Abstract
Quantitative analysis of fatty acids (FAs) is an important area of analytical biochemistry. Ultra high sensitivity FA analysis usually is done with gas chromatography of pentafluorobenzyl esters coupled to an electron-capture detector. With the popularity of electrospray ionization (ESI) mass spectrometers coupled to liquid chromatography, it would be convenient to develop a method for ultra high sensitivity FA detection using this equipment. Although FAs can be analyzed by ESI in negative ion mode, this method is not very sensitive. In this study, we demonstrate a new method of FA analysis based on conversion of the carboxylic acid to an amide bearing a permanent positive charge, N-(4-aminomethylphenyl)pyridinium (AMPP) combined with analysis on a reverse-phase liquid chromatography column coupled to an ESI mass spectrometer operating in positive ion mode. This leads to an ∼60,000-fold increase in sensitivity compared with the same method carried out with underivatized FAs. The new method is about 10-fold more sensitive than the existing method of gas chromatography/electron-capture mass spectrometry of FA pentafluorobenzyl esters. Furthermore, significant fragmentation of the precursor ions in the nontag portion improves analytical specificity. We show that a large number of FA molecular species can be analyzed with this method in complex biological samples such as mouse serum.
Keywords: lipid analysis, lipidomics, metabolomics
The analysis of fatty acids (FAs) is of considerable importance to both the clinical and biomedical research communities. From the clinical perspective, perturbations of FA metabolism have important physiological implications for a variety of medical conditions such as obesity, cardiovascular disease, and diabetes mellitus (1–3). Attention from the biomedical community is largely derived from the observation that some FAs, in particular the nonesterified fractions of polyunsaturated species such as arachidonic acid (AA) and docosahexaenoic acid (DHA), have distinct roles as precursors to important lipid signaling molecules (4, 5). Given their diverse biological roles and implication in a host of pathological conditions, considerable effort is dedicated to the development of methodologies to reliably and accurately assess FA composition and metabolism in a host of biological contexts. To meet these ends, tandem mass spectrometry (MS/MS) has emerged as the premier analytical platform due to its sensitivity, specificity, and ability to be directly coupled to chromatography systems (6).
Early quantitation methods for free FAs typically relied on gas chromatography with flame ionization detection or coupled to a mass spectrometer via electron ionization. The advantages of gas chromatography include high specificity, sensitivity, and good reproducibility (7). Resolution of FAs requires prior derivatization to increase their volatility and thermal stability. This has been typically accomplished by esterification to methyl (8), trimethylsilyl (9), or pentafluorobenzyl esters (10). The utility of these methods was greatly enhanced through the development of novel ionization sources and MS/MS instrumentation capable of selected reaction monitoring (SRM) experiments. SRM detects fragmentation products of specific chemical species at the exclusion of potential interference from chemical noise and coeluting compounds with identical masses. The analytical specificity of these experiments enables the direct quantitative analysis of species from very complex biological mixtures. Although useful, these methods are still limited by dynamic range limitations and compound volatility considerations (11). Although electron-capture detection of pentafluorobenzyl esters of FAs provides exceptionally high sensitivity, there are many laboratories worldwide that now have access to electrospray ionization (ESI) machines rather than electron-capture instruments. However, a major obstacle to the ESI technique is that FAs undergo less than ideal fragmentation behavior in negative ion mode via collision-induced dissociation (CID). Under low-energy (<100 eV) CID conditions typical to most commercial instruments, fragmentation of the featureless backbone of a saturated FA is minimal. Furthermore, the most prominent fragments originate from the loss of CO2 (−44 Da) and elimination of water (−18 Da) from the carboxylic acid group, neither of which are specific enough for reliable quantitation in complex matrices. Unsaturated FAs do undergo, to some extent, fragmentations that are specific to their structure. However, the abundances of these fragmentations are relatively weak and result in SRM measurements of poor sensitivity. Another major limitation of this approach is related to a FA's relative ionization efficiency and the manner in which the ions are analyzed. For compounds that contain free carboxylates, such as FAs, ionization is best achieved in negative ion mode under basic pH conditions where the carboxylate is ionized (12–15). Unfortunately, optimal LC resolution is facilitated by acidic pH conditions, to keep the carboxyl group protonated where ionization of the carboxylate is suppressed. Post-column addition of base could potentially alleviate this problem at the expense of the method's simplicity and sensitivity.
One group recently reported a LC-ESI-MS/MS method of FA analysis in plasma using post-column infusion of a barium ion solution (16). The formation of positively charged adduct ions promotes diagnostic fragmentation reactions of unsaturated FA species with enhanced SRM detection sensitivity. Other cation reagents, including alkaline earth metals and copper ions, also proved suitable for enhanced sensitivity for FA analysis in the SRM mode (17, 18). An alternative strategy for enhanced sensitivity is to improve the ionization efficiency of FAs via specific derivatization with reagents that introduce either readily chargeable or fixed charge groups such as tertiary or quaternary amines, respectively. Many derivatives of this nature have been reported including: pyrolidides (19–21), picolinyl esters (22, 23), dimethyloxazolines (24–28), benzofurazans (29), pyridiniums (30), and cholines (31). The advantages of these derivatives include improved MS sensitivity and reproducible chromatography profiles. A major limitation of these methodologies is the relatively harsh conditions usually required for derivatization, which can result in unwanted oxidation, isomerization, or degradation of some FAs. This limitation could potentially be addressed by the development of robust derivatization procedures that require milder conditions. Another major limitation is the tendency of these derivatives to fragment via CID in immediate proximity to the chargeable/cationic site. Fragmentation in the derivatization tag is undesirable due to the fact that analytes that form isobaric precursor ions and coelute during LC will not be distinguished in the mass spectrometer if they give rise to the same detected fragment ion, essentially eliminating any advantage a MS/MS experiment has over a MS experiment. This loss of specificity represents a significant limitation when analyzing complex biological samples.
We recently reported a straightforward LC-ESI-MS/MS derivatization procedure for the targeted lipidomic analysis of eicosanoids via stable isotope dilution (32). The carboxyl group is derivatized with a newly developed reagent, N-(4-aminomethylphenyl)pyridinium (AMPP), that results in a permanent positive charge (charge reversal). This derivatization results in a 10- to 20-fold improvement in detection sensitivity by LC-ESI-MS/MS (32). Our methodology employed a simple solid-phase extraction procedure of eicosanoids from a variety of biological matrices followed by a mild quantitative derivatization step with AMPP. The resulting derivatives can be directly submitted to LC-ESI-MS/MS and display robust fragmentations in their analyte segments making them attractive candidates for high-sensitivity/specificity SRM experiments. Here we utilize a similar approach, with the exception of an alternative extraction method, to monitor the free FA profiles in complex biological samples. We developed and validated a stable isotope dilution LC-ESI-MS/MS method that is able to detect essentially all saturated and unsaturated FAs in a single chromatographic run. Sensitivity improvement over LC-ESI-MS/MS of underivatized FAs in negative ion mode is ∼60,000-fold.
METHODS
Preparation of FA-free glassware and reagents
Low abundant FAs such as AA are usually not present as a contaminant in glassware and reagents; however, abundant FAs such as oleic, palmitic, and stearic acids are present as common contaminants. It has not been possible to remove these contaminants to a level below the FA detection limit for the method described in this paper. The procedure described here reduces abundant FA contamination to a level usually below the amounts to be detected in the sample of interest.
All glassware used for extraction and pre-LC-ESI-MS/MS work-up was baked overnight in a high temperature oven at 450°C to remove any residual FA contamination. Similarly, isooctane (Sigma Chromasolv Plus, catalog #650439), dimethylformamide (DMF) (Sigma, catalog #227056), Milli-Q water, ethanol, and acetonitrile (Fisher Optima grade, catalog #L-14338) were distilled in-house (DMF distilled under vacuum) with an oven-baked (450°C, overnight) distillation apparatus into oven-baked glass-stoppered flasks. Finally, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI) (TCI America, catalog #D1601), 1-hydroxy-7-azabenzotriazole (HOAt) (Sigma, catalog #44545-2), and AMPP (32) were triturated with distilled isooctane to remove any residual FA contamination.
Preparation of FA stock solutions
The following FA standards from Cayman Chemicals were used (d14-palmitoleic acid, d14-α-linolenic acid, d4-linoleic acid, d5-eicosapentaenoic acid, d8-AA, d17-oleic acid, d6-dihomo-γ-linolenic acid, d5-DHA, stearidonic acid, and AA (ω-3). d31-Palmitic acid and d35-stearic acid were from Sigma-Aldrich. GLC-463 standard (Nu-Check Prep, Inc.), containing 52 distinct FA molecular species, was used for the rest of the calibration standards. Stock solutions of FAs were prepared at concentration of 25–100 pg/μl in absolute ethanol and stored at −80°C under Ar in 1.5 ml amber vials (Agilent, catalog #5182-0716) with polytetrafluoroethylene/silicone septum screw caps (Agilent, catalog #5185-5838). Serial dilutions of the stock solutions were made in absolute ethanol for standard curve and extraction recovery analyses. Internal standards were diluted to a working stock of 100 pg/μl in absolute ethanol.
Preparation of samples and derivatization with AMPP
Standard curves.
Each sample contained 1 ng of each internal standard and various amounts of nondeuterated FAs (added from serial dilutions of the accurate concentration stock solution made from milligram amounts of FA as described above) transferred to a glass auto-sampler vial (Waters Total Recovery screw cap vial, catalog #186002805). Solvent was removed with a stream of nitrogen, and the residue was derivatized with AMPP as described below.
Extraction of FAs from mouse serum.
Analysis of endogenous FAs in serum was carried out with commercial mouse serum (Atlantic Biologicals, catalog #S18110). A 10 μl aliquot of serum was transferred to a 12 × 75 mm glass culture tube. To each culture tube, 50 μl of absolute ethanol containing 1 ng of each internal standard was added. The sample was adjusted to 125 μl by adding purified water (Milli-Q, Millipore Corp.). Aliquots of 250 μl of methanol (Fisher Optima grade, catalog #A456-4) and 12.5 μl of 1 N HCl were added to each sample. A bi-phasic solution was formed via addition of 750 μl of isooctane. This solution was vortexed for 60 s, and the phases were separated by centrifugation at 3,000 rpm for 60 s. The upper isooctane phase was removed via an oven-baked glass Pasteur pipette and transferred to an oven-baked Waters Total Recovery vial. The remaining aqueous phase was extracted once more with an additional 750 μl of isooctane. The combined isooctane phases were evaporated to dryness under a stream of filtered N2 and derivatized with AMPP as described below.
Derivatization with AMPP.
AMPP was synthesized in-house as described previously (32). Subsequent to our lead publication, the AMPP reagent was made commercially available by Cayman Chemical Company (catalog #710000) under the product name AMP+ Mass Spectrometry Kit.
To the residue in the oven-baked Waters Total Recovery auto-sampler vial was added 10 μl of ice-cold acetonitrile/DMF (4:1, v/v). Ten microliters of ice-cold 1 M EDCI in distilled Milli-Q water (freshly prepared daily) was added. The vial was briefly mixed on a vortex mixer and placed on ice while other samples were processed as above. To each vial was added 20 μl of 5 mM HOAt/15 mM AMPP in distilled acetonitrile (stored at −20°C and warmed to 65°C immediately prior to use). The vials were mixed briefly on a vortex mixer, capped with a split-septum screw cap (Agilent, catalog #5185-5824), and placed in a 60°C incubator for 30 min. Samples were analyzed on the same day and kept in the auto-sampler rack at 10°C while queued for injection.
LC/ESI-MS/MS analysis
Studies were carried out on a Waters Xevo TQ triple quadrupole mass spectrometer interfaced to an Acquity UPLC. The MassLynx 4.1 software package was used for data collection and analysis. Chromatography was carried out with a C18 reverse-phase column (Waters Acquity UPLC BEH Shield RP18, 2.1 × 100 mm, 1.7 μm, catalog #186002854). Solvent A was 100% water (Fisher Optima grade, catalog #L-13780)/0.1% formic acid (Fisher Optima grade, catalog #A117-50), and solvent B was acetonitrile (Fisher Optima grade, catalog #L-14338)/0.1% formic acid. The solvent program was (linear gradients): 0–0.5 min, 90% A; 0.5–0.51 min, 90–80% A; 0.51–10.0 min, 80–30% A; 10.0–10.1 min, 30–0% A; 10.1–12.0 min, 0% A; 12.0–12.1 min, 0–90% A; 12.1–15.0 min, 90% A. The flow rate was 0.4 ml/min and column temperature was 45°C. Supplementary Table I summarizes the auto-sampler and ESI-MS/MS parameters for data collection, respectively.
RESULTS AND DISCUSSION
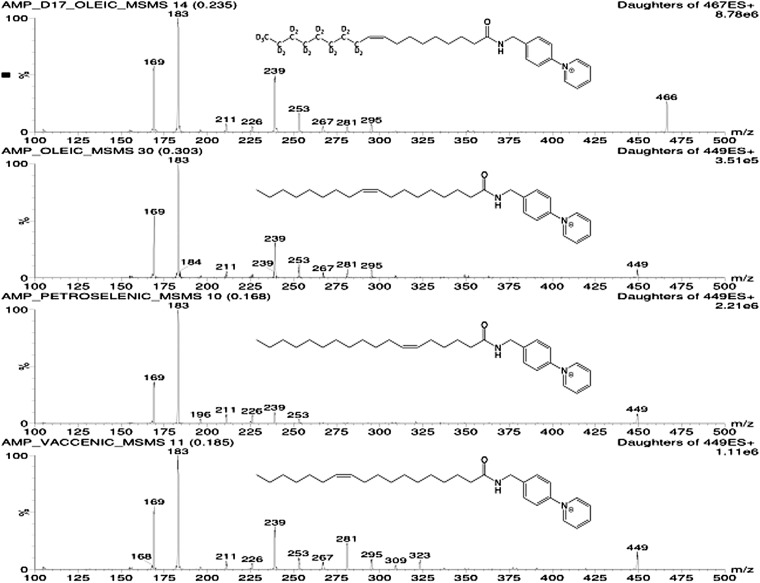
As noted in the introduction, conversion of the carboxyl group of lipids, such as eicosanoids and FAs, to the AMPP amide results in an analyte with a permanent positive charge, which can be analyzed by LC-ESI-MS/MS in positive ion mode. This is more sensitive than negative ion mode detection of the underivatized caboxylate anion because ionization of the latter is greatly suppressed by the protonation resulting from the addition of weak organic acid such as acetic or formic acid, which is necessary for optimal LC on reverse-phase columns. As shown in Fig. 1, AMPP amides of FAs give rise to spectral signature ions at m/z 169 and 183 due to CID of the AMPP tag. Additionally, abundant high molecular weight fragments are also generated, for example, m/z 239 for fragmentation between C3 and C4 in most FA species (Fig. 1). AMPP amides of oleic acid and its deuterated analog show an abundant product ion at m/z 295 due to cleavage between C7 and C8, thus leaving a relatively stable allylic radical. This ion is not present in the spectrum of the AMPP amide of petroselenic acid (Fig. 1). Likewise, vaccenic AMPP amide shows a major product ion at m/z 323, due to cleavage of the C9-C10 bond to generate an allylic radical. This species is not present in the other 18:1 spectra. These high molecular weight product ions provide for high analytical specificity, which may be important for analysis of FAs in complex biological samples. If a product ion resulting from cleavage of the AMPP tag is used for MS/MS, it would not be possible to distinguish isobaric AMPP-labeled species that coelute during LC. High molecular weight product ions were observed for all FAs analyzed. Precursor and product m/z values for all FAs are given in Table 1, and product ion mass spectra are shown in Fig. 1 and in supplementary Fig. I.
Fig. 1.
Production mass spectra for d17-oleic acid AMPP amide (first panel), oleic acid AMPP amide (second panel), petroselenic acid AMPP amide (third panel) and vacenic acid AMPP amide (fourth panel).
TABLE 1.
Liquid chromatography retention times and MS/MS parameters for analysis of FA AMPP amide molecular species
| FA Molecular Species | LC Retention Time (min)a | Retention Windowb | Internal Standard | Precursor Ionc (m/z) | Product Ionc (m/z) | Cone Voltaged (V) | Collision Energyd (eV) |
| Dodecenoic (11Z-12:1) | 4.37 | 1 | A | 365 | 239 | 56 | 42 |
| Lauric (12:0) | 4.96 | 1 | A | 367 | 239 | 60 | 44 |
| Myristoleic (9Z-14:1) | 5.35 | 1 | A | 393 | 239 | 58 | 44 |
| Myristic (14:0) | 6.04 | 1 | A | 395 | 239 | 62 | 47 |
| Palmitoleic (9Z-16:1) | 6.34 | 1 | A | 421 | 239 | 60 | 47 |
| Palmitoleic (9E-16:1) | 6.5 | 1 | A | 421 | 239 | 60 | 47 |
| Palmitic (16:0) | 7.02 | 1 | B | 423 | 239 | 65 | 49 |
| Stearidonic (6Z,9Z,12Z,15Z-18:4) | 5.8 | 1 | A | 443 | 239 | 64 | 42 |
| α-Linolenic (9Z,12Z,15Z-18:3) | 6.21 | 1 | C | 445 | 239 | 64 | 46 |
| 337 | 64 | 38 | |||||
| γ-Linolenic (6Z,9Z,12Z-18:3) | 6.29 | 1 | C | 445 | 239 | 64 | 45 |
| 347 | 64 | 38 | |||||
| Linoleic (9Z,12Z-18:2) | 6.7 | 1 | D | 447 | 239 | 65 | 48 |
| Linoleic (9E,12E-18:2) | 6.96 | 1 | D | 447 | 239 | 65 | 48 |
| Oleic (9Z-18:1) | 7.26 | 1 | E | 449 | 239 | 64 | 45 |
| Petroselinic (6Z-18:1) | 7.26 | 1 | E | 449 | 239 | 64 | 45 |
| Vaccenic (11Z-18:1) | 7.26 | 1 | E | 449 | 239 | 64 | 45 |
| Stearic (18:0) | 7.91 | 2 | G | 451 | 239 | 66 | 45 |
| Eicosapentaenoic (5Z,8Z,11Z,14Z,17Z-20:5) | 6.26 | 1 | F | 469 | 239 | 62 | 42 |
| Arachidonic (5Z,8Z,11Z,14Z-20:4) | 6.73 | 1 | H | 471 | 239 | 70 | 50 |
| 373 | 70 | 40 | |||||
| ω3-Arachidonic (8Z,11Z,14Z,17Z-20:4) | 6.6 | 1 | H | 471 | 239 | 70 | 50 |
| 363 | 70 | 40 | |||||
| Eicosatrienoic (11Z,14Z,17Z-20:3) | 7.07 | 1 | I | 473 | 239 | 70 | 50 |
| 365 | 70 | 40 | |||||
| Dihomo-γ-linolenic (8Z,11Z,14Z-20:3) | 7.07 | 1 | I | 473 | 239 | 70 | 50 |
| 375 | 70 | 38 | |||||
| Eicosadienoic (11Z,14Z-20:2) | 7.53 | 1 | E | 475 | 239 | 65 | 52 |
| 5-Eicosenoic (5Z-20:1) | 8.29 | 2 | G | 477 | 239 | 70 | 50 |
| 8-Eicosenoic (8Z-20:1) | 8.15 | 2 | G | 477 | 239 | 70 | 50 |
| 11-Eicosenoic (11Z-20:1) | 8.08 | 2 | G | 477 | 239 | 70 | 50 |
| Arachidic (20:0) | 8.74 | 2 | G | 479 | 239 | 66 | 55 |
| Docosahexaenoic (4Z,7Z,10Z,13Z,16Z,19Z-22:6) | 6.74 | 1 | J | 495 | 239 | 64 | 36 |
| Docosapentaenoic (7Z,10Z,13Z,16Z,19Z-22:5) | 6.98 | 1 | J | 497 | 239 | 65 | 55 |
| Docosapentaenoic (4Z,7Z,10Z,13Z,16Z-22:5) | 6.98 | 1 | J | 497 | 239 | 65 | 55 |
| Docosatetraenoic (7Z,10Z,13Z,16Z-22:4) | 7.41 | 1 | J | 499 | 239 | 65 | 55 |
| Docosatrienoic (13Z,16Z,19Z-22:3) | 7.88 | 2 | G | 501 | 239 | 65 | 55 |
| Docosadienoic (13Z,16Z-22:2) | 8.33 | 2 | G | 503 | 239 | 65 | 55 |
| Erucic (13Z-22:1) | 8.85 | 2 | G | 505 | 239 | 65 | 55 |
| Behenic (22:0) | 9.51 | 2 | G | 507 | 239 | 65 | 55 |
| Nervonic (15Z-24:1) | 9.59 | 2 | G | 533 | 239 | 70 | 60 |
| Lignoceric (24:0) | 10.22 | 2 | G | 535 | 239 | 65 | 55 |
| Internal Standards | |||||||
| (A) D14 palmitoleic (9Z-16:1) | 6.3 | 1 | A | 435 | 242 | 60 | 47 |
| (B) D31 palmitic (16:0) | 6.92 | 1 | B | 455 | 242 | 65 | 55 |
| (C) D14 α-linolenic (9Z,12Z,15Z-18:3) | 6.17 | 1 | C | 459 | 242 | 64 | 48 |
| (D) D4 linoleic (9Z,12Z-18:2) | 6.75 | 1 | D | 451 | 239 | 68 | 44 |
| (E) D17 oleic (9Z-18:1) | 7.28 | 1 | E | 466 | 239 | 68 | 48 |
| (F) D5 eicosapentaenoic (5Z,8Z,11Z,14Z,17Z-20:5) | 6.31 | 1 | F | 474 | 239 | 62 | 43 |
| (G) D35 stearic (18:0) | 7.81 | 2 | G | 487 | 242 | 65 | 58 |
| (H) D8 arachidonic (5Z,8Z,11Z,14Z-20:4) | 6.77 | 1 | H | 479 | 239 | 65 | 45 |
| (I) D6 Dihomo-γ-linolenic (8Z,11Z,14Z-20:3) | 7.04 | 1 | I | 479 | 239 | 70 | 46 |
| (J) D5 Docosahexaenoic (4Z,7Z,10Z,13Z,16Z,19Z-22:6) | 6.73 | 1 | J | 501 | 239 | 64 | 36 |
Fatty acyl chains are abbreviated with the number of carbons and double bonds and the double bond positions, i.e. 5,9-18:2 is an 18 carbon fatty acyl chain with 2 double bonds starting at carbons 5 and 9 from the carboxyl end. All double bonds have the cis (Z) configuration unless stated otherwise.
Retention times listed are derived from the LC protocol detailed in the Methods section.
Data for retention window 1 was collected from minutes 4.0 to 7.65. Data for retention window 2 was collected from minutes 7.65 to 10.
The m/z values listed are calculated monoisotopic values. The actual center mass values used are derived from instrument tuning, which is instrument dependent.
Cone voltages and collision energies were optimized for each analyte. These numbers are instrument dependent.
Isobaric species (i.e., cis/trans isomers or double bond positional isomers) were addressed via LC retention times. This was true for all species with the exception of the 18:1 isomers, which were not completely resolved. Although not applicable to the current study, alternative SRM transitions for the isobaric species could also be used as a method to resolve these species as each isomer has a distinct fragmentation pattern.
The limits of quantification for the AMPP amide method were all on the order of 50–100 femtograms on-column, as determined by standard curve analysis. We used accurate concentration FA stock solutions made from milligram amounts of FAs and carried out serial dilution to obtain low concentration stock solutions. AMPP derivatization and pre-MS/MS sample clean-up were carried out on fully diluted FA solutions, so the limits-of-quantification we report include any losses due to AMPP derivatization and pre-MS/MS sample clean-up. The limit-of-quantification of FA by gas chromatography/electron-capture mass spectrometry of pentafluorobenzyl esters is reported to be about 10 femtomoles (3,000 femtograms) (33). Thus, our method is about 10-fold more sensitive than this previous method of FA analysis.
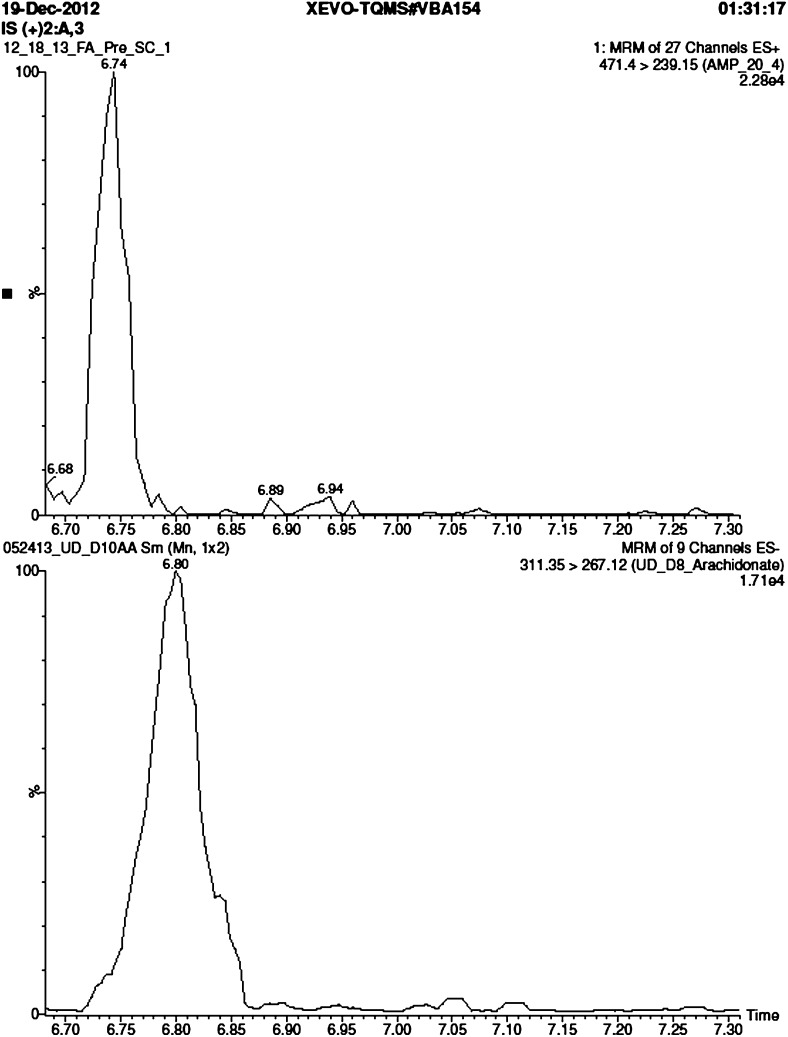
To gauge the increase in FA detection sensitivity, we analyzed various amounts of d8-20:4 AMPP amide in positive ion mode and various amounts of d8-20:4 free acid in negative ion mode. For the latter we monitored a major high mass product ion due to the loss of CO2. We also tuned the instrument to optimize the cone voltage and collision energy for this transition in negative ion mode. Results are shown in Fig. 2. Injection of 0.78 pg of d8-20:4 AMPP amide gives rise to a peak area of 22,800 in positive ion mode versus 17,100 for 50 ng of d8-20:4 free acid in negative ion mode. Thus, the increase in sensitivity for the AMPP derivatization method is 64,000-fold.
Fig. 2.
Selected-ion trace for (top panel) d8-20:4 AMPP amide in positive ion mode (0.78 pg injected, 471 > 239 transition) and (bottom panel) d8-20:4 free acid in negative ion mode (50 ng injection, 311 > 267 transition). Both peaks integrate to similar area (22,800 for d8-20:4 AMPP amide and 17,100 for d8-20:4 free acid).
Next we analyzed the FAs present in mouse serum, and the results are summarized in Table 2. Intra-assay coefficients of variation based on five injections of the same sample were typically less than 4%. Inter-assay coefficients of variations based on injections of six independent extractions of the same serum were typically less than 6%. Thus, the method is highly reproducible. For these studies we used the m/z 239 production ion. As noted above, this is present in all of the FAs, but its use is adequate in the case of mouse serum. Additional analytical specificity can be obtained by monitoring analyte-specific precursor ions, such as those noted above for the 18:1 species.
TABLE 2.
Calculated concentrations (pg/μl) and coefficients of variation (%) for LC/ESI-MS/MS analysis of FA AMPP amides in commercial mouse serum
| FA (pg/μl) | %CV Intra-sample (10 μl of Serum)a | %CV Inter-sample (10 μl of Serum)b | |
| Lauric | 143.2 | 3.1 | 7.6 |
| Myristoleic | 12.9 | 1.2 | 5.8 |
| Myristic | 299.4 | 2.2 | 3.0 |
| Palmitoleic | 295.8 | 0.8 | 2.6 |
| Palmitic | 1286.9 | 1.2 | 5.3 |
| Linolenic | 185.2 | 1.6 | 2.7 |
| Linoleic | 462.0 | 0.6 | 3.2 |
| Oleic | 2066.1 | 1.1 | 4.3 |
| Stearic | 1820.4 | 1.8 | 6.1 |
| Eicosapentaenoic | 72.0 | 0.9 | 3.7 |
| Arachidonic | 873.0 | 1.7 | 5.2 |
| ω3-Arachidonic | 24.2 | 4.8 | 5.9 |
| Eicosatrienoic | 147.0 | 2.4 | 1.5 |
| Eicosadienoic | 53.9 | 1.7 | 3.8 |
| Eicosenoic Acid | 98.7 | 1.4 | 2.4 |
| Arachidic | 21.4 | 2.9 | 8.1 |
| Docosahexaenoic | 605.6 | 4.4 | 6.9 |
| Docosapentaenoic | 148.2 | 3.9 | 7.9 |
| Docosatetraenoic | 66.9 | 3.5 | 9.0 |
| Docosatrienoic | 14.5 | 2.4 | 2.7 |
| Docosadienoic | 0.8 | 3.1 | 2.7 |
| Erucic | 8.3 | 3.0 | 10.4 |
| Behenic | 7.6 | 1.7 | 5.3 |
| Nervonic | 20.6 | 3.5 | 6.0 |
| Lignoceric | 18.6 | 1.9 | 7.8 |
A trace amount of 12:1 fatty acid was seen but variability was high due to its low level. %CV, percent coefficient of variation.
Coefficient of variation for the analysis of the same sample of extracted and derivatized serum injected five times onto the LC/ESI-MS/MS.
Coefficient of variation for the analysis of six independently extracted and derivatized serum samples.
It should be mentioned that accurate quantification of the absolute amount of any particular FA species requires a chemically identical isotopic substituted internal standard. Only in this way can one account for differences in ionization efficiencies in the mass spectrometer source and in differences in precursor-to-product ion generation for the different FA molecular species. Also, if a deuterated FA is used as in internal standard, deuterium should not be present at a site that leads to an isotope effect on the amount of product ion generated. Another option is to use a limited number of heavy atom substituted FA internal standards and to determine the relative MS/MS signal intensities of each FA molecular species by using standard curves for the appropriate species. This is not as accurate as an internal standard for absolute quantification.
Background contamination of solvents was particularly bad for the saturated series of chains 12–18 carbons in length as well as for the monounsaturated 18 carbon series. Baking of glassware and trituration of reagents improved background levels significantly.
Large amounts of derivatization reagents relative to FAs are used to ensure quantitative conversion to AMPP amides. All reagents and their products elute in the void volume of the LC run and do not enter the ESI-MS/MS source because a diversion value is used to direct LC output to waste during the initial part of the run. Thus, the method does not lead to excessive loading of the ESI-MS/MS source.
In summary, we have developed a new FA quantitative analysis using readily available LC/ESI-MS/MS equipment that provides a sensitivity close to that of the most sensitive FA method so far developed (gas chromatography of pentafluorobenzyl esters with electron-capture detection). Although LC does not provide the resolving power of capillary gas chromatography, the use of unique MS/MS channels is usually sufficient to resolve isobaric species that coelute during LC. The new method should find widespread use given the relatively large number of ESI-MS/MS instruments available in modern analytical laboratories.
Supplementary Material
Footnotes
Abbreviations:
- AA
- arachidonic acid
- AMPP
- N-(4-aminomethylphenyl)pyridinium
- CID
- collision-induced dissociation
- DHA
- docosahexaenoic acid
- DMF
- dimethylformamide
- EDCI
- 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- HOAt
- 1-hydroxy-7-azabenzotriazole
- SRM
- selected reaction monitoring
This work was supported by National Institutes of Health Grants HL-36235 and HL-50040. The AMPP derivatization reagent is commercially available from Cayman Chemicals under the name AMP+ Mass Spectrometry Kit (catalog #710000). The University of Washington derives royalty revenue from the net sales of this product.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and one table.
REFERENCES
- 1.Rasmussen L. B., Kiens B., Pedersen B. K., Richter E. A. 1994. Effect of diet and plasma fatty acid composition on immune status in elderly men. Am. J. Clin. Nutr. 59: 572–577 [DOI] [PubMed] [Google Scholar]
- 2.Schaefer E. J., Lichtenstein A. H., Lamon-Fava S., McNamara J. R., Ordovas J. M. 1995. Lipoproteins, nutrition, aging, and atherosclerosis. Am. J. Clin. Nutr. 61: 726S–740S [DOI] [PubMed] [Google Scholar]
- 3.Simopoulos A. P. 1991. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 54: 438–463 [DOI] [PubMed] [Google Scholar]
- 4.Funk C. D. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294: 1871–1875 [DOI] [PubMed] [Google Scholar]
- 5.Serhan C. N. 2007. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 25: 101–137 [DOI] [PubMed] [Google Scholar]
- 6.Feng L., Prestwich G. D., editors. 2006. Functional Lipidomics. Taylor and Francis. Boca Raton, FL. [Google Scholar]
- 7.Bicalho B., David F., Rumplel K., Kindt E., Sandra P. 2008. Creating a fatty acid methyl ester database for lipid profiling in a single drop of human blood using high resolution capillary gas chematography and mass spectrometry. J. Chromatogr. A. 1211: 120–128 [DOI] [PubMed] [Google Scholar]
- 8.Eder K. 1995. Gas chromatographic analysis of fatty acid methyl esters. J. Chromatogr. B Biomed. Appl. 671: 113–131 [DOI] [PubMed] [Google Scholar]
- 9.Schmitz B., Egge H., Murawski U. 1976. Capillary gas chromatography-mass spectrometry analysis of isomeric polyenoic FA as their poly-O-trimethylsilyl ether derivatives. Fres. Z. Anal. Chem. 279: 166–167 [Google Scholar]
- 10.Lagerstedt S. A., Hinrichs D. R., Batt S. M., Magera M. J., Rinaldo P., McConnell J. P. 2001. Quantitative determination of plasma C8-C26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol. Genet. Metab. 73: 38–45 [DOI] [PubMed] [Google Scholar]
- 11.Roberts L. D., McCombie G., Titman C. M., Griffin J. L. 2008. A matter of fat: an introduction to lipidomic profiling methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 871: 174–181 [DOI] [PubMed] [Google Scholar]
- 12.Carrier A., Parent J. 2001. Liquid chromatography-mass spectrometry determination of free fatty acids in phospholipid-based formulations. J. Liq. Chromatogr. Relat. Technol. 24: 97–107 [Google Scholar]
- 13.Nagy K., Jakab A., Fekete J., Vekey K. 2004. An HPLC-MS approach for analysis of very long chain fatty acids and other apolar compounds on octadecyl-silica phase using partly miscible solvents. Anal. Chem. 76: 1935–1941 [DOI] [PubMed] [Google Scholar]
- 14.Perret D., Gentili A., Marchese S., Sergi M., Caporossi L. 2004. Determination of free fatty acids in chocolate by liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 18: 1989–1994 [DOI] [PubMed] [Google Scholar]
- 15.Sajiki J., Yonekubo J. 2002. Determination of free polyunsaturated fatty acids and their oxidative metabolites by high-performance liquid chromatography (HPLC) and mass spectrometry (MS). Anal. Chim. Acta. 465: 417–426 [Google Scholar]
- 16.Zehethofer N., Pinto D. M., Volmer D. A. 2008. Plasma free fatty acid profiling in a fish oil human intervention study using ultra-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 22: 2125–2133 [DOI] [PubMed] [Google Scholar]
- 17.Afonso C., Riu A., Xu Y., Fournier F., Tabet J-C. 2005. Structural characterization of fatty acids cationized with copper by electrospray ionization mass spectrometry under low-energy collision-induced dissociation. J. Mass Spectrom. 40: 342–349 [DOI] [PubMed] [Google Scholar]
- 18.Hsu F-F., Turk J. 1999. Distinction among isomeric unsaturated fatty acids as lithiated adducts by electrospray ionization mass spectrometry using low energy collisionally activated dissociation on a triple stage quadrupole instrument. J. Am. Soc. Mass Spectrom. 10: 600–612 [DOI] [PubMed] [Google Scholar]
- 19.Andersson B. A. 1978. Mass spectrometry of fatty acid pyrrolidides. Prog. Chem. Fats Other Lipids. 16: 279–308 [DOI] [PubMed] [Google Scholar]
- 20.Andersson B. A., Heimermann W. H., Holman R. T. 1974. Comparison of pyrrolidides with other amides for mass spectral determination of structure of unsaturated fatty acids. Lipids. 9: 443–449 [DOI] [PubMed] [Google Scholar]
- 21.Vetter W., Walther W., Vecchi M. 1971. Pyrrolidides as derivatives for structural analysis of aliphatic and alicyclic fatty acids by mass spectrometry. Helv. Chim. Acta. 54: 1599–1605 [Google Scholar]
- 22.Destaillats F., Angers P. 2002. One-step methodology for the synthesis of FA picolinyl esters from intact lipids. J. Am. Oil Chem. Soc. 79: 253–256 [Google Scholar]
- 23.Dubois N., Barthomeuf C., Berge J-P. 2006. Convenient preparation of picolinyl derivatives from fatty acid esters. Eur. J. Lipid Sci. Technol. 108: 28–32 [Google Scholar]
- 24.Christie W. W., Robertson G. W., McRoberts W. C., Hamilton J. T. G. 2000. Mass spectrometry of the 4,4-dimethyloxazoline derivatives of isomeric octadecenoates (monoenes). Eur. J. Lipid Sci. Technol. 102: 23–29 [Google Scholar]
- 25.Fay L., Richli U. 1991. Location of double bonds in polyunsaturated fatty acids by gas chromatography-mass spectrometry after 4,4-dimethyloxazoline derivatization. J. Chromatogr. A. 541: 89–98 [Google Scholar]
- 26.Hamilton J. T. G., Christie W. W. 2000. Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4,4-dimethyloxazoline derivatives of fatty acids. Chem. Phys. Lipids. 105: 93–104 [DOI] [PubMed] [Google Scholar]
- 27.Wolff R. L., Christie W. W., Pedrono F., Marpeau A. M. 1999. Arachidonic, eicosapentaenoic, and biosynthetically related fatty acids in the seed lipids from a primitive gymnosperm, Agathis robusta. Lipids. 34: 1083–1097 [DOI] [PubMed] [Google Scholar]
- 28.Zhuang W., McKague B., Reeve D. 2004. Mass spectrometric elucidation of chlorine location in dichloro fatty acids following 4,4-dimethyloxazoline derivatization, and its application to chlorinated fatty acids in fish. Int. J. Mass Spectrom. 232: 127–137 [Google Scholar]
- 29.Tsukamoto Y., Santa T., Saimaru H., Imai K., Funatsu T. 2005. Synthesis of benzofurazan derivatization reagents for carboxylic acids and its application to analysis of fatty acids in rat plasma by high-performance liquid chromatography-electrospray ionization mass spectrometry. Biomed. Chromatogr. 19: 802–808 [DOI] [PubMed] [Google Scholar]
- 30.Barry S. J., Carr R. M., Lane S. J., Leavens W. J., Monté S., Waterhouse I. 2003. Derivatisation for liquid chromatography/electrospray mass spectrometry: synthesis of pyridinium compounds and their amine and carboxylic acid derivatives. Rapid Commun. Mass Spectrom. 17: 603–620 [DOI] [PubMed] [Google Scholar]
- 31.Lamos S. M., Shortreed M. R., Frey B. L., Belshaw P. J., Smith L. M. 2007. Relative quantification of carboxylic acid metabolites by liquid chromatography-mass spectrometry using isotopic variants of cholamine. Anal Chem. 79: 5143–5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bollinger J. G., Thompson W., Lai Y., Oslund R. C., Hallstrand T. S., Sadilek M., Turecek F., Gelb M. H. 2010. Improved sensitivity mass spectrometric detection of eicosanoids by charge reversal derivatization. Anal. Chem. 82: 6790–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Major C., Wolf B. A. 1994. Quantitation of the fatty acid composition of phosphatidic acid by capillary gas chromatography electron-capture detection with picomole sensitivity. J. Chromatogr. B Biomed. Appl. 658: 233–240 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.