Abstract
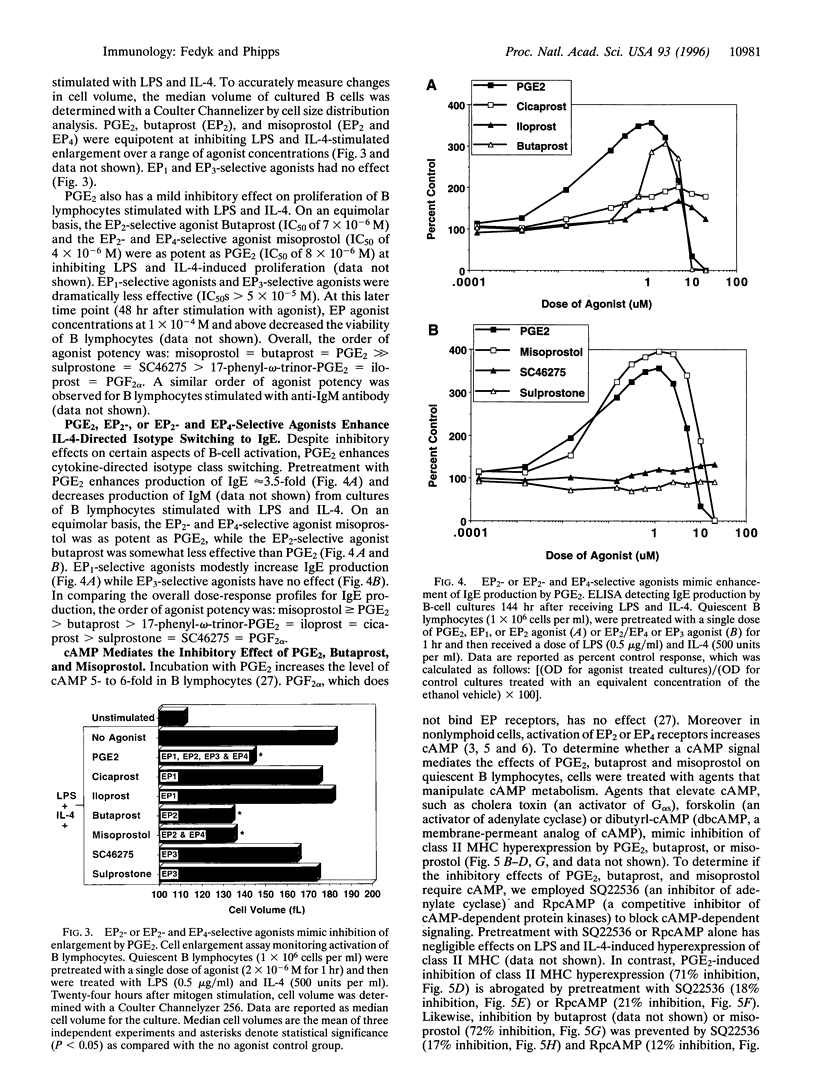
Prostaglandin E2 (PGE2) is a potent lipid molecule with complex proinflammatory and immunoregulatory properties. PGE2 can shape the immune response by stimulating the production of IgE antibody by B lymphocytes and the synthesis of T-helper type 2 cytokines [e.g., interleukin (IL)-4, IL-10], while inhibiting production of Th1 cytokines (e.g., interferon-gamma, IL-12). It is unknown what type of receptor binds PGE2 and modulates these responses. Recent analyses in nonhematopoietic cells have identified six PGE2 receptors (EP1, EP2, EP3 alpha, EP3 beta, EP3 gamma, and EP4). This investigation examines quiescent B lymphocytes and reports that these cells express mRNA encoding EP1, EP2, EP3 beta, and EP4 receptors. The immunoregulatory functions of each receptor were investigated using small molecule agonists that preferentially bind EP receptor subtypes. Unlike agonists for EP1 and EP3, agonists that bound EP2 or EP2 and EP4 receptors strongly inhibited expression of class II major histocompatibility complex and CD23 and blocked enlargement of mouse B lymphocytes stimulated with IL-4 and/or lipopolysaccharide. PGE2 promotes differentiation and synergistically enhances IL-4 and lipopolysaccharide-driven B-cell immunoglobulin class switching to IgE. Agonists that bound EP2 or EP2 and EP4 receptors also strongly stimulated class switching to IgE. Experiments employing inhibitors of cAMP metabolism demonstrate that the mechanism by which EP2 and EP4 receptors regulate B lymphocyte activity requires elevation of cAMP. In conclusion, these data suggest that antagonists to EP2 and EP4 receptors will be important for diminishing allergic and IgE-mediated asthmatic responses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. M., Phipps R. P. Characterization and regulation of prostaglandin E2 receptors on normal and malignant murine B lymphocytes. Cell Immunol. 1995 Mar;161(1):79–87. doi: 10.1006/cimm.1995.1011. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Eglen R. M., Jones R. L., Narumiya S., Shimizu T., Smith W. L., Dahlén S. E., Drazen J. M., Gardiner P. J., Jackson W. T. Prostanoid and leukotriene receptors: a progress report from the IUPHAR working parties on classification and nomenclature. Adv Prostaglandin Thromboxane Leukot Res. 1995;23:283–285. [PubMed] [Google Scholar]
- Elkashab M., Lala P. PGE2 receptors on murine splenic lymphocytes: effects of tumor bearing. Immunol Lett. 1991 Sep;30(1):7–15. doi: 10.1016/0165-2478(91)90082-l. [DOI] [PubMed] [Google Scholar]
- Fedyk E. R., Phipps R. P. Reactive oxygen species and not lipoxygenase products are required for mouse B-lymphocyte activation and differentiation. Int J Immunopharmacol. 1994 Jul;16(7):533–546. doi: 10.1016/0192-0561(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Fedyk E. R., Ripper J. M., Brown D. M., Phipps R. P. A molecular analysis of PGE receptor (EP) expression on normal and transformed B lymphocytes: coexpression of EP1, EP2, EP3beta and EP4. Mol Immunol. 1996 Jan;33(1):33–45. doi: 10.1016/0161-5890(95)00130-1. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., An S., Smith W. L. Specificity of expression and effects of eicosanoid mediators in normal physiology and human diseases. FASEB J. 1995 Aug;9(11):1051–1058. doi: 10.1096/fasebj.9.11.7649404. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., An S., Zeng L. Specific suppression by prostaglandin E2 of activation-induced apoptosis of human CD4+CD8+ T lymphoblasts. J Immunol. 1995 Feb 1;154(3):1041–1047. [PubMed] [Google Scholar]
- Haraguchi S., Good R. A., Day N. K. Immunosuppressive retroviral peptides: cAMP and cytokine patterns. Immunol Today. 1995 Dec;16(12):595–603. doi: 10.1016/0167-5699(95)80083-2. [DOI] [PubMed] [Google Scholar]
- Hikida M., Takai T., Ohmori H. Selective regulation of antigen-specific IgE response by cyclic AMP level in murine lymphocytes. Immunol Lett. 1992 Aug;33(3):301–306. doi: 10.1016/0165-2478(92)90077-2. [DOI] [PubMed] [Google Scholar]
- Holter W., Spiegel A. M., Howard B. H., Weber S., Brann M. R. Expression of GTP-binding proteins and prostaglandin E2 receptors during human T cell activation. Cell Immunol. 1991 May;134(2):287–295. doi: 10.1016/0008-8749(91)90303-s. [DOI] [PubMed] [Google Scholar]
- Irie A., Sugimoto Y., Namba T., Harazono A., Honda A., Watabe A., Negishi M., Narumiya S., Ichikawa A. Third isoform of the prostaglandin-E-receptor EP3 subtype with different C-terminal tail coupling to both stimulation and inhibition of adenylate cyclase. Eur J Biochem. 1993 Oct 1;217(1):313–318. doi: 10.1111/j.1432-1033.1993.tb18248.x. [DOI] [PubMed] [Google Scholar]
- Ivashkiv L. B., Ayres A., Glimcher L. H. Inhibition of IFN-gamma induction of class II MHC genes by cAMP and prostaglandins. Immunopharmacology. 1994 Jan-Feb;27(1):67–77. doi: 10.1016/0162-3109(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Kambayashi T., Alexander H. R., Fong M., Strassmann G. Potential involvement of IL-10 in suppressing tumor-associated macrophages. Colon-26-derived prostaglandin E2 inhibits TNF-alpha release via a mechanism involving IL-10. J Immunol. 1995 Apr 1;154(7):3383–3390. [PubMed] [Google Scholar]
- Katsuyama M., Nishigaki N., Sugimoto Y., Morimoto K., Negishi M., Narumiya S., Ichikawa A. The mouse prostaglandin E receptor EP2 subtype: cloning, expression, and northern blot analysis. FEBS Lett. 1995 Sep 25;372(2-3):151–156. doi: 10.1016/0014-5793(95)00966-d. [DOI] [PubMed] [Google Scholar]
- Negishi M., Sugimoto Y., Namba T., Irie A., Narumiya S., Ichikawa A. Signal transductions of three isoforms of mouse prostaglandin E receptor EP3 subtype. Adv Prostaglandin Thromboxane Leukot Res. 1995;23:255–257. [PubMed] [Google Scholar]
- Nishigaki N., Negishi M., Honda A., Sugimoto Y., Namba T., Narumiya S., Ichikawa A. Identification of prostaglandin E receptor 'EP2' cloned from mastocytoma cells EP4 subtype. FEBS Lett. 1995 May 15;364(3):339–341. doi: 10.1016/0014-5793(95)00421-5. [DOI] [PubMed] [Google Scholar]
- Phipps R. P., Stein S. H., Roper R. L. A new view of prostaglandin E regulation of the immune response. Immunol Today. 1991 Oct;12(10):349–352. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- Roper R. L., Brown D. M., Phipps R. P. Prostaglandin E2 promotes B lymphocyte Ig isotype switching to IgE. J Immunol. 1995 Jan 1;154(1):162–170. [PubMed] [Google Scholar]
- Roper R. L., Conrad D. H., Brown D. M., Warner G. L., Phipps R. P. Prostaglandin E2 promotes IL-4-induced IgE and IgG1 synthesis. J Immunol. 1990 Oct 15;145(8):2644–2651. [PubMed] [Google Scholar]
- Roper R. L., Ludlow J. W., Phipps R. P. Prostaglandin E2 inhibits B lymphocyte activation by a cAMP-dependent mechanism: PGE-inducible regulatory proteins. Cell Immunol. 1994 Apr 1;154(1):296–308. doi: 10.1006/cimm.1994.1079. [DOI] [PubMed] [Google Scholar]
- Roper R. L., Phipps R. P. Prostaglandin E2 and cAMP inhibit B lymphocyte activation and simultaneously promote IgE and IgG1 synthesis. J Immunol. 1992 Nov 1;149(9):2984–2991. [PubMed] [Google Scholar]
- Roper R. L., Phipps R. P. Prostaglandin E2 regulation of the immune response. Adv Prostaglandin Thromboxane Leukot Res. 1994;22:101–111. [PubMed] [Google Scholar]
- Savage M. A., Moummi C., Karabatsos P. J., Lanthorn T. H. SC-46275: a potent and highly selective agonist at the EP3 receptor. Prostaglandins Leukot Essent Fatty Acids. 1993 Dec;49(6):939–943. doi: 10.1016/0952-3278(93)90179-z. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Meade E. A., DeWitt D. L. Pharmacology of prostaglandin endoperoxide synthase isozymes-1 and -2. Ann N Y Acad Sci. 1994 Apr 18;714:136–142. doi: 10.1111/j.1749-6632.1994.tb12037.x. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Watabe A., Sugimoto Y., Honda A., Irie A., Namba T., Negishi M., Ito S., Narumiya S., Ichikawa A. Cloning and expression of cDNA for a mouse EP1 subtype of prostaglandin E receptor. J Biol Chem. 1993 Sep 25;268(27):20175–20178. [PubMed] [Google Scholar]
- Watanabe S., Yssel H., Harada Y., Arai K. Effects of prostaglandin E2 on Th0-type human T cell clones: modulation of functions of nuclear proteins involved in cytokine production. Int Immunol. 1994 Apr;6(4):523–532. doi: 10.1093/intimm/6.4.523. [DOI] [PubMed] [Google Scholar]
- Yang J., Xia M., Goetzl E. J., An S. Cloning and expression of the EP3-subtype of human receptors for prostaglandin E2. Biochem Biophys Res Commun. 1994 Feb 15;198(3):999–1006. doi: 10.1006/bbrc.1994.1142. [DOI] [PubMed] [Google Scholar]
- van der Pouw Kraan T. C., Boeije L. C., Smeenk R. J., Wijdenes J., Aarden L. A. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995 Feb 1;181(2):775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]






