Abstract
Objectives
To examine the effect of chemotherapy for ovarian cancer on immunologic function and to define the effect on the serologic response to the influenza vaccine.
Methods
Under IRB approved protocols, patients with ovarian cancer were administered seasonal trivalent killed influenza vaccines. Peripheral blood was collected for immunologic assessments. Serum was analyzed for hemagglutination inhibition (HAI) antibody titers. Peripheral blood mononuclear cells were isolated to characterize T and B cell populations and function.
Results
Thirty-one patients were recruited: 13 in remission receiving a dendritic cell vaccine with or without a single dose of low-dose cyclophosphamide, 3 in remission not receiving treatment, and 15 undergoing standard therapy. Significant effects on T cell and B cell subset distributions were seen. Functional effects were also seen. Few patients were able to mount a 4-fold HAI antibody response. A 4-fold response was observed for H1N1 in 20%, for H3N2 in 26%, and for influenza B in 6%. Pre-existing exposure to influenza was predictive of responders.
Conclusions
Despite CDC recommendations that patients undergoing chemotherapy receive influenza vaccine, there is little evidence to support its serologic effectiveness in this population. Patients with ovarian cancer are almost uniformly unable to mount a meaningful antibody response. These findings have serious implications for future resource allocation for both seasonal and novel pandemic influenza outbreak and understanding the immunologic deficits as a result of chemotherapy may improve patient care.
Keywords: Treg, monoclonal B cell lymphocytosis, DC vaccine, ELISpot, immune compromised, cyclophosphamide, antibody
Introduction
An average of 36,000 people die in the United States each year from seasonal influenza [1]. In the general population, attack rates are as high as 10-20% though surveillance data suggests that 10-40% of oncology patients may be infected [2]. The Centers for Disease Control recommend that all immunocompromised patients receive the seasonal influenza vaccine, given the increased risk for flu-related complications. However, there are scant data regarding the efficacy of the vaccine in patients with generalized malignancies, and in particular, in those undergoing chemotherapy. Cancer patients represent an ever-increasing proportion of the population with over 1.5 million new cases per year [3]. These patients are not only at increased risk of influenza related morbidity, but serve as a potential reservoir of virus that may lead to spread in the non-immunocompromised population.
Patients with ovarian cancer present a unique challenge. It has been estimated that 21,880 new cases of ovarian cancer will be diagnosed each year in the United States, accounting for 13,850 deaths, making ovarian cancer the most common cause of death from a gynecologic malignancy [4]. With a 5-year survival rate of 45%, there are increasing efforts directed at better therapies. The majority of patients are treated by surgical excision of tumor, followed by adjuvant chemotherapy. Despite initial response rates of 70-80%, nearly all patients with advanced disease pursue a course of relapsing disease, treated with multiple sequential regimens of chemotherapy during their lifetime, used for both maintenance, as well as salvage.
There is no quantitative information available on the immunogenicity of the influenza vaccine in patients with ovarian cancer and limited data on overall immunologic function. Ovarian cancer itself is in part, an immune-mediated disease [5]. Excess numbers and function of regulatory T cells (Treg) as well as dendritic cell (DC) dysfunction contribute to tumor-related immune suppression [6-10]. These same factors are predicted to affect influenza vaccine efficacy. This study was designed to characterize the immunologic status of women with ovarian cancer and to define the immunogenicity of the killed seasonal trivalent influenza vaccine.
Methods
Study Design and Patients
From 2005-2009, subjects were recruited under 2 protocols approved by the University of Pennsylvania Institutional Review Board. Signed written informed consent was obtained from each subject and the study was carried out in accordance with the Declaration of Helsinki. The first protocol enrolled patients with advanced epithelial ovarian or primary peritoneal cancer in first or second remission after primary surgery and chemotherapy, within 6 months of completion of chemotherapy, to receive an autologous dendritic cell vaccine loaded with Her2/neu, hTERT, and PADRE peptides. These patients were randomized to receive a single low dose of 300 mg/m2 of cyclophosphamide 2 days prior to vaccination with both DC and influenza vaccine to assess the affect upon regulatory T cells (Treg). To be eligible, patients had to be ≥ 18 years of age with a baseline Eastern Cooperative Oncology Group Clinical performance status of 0-2. Patients demonstrated adequate hematologic function, renal function, hepatic function, and cardiac function. Patients were required to demonstrate no radiologic evidence of disease by contrast computed tomography (CT) or magnetic resonance imaging (MRI) scans, as well as a normal serum CA125 level of ≤ 35 IU/ml. The second protocol enrolled a much broader spectrum of patients with ovarian or primary peritoneal cancer at any point in their treatment course. Patients were required to be ≥ 18 years of age, with life expectancy of > 6 months.
Patients on both protocols were excluded for history of prior invasive malignancy (except basal cell or squamous cell skin cancer) within the past 5 years; evidence of active central nervous system disease; serious systemic disease; active viral, fungal, or bacterial infection; or active autoimmunity or immunosuppression. Pregnant or breastfeeding subjects were excluded. Controls were recruited from the local community (n=21). The controls were age-matched to the patients and received the same year-specific vaccines as the patients. Vaccine dose and time of administration were parallel to those of the patients. Post-vaccine laboratory studies were performed at one month.
Vaccine
All patients received the World Health Organization designated trivalent killed influenza vaccine for the appropriate season in a single intramuscular injection of 0.5 ml. Patients on the DC vaccine protocol received influenza vaccine on the same day as dose 1 of the DC vaccine, and patients on the general protocol received influenza vaccine on the day of their presentation to the clinic—generally on day 1 of their current cycle of chemotherapy, or if not undergoing active chemotherapy, on the day of their routine surveillance visit.
Immune monitoring
Peripheral blood was collected at day 0, 3 months, 4 months, 9 months, and 12 months. The hemagglutinin inhibition assays (HAI) were performed as previously described using the season-specific HA antigen [10]. For B cell analyses, fresh venous whole blood anti-coagulated with EDTA was prepared and stained with antibodies (all from BD Pharmingen, San Diego, CA), as described previously [11]. Analyses were performed on a FACSCalibur with CellQuest software (Version 5.2.1, Becton Dickenson, San Jose, CA). B cells were identified on the basis of CD19 expression and physical characteristics. CD19+ lymphocytes were analyzed for CD27, CD38, CD5, lambda, CD20, IgD and IgM expression. The cells were separated into the following subsets in rough order of maturity, starting with the least mature: TR (transitional, CD27−, CD38++), MN (mature naive, CD27−, CD38+), MA (mature activated, CD27+, CD38+), PB (plasmablast, CD27++, CD38++), RM (resting memory, CD27+, CD38−). Differential staining with CD27 and IgM was used to identify Naïve, IgM memory, and isotype switched memory B cells. Whenever possible, 5,000 - 10,000 CD19+ events were analyzed per tube. Counts were determined from a simultaneous CBC and electronic differential.
For proliferation analyses, peripheral blood mononuclear cells (PBMC) were loaded with CFSE dye (Invitrogen, Carlsbad, CA) and washed extensively. The cells were incubated with PHA (Sigma-Aldrich, St Louis, MO) or influenza virions specific for the year of the vaccine (FluMist, MedImmune, Gaithersburg, MD). Five days after stimulation, the cells were washed again and stained with CD3. Flow cytometry (FacsCalibur) was performed to quantitate the responding cells. The gating included CD3+ blasts. FloJo (TreeStar Ashland, OR) was used to define the Proliferation Index, Division Index, and the Percent Divided cells.
T cell ELISPOTs and B cell ELISPOTs were performed as a measure of cell function. A cocktail of influenza proteins (Protein Sciences, Meriden, CT) based on the year-specific vaccine was used as specific antigen (at 5 μg/mL) in a standard γ-interferon T cell ELISpot assay [12]. CD8 depletion was used to define CD4-specific responses. PMA and ionomycin (combined at 5 μg/mL each) were used as non-specific stimuli. The B cell ELISpot defined the frequency of memory B cells activated by influenza to produce antibody and total IgG-producing B cells [13]. Pokeweed mitogen, Staphylococcus aureus (SAC) and CpG-2006 were used as priming stimuli as previously described [11].
Cytometric Fingerprinting
Cytometric fingerprinting [14, 15] was used as an unbiased method to discover phenotypic B-cell subsets that were significantly differentially regulated among subjects with ovarian cancer compared to healthy controls. List mode data for two tubes (non-B cell dump FITC/IgM PE/CD20 PerCP/CD19 APC and IgD FITC/lambda PE/CD19 PerCP-Cy5.5/CD5 APC) were subjected to automated gating, which selected the CD19+ lymphocytes. The gated data were then normalized based upon warping functions computed on high-density region landmarks for individual parameters. The Bioconductor package flowFP version 1.12.1 was used to compute the cytometric fingerprints [15]. Probability binning models were computed for each tube by aggregating the data of all of the healthy control subjects [16]. Fingerprints were then computed based upon these models for each individual sample from both the healthy controls and the ovarian cancer patients.
Statistical methods
Data were examined using descriptive statistics and are presented by mean, median, standard deviation, frequency distributions and range. The independent two sample t-test or the non-parametric Wilcoxon rank test were used for the comparison between two groups and Analysis of Variance (ANOVA) or the non-parametric Kruskal-Wallis test were used. The Spearman Correlation method was used for defining the associations of baseline values and outcome. The Chi-Square test was used to examine the association between grouping variable and categorical variables. Statistical analyses were performed using SAS 9.2. For cytometric fingerprinting analysis individual bins were compared between control and ovarian cancer patient groups using the two-sided Wilcoxon rank sum test and P-values were corrected for multiple comparisons using the Benjamini-Hochberg correction. Statistical significance was define by p-values < 0.05.
Results
Patient population
A total of 31 subjects were enrolled on the combined protocols: 6 receiving DC vaccine without cyclophosphamide (DC-NC), 7 receiving DC vaccine with cyclophosphamide (DC-C), and 18 undergoing general off-protocol therapy (OVCA) (Supplemental Table 1). Of these 18, 3 were in remission receiving no therapy, 7 were undergoing primary adjuvant chemotherapy with paclitaxel/platinum, 2 were in remission after completion of primary therapy receiving bevacizumab for consolidation, and 6 were receiving salvage therapy for recurrent disease. The patients with recurrent disease had received 2-5 prior regimens (median 4), and each was being treated with different therapy: weekly paclitaxel, bevacizumab + sorafenib, liposomal doxorubicin + IL-18, paclitaxel + bevacizumab, paclitaxel + carboplatin + bevacizumab, and bevacizumab + cyclophosphamide. The median stage at diagnosis and the prior number of prior chemotherapy regimens were similar across all groups. However, the mean age was different: the OVCA group (61.2 years) was significantly older than those in the DC-NC arm (49.3 years) (p = 0.042). The controls were age-matched to the overall study population (n=21).
HAI Titers
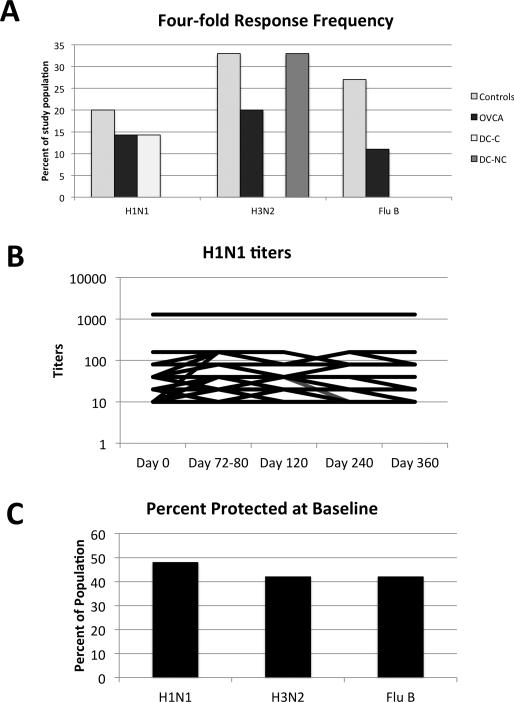
To characterize the function of the immune system, we examined antibody responses to the influenza vaccine. HAI titers were determined for each serotype at defined time points through 1-year post vaccination. Patients were examined for a four-fold rise in baseline titers against any of the 3 strains included in the vaccine (Figure 1A). In general, fewer than half of the patients could mount a four-fold rise in titer after vaccination, and the difference between the groups was not significant. There was remarkably little change in titers over time (Figure 1B). We stratified patients according to the number of prior chemotherapy cycles (1 Prior cycle or >1 prior cycles) and by age (<50y and >50y) and saw no differences in the responder frequency.
Figure 1.
HAI titer responses of subjects. (A) The percent of patients able to demonstrate at least a four-fold rise in titer to any one of the three influenza strains after vaccination. (B) H1N1 titers across all time points do not vary dramatically. (C) The percentage of the entire cohort with baseline titers ≥1:40. OVCA=ovarian cancer patients off protocol, DC-C=dendritic cell vaccine recipients with Cytoxan, DC-NC-dendritic cell vaccine recipients without Cytoxan.
Though the response to vaccination was minimal, approximately half of the patients were noted to have antibody titers of >1:40 at baseline against the three strains (Figure 1C). For unclear reasons, patients on the DC-C arm appeared to have the highest rate of baseline immunity, and OVCA patients displayed the lowest levels. Because the three cohorts were not equivalent at baseline, all subsequent analyses compare the entire ovarian cancer cohort with healthy controls.
T cell subsets
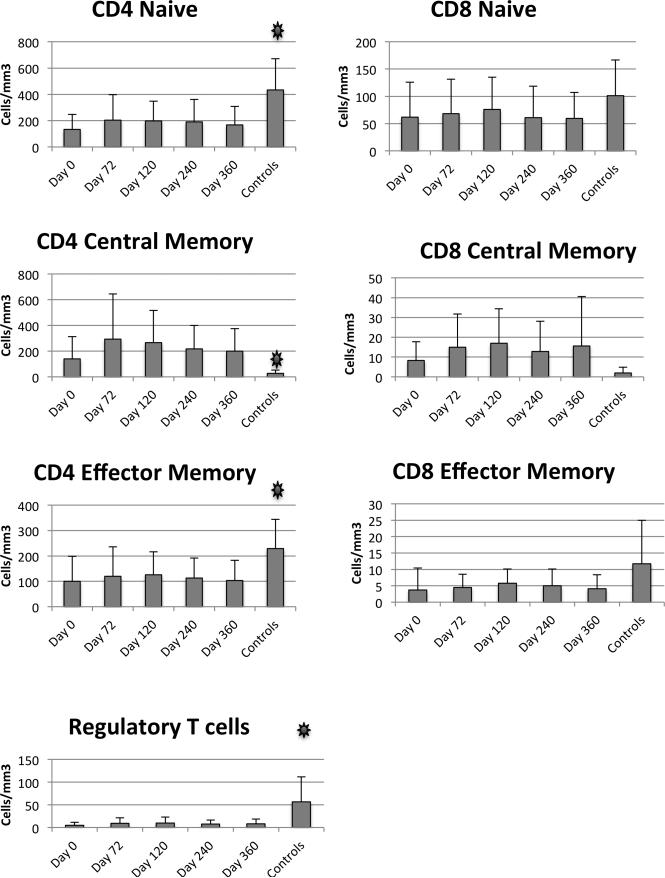
We measured the distribution of various T cell subsets in patients to assess the impact of chemotherapy (Figure 2). CD4 naïve cells were significantly diminished in patients compared to controls. Similarly, Regulatory T cells and Effector Memory CD4 cells were diminished in patients compared to controls. In contrast, the CD4 Central Memory T cells were increased in patients compared to controls. Over the one year time frame of the study, we saw little change overall in the distribution of T cell subsets.
Figure 2.
T cell subsets. Flow cytometry was used to define T cell subsets in patients and controls. Mean and standard deviation are shown. Asterisks indicate p<0.05 comparing baseline and controls.
T cell function
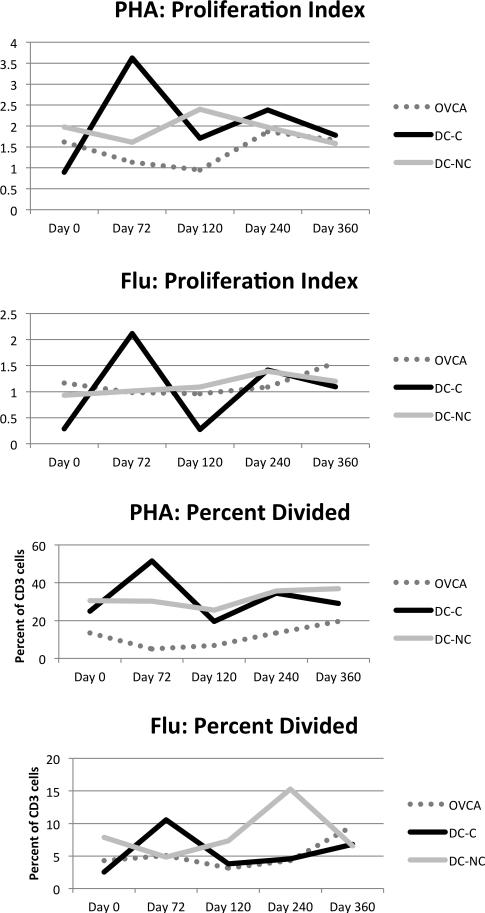
The subjects who received the combination of cyclophosphamide and the DC vaccine (DC-C) had a clear boost in their T cell proliferation (Figure 3). This was statistically significant with p<0.05. The Proliferation Index measures the number of cycles of cell division experienced by the cells that committed to cell division. The number of cells that could be recruited to divide (percent divided) was also increased after cyclophosphamide. Previous studies have demonstrated that low dose cyclophosphamide can augment immunity, however, this study demonstrates that T cell proliferation can be improved [7]. Across all three subsets, however, the groups were not statistically significantly different than controls (not shown). We also analyzed the function of the T cells using an ELISPOT assay. There was little rise in influenza responses after vaccination and no significant difference from controls in either global responses or influenza-specific responses (data not shown).
Figures 3.
DC-C patients have improved T cell proliferation after cyclophosphamide. Proliferative responses were measured by CFSE. The mean for each population is shown. OVCA=ovarian cancer patients off protocol, DC-C=dendritic cell vaccine recipients with Cytoxan, DC-NC-dendritic cell vaccine recipients without Cytoxan.
B Cell Subsets
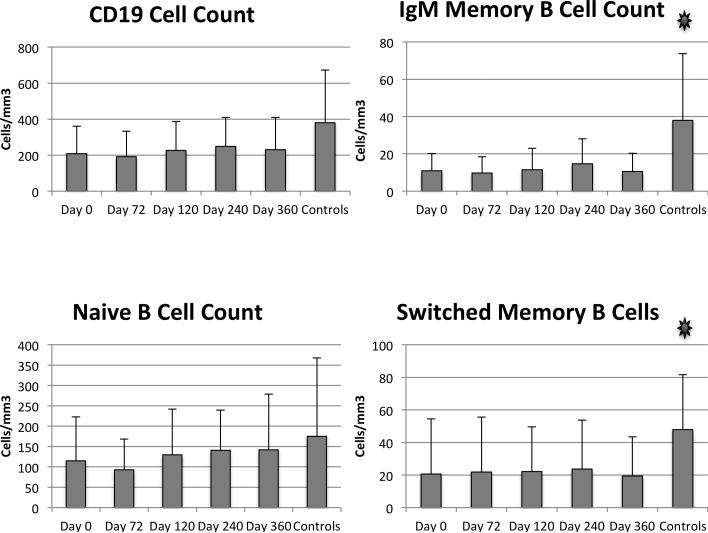
The average B cell count of all of the ovarian cancer subjects was approximately 150/microliter, slightly lower than historical reference ranges for healthy adults and lower than our controls [17] (Figure 4). The absolute numbers of memory B cells were significantly lower than for our age-matched controls. Both IgM memory and switched memory B cells were lower in patients than controls.
Figure 4.
B cell subsets. Flow cytometry was used to define B cell subsets in patients and controls. Mean and standard deviation are shown. Asterisks indicate p<0.05 comparing baseline and controls.
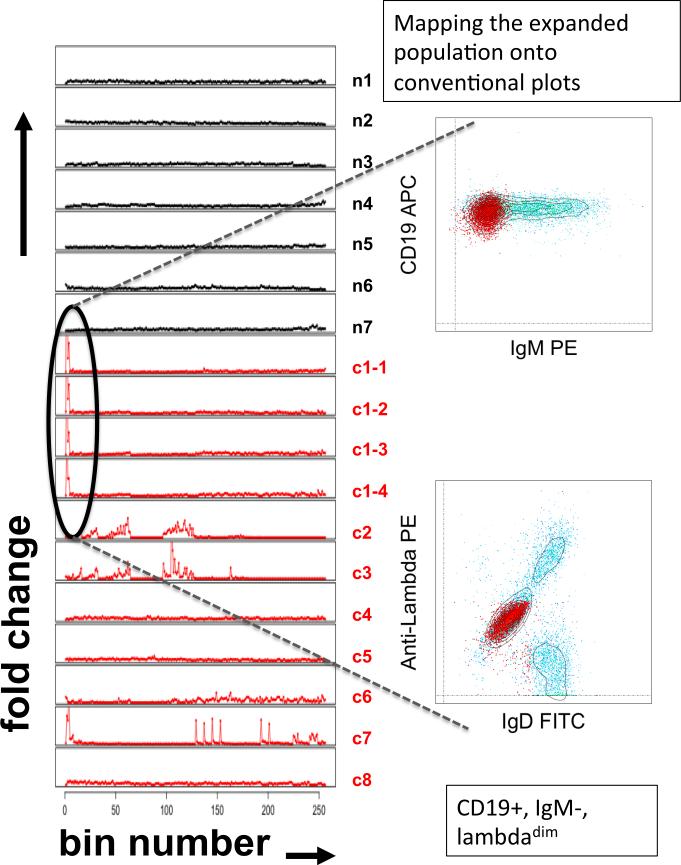
Of interest, at least five of the patients studied had evidence of atypical B cell populations at one or more time points. Two patients showed evidence of monoclonal B cell lymphocytosis (MBL), characterized by CD5+, light chain-restricted cells. In one patient, the light chain restricted MBL population comprised approximately 45% of her circulating cells with a phenotype of: CD19+, CD20dim, IgM−, CD27+, CD38+, lambdadim. Cytometric fingerprinting analysis revealed that this expanded clone was significantly different from the B cell subsets found in control subjects and was present at all of the time points (Figure 5).
Figure 5.
Monoclconal B cell expansions. Top panel: CD20+ lymphocytes (B cells) from whole blood are stained with CD27 and CD38 and separated into the following subsets in rough order of maturity, starting with the least mature: Tr (transitional, CD27−, CD38++), MN (mature naive, CD27−, CD38+), MA (mature activated, CD27+, CD38+), PB (plasmablast, CD27++, CD38++), RM (resting memory, CD27+, CD38−), LL (left lower quadrant, CD27−, CD38−). Lower panel: Persistent monoclonal B cell lymphocytosis in a patient with ovarian cancer. Cytometric fingerprinting of B cell subsets was performed as described in the methods section. The y-axis indicates the fold change in the frequency of events vs. the binning model (produced from the aggregated data of the normal subjects) and the x-axis indicates the bins. Black lines = healthy subjects. Red lines = ovarian cancer patients. Data from ovarian cancer patient #1, analyzed at four separate time points (c1-1, c1-2, c1-3 and c1-4) over a 1-year period, reveal the persistence of an expanded population. The cells in the corresponding bins were mapped onto conventional flow cytometry plots and reveal a light chain restricted, class-switched B cell population.
B Cell Immunoglobulin Production
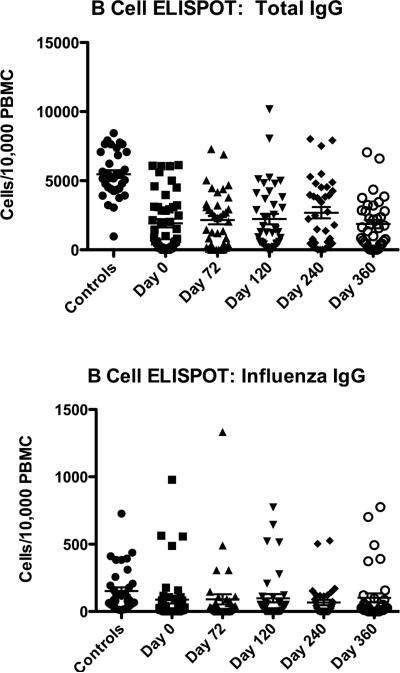
We used a B cell ELISpot to identify deficits in antibody secretion (Figure 6). We measured total IgG production and influenza-specific IgG. Patients had significantly lower total IgG responses to in vitro stimulation than controls. This is commensurate with the decrement in memory B cells seen on flow cytometry. IgG responses to influenza antigens were highly variable across the population but did not differ significantly from controls.
Figure 6.
B cell function. Total immunoglobulin producing B cells were enumerated in an ELISPOT as well as influenza-specific IgG producing B cells. Means are shown as a horizontal line. The controls are significantly different than the patient populations for total IgG at all time points but the difference between patients and controls for influenza-specific IgG is not significant.
Laboratory Associations With Influenza Vaccine Responses
The data demonstrated that the patients exhibited far ranging immunologic deficits. The most substantial functional deficits appeared confined to the B cell compartment. To determine whether a laboratory variable might be predictive of antibody responses to the influenza vaccine, we performed a Spearman Correlation analysis. For this analysis, we used the delta HAI (the sum of the change in H1N1, H3N2 and B HAI titers from baseline to Day 72). The variables tested were lymphocyte counts and ELISpot results at baseline. From this analysis, previous T cell exposure to influenza as detected by the ELISpot at baseline was the only variable that was significantly associated with subsequent antibody response (Supplemental Table 2).
Discussion
Our bulwark against influenza is our ability to rapidly develop a vaccine appropriate to the infecting strain [18, 19]. Immunocompromised people have not traditionally represented a significant population in the United States. However, over 1 million patients receive chemotherapy each year, and numerous patients with autoimmune or inflammatory disorders are treated with immunosuppressive medications. This represents a significant population of people with unique vulnerabilities to influenza and unclear responsiveness to the vaccine.
Our study prospectively characterized the immunogenicity of the seasonal influenza vaccine in an immunosuppressed population of women with ovarian cancer. We have predominantly focused on HAI titers, given that those values have correlated most closely to protection from infection [20-23]. Patients were infrequently able to mount an adequate humoral immune response. This was true for both lightly and heavily pretreated patients, suggesting that either the malignant state itself mayimpair responses, or that the effect of chemotherapy is extremely durable. The patients undergoing DC vaccination did appear to have the best immunity to influenza at baseline. These patients would also be predicted to have more intact immune systems than their more heavily treated counterparts—the DC vaccinated patients had only received one prior regimen of cytotoxic therapy and were in clinical remission.
We must note that while a titer of 1:40 is deemed protective in young healthy adults, it is unclear if this level truly represents a protective threshold in this population. In other immunosuppressed populations (elderly, infants) higher titers may be necessary [24-26]. On the other hand, the protection conferred by cross-reactive antibodies that appear to arise due to successive rounds of vaccination, could mean that lower titers of the right kinds of antibodies might still be protective, [27]. Indeed, pre-existing immunity may contribute to the lack of increase in total HAI titers after vaccination [28]. Yet, pre-existing immunity may not be protective, as domination of the antibody repertoire by high-affinity clones could result in susceptibility to viral escape mutants [29]. Notably, none of the patients on the study developed a documented influenza infection despite low level immune responses, though certainly it is well known that influenza is under-diagnosed [30].
Protection of immunologically vulnerable patients has received increasing attention. Various strategies have been studied in an effort to augment vaccine responses since many of the same characteristics that render patients vulnerable to infection, also compromise vaccine responses. More powerful adjuvants, the use of multiple doses or higher antigen doses, and identification of universal epitopes show promise for populations such as this [31-33]. Additionally, personalizing protection by targeting vaccines to specific time points during therapy may also maximize benefit [34-37]. In cases where vaccination is unlikely to benefit the patient, hygiene strategies, antiviral medications and other approaches can be considered.
This study was small, covering a very diverse population of patients both with disease and in remission, ranging from stages 1-4, receiving 1-5 prior regimens of chemotherapy. They also had diverse ages. It is well known that the immune system undergoes age-related declines [26, 38, 39]. Reduced frequency of mature lymphocytes could be a consequence of failure of reconstitution after myelosuppressive therapy in some of the patients studied. Given the decrement in primary bone marrow output associated with age and the prospect for continued regimens of chemotherapy, patients may never achieve pretreatment subset homeostasis.
Of note, our study also found the presence of atypical B cell populations, including monoclonal B cell lymphocytosis (MBL). The frequency of MBL increases with age [40]. The persistence of MBL clones (as was documented here for one of the patients by cytometric fingerprinting) has been associated with the subsequent development of chronic lymphocytic leukemia (which is often also CD5+) or lymphomas in a small fraction of patients [41]. It is intriguing to speculate if the MBL itself might reflect an underlying immune defect that predisposes to cancer development in these patients, or whether it is merely a consequence of therapy or disease.
Despite its shortcomings, this is the only study to date to examine vaccine responsiveness in this population. The killed vaccine is safe, but may do little to provide protective immunity in patients with ovarian cancer, particularly in those undergoing chemotherapy. Larger studies are needed to better identify individuals who may be the best candidates to allow focused efforts to encourage vaccination, as well as to define optimal schedules of administration in these patients who are undergoing cyclic immunodepletion and repletion.
Supplementary Material
Research highlights.
Patients undergoing treatment for ovarian cancer are profoundly immunosuppressed and unable to mount an adequate immune response to seasonal influenza vaccine
The B cell compartment was significantly functionally compromised
Monoclonal B cell lymphocytosis was seen
Acknowledgements
Supported in part by grant NO1-AI-50024 (to KS). The authors acknowledge the patients, physicians, nurses and research staff.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Specifically, Adriana Weinberg, Ximena Rivera, Tiketta McIntyre, Yang-Zhu Du, Naseem Kerr, and Kelly Maurer are thanked for their contributions.
Conflict of Interest:
The authors declare no conflict of interest.
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Hicks KL, Chemaly RF, Kontoyiannis DP. Common community respiratory viruses in patients with cancer: more than just “common colds”. Cancer. 2003 May 15;97(10):2576–87. doi: 10.1002/cncr.11353. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010 Sep-Oct;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse S, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, et al. SEER Cancer Statistics Review. National Cancer Institute. 2011 [Google Scholar]
- 5.Berd D, Maguire HC., Jr. Mastrangelo MJ. Impairment of concanavalin A-inducible suppressor activity following administration of cyclophosphamide to patients with advanced cancer. Cancer Research. 1984;44(3):1275–80. [PubMed] [Google Scholar]
- 6.Berd D, Maguire HC, Jr., McCue P, Mastrangelo MJ. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunologic results in 64 patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1990 Nov;8(11):1858–67. doi: 10.1200/JCO.1990.8.11.1858. [DOI] [PubMed] [Google Scholar]
- 7.Berd D, Mastrangelo MJ, Engstrom PF, Paul A, Maguire H. Augmentation of the human immune response by cyclophosphamide. Cancer Research. 1982;42(11):4862–6. [PubMed] [Google Scholar]
- 8.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004 Sep;10(9):942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 9.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. Journal of immunology. 2002 May 1;168(9):4272–6. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 10.Levin MJ, Song LY, Fenton T, Nachman S, Patterson J, Walker R, et al. Shedding of live vaccine virus, comparative safety, and influenza-specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV-infected children. Vaccine. 2008 Aug 5;26(33):4210–7. doi: 10.1016/j.vaccine.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stadtmauer EA, Vogl DT, Luning Prak E, Boyer J, Aqui NA, Rapoport AP, et al. Transfer of influenza vaccine-primed costimulated autologous T cells after stem cell transplantation for multiple myeloma leads to reconstitution of influenza immunity: results of a randomized clinical trial. Blood. 2011 Jan 6;117(1):63–71. doi: 10.1182/blood-2010-07-296822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Fabio S, Mbawuike IN, Kiyono H, Fujihashi K, Couch RB, McGhee JR. Quantitation of human influenza virus-specific cytotoxic T lymphocytes: correlation of cytotoxicity and increased numbers of IFN-gamma producing CD8+ T cells. International Immunology. 1994;6(1):11–9. doi: 10.1093/intimm/6.1.11. [DOI] [PubMed] [Google Scholar]
- 13.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. Journal of Immunological Methods. 2004;286(1-2):111–22. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Rogers WT, Moser AR, Holyst HA, Bantly A, Mohler ER, 3rd, Scangas G, et al. Cytometric fingerprinting: quantitative characterization of multivariate distributions. Cytometry A. 2008 May;73(5):430–41. doi: 10.1002/cyto.a.20545. [DOI] [PubMed] [Google Scholar]
- 15.Rogers WT, Holyst HA. FlowFP: A Bioconductor Package for Fingerprinting Flow Cytometric Data. Adv Bioinformatics. 2009:193947. doi: 10.1155/2009/193947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roederer M, Moore W, Treister A, Hardy RR, Herzenberg LA. Probability binning comparison: a metric for quantitating multivariate distribution differences. Cytometry. 2001 Sep 1;45(1):47–55. doi: 10.1002/1097-0320(20010901)45:1<47::aid-cyto1143>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 17.Jentsch-Ullrich K, Koenigsmann M, Mohren M, Franke A. Lymphocyte subsets’ reference ranges in an age- and gender-balanced population of 100 healthy adults--a monocentric German study. Clinical Immunology. 2005 Aug;116(2):192–7. doi: 10.1016/j.clim.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003 May 1;21(16):1776–9. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 19.Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007 Sep 28;25(39-40):6852–62. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979 Jan;35(1):69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- 21.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. Journal of Clinical Microbiology. 1986;24(1):157–60. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. Journal of Infectious Diseases. 2000;181(3):1133–7. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- 23.Edmondson WP, Jr., Rothenberg R, White PW, Gwaltney JM., Jr A comparison of subcutaneous, nasal, and combined influenza vaccination. II. Protection against natural challenge. American journal of epidemiology. 1971 Jun;93(6):480–6. doi: 10.1093/oxfordjournals.aje.a121282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster SL, Moore WP. High-dose influenza vaccination in the elderly. J Am Pharm Assoc (2003) 2010 Jul-Aug;50(4):546–7. doi: 10.1331/JAPhA.2010.10529. [DOI] [PubMed] [Google Scholar]
- 25.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. Journal of virology. 2001 Dec;75(24):12182–7. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus research. 2004 Jul;103(1-2):133–8. doi: 10.1016/j.virusres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 27.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. The Journal of experimental medicine. 2011 Jan 17;208(1):181–93. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3(8):e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hensley SE, Das SR, Bailey AL, Schmidt LM, Hickman HD, Jayaraman A, et al. Hemagglutinin receptor binding avidity drives influenza A virus antigenic drift. Science. 2009 Oct 30;326(5953):734–6. doi: 10.1126/science.1178258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrat F, Tachet A, Rouzioux C, Housset B, Valleron AJ. Evaluation of clinical case definitions of influenza: detailed investigation of patients during the 1995-1996 epidemic in France. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1999 Feb;28(2):283–90. doi: 10.1086/515117. [DOI] [PubMed] [Google Scholar]
- 31.De Donato S, Granoff D, Minutello M, Lecchi G, Faccini M, Agnello M, et al. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine. 1999;17(23-24):3094–101. doi: 10.1016/s0264-410x(99)00138-3. [DOI] [PubMed] [Google Scholar]
- 32.Tetsutani K, Ishii KJ. Adjuvants in influenza vaccines. Vaccine. 2012 Dec 14;30(52):7658–61. doi: 10.1016/j.vaccine.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Subbarao K, Matsuoka Y. The prospects and challenges of universal vaccines for influenza. Trends in microbiology. 2013 Jul;21(7):350–8. doi: 10.1016/j.tim.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kersun LS, Reilly A, Coffin SE, Boyer J, Luning Prak ET, McDonald K, et al. A prospective study of chemotherapy immunologic effects and predictors of humoral influenza vaccine responses in a pediatric oncology cohort. Influenza and other respiratory viruses. 2012 Nov 30; doi: 10.1111/irv.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kersun LS, Reilly AF, Coffin SE, Sullivan KE. Protecting pediatric oncology patients from influenza. The oncologist. 2013;18(2):204–11. doi: 10.1634/theoncologist.2012-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reilly A, Kersun LS, McDonald K, Weinberg A, Jawad AF, Sullivan KE. The efficacy of influenza vaccination in a pediatric oncology population. Journal of pediatric hematology/oncology. 2010 Jul;32(5):e177–81. doi: 10.1097/MPH.0b013e3181d869f3. [DOI] [PubMed] [Google Scholar]
- 37.Pollyea DA, Brown JM, Horning SJ. Utility of influenza vaccination for oncology patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 May 10;28(14):2481–90. doi: 10.1200/JCO.2009.26.6908. [DOI] [PubMed] [Google Scholar]
- 38.Bender BS, Tallman E. The heterogeneity of the age-related decline in immune response: impairment in delayed-type hypersensitivity and cytotoxic T-lymphocyte activity occur independently. Experimental gerontology. 1992;27(3):347–54. doi: 10.1016/0531-5565(92)90061-4. [DOI] [PubMed] [Google Scholar]
- 39.Castle SC. Impact of age-related immune dysfunction on risk of infections. Z Gerontol Geriatr. 2000 Oct;33(5):341–9. doi: 10.1007/s003910070030. [DOI] [PubMed] [Google Scholar]
- 40.Ghia P, Prato G, Scielzo C, Stella S, Geuna M, Guida G, et al. Monoclonal CD5+ and CD5- B-lymphocyte expansions are frequent in the peripheral blood of the elderly. Blood. 2004 Mar 15;103(6):2337–42. doi: 10.1182/blood-2003-09-3277. [DOI] [PubMed] [Google Scholar]
- 41.Marti GE, Rawstron AC, Ghia P, Hillmen P, Houlston RS, Kay N, et al. Diagnostic criteria for monoclonal B-cell lymphocytosis. British journal of haematology. 2005 Aug;130(3):325–32. doi: 10.1111/j.1365-2141.2005.05550.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.