Abstract
Rationale: Children with obstructive sleep apnea syndrome (OSAS) have impaired cortical processing of respiratory afferent stimuli, manifested by blunted sleep respiratory-related evoked potentials (RREP). However, whether this impairment is limited to respiratory stimuli, or reversible after successful treatment, is unknown. We hypothesized that, during sleep, children with OSAS have (1) abnormal RREP, (2) normal cortical processing of nonrespiratory stimuli, and (3) persistence of abnormal RREP after treatment.
Objectives: To measure sleep RREP and auditory evoked potentials in normal control subjects and children with OSAS before and after treatment.
Methods: Twenty-four children with OSAS and 24 control subjects were tested during N3 sleep. Thirteen children with OSAS repeated testing 4–6 months after adenotonsillectomy.
Measurements and Main Results: RREP were blunted in OSAS compared with control subjects (N350 at Cz −27 ± 15.5 vs. −47.4 ± 28.5 μV; P = 0.019), and did not improve after OSAS treatment (N350 at Cz pretreatment −25.1 ± 7.4 vs. −29.8 ± 8.1 post-treatment). Auditory evoked potentials were similar in OSAS and control subjects at baseline (N350 at Cz −58 ± 33.1 vs. −66 ± 31.1 μV), and did not change after treatment (N350 at Cz −67.5 ± 36.8 vs. −65.5 ± 20.3).
Conclusions: Children with OSAS have persistent primary or irreversible respiratory afferent cortical processing deficits during sleep that could put them at risk of OSAS recurrence. OSAS does not seem to affect the cortical processing of nonrespiratory (auditory) afferent stimuli during sleep.
Keywords: evoked potentials, children, auditory, respiratory, obstructive sleep apnea syndrome
At a Glance Commentary
Scientific Knowledge on the Subject
Children with obstructive sleep apnea syndrome have impaired respiratory afferent cortical processing during sleep. However, it is unknown whether this deficit is limited to respiratory stimuli or is reversible after treatment.
What This Study Adds to the Field
This study shows that, during sleep, children with obstructive sleep apnea syndrome have normal cortical processing of auditory afferent stimuli, and respiratory afferent cortical processing impairment. Moreover, this condition does not resolve after treatment.
The pathophysiology of the childhood obstructive sleep apnea syndrome (OSAS) is not clear. In most children, OSAS is related to adenotonsillar hypertrophy or obesity. However, these structural factors cannot fully explain the degree of upper airway collapsibility (1, 2). Studies have shown that upper airway neuromotor tone and reflexes during sleep play an important role in maintaining airway patency during sleep in the pediatric population (3, 4). Recently, it has been shown that central nervous system processing of upper airway respiratory stimuli is abnormal during sleep in children with OSAS (5). However, it is not known whether this abnormal afferent processing is limited to respiratory stimuli, or whether it is an indicator of broader abnormalities in stimulus processing, secondary to the hypoxemia, hypercapnia, and sleep fragmentation of OSAS. Furthermore, it is not known whether this deficit resolves after treatment of OSAS. Determining reversibility would help elucidate whether the blunted responses were a primary and perhaps predisposing abnormality, or were secondary to the OSAS.
Respiratory-related evoked potentials (RREP) have been used to study the central nervous system processing of upper airway stimuli (6, 7). Auditory evoked potentials (AEP) are a useful tool to investigate cortical responses to nonrespiratory stimuli and produce the same set of longer-latency evoked response components as those seen in the sleep RREP (8, 9). Therefore, we decided to use event-related evoked potentials to test the following hypotheses: (1) children with OSAS have abnormal RREP during sleep compared with control subjects; (2) children with OSAS have normal cortical processing of nonrespiratory (auditory) stimuli, manifested by normal AEP during sleep; and (3) assuming that children with OSAS have a primary congenital sensory afferent processing, abnormal RREP does not normalize after treatment of OSAS. We performed RREP and AEP in children with OSAS and age-matched control subjects during sleep, and repeated these after surgical treatment of OSAS.
Methods
Additional method details are provided in the online supplement. Children with OSAS and age-matched control subjects were studied. RREP and AEP were obtained from surface EEG during stage N3 sleep. Some children with OSAS underwent surgical treatment (adenoidectomy and/or tonsillectomy) as per standard clinical care, followed 4–6 months later by repeat RREP-AEP testing.
The Institutional Review Board at the Children’s Hospital of Philadelphia approved the study. Informed consent was obtained from the parents or legal guardians of the subjects, and assent from subjects older than 7 years of age.
Study Group
Subjects with OSAS and control subjects, aged 6–16 years, underwent baseline polysomnography using standard pediatric techniques and scoring (10). OSAS was defined as having an apnea–hypopnea index (AHI) greater than or equal to 2 per hour, and control subjects were included if they were asymptomatic and had an AHI less than 1.5 per hour (11–14). Subjects with OSAS were recruited after a clinical polysomnogram, and healthy nonsnorer control subjects were recruited from the community by means of advertisements.
Sleep RREP and AEP
Sleep AEP and RREP were performed on the same night during stage N3. Subjects slept wearing a snug face mask and ear pieces. RREP were elicited as previously described (5). For AEP, auditory stimuli were applied 500 times by the ear inserts as a monotonous series of 80-dB, 1,000-Hz, and 50-ms tone pips. Data were obtained and analyzed as previously described (5). RREP and AEP were calculated at Fz, Cz, and Pz. P2, N350, N550, and P900 components were determined (15). All amplitudes were expressed relative to the average of activity in the prestimulus baseline period. Latencies of auditory components were expressed relative to stimulus onset. Latencies of respiratory components were expressed relative to the start of the change in mask pressure after occlusion onset.
Statistical Analysis
Statistical analysis was performed with SPSS software version 17.0 for Windows (SPSS Inc., Chicago, IL). The Kolmogorov-Smirnov test was used to test for normality. Categorical data were compared using the chi-square or Fisher exact test. Continuous data were compared using the paired or unpaired Student t test or Mann-Whitney rank sum test, as appropriate. A P value less than 0.05 was considered statistically significant.
Results
Study Group
Twenty-four subjects with OSAS and 24 control subjects were studied (Table 1). Subjects with OSAS were of similar age to control subjects but were more obese. There was a wide range of severity of OSAS. Thirteen participants were reevaluated after surgical treatment.
TABLE 1.
STUDY GROUP DEMOGRAPHICS AND POLYSOMNOGRAPHY RESULTS
| Control Subjects | OSAS (Baseline) | |
|---|---|---|
| N |
24 |
24 |
| Age, yr |
12 ± 3 |
11 ± 3 |
| Males, % |
13 (54) |
17 (71) |
| Body mass index z score |
0.4 ± 1.1 |
1.8 ± 1.0* |
| Obese, % |
5 (20.8) |
15 (62.5)* |
| Apnea–hypopnea index, n/h |
0.3 ± 0.3 |
24.8 ± 26.7† |
| Apnea–hypopnea index range, n/h |
0–1 |
2–103.7 |
| SpO2 nadir, % |
94 ± 2 |
84 ± 8* |
| Time with SpO2 < 90%, % TST |
0 ± 0.1 |
2.8 ± 7.0* |
| Peak end-tidal CO2, mm Hg |
53 ± 4 |
57 ± 5‡ |
| Time with end-tidal Pco2 ≥ 50 mm Hg, %TST | 3.9 ± 9.5 | 17.9 ± 26.2§ |
Definition of abbreviations: OSAS = obstructive sleep apnea syndrome; SpO2 = oxygen saturation as measured by pulse oximetry; TST = total sleep time.
Data shown as mean ± SD or n (%).
P < 0.001.
P = 0.008.
P = 0.006.
P = 0.005.
RREP Stimulus Intensity
The magnitude of the mouth pressure change elicited by occlusions did not vary significantly as a function of diagnosis or treatment. The pressure differences for the subjects with OSAS were 4.2 ± 1.3 cm H2O at baseline and 3.8 ± 1.2 cm H2O after surgery. The pressure differences for control subjects were 3.3 ± 1.8 cm H2O.
Sleep RREP
One white male subject with OSAS, aged 12.6 years, whose AHI was 11.6 events per hour did not have identifiable RREP waveforms at baseline, as compared with zero control subjects. Two control subjects refused to wear the mask during sleep, and therefore did not have RREP data during sleep. AEP data only was collected from these participants.
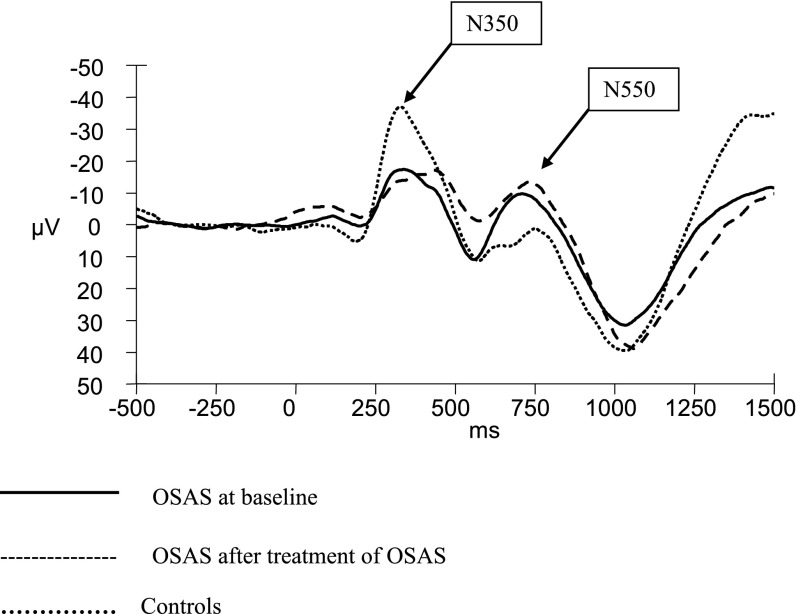
N350 amplitudes at Cz and Pz were significantly reduced in OSAS compared with control subjects but latencies were not significantly different between the two groups (Table 2). Children with OSAS had reduced N550 amplitudes at Fz and Cz, and longer N550 latencies at all sites (Figure 1). Similarly, they had longer P900 latency at Cz compared with control subjects. As previously shown (5), neither P2 amplitude nor latency displayed any significant effects of diagnosis or electrode site.
TABLE 2.
SLEEP RESPIRATORY-RELATED EVOKED POTENTIALS DURING N3 IN CHILDREN WITH OBSTRUCTIVE SLEEP APNEA SYNDROME VERSUS CONTROL SUBJECTS
| Peak | Fz |
Cz |
Pz |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OSAS | Control Subjects | P | OSAS | Control Subjects | P | OSAS | Control Subjects | P | |
| P2 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
7.7 ± 7.9 |
11.1 ± 9.2 |
0.31 |
−0.2 ± 28.5 |
9.2 ± 9.4 |
0.27 |
5 ± 7 |
6.5 ± 7.3 |
0.62 |
| Latency, ms |
188.8 ± 145.1 |
144.8 ± 37.5 |
0.30 |
198.6 ± 155.4 |
147.8 ± 33.8 |
0.26 |
134.1 ± 21.5 |
160.1 ± 39.6 |
0.29 |
| N350 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
−21.8 ± 11.1 |
−28.8 ± 12.2 |
0.11 |
−27 ± 15.5 |
−47.4 ± 28.5 |
0.019 |
−20.9 ± 10.4 |
−33.3 ± 20.9 |
0.05 |
| Latency, ms |
356.5 ± 117 |
298.6 ± 47.4 |
0.1 |
312.7 ± 106.6 |
283.5 ± 47.7 |
0.35 |
293 ± 49.7 |
275.1 ± 52.8 |
0.36 |
| N550 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
−18 ± 14.6 |
−31 ± 17.3 |
0.036 |
−19.9 ± 17.2 |
−34.6 ± 21.8 |
0.053 |
−16 ± 11 |
−22.3 ± 19.4 |
0.27 |
| Latency, ms |
622.2 ± 124.6 |
466.1 ± 136.4 |
0.003 |
583.6 ± 125.7 |
456.4 ± 134.1 |
0.013 |
557.9 ± 115.8 |
448.2 ± 111.2 |
0.017 |
| P900 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
30.1 ± 17.8 |
49.8 ± 36 |
0.07 |
35.6 ± 21.9 |
54.2 ± 39 |
0.09 |
27.2 ± 19.4 |
36.1 ± 37.5 |
0.42 |
| Latency, ms | 997.2 ± 112.9 | 935 ± 159.3 | 0.24 | 978.7 ± 73.5 | 886.7 ± 171.4 | 0.049 | 957.5 ± 98.2 | 883.7 ± 180.2 | 0.17 |
Definition of abbreviation: OSAS = obstructive sleep apnea syndrome.
Data shown as mean ± SD.
Figure 1.
Respiratory-related evoked potentials Cz during sleep stage N3 in children with obstructive sleep apnea syndrome (OSAS) at baseline compared with children with OSAS after treatment and control subjects. Children with OSAS had blunted N350 and more prominent N550 at Cz at baseline compared with control subjects. Respiratory-related evoked potentials did not change after OSAS treatment.
Sleep AEP
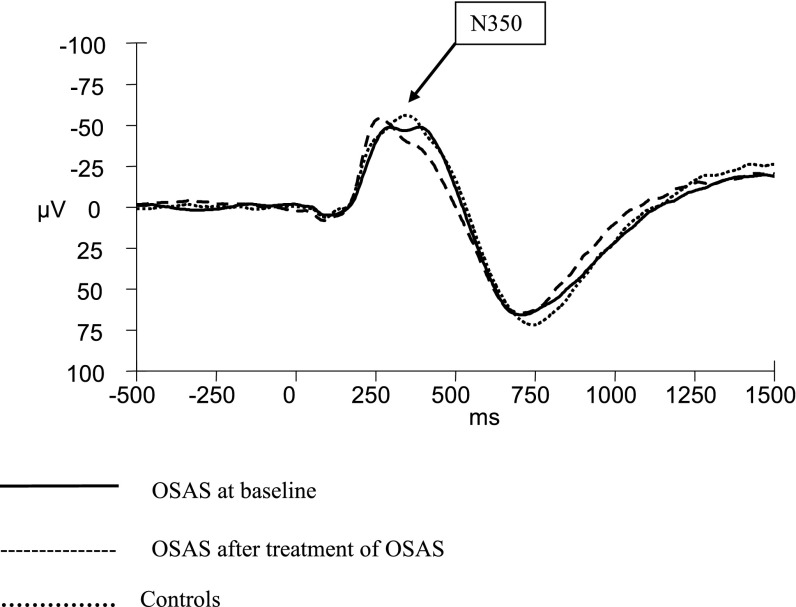
There were no significant amplitude or latency differences between OSAS and control subjects (Table 3). The amplitudes of N550 at Fz and P900 at Cz showed nonsignificant trends toward blunted responses in the OSAS group.
TABLE 3.
SLEEP AUDITORY EVOKED POTENTIALS DURING N3 IN CHILDREN WITH OBSTRUCTIVE SLEEP APNEA SYNDROME VERSUS CONTROL SUBJECTS
| Peak | Fz |
Cz |
Pz |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OSAS | Control Subjects | P | OSAS | Control Subjects | P | OSAS | Control Subjects | P | |
| P2 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
6.3 ± 9.9 |
7.1 ± 9.1 |
0.82 |
9 ± 10.2 |
9.8 ± 9 |
0.79 |
7.1 ± 10 |
7.1 ± 6.6 |
0.99 |
| Latency, ms |
158.1 ± 27.4 |
149.1 ± 42.7 |
0.43 |
139.7 ± 33.3 |
146 ± 29.4 |
0.50 |
151.2 ± 29.6 |
147.6 ± 26.1 |
0.67 |
| N350 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
−46.6 ± 31.3 |
−55.3 ± 27.3 |
0.32 |
−58 ± 33.1 |
−66 ± 31.1 |
0.4 |
−43.6 ± 33.9 |
−41.6 ± 21.2 |
0.81 |
| Latency, ms |
348.4 ± 64 |
328 ± 66 |
0.3 |
307.2 ± 52.5 |
291.7 ± 61.5 |
0.25 |
298.1 ± 52 |
288.1 ± 62.2 |
0.36 |
| N550 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
−38.9 ± 29.1 |
−56.5 ± 32 |
0.07 |
−46.4 ± 30.1 |
−54.6 ± 32.9 |
0.42 |
−35.6 ± 33.5 |
−31.1 ± 25.8 |
0.62 |
| Latency, ms |
434.4 ± 65.1 |
411 ± 64 |
0.56 |
409 ± 58.1 |
389.4 ± 67.7 |
0.25 |
421.3 ± 70.3 |
415.8 ± 88 |
0.32 |
| P900 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
58.7 ± 28.5 |
76.2 ± 37.7 |
0.09 |
65.1 ± 33.8 |
85.7 ± 40.5 |
0.07 |
47 ± 38 |
53.1 ± 31.3 |
0.55 |
| Latency, ms | 769.3 ± 111.8 | 727.5 ± 70.9 | 0.83 | 721.6 ± 57.6 | 719.1 ± 75.8 | 0.14 | 761.5 ± 124.1 | 735.2 ± 96.2 | 0.9 |
Definition of abbreviation: OSAS = obstructive sleep apnea syndrome.
Data shown as mean (SD).
Response to Treatment of OSAS
Subjects underwent surgical treatment as per the discretion of their clinical physician. Thirteen of the 24 subjects underwent surgery and were reevaluated postoperatively. Eleven of these underwent adenotonsillectomy, one underwent adenoidectomy and turbinectomy, and one underwent adenoidectomy alone. Of the remaining subjects, three were too mild to be treated surgically, three declined further research, two received continuous positive airway pressure therapy rather than surgery, and one moved away. As anticipated, those undergoing treatment had more severe OSAS (Table 4), but no other clinical differences, compared with those who did not.
TABLE 4.
BASELINE DEMOGRAPHICS AND POLYSOMNOGRAPHIC RESULTS FOR SUBJECTS WITH OBSTRUCTIVE SLEEP APNEA SYNDROME WHO UNDERWENT POSTOPERATIVE ASSESSMENTS VERSUS THOSE WHO DID NOT
| Postoperative Assessment | No Postoperative Assessment | |
|---|---|---|
| N |
13 |
11 |
| Age, yr |
10 ± 3 |
12 ± 4 |
| Males, % |
9 (69) |
8 (73) |
| Body mass index z score |
1.8 ± 1.0 |
1.8 ± 1.0 |
| Obese |
9 (69) |
6 (55) |
| Apnea–hypopnea index, n/h | 24.8 ± 26.7 | 11.3 ± 14.4* |
Data shown as mean ± SD or n (%).
P = 0.028.
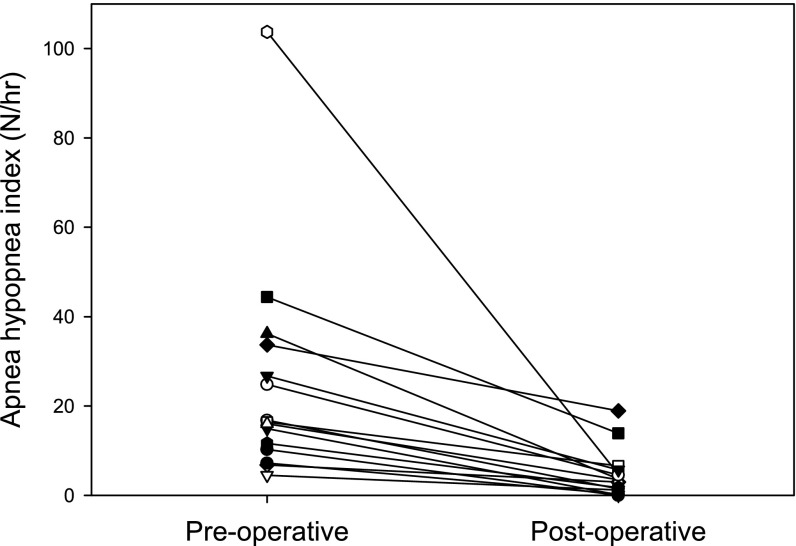
Baseline polysomnography, RREP, and AEP were repeated 170 ± 71 days after the initial study, and 137 ± 52 days after surgery. There was no significant change in body mass index z score postoperatively (P = 0.62). The AHI decreased considerably in all subjects, from 24.8 ± 26.7 to 4.5 ± 5.7 (P < 0.001), with the decrease in AHI varying from 44–100% of the initial AHI. However, seven subjects still had an AHI in the abnormal range (Figure 2).
Figure 2.
Change in apnea–hypopnea index after surgery.
Sleep RREP
One of the 13 subjects tested post-treatment did not have identifiable preoperative RREP data. However, this subject had identifiable components post-treatment. Another subject refused to wear the mask. Therefore, the paired comparisons were based on 11 subjects.
Amplitudes and latencies did not significantly change after treatment (Table 5, Figure 1). RREP changes post-treatment of OSAS did not correlate with OSAS improvement as measured by AHI reduction (r = 0.324; P = 0.395).
TABLE 5.
SLEEP RESPIRATORY-RELATED EVOKED POTENTIALS DURING N3 IN CHILDREN WITH OBSTRUCTIVE SLEEP APNEA SYNDROME BEFORE AND AFTER TREATMENT
| |
Fz |
Cz |
Pz |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak | Before | After | P | Before | After | P | Before | After | P |
| P2 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
9.6 ± 13.2 |
2.7 ± 1.6 |
0.49 |
3.5 ± 10.2 |
7.4 ± 5.7 |
0.57 |
0.4 ± 7.2 |
2.8 ± 14 |
0.78 |
| Latency, ms |
156.2 ± 34.7 |
194 ± 116.1 |
0.71 |
206.3 ± 142.3 |
248.4 ± 163.5 |
0.74 |
218.8 ± 154.4 |
252.3 ± 163.2 |
0.79 |
| N350 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
−16.6 ± 10.2 |
−21.6 ± 12.3 |
0.43 |
−25.1 ± 7.4 |
−29.8 ± 8.1 |
0.37 |
−27.2 ± 9.6 |
−22.9 ± 12.5 |
0.55 |
| Latency, ms |
389.6 ± 150.3 |
399.4 ± 157.7 |
0.91 |
340.3 ± 145 |
408.7 ± 144.9 |
0.45 |
287.4 ± 57.7 |
369.8 ± 160.5 |
0.32 |
| N550 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
−11.4 ± 15.9 |
−19.5 ± 10.3 |
0.18 |
−16.2 ± 14.7 |
−20.7 ± 12.9 |
0.41 |
−19.7 ± 10.3 |
−19.6 ± 12.7 |
0.98 |
| Latency, ms |
654.8 ± 115.6 |
548.3 ± 159.8 |
0.074 |
612.8 ± 146.6 |
622.4 ± 131.8 |
0.9 |
569.3 ± 146.7 |
587.4 ± 161.7 |
0.80 |
| P900 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
22.4 ± 13.8 |
25.6 ± 13.1 |
0.67 |
30.8 ± 20 |
43.1 ± 19 |
0.2 |
25.6 ± 18.1 |
45.6 ± 29 |
0.13 |
| Latency, ms | 1036.3 ± 94.4 | 1000.6 ± 29.7 | 0.41 | 1003.5 ± 71 | 958.2 ± 82.1 | 0.12 | 998 ± 71.2 | 954.6 ± 82 | 0.16 |
Sleep AEP
Amplitudes and latencies did not significantly change after treatment (Table 6, Figure 3). AEP changes post-treatment of OSAS did not correlate with OSAS improvement as measured by AHI reduction (r = −0.246; P = 0.557) (16).
TABLE 6.
SLEEP AUDITORY-RELATED EVOKED POTENTIALS DURING N3 IN CHILDREN WITH OBSTRUCTIVE SLEEP APNEA SYNDROME BEFORE AND AFTER TREATMENT
| Peak | Fz |
Cz |
Pz |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | P | Before | After | P | Before | After | P | |
| P2 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
6.2 ± 6.2 |
7.7 ± 9.2 |
0.7 |
10.3 ± 11.6 |
9.6 ± 10 |
0.86 |
9 ± 10.4 |
8.2 ± 6.1 |
0.78 |
| Latency, ms |
153.2 ± 29.7 |
135.8 ± 48.8 |
0.37 |
138.3 ± 29.6 |
118 ± 42.5 |
0.21 |
155.9 ± 25.6 |
130.7 ± 47.2 |
0.05 |
| N350 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
−51.6 ± 35.4 |
−40.1 ± 13.2 |
0.24 |
−67.5 ± 36.8 |
−65.5 ± 20.3 |
0.87 |
−54.6 ± 40.3 |
−57.8 ± 27.5 |
0.77 |
| Latency, ms |
363.3 ± 60.9 |
342.3 ± 60.5 |
0.29 |
327.1 ± 53 |
288.7 ± 46.9 |
0.06 |
316.7 ± 52 |
289.1 ± 43.1 |
0.08 |
| N550 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
−43.9 ± 30.1 |
−36.3 ± 18.6 |
0.27 |
−52.6 ± 33.9 |
−50.3 ± 29 |
0.82 |
−49.1 ± 42.9 |
−44.8 ± 36.8 |
0.7 |
| Latency, ms |
450.4 ± 55.9 |
448.8 ± 73.8 |
0.95 |
419.1 ± 52 |
395.3 ± 64 |
0.21 |
414.1 ± 70.4 |
407 ± 69.8 |
0.7 |
| P900 |
|
|
|
|
|
|
|
|
|
| Amplitude, μV |
63.9 ± 30.1 |
58.6 ± 19.2 |
0.44 |
73.1 ± 37 |
77.4 ± 22.3 |
0.67 |
60.9 ± 42.2 |
62.6 ± 33.1 |
0.9 |
| Latency, ms | 784.8 ± 138.7 | 800.8 ± 129 | 0.63 | 726.2 ± 64 | 718.4 ± 97 | 0.81 | 724.3 ± 64.6 | 740.6 ± 95.4 | 0.47 |
Figure 3.
Auditory evoked potentials Cz during sleep stage N3 in children with obstructive sleep apnea syndrome (OSAS) at baseline compared with children with OSAS after treatment and control subjects. Children with OSAS and control subjects had similar auditory evoked potentials during sleep before treatment. However, the latency of N350 at Cz tended to be shorter in children with OSAS after treatment.
Discussion
This study has confirmed previous findings that children with untreated OSAS have blunted RREP responses during sleep (5). Furthermore, this study has shown that these RREP abnormalities do not improve after treatment of OSAS. This study has also demonstrated that children with OSAS and control subjects have similar AEP during sleep. These data suggest that the abnormalities in central nervous system processing of afferent signals in children with OSAS during sleep are limited to respiratory stimuli. This is similar to adults with OSAS, who have been shown to have normal early RREP components during wakefulness, blunted longer latency RREP components during sleep, but to have normal AEP components during wakefulness and sleep (17–20).
Upper Airway Neuromotor Control
Upper airway muscles are activated in response to subatmospheric pressure, such as that generated during inspiration. This response is considered to be a centrally mediated reflex, as suggested by the following: (1) functional magnetic resonance imaging studies showing activation of central nervous system centers in response to upper airway loading (21); (2) the fact that the upper airway response to disparate stimuli, such as hypercapnia and inspiratory loading, is similar (22); (3) the rapid timing of the response compared with voluntary activation (23); and (4) changes in the response to loading during sleep compared with wakefulness (24). The upper airway contains pressure receptors in the mucosa of the nasopharynx and larynx (25). Afferent stimuli are conducted along the trigeminal, glossopharyngeal, and vagus nerves (26). Central pathways are thought to involve the nucleus of the tractus solitarius, locus coeruleus, caudal raphe, mesopontine tegmentum, and medullary reticular formation (27). Stimulation of these upper airway negative-pressure receptors results in inspiratory and expiratory activation of numerous upper airway dilator muscles (26), thereby preventing airway collapse. In children with OSAS, the dilatory response to subatmospheric pressure is diminished (1, 4). The reason for this is unclear, but one possibility is that the afferent limb of this reflex is abnormal (i.e., that the children do not mount a compensatory [efferent] neuromotor response to airway collapse in part because of lack of an afferent stimulus or a central neural processing deficit). Our data support this hypothesis.
RREP during Sleep
We have previously shown blunting of the RREP N350 during N3 sleep in children with OSAS and control subjects (5). In the present study, we confirmed those results and also showed blunting of the N550 peak and delayed latency, which has been well documented in adults (17, 28). Importantly, this study demonstrated no RREP improvement after treatment of OSAS. This implies that the RREP deficits observed during sleep represent either a primary congenital abnormality or an irreversible one, secondary to OSAS. However, it is important to point out that one of our subjects showed significant RREP improvement after OSAS treatment. Specifically, he did not have identifiable RREP components before treatment but peaks were present after considerable OSAS improvement (baseline AHI = 11.7 and post-treatment AHI = 1.7 events per hour). Therefore, reversibility may be possible in certain children with a shorter OSAS course. Further research is warranted.
AEP during Sleep
To determine whether the cortical processing of afferent stimuli impairment was limited to respiratory stimuli or was broadly affected (5), we tested the hypothesis that auditory cortical processing would be normal in children with OSAS compared with control subjects. We have previously demonstrated that children with OSAS had blunted arousal responses to hypercapnia (29), and impaired arousal responses to inspiratory loading during sleep compared with control subjects (16) but had a similar acoustic arousal threshold (30). This study, using a more sophisticated measure of respiratory and auditory processing stimuli, has confirmed our previous findings. Among the non-REM–specific auditory evoked response potentials components, N350, N550, and P900 have been reported to be elevated in response to stimuli occurring less frequently (31, 32). These waveforms do not merely reflect a general response to sensory stimuli but are associated with at least a minimum level of discrimination in the processing of information. Because all these peaks were present in OSAS and control subjects, this finding suggests that OSAS does not affect the children’s ability to properly process auditory information during sleep. This is similar to a previous study in adults with OSAS, in which the main AEP outcome was N550 (17). In the current study we documented the presence of a P900 as well, which has been reported to increase with the deepening of sleep, and has been associated with maintenance of sleep (33, 34). Therefore, the presence of this peak in children with OSAS is consistent with one of the main differences between adult and pediatric OSAS: the conserved sleep architecture in children with OSAS (35).
Based on the foregoing, we can infer that the auditory pathway is not affected in children with OSAS. Of note, AEP are generated in the cochlea and travel through the cochlear nerve, cochlear nucleus, superior olivary complex, and lateral lemniscus, to the inferior colliculus in the midbrain, and on to the medial geniculate body, to finally arrive to the cortex (36). Any of these structures could theoretically be affected by hypoxia or hypercapnia secondary to OSAS. However, this was not shown by our results. Interestingly, results in adults have showed blunted cortical AEP during wakefulness that does not resolve after a 3-month treatment with CPAP with adequate adherence (37). These results have been linked to behavioral daytime symptoms as attention is required to comply with research procedures because subjects were asked to identify oddball sounds. AEP during wakefulness have yet to be studied in children.
Limitations
A limitation of this study was that the subjects with OSAS were more obese than the control subjects. It is theoretically possible that adipose tissue could alter the respiratory mechanics during ventilation and load compensation, impairing afferent transduction of occlusion-related pressure changes. However, no data have established a link between obesity and impaired RREP. Further, our previous research showed that body mass index z score was not a predictor of RREP amplitude or latency (5). Nevertheless, this study evaluated children across a wide age-spectrum. It is possible that nonobese younger children may represent a different phenotype of OSAS than obese adolescents, who may be more similar to adult patients with OSAS. Future studies may be able to clarify this issue.
The number of males was greater in the control group but this difference was not statistically significant. However, because this study evaluated children across a wide age-spectrum, it is possible that adolescent females differ in their response from adolescent males depending on the degree of sexual maturation. Further research in adolescents distributed according to Tanner stage is warranted.
Conclusions
This study has shown that RREP during sleep were impaired in children with OSAS, and that these deficits did not improve after treatment. Children with OSAS and control subjects had similar AEP during sleep. These findings suggest that children with OSAS have a primary or a secondary but irreversible respiratory cortical processing deficit during sleep. This impairment may put them at risk of recurrence of OSAS, despite initial resolution after treatment as measured by the AHI, if they develop further risk factors later in life.
Acknowledgments
Acknowledgment
The authors thank the patients and their families who participated in this study; the research coordinators, Ruth Bradford and Mary Anne Cornaglia; and the sleep technologists, John Samuel and Michelle DiMaria at the Sleep Center of Children’s Hospital of Philadelphia, for their help with this study.
Footnotes
Supported by NIH grant UL1 RR-024134 and American Heart Association grant 0825666D.
Author Contributions: J.H., C.L.M., and I.E.T. contributed to the conception, design, analysis, and interpretation of the data; the drafting of the article; and final approval of the version to be published. P.W.D. contributed to critical revision of the article for important intellectual content, and final approval of the version to be published. I.M.C. contributed to the conception, design, critical revision of the article for important intellectual content, and final approval of the version to be published. P.R.G. contributed to statistical analysis and interpretation of the data; the drafting of the article; and final approval of the version to be published.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201307-1257OC on August 15, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA.Upper airway dynamic responses in children with the obstructive sleep apnea syndrome Pediatr Res 20055799–107.Published erratum appears in Pediatr Res 2008;63:327 [DOI] [PubMed] [Google Scholar]
- 2.Arens R, McDonough JM, Costarino AT, Mahboubi S, Tayag-Kier CE, Maislin G, Schwab RJ, Pack AI. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164:698–703. doi: 10.1164/ajrccm.164.4.2101127. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Fernandes Do Prado LB, Lutz J, Katz ES, Black CA, Galster P, Carson KA. Developmental changes in upper airway dynamics. J Appl Physiol. 2004;97:98–108. doi: 10.1152/japplphysiol.00462.2003. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Pinto SJ, Yuan H, Katz ES, Karamessinis LR, Bradford RM, Gallagher PR, Hannigan JT, Nixon T, Ward MB, et al. Upper airway collapsibility and genioglossus activity in adolescents during sleep. Sleep. 2012;35:1345–1352. doi: 10.5665/sleep.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Colrain IM, Melendres MC, Karamessinis LR, Pepe ME, Samuel JM, Abi-Raad RF, Trescher WH, Marcus CL. Cortical processing of respiratory afferent stimuli during sleep in children with the obstructive sleep apnea syndrome. Sleep. 2008;31:403–410. doi: 10.1093/sleep/31.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davenport PW, Friedman WA, Thompson FJ, Franzén O. Respiratory-related cortical potentials evoked by inspiratory occlusion in humans. J Appl Physiol. 1986;60:1843–1848. doi: 10.1152/jappl.1986.60.6.1843. [DOI] [PubMed] [Google Scholar]
- 7.Davenport PW, Colrain IM, Hill PM. Scalp topography of the short-latency components of the respiratory-related evoked potential in children. J Appl Physiol. 1996;80:1785–1791. doi: 10.1152/jappl.1996.80.5.1785. [DOI] [PubMed] [Google Scholar]
- 8.Colrain IM, Webster KE, Hirst G, Campbell KB. The roles of vertex sharp waves and K-complexes in the generation of N300 in auditory and respiratory-related evoked potentials during early stage 2 NREM sleep. Sleep. 2000;23:97–106. [PubMed] [Google Scholar]
- 9.Colrain IM, Webster KE, Hirst G. The N550 component of the evoked K-complex: a modality non-specific response? J Sleep Res. 1999;8:273–280. doi: 10.1046/j.1365-2869.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 11.Marcus CL, Omlin KJ, Basinki DJ, Bailey SL, Rachal AB, Von Pechmann WS, Keens TG, Ward SL.Normal polysomnographic values for children and adolescents Am Rev Respir Dis 19921461235–1239.see comment [DOI] [PubMed] [Google Scholar]
- 12.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values [letter] Am J Respir Crit Care Med. 2003;168:1540. doi: 10.1164/ajrccm.168.12.954. [DOI] [PubMed] [Google Scholar]
- 13.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R.Polysomnographic values in children 2-9 years old: additional data and review of the literature Pediatr Pulmonol 20054022–30.see comment [DOI] [PubMed] [Google Scholar]
- 14.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–878. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 15.Chan PY, Davenport PW. Respiratory-related evoked potential measures of respiratory sensory gating. J Appl Physiol. 2008;105:1106–1113. doi: 10.1152/japplphysiol.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus CL, Moreira GA, Bamford O, Lutz J. Response to inspiratory resistive loading during sleep in normal children and children with obstructive apnea. J Appl Physiol. 1999;87:1448–1454. doi: 10.1152/jappl.1999.87.4.1448. [DOI] [PubMed] [Google Scholar]
- 17.Afifi L, Guilleminault C, Colrain IM. Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir Physiol Neurobiol. 2003;136:221–234. doi: 10.1016/s1569-9048(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 18.Gora J, Trinder J, Pierce R, Colrain IM. Evidence of a sleep-specific blunted cortical response to inspiratory occlusions in mild obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166:1225–1234. doi: 10.1164/rccm.2106005. [DOI] [PubMed] [Google Scholar]
- 19.Eckert DJ, Lo YL, Saboisky JP, Jordan AS, White DP, Malhotra A. Sensorimotor function of the upper-airway muscles and respiratory sensory processing in untreated obstructive sleep apnea. J Appl Physiol. 2011;111:1644–1653. doi: 10.1152/japplphysiol.00653.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grippo A, Carrai R, Romagnoli I, Pinto F, Fanfulla F, Sanna A. Blunted respiratory-related evoked potential in awake obstructive sleep apnoea subjects: a NEP technique study. Clin Neurophysiol. 2011;122:1562–1568. doi: 10.1016/j.clinph.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Gozal D, Omidvar O, Kirlew KA, Hathout GM, Hamilton R, Lufkin RB, Harper RM. Identification of human brain regions underlying responses to resistive inspiratory loading with functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1995;92:6607–6611. doi: 10.1073/pnas.92.14.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol. 1991;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aronson RM, Onal E, Carley DW, Lopata M. Upper airway and respiratory muscle responses to continuous negative airway pressure. J Appl Physiol. 1989;66:1373–1382. doi: 10.1152/jappl.1989.66.3.1373. [DOI] [PubMed] [Google Scholar]
- 25.Sant’Ambrogio G, Tsubone H, Sant’Ambrogio FB. Sensory information from the upper airway: role in the control of breathing. Respir Physiol. 1995;102:1–16. doi: 10.1016/0034-5687(95)00048-i. [DOI] [PubMed] [Google Scholar]
- 26.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–853. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- 27.Widdicombe J. Upper airway reflexes. Curr Opin Pulm Med. 1998;4:376–382. doi: 10.1097/00063198-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Gora J, Colrain IM, Trinder J. The investigation of K-complex and vertex sharp wave activity in response to mid-inspiratory occlusions and complete obstructions to breathing during NREM sleep. Sleep. 2001;24:81–89. doi: 10.1093/sleep/24.1.81. [DOI] [PubMed] [Google Scholar]
- 29.Marcus CL, Lutz J, Carroll JL, Bamford O. Arousal and ventilatory responses during sleep in children with obstructive sleep apnea. J Appl Physiol. 1998;84:1926–1936. doi: 10.1152/jappl.1998.84.6.1926. [DOI] [PubMed] [Google Scholar]
- 30.Moreira GA, Tufik S, Nery LE, Lutz J, Verfaille K, Luan X, Marcus CL. Acoustic arousal responses in children with obstructive sleep apnea. Pediatr Pulmonol. 2005;40:300–305. doi: 10.1002/ppul.20219. [DOI] [PubMed] [Google Scholar]
- 31.Bastien CH, Crowley KE, Colrain IM. Evoked potential components unique to non-REM sleep: relationship to evoked K-complexes and vertex sharp waves. Int J Psychophysiol. 2002;46:257–274. doi: 10.1016/s0167-8760(02)00117-4. [DOI] [PubMed] [Google Scholar]
- 32.Bastien C, Campbell K. Effects of rate of tone-pip stimulation on the evoked K-complex. J Sleep Res. 1994;3:65–72. doi: 10.1111/j.1365-2869.1994.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 33.Yang CM, Lo HS. ERP evidence of enhanced excitatory and reduced inhibitory processes of auditory stimuli during sleep in patients with primary insomnia. Sleep. 2007;30:585–592. doi: 10.1093/sleep/30.5.585. [DOI] [PubMed] [Google Scholar]
- 34.Yang CM, Wu CS. The effects of sleep stages and time of night on NREM sleep ERPs. Int J Psychophysiol. 2007;63:87–97. doi: 10.1016/j.ijpsycho.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:682–686. doi: 10.1164/ajrccm.162.2.9908058. [DOI] [PubMed] [Google Scholar]
- 36.Musiek FE, Baran JA. Boston, MA: Pearson Education, Inc; 2007. The auditory system. [Google Scholar]
- 37.Vakulin A, Catcheside PG, Baulk SD, Antic NA, van den Heuvel CJ, Banks S, McEvoy RD. Auditory evoked potentials remain abnormal after CPAP treatment in patients with severe obstructive sleep apnoea. Clin Neurophysiol. 2012;123:310–317. doi: 10.1016/j.clinph.2011.07.004. [DOI] [PubMed] [Google Scholar]