, and then asked to write down as much of the story as he or she can remember in two minutes. Parallel versions of the HVLT, AVLT, and RBMT-Paragraph Recall were administered at each visit.
In ACTIVE, protocols for administering the HVLT, AVLT, and RBMT were modified from standard clinical administration in three important ways. To standardize test administration, test stimuli (i.e., words for HVLT and AVLT, paragraph for Rivermead) were presented by audiotape, rather than being read by each examiner. In addition, participants wrote down responses instead of speaking them. Further, delayed recall trials were not administered due to time constraints. The three tests were always administered in the same order, and they were spaced over two days to avoid fatigue and interference effects. The HVLT was administered on the first day of assessments while the AVLT and the Rivermead were administered on the second day of assessments. All tests were scored by certified personnel.
A memory composite score was calculated for each assessment, and this composite was the dependent variable of interest in the present study. To compute the composite score, the total scores from each of the three memory measures were equally weighted, pooled together, and Blom transformed (Blom, 1958), producing more normally distributed scores. When computing the composite scores, test scores at each time point were standardized to the baseline mean and standard deviation (see (K. Ball et al., 2002), for a full description). If a participant was missing one or more tests for the composite, their score for that test was calculated as the average of all non-missing tests (K. Ball et al., 2002).
Importantly, the ACTIVE study design used parallel but nonequivalent forms of the memory assessments at each study visit to reduce practice effects. Because HVLT and AVLT forms used were nonequivalent, the scale of the outcome (i.e., the memory composite score) varied across visits for methodological reasons unrelated to training or aging. To make valid within-person comparisons for the present study, alternate forms were placed on an equivalent metric using an equipercentile equating procedure (Kolen & Brennan, 1995). Equipercentile equating uses a test score's percentile rank at follow-up occasions to define a non-parametric transformation that puts the test on the same scale (i.e., same mean, standard deviation, skew, and kurtosis) as the baseline test distribution. In equipercentile equating, test scores at follow-up visits are scaled to baseline scores with the same percentile rank. To preserve aging and cohort differences over time in ACTIVE, we tailored a weighted version of this procedure for the present study using a two-stage approach. First, we identified a restricted sample from which to calculate test score percentiles and derive the equating algorithm. Second, we applied the equating algorithm to the full study sample. Details and diagnostic procedures are provided in a recently published article (A. Gross et al., 2012). Briefly, the restricted sample was defined by control group participants who were seen at all study visits and to an age range observable at all study visits (70 to 85 years). We estimated analytic weights using a direct adjustment procedure for age to ensure the same age distribution at each study wave; although this removes an aging effect in the equating sample (that was comprised of a highly restricted group), the aging effect is preserved in the full sample for analyses of individual differences.
The equipercentile-equated memory composite retains sensitivity to aging and training effects, which are the focus of the current study..
Analysis
We used a multiple-group latent growth curve analysis (McArdle & Hamagami, 1996; B. Muthén & Curran, 1997) to model the effect of booster training, adherence, and participant characteristics on memory performance over time, as measured by the memory composite score. The multiple-group approach enabled us to examine the effects of booster training and adherence on trajectories of memory performance. The adherence variable as it stands in the present study is necessary because being randomized to booster training was dependent on whether the participant was adherent to the initial training session. We defined adherence as the number of sessions attended during the initial training as well as during booster training. To be considered adherent during initial training (Adherent A), participants had to complete at least 8 out of 10 sessions. Participants completing 7 or fewer of the 10 sessions were considered non-adherent (Non-adherent A). Participants who completed the initial training were eligible for booster training. Booster training was provided to a random subsample of individuals in each intervention group. To be considered adherent to booster training (Adherent B), participants had to complete at least 3 of 4 booster sessions prior to the first and third annual assessments (see Table 1). Participants completing only 1 or 2 booster sessions prior to the first and third annual assessments were considered non-adherent (Non-adherent B). Participants were assigned using these criteria to one of four groups: Non-adherent A, Adherent A, Non-adherent B, and Adherent B. Final model selection was based on traditional structural equation modeling model fit statistics, including χ2 tests, comparative fit index (CFI; (Bentler, 1990)), root mean square error of approximation (RMSEA; (Steiger, 1989)), and the standardized root mean square residual (SRMR; (Browne & Cudeck, 1993)).
Table 1. Baseline Characteristics of Memory-Trained Participants: Results from ACTIVE (n = 629).
| Characteristic | |
|---|---|
| Mean age (years) [range 65, 93] | 73.5 ± 6.0 |
| Sex (female) – N (%) | 485 (77) |
| Race (White) – N (%) | 481 (76) |
| Education (years) [range 5, 20] | 13.7 ± 2.7 |
| MMSE Score [range 23, 30] | 27.3 ± 2.0 |
| Self-reported health status | |
| Excellent/very good/good – N (%) | 534 (85) |
| Adherence level – N (%) | |
| Non-adherent A | 77 (12) |
| Adherent A | 219 (35) |
| Non-adherent B | 131 (21) |
| Adherent B | 202 (32) |
Note. MMSE: Mini-Mental State Examination (Folstein et al., 1975). Non-adherent A: completed <8 of 10 initial training sessions; Adherent A: completed ≥8 of 10 initial training sessions; Non-adherent B: completed <3 of 4 booster training sessions; Adherent B: completed ≥3 of 4 booster training sessions.
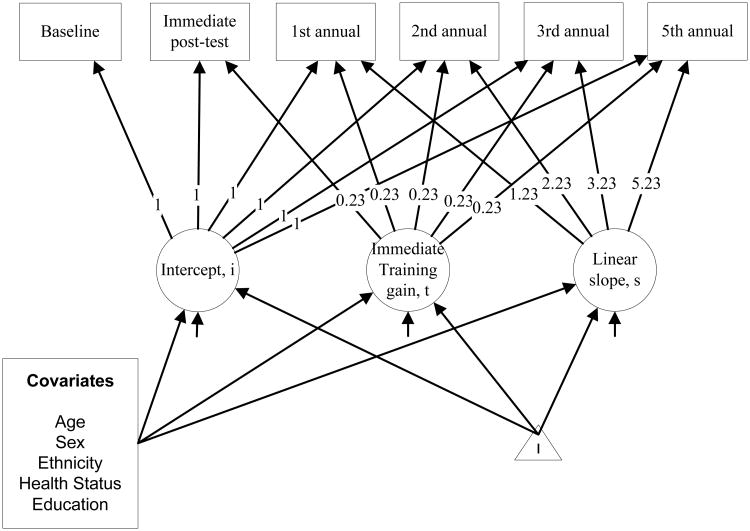
We conducted the analysis in two steps. In the first step, the first model (Figure 1) consisted of latent intercept and slope factors estimated separately in each adherence group to account for individual differences in overall level of memory ability and change in memory performance over time, independent of training-induced changes. Factor loading paths from the intercept to the observed memory composite scores were fixed at 1.0 for each assessment, and paths from the linear slope to the memory composite scores were fixed at 0, 0.23, 1.23, 2.23, 3.23, and 5.23 for baseline, immediate posttest, and first, second, third, and fifth annual assessments, respectively. These fixed loadings reflect the per-protocol amount of time in years that elapsed between baseline and each assessment occasion. We used a latent retest factor to assess the immediate training effect (baseline to immediate post-training) on changes in memory; the factor loading from this latent factor to the baseline time-point was fixed at 0 and at 1.0 for observed variables at subsequent occasions. Means, variances, and covariances among latent growth factors were allowed to vary between adherence groups. A second model (not shown) consisted of the same factors plus the addition of two latent effects for booster training just prior to the first and third annual assessments in the two adherence groups that received booster training. Specifically, the first booster factor loaded onto the first, second, third, and fifth annual visits with unit weight, and the second booster factor loaded onto the third and fifth annual visits with unit weight. Wald tests of whether the means of these latent factors differ from zero were used to evaluate booster effects.
Figure 1. Multiple Group Latent Growth Curve Model of Composite Memory Performance.
Note: This figure represents the parameterization of the latent growth model for Model 1. Observed memory composite scores are displayed in squares. Three latent variables capturing change over time are illustrated: i captures baseline (pre-training) and loads in each study visit with unit weight; t captures the immediate effect of training and practice, loading in study visits with unit weight; and s captures the annual change in composite performance, loading in study visits with fixed time steps reflecting years from baseline. The level (i) and linear slope (s) factors are handled as random effects. The triangle represents a constant effect, illustrating that we are modeling means in i, s, and t. Means (and variances/covariances) are estimated separately for the Non-adherent A, Adherent A, Non-adherent B, and Adherent B groups. In the final model-building step, latent growth factors were regressed on demographic covariates.
In the second step, the effects of participant characteristics at baseline (age, sex, education, MMSE, health status, reasoning ability, and speed of processing ability) on the latent intercept, slope, and training effect factors were assessed. Binary covariates included sex (0=male; 1=female) and baseline self-reported health status (excellent/very good/good vs. fair/poor). To study whether covariate effects on latent factors differ by adherence group, the Satorra-Bentler scaled χ2 difference test was used to compare a model that constrained regression parameters between latent factors and covariates to be the same across groups with a model that allowed them to vary by group (Satorra & Bentler, 1994).
Descriptive analyses were conducted using Stata v11 (Stata Corp., College Station, TX). LGM analyses were conducted using Mplus v6.1 (L. Muthén & Muthén, 1998-2010). Missing data in the outcome variable were included using a robust maximum likelihood estimator under the assumption that the missing data are missing at random (Little & Rubin, 1987).
Results
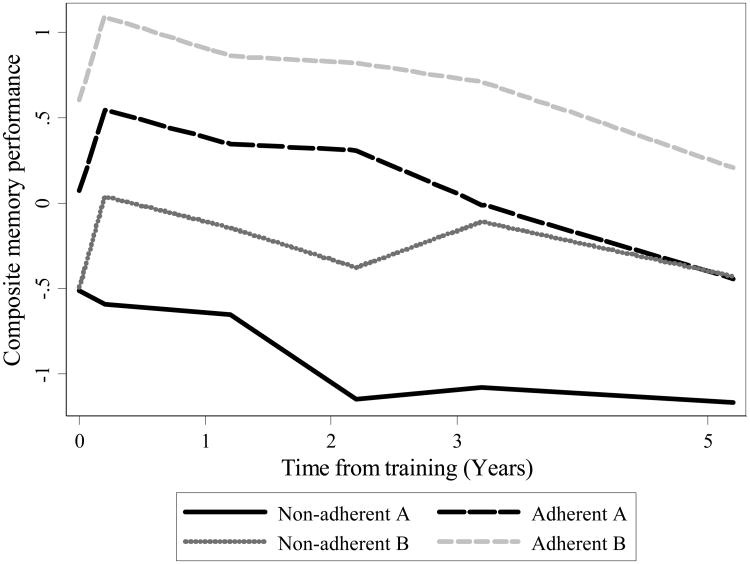
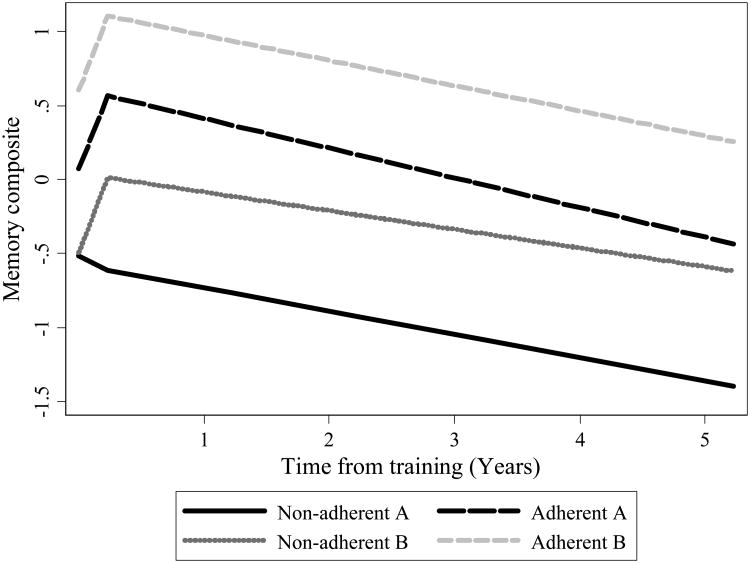
At baseline, participants, on average, were 73.5 years of age (SD = 6.0; range = 65 to 93 years), had 13.6 years of education (SD = 2.7; range 5 to 20 years), and did not exhibit signs of cognitive impairment as indicated by a score of 23 or greater on the MMSE (M = 27.3; SD = 2.1; range = 23 to 30) (Table 1). Mean-estimated equipercentile memory composite scores at each study timepoint by adherence group are displayed graphically in Figure 2. For all boosted groups, regardless of adherence, mean memory composite scores are higher at post-training than at baseline. The mean memory score in the Adherent A, Non-adherent B, and Adherent B groups showed a large improvement after training that remained high through five years following training, whereas the mean score of participants in the Non-adherent A group showed decline through the study period. Model-estimated trajectories of composite memory performance by adherence group are shown in Figure 3.
Figure 2. Mean-estimated Trajectories of Composite Memory Performance by Adherence: Results from ACTIVE (n = 629).
Note. Mean trajectories of composite memory performance by adherence group. Non-adherent A: completed <8 of 10 initial training sessions; Adherent A: completed >8 of 10 initial training sessions; Non-adherent B: completed <3 of 4 booster training sessions; Adherent B: completed ≥3 of 4 booster training sessions.
Figure 3. Model-estimated Trajectories of Composite Memory Performance by Adherence: Results from ACTIVE (n = 629).
Note. Model-estimated trajectories of composite memory performance by adherence group. Non-adherent A: completed <8 of 10 initial training sessions; Adherent A: completed >8 of 10 initial training sessions; Non-adherent B: completed <3 of 4 booster training sessions; Adherent B: completed ≥3 of 4 booster training sessions.
Estimates from the four-group LGM in step one are displayed in Table 2. Model fit was excellent, confirming inferences from Figure 2 that person-level trajectories generally followed a linear pattern following the immediate post-training visit. Mean memory performance at baseline in the fully adherent group (Adherent B) was highest compared to all the other groups, followed by the initially adherent group (Adherent A) not assigned to booster training. The initially non-adherent group (Non-adherent A) and the initially adherent group that did not complete 75% of the booster sessions (Non-adherent B) did not differ from one another and had the lowest mean memory performance at baseline. All groups had a significant negative slope, indicating worsening memory performance over time. The magnitude of the slope did not differ across groups (all p > 0.19). In contrast, the factor representing the immediate pre-post training effect on memory performance was significantly positive for all participants who were adherent to the initial training, regardless of randomization to booster training or adherence to booster training. Memory pre-post training change in the initially non-adherent group (Non-adherent A) was significantly lower than all other groups and not significantly different from 0.
Table 2. Four-Group Latent Growth Model of Composite Memory Performance: Results from ACTIVE (n = 629).
| Initial training | Booster training | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Non-adherent A | Adherent A | Non-adherent B | Adherent B | |||||
|
| ||||||||
| β (SE) | Effect size | β (SE) | Effect size | β (SE) | Effect size | β (SE) | Effect size | |
| Means | ||||||||
| Intercept | -0.51 (0.29) | (REF) | 0.07 (0.16) | 2.86 | -0.49 (0.21)* | 0.08 | 0.61 (0.14)* | 5.77 |
| Slope (normal aging) | -0.16 (0.06)* | (REF) | -0.20 (0.03)* | 1.00 | -0.13 (0.05)* | 0.55 | -0.17 (0.03)* | 0.25 |
| Training effect | -0.06 (0.21) | (REF) | 0.54 (0.09)* | 4.52 | 0.53 (0.11)* | 3.79 | 0.54 (0.09)* | 4.45 |
| Variances | ||||||||
| Intercept | 4.57 (0.93)* | 4.64 (0.40)* | 5.20 (0.49)* | 3.58 (0.36)* | ||||
| Slope (normal aging) | 0.02 (0.03) | 0.02 (0.01) | 0.06 (0.03)* | 0.04 (0.01)* | ||||
| Training effect | @0 | 0.28 (0.20) | 0.82 (0.30)* | 0.33 (0.18) | ||||
| Covariance(Intercept, Slope) | 0.10 (0.14) | 0.08 (0.06) | -0.04 (0.08) | 0.09 (0.04)* | ||||
| Model fit statistics | ||||||||
| Model χ2 (df) | 85.93 (57), p=0.01 | |||||||
| Contribution to χ2 | 14.76 | 15.86 | 37.55 | 17.77 | ||||
| CFI | 0.988 | |||||||
| RMSEA | 0.057 | |||||||
| SRMR | 0.047 | |||||||
Note. Results from a four-group latent growth curve model of equipercentile-equated composite memory performance for each adherence group. The model setup is displayed graphically in Figure 1. Means of latent variable intercepts and slopes are free to vary by adherence group. Asterisks indicate parameter estimates are significantly different from zero (p < 0.05). Effect sizes represent Cohen's d effect sizes for group differences in a parameter relative to the Non-adherent-A group.
0 indicates the variance of the training effect in the Non-adherent A group was fixed at 0 for model convergence. Residual variances of observed dependent variables were freely estimated, results of which are not shown. SE: standard error; CFI: Comparative fit index; RMSEA: Root mean square error of approximation; SRMR: Standardized root mean square residual. Mean intercept values differed significantly (p < 0.05) by group: Non-adherent A < Adherent A = Non-adherent B < Adherent B. Mean slopes did not differ across group. For the training effect, Non-adherent A < Adherent A = Non-adherent B = Adherent B.
The effect size estimates for the intercept, slope, and training effect relative to the Non-adherent-A group are shown in Table 2. The results indicate that adherent participants demonstrated higher memory function by between 2.8 and 5.8 standard deviations over the Non-adherent A and Non-adherent B groups (intercepts). Further, there is a robust immediate training effect among all groups that were initially adherent to training (training effects). The effect sizes for the slope show the annual pace of change, or trajectory, is 1, 0.55, and 0.25 standard deviations per year in the groups. The latter two slopes are different from 0.
A second model was similar to Model 1 but included factors for booster training just prior to the first and third annual assessments. Model fit remained excellent (CFI = 0.991; RMSEA = 0.055; SRMR = 0.046). Results from this model paralleled those of the first model. The effect of booster training was not significant in either the Non-adherent B group (first annual booster training effect: β = −0.05, p = 0.75; third annual booster training effect: β = 0.45, p = 0.07) or the Adherent B group (first annual booster training effect: β = 0.07, p = 0.51; third annual booster training effect: β = 0.18, p = 0.21).
Because effects of booster training were not significant in the second model, we retained the first model. In step 2 of the analysis, the first model in step 1 was extended by regressing latent intercepts, slopes, and training effect factors on covariates. A model that allowed regression parameters to vary by adherence group did not differ significantly from a model in which associations were invariant across groups (Satorra-Bentler scaled χ2df=45 = 54.95, p = 0.15), suggesting that differences in associations by adherence group are negligible. Results from an LGM in which covariate effects were held constant across all groups are provided in Table 3. Coefficients for intercept predictors represent the difference in baseline composite memory performance per unit difference in the predictor. Focusing on the covariate effects on baseline memory performance levels (intercept predictors), results indicate that younger age, higher education, better general cognitive function, female sex, reasoning ability, and speed of cognitive processing were associated with higher memory performance at baseline. In terms of change in memory performance over time independent of the effect of training (slope predictors), coefficients are interpretable as differences in the trajectory or slope of composite memory performance per unit difference in the predictor. Results indicate that only age was associated with change in memory performance independent of training. We also tested for a non-linear effect of age by including a quadratic term for age in the model. The quadratic term was not associated with slope or training effect but is associated with a higher intercept (β = 0.006; SE = 0.002, p = 0.001). With regard to the immediate effect of training on the memory composite score, individuals reporting a greater number of years of education and better self-rated health demonstrated a greater gain in memory performance immediately after training (Table 3). With regard to baseline cognitive status, better inductive reasoning and processing speed at baseline were not associated with the pace of memory change or training benefit.
Table 3. Latent Growth Model of Composite Memory Performance with Covariates: Results from ACTIVE (n = 629).
| β | 95% CI | |
|---|---|---|
| Intercept Predictors | ||
| Age | −0.10 | (−0.13, −0.07) |
| Education | 0.16 | (0.09, 0.22) |
| MMSE | 0.43 | (0.36, 0.51) |
| Sex (1=Female) | 0.94 | (0.59, 1.29) |
| Self-rated Health Status | 0.37 | (−0.05, 0.80) |
| Reasoning | 0.34 | (0.26, 0.41) |
| Speed of processing | −0.10 | (−0.17, −0.03) |
| Slope (Normal aging) predictors | ||
| Age | −0.01 | (−0.02, −0.01) |
| Education | −0.01 | (−0.02, 0.00) |
| MMSE | 0.01 | (0.00, 0.03) |
| Sex (1=Female) | −0.03 | (−0.11, 0.05) |
| Self-rated Health Status | 0.04 | (−0.05, 0.13) |
| Reasoning | 0.01 | (0.00, 0.03) |
| Speed of processing | −0.01 | (−0.02, 0.00) |
| Training effect predictors | ||
| Age | 0.00 | (−0.02, 0.02) |
| Education | 0.05 | (0.01, 0.09) |
| MMSE | 0.02 | (−0.03, 0.08) |
| Sex (1=Female) | 0.17 | (−0.06, 0.40) |
| Self-rated Health Status | 0.38 | (0.16, 0.59) |
| Reasoning | −0.05 | (−0.11, 0.01) |
| Speed of processing | 0.01 | (−0.05, 0.07) |
Note. Results from a single-group latent growth curve model of equipercentile-equated composite memory performance. Latent variable growth parameters are regressed on demographic predictors. Residual variances of observed dependent variables were freely estimated, results of which are not shown. MMSE: Mini-Mental State Examination; 95% CI: 95% confidence interval.
Discussion
In the present study of community-dwelling older adults who participated in the ACTIVE trial, we found that adherence to initial memory training was associated with immediate training benefits, but the impact of memory booster training on the long-term training effect after five years was negligible. Further, neither adherence nor booster training was associated with attenuated rates of normal age-related memory decline. The effects of covariates on the intercept, slope, and training effect factors did not differ significantly by booster training status or adherence to training. Although age, sex, education, and baseline cognitive status each were significantly associated with baseline memory performance, only baseline age was associated with change in memory performance attributable to aging, such that older individuals declined faster, and more education and higher self-rated health were the only participant characteristics associated with improvement in memory performance attributable to training.
The findings from the present study complement results from previous ACTIVE reports (K. Ball et al., 2002; S. L. Willis et al., 2006) by providing new information regarding the rate of change in memory performance that is due to time (aging) and training. Although memory composite scores decrease over time, the retest factor, which assesses the actual effect of training over a five-year time period, indicates that both the boosted and the non-boosted groups experienced an overall increase in memory performance over five years. The fact that the performance gains following memory training did not diminish after five years argues against Salthouse's (Salthouse, 2006) conclusion from a secondary analysis of ACTIVE training data suggesting that the pace of change over time in trained abilities is actually accelerated for persons who participated in the intervention. Although several plausible explanations may exist, our use of sophisticated modeling techniques to capture change over time in three different latent variables may account for the differences between the present study's findings and those from Salthouse's (2006) analysis. The reasons for the long-term maintenance of memory training gains in ACTIVE may be related to the frequent opportunities for practice of the learned mnemonics, the inclusion of group practice exercises involving everyday memory abilities, and the increased emphasis on how to apply memory skills learned in the laboratory to daily life ((K. Ball et al., 2002; Langbaum et al., 2009; S. L. Willis et al., 2006)). However, with the current ACTIVE data, we are not able to explore this last issue directly. One of the challenges for future research on durability of training-related gains in memory will be to explore everyday activities that are most effective in enhancing and maintaining memory function over decades and across different periods of older adulthood.
The results for the booster memory training arm of the ACTIVE trial are in line with previous reports of the ACTIVE memory training data, but, as reported in this issue, booster training for both inductive reasoning and speed of processing had beneficial effects on their targeted cognitive outcomes (K Ball, Ross, Roth, & Edwards, under review; S. Willis & Caskie, under review). All participants in the memory intervention benefited from initial training, regardless of whether or not they received the additional booster training. Indeed, there did not appear to be any additional benefit of having participated in booster sessions beyond the benefit received from the initial training sessions. One interpretation of this result is that the training optimized memory performance so that there was little room left for improvement beyond the initial training gain. However, an examination of the pre- and post-training scores on the memory measures that comprise the memory composite suggests that, while scores increased as a function of training, they remained well below ceiling levels (data not shown). A second explanation involves the dosage level needed to provide a booster training effect. Perhaps four booster sessions were not enough to refresh subjects in the use of the four different mnemonic techniques, especially the more cognitively demanding techniques such as the method of loci. A third possibility is that, compared to the strategies taught in reasoning and speed of processing training, mastery of mnemonic strategies requires greater practice and experience. Further studies are needed to determine whether changes in the dosage, content, or the format of memory booster training sessions increase training effectiveness.
An important question for memory training research is to determine the amount of and adherence to training that is needed to produce a significant improvement in memory. Results of this study indicate that adherent non-boosted participants (Adherent A) showed a significant increase in verbal episodic memory, suggesting that completing at least eight training sessions of the type used in ACTIVE was sufficient to produce a reliable and sustained gain. As discussed above, the non-adherent booster participants (Non-adherent B) also showed significant training gains, further supporting the conclusion that additional booster sessions may not produce added benefits for those adherent to the initial training. Whether additional sessions of the initial training beyond the eight required to meet adherence criteria would have resulted in larger training gains cannot be definitively answered by the present study. However, it has been noted that many, if not most, memory training programs with older adults are seriously underdosed, and may not be sufficiently intensive to offset a lifetime of poor memory habits or disuse (G. W. Rebok et al., 2007).
In contrast to reasoning and speed training (K Ball et al., under review; S. Willis & Caskie, under review), adherence to training in the present study was not associated with greater benefits of memory training, perhaps because the measurement is not sensitive enough to capture such dynamics as whether participants practiced the mnemonic strategies outside of training in their daily life or retained the strategies after training. Nor do the ACTIVE data presently allow us to investigate the issue of adherence by analyzing which sessions the participant attended; we can only examine the total number of sessions. Raw data sheets from the ACTIVE training sessions are currently being examined to determine which strategies were employed at each session (A. L. Gross & Rebok, 2011; Sisco, Marsiske, Gross, & Rebok, under review). This work should allow for further exploration of the adherence issue in the future. Nonetheless, the lack of a significant finding for adherence in the present study should not be taken to indicate that memory training interventions are effective even if a participant does not show up for many sessions. In ACTIVE, over 85% of the memory training participants were adherent to initial training, suggesting that the majority of the participants were at least exposed to the intervention.
Results from the covariates analysis show that age, sex, education, and MMSE score each are significantly associated with baseline memory performance. These effects replicate previous findings that have been reported in memory aging research. Specifically, being younger, female, having more years of education, and a higher MMSE score are associated with better memory performance at baseline. For change in memory performance attributable to training, only education and self-rated health status have a significant impact. These results are in contrast to previous studies that report that higher baseline cognitive status (R. D. Hill, Sheikh, & Yesavage, 1988; Yesavage et al., 1990) is associated with greater ability to benefit from mnemonic training. It is conceivable that data from these studies were not sufficiently analyzed to determine the effect of covariates on the pace of change.
The present results using the LGM approach allow us to draw specific conclusions regarding how training improves older adults' performance on verbal episodic memory tasks. The LGM analyses reveal that memory training is associated with improved memory performance for at least five years following training, and that neither booster training nor adherence to training appear to significantly influence this effect above the overall effects of training. Who benefits from memory training? In the present study, only education and self-rated health were found to influence the benefits of memory training. The impact of other factors such as sex and cognitive status on training responsiveness remains unclear and requires additional research. Another key question that remains is whether the training effects present five years after initial training will continue to persist after another five years. We recently completed 10-year follow-up assessments on the current ACTIVE cohort that hopefully will provide answers to these important questions.
Supplementary Material
Acknowledgments
ACTIVE is supported by grants from the National Institute on Aging and the National Institute of Nursing Research to Hebrew Senior Life (U01NR04507), Indiana University School of Medicine (U01NR04508), Johns Hopkins University (U01AG14260), New England Research Institutes (U01AG14282), Pennsylvania State University (U01AG14263), University of Alabama at Birmingham (U01AG14289), University of Florida (U01AG14276). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research, National Institute on Aging, or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
References
- Anschutz L, Camp CJ, Markley RP, Kramer JJ. Remembering mnemonics: a three-year follow-up on the effects of mnemonics training in elderly adults. Exp Aging Res. 1987;13(3):141–143. doi: 10.1080/03610738708259315. [DOI] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Ross L, Roth D, Edwards J. Speed of Processing Training in the ACTIVE Study: How Much is Needed and Who Benefits? Journal of Aging and Health. doi: 10.1177/0898264312470167. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler P. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Blom G. Statistical Estimates and Transformed Beta Variables. New York: John Wiley and Sons; 1958. [Google Scholar]
- Borella E, Carretti B, Riboldi F, De Beni R. Working memory training in older adults: evidence of transfer and maintenance effects. Psychol Aging. 2010;25(4):767–778. doi: 10.1037/a0020683. [DOI] [PubMed] [Google Scholar]
- Brandt J. The hopkins verbal learning test: Development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5(2):125–142. [Google Scholar]
- Brooks JO, 3rd, Friedman L, Pearman AM, Gray C, Yesavage JA. Mnemonic training in older adults: effects of age, length of training, and type of cognitive pretraining. Int Psychogeriatr. 1999;11(1):75–84. doi: 10.1017/s1041610299005608. [DOI] [PubMed] [Google Scholar]
- Browne M, Cudeck R. Alternative ways of assessing model fit. In: Bollen K, Long J, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Dorfman C, Ager C. Memory and memory training: Some treatment implications for use with the well elderly. Physical & Occupational Therapy in Geriatrics. 1989;7:21–41. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gross A, Inouye S, Rebok G, Brandt J, Crane P, Parisi J, et al. Parallel but not equivalent: Challenges and solutions for repeated assessment of cognition over time. J Clin Exp Neuropsychol. 2012 Apr 30; doi: 10.1080/13803395.2012.681628. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Rebok GW. Memory training and strategy use in older adults: results from the ACTIVE study. Psychol Aging. 2011;26(3):503–517. doi: 10.1037/a0022687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings EC, West RL. The relative success of a self-help and a group-based memory training program for older adults. Psychol Aging. 2009;24(3):586–594. doi: 10.1037/a0016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Yesavage J, Sheikh J, Friedman L. Mental status as a predictor of response to memory training in older adults. Educational Gerontology. 1989;15:633–639. [Google Scholar]
- Hill RD, Sheikh JI, Yesavage JA. Pretraining enhances mnemonic training in elderly adults. Exp Aging Res. 1988;14(4):207–211. doi: 10.1080/03610738808259749. [DOI] [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL, et al. ACTIVE: a cognitive intervention trial to promote independence in older adults. Control Clin Trials. 2001;22(4):453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegl R, Smith J, Baltes P. On the locus and process of magnification of age differences during mnemonic training. Dev Psychol. 1990;26(6):894–904. [Google Scholar]
- Kolen M, Brennan R. Test equating: Methods and practices. New York: Springer; 1995. [Google Scholar]
- Langbaum JB, Rebok GW, Bandeen-Roche K, Carlson MC. Predicting memory training response patterns: results from ACTIVE. J Gerontol B Psychol Sci Soc Sci. 2009;64(1):14–23. doi: 10.1093/geronb/gbn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R, Rubin D. Statistical analysis with missing data. New York: John Wiley & Sons; 1987. [Google Scholar]
- McArdle J, Hamagami F. Multilevel models from a multiple group structural equation perspective. In: Marcoulides G, Schumaker R, editors. Advanced structural equpation modeling: Issues and techniques. Mahwah, NJ: Erlbaum; 1996. pp. 89–124. [Google Scholar]
- McDougall G, Becker H, Pituch K, Acee T, Vaughan P, Delville C. The SeniorWise study: Improving everyday memory in older adults. Archives of Psychiatric Nursing. 2010;24:291–306. doi: 10.1016/j.apnu.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKitrick LA, Friedman LF, Brooks JO, 3rd, Pearman A, Kraemer HC, Yesavage JA. Predicting response of older adults to mnemonic training: who will benefit? Int Psychogeriatr. 1999;11(3):289–300. doi: 10.1017/s1041610299005852. [DOI] [PubMed] [Google Scholar]
- Muthén B, Curran P. General longitudinal modeling of individual differences in experimental designs: A latent variable framework for analysis and power estimation. Psychological Methods. 1997;2:371–402. [Google Scholar]
- Muthén L, Muthén B. Mplus user's guide. Sixth. Los Angeles, CA: Muthén & Muthén; 1998-2010. [Google Scholar]
- Neely A, Bäckman L. Maintenance of gains following multifactorial and unifactorial memory training in late adulthood. Educational Gerontology. 1993b;19:105–117. [Google Scholar]
- Neely AS, Backman L. Long-term maintenance of gains from memory training in older adults: two 3 1/2-year follow-up studies. J Gerontol. 1993a;48(5):P233–237. doi: 10.1093/geronj/48.5.p233. [DOI] [PubMed] [Google Scholar]
- O'Hara R, Brooks J, Friedman L, Schroder C, Morgan K, Kraemer H. Long-term effects of mnemonic training in community-dwelling older adults. Journal of Psychiatric Research. 2007;(41):585–590. doi: 10.1016/j.jpsychires.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Rasmusson D, Rebok G, Bylsma F, Brandt J. Effects of three types of memory training in normal elderly. Aging Neuropsychol Cognit. 1999;6:56–66. [Google Scholar]
- Rebok G, Balcerak L. Memory self-efficacy and performance differences in young and old adults: The effect of mnemonic training. Dev Psychol. 1989;25(5):714–721. [Google Scholar]
- Rebok GW, Carlson MC, Langbaum JB. Training and maintaining memory abilities in healthy older adults: traditional and novel approaches. J Gerontol B Psychol Sci Soc Sci. 2007;62 Spec No 1:53–61. doi: 10.1093/geronb/62.special_issue_1.53. [DOI] [PubMed] [Google Scholar]
- Rey A. L'examen psychologique dans les cas d'encéphalopathie traumatique. (Les problems.)./The psychological examination in cases of traumatic encepholopathy. Problems. Archives de Psychologie. 1941;28:215–285. [Google Scholar]
- Salthouse T. Mental exercise and mental aging: Evaluating the validity of the “use it or lose it” hypothesis. Perspectives on Psychological Science. 2006;1:68–87. doi: 10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Satorra A, Bentler P. Corrections to test statistics and standard errors in covariance structure analysis. In: Eye Av, Clogg C., editors. Latent variables analysis: Applications for developmental research. Thousand Oaks, CA: Sage; 1994. pp. 399–419. [Google Scholar]
- Schaie K. Intellectual Development in Adulthood The Seattle Longitudinal Study. New York: Cambridge University Press; 1996. [Google Scholar]
- Schmidt I, Dijkstra H, Berg I, Deelman B. Memory training for remembering names in older adults. Clinical Gerontologist. 1999;20(2):57–73. [Google Scholar]
- Scogin F, Bienias JL. A three-year follow-up of older adult participants in a memory-skills training program. Psychol Aging. 1988;3(4):334–337. doi: 10.1037//0882-7974.3.4.334. [DOI] [PubMed] [Google Scholar]
- Sisco S, Marsiske M, Gross A, Rebok G. The influence of cognitive training on older adults' recall for short stories. Journal of Aging and Health. doi: 10.1177/0898264313501386. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger J. EZPATH: A supplementary module for SYSTAT and SYGRAPH. Evanston, IL: Systat; 1989. [Google Scholar]
- Stigsdotter A, Backman L. Multifactorial memory training with older adults: how to foster maintenance of improved performance. Gerontology. 1989;35(5-6):260–267. doi: 10.1159/000213035. [DOI] [PubMed] [Google Scholar]
- Unverzagt FW, Smith DM, Rebok GW, Marsiske M, Morris JN, Jones R, et al. The Indiana Alzheimer Disease Center's Symposium on Mild Cognitive Impairment. Cognitive training in older adults: lessons from the ACTIVE Study. Curr Alzheimer Res. 2009;6(4):375–383. doi: 10.2174/156720509788929345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A. On the mechanisms of plasticity in young and older adults after instruction in the method of loci: evidence for an amplification model. Psychol Aging. 1996;11(1):164–178. doi: 10.1037//0882-7974.11.1.164. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Improving memory performance in the aged through mnemonic training: a meta-analytic study. Psychol Aging. 1992;7(2):242–251. doi: 10.1037//0882-7974.7.2.242. [DOI] [PubMed] [Google Scholar]
- Willis S, Caskie G. Reasoning training in the ACTIVE study: How much is needed and who benefits? Journal of Aging and Health. doi: 10.1177/0898264313503987. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis S, Nesselroade C. Long term effects of fluid ability training in old-old age. Dev Psychol. 1990;26:905–910. [Google Scholar]
- Willis S, Schaie K. Training the elderly on the ability factors of spatial orientation and inductive reasoning. Psychol Aging. 1986;1:239–247. doi: 10.1037//0882-7974.1.3.239. [DOI] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Baddeley A. The Rivermead Behavioural Memory Test. 34 The Square, Titchfield, Fareham, Hampshire PO14 4AF: Thames Valley Test Company; 1985. [Google Scholar]
- Wolters G, Theunissen I, Bemelmans K, Does Avd, Spinhoven P. Immediate and intermediate-term effectiveness of a memory training program for the elderly. Journal of Cognitive Rehabilitation. 1996;14:16–22. [Google Scholar]
- Yesavage JA, Sheikh JI, Friedman L, Tanke E. Learning mnemonics: roles of aging and subtle cognitive impairment. Psychol Aging. 1990;5(1):133–137. doi: 10.1037//0882-7974.5.1.133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.