Abstract
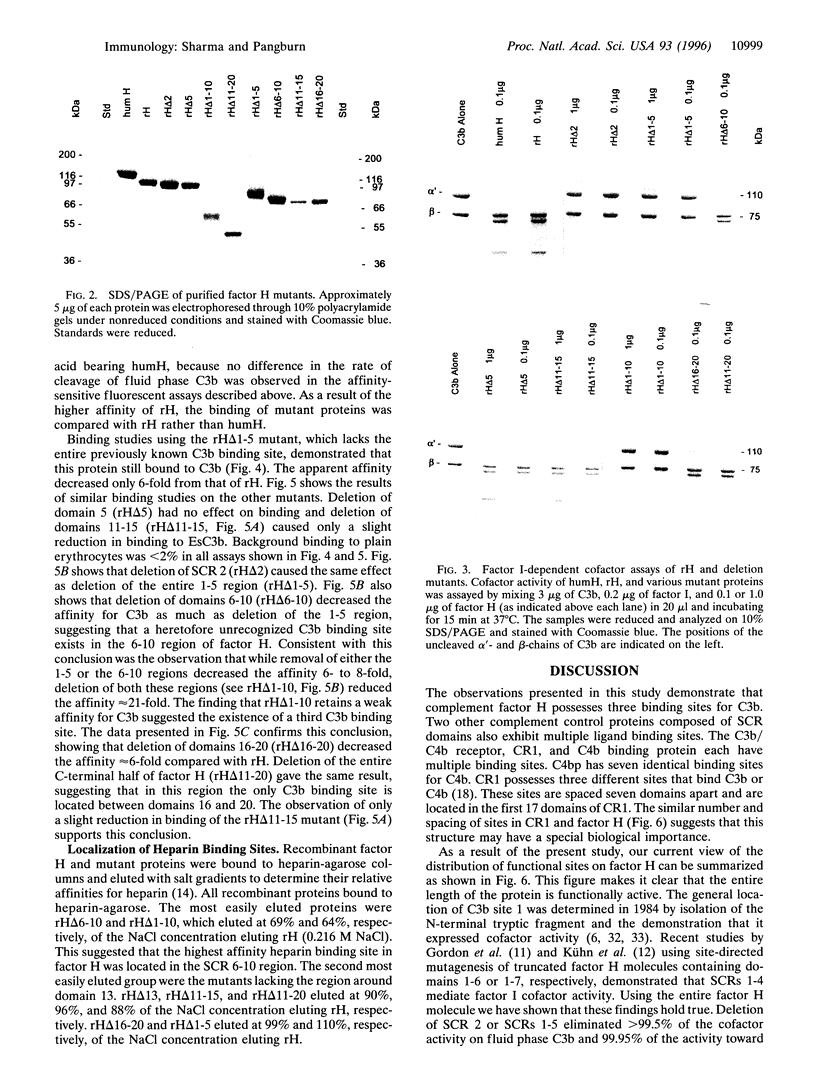
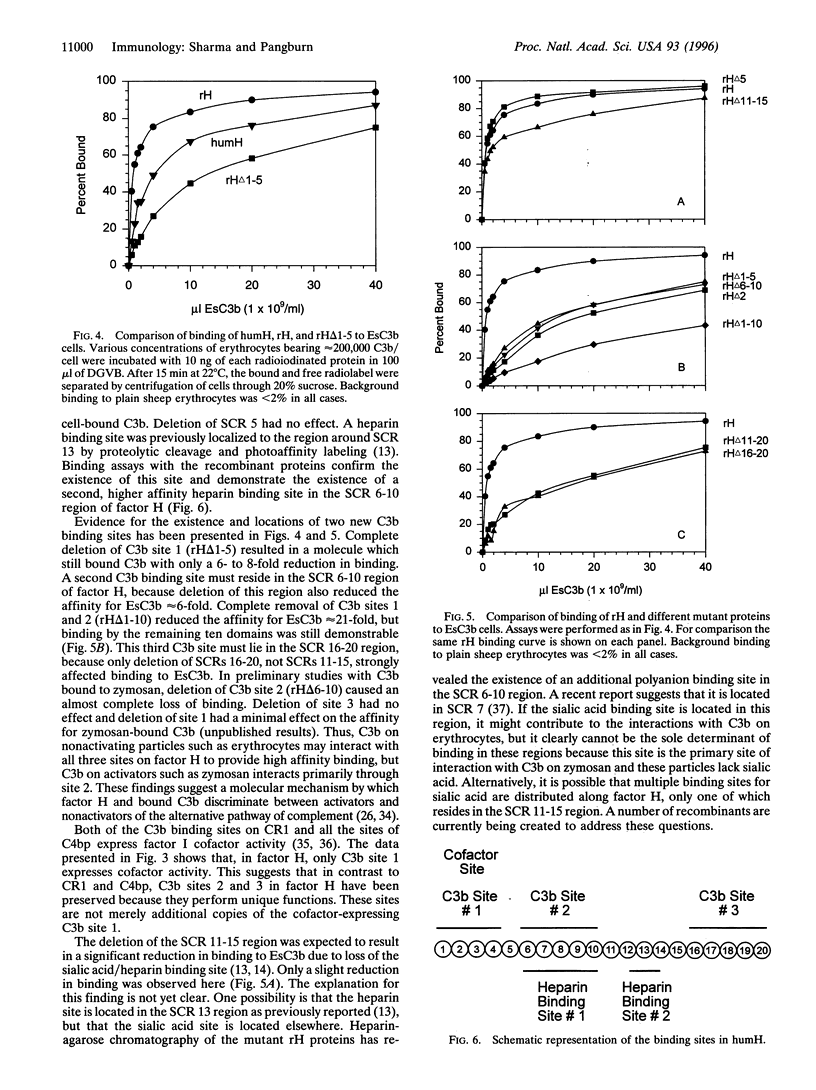
Human complement factor H controls spontaneous activation of complement in plasma and appears to play a role in distinguishing host cells from activators of the alternative pathway of complement. In both mice and humans, the protein is composed of 20 homologous short consensus repeat (SCR) domains. The size of the protein suggests that portions of the structure outside the known C3b binding site (SCR 1-4) possess a significant biological role. We have expressed the full-length cDNA of factor H in the baculovirus system and have shown the recombinant protein to be fully active. Mutants of this full-length protein have now been prepared, purified, and examined for cofactor activity and binding to C3b and heparin. The results demonstrate (i) that factor H has at least three sites that bind C3b, (ii) that one of these sites is located in SCR domains 1-4, as has been shown by others, (iii) that a second site exists in the domain 6-10 region, (iv) that a third site resides in the SCR 16-20 region, and (v) that two heparin binding sites exist in factor H, one near SCR 13 and another in the SCR 6-10 region. Functional assays demonstrated that only the first C3b site located in SCR 1-4 expresses factor I cofactor activity. Mutant proteins lacking any one of the three C3b binding sites exhibited 6- to 8-fold reductions in affinity for C3b on sheep erythrocytes, indicating that all three sites contribute to the control of complement activation on erythrocytes. The identification of multiple functionally distinct sites on factor H clarifies many of the heretofore unexplainable behaviors of this protein, including the heterogeneous binding of factor H to surface-bound C3b, the effects of trypsin cleavage, and the differential control of complement activation on activators and nonactivators of the alternative pathway of complement.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsenz J., Lambris J. D., Schulz T. F., Dierich M. P. Localization of the complement-component-C3b-binding site and the cofactor activity for factor I in the 38kDa tryptic fragment of factor H. Biochem J. 1984 Dec 1;224(2):389–398. doi: 10.1042/bj2240389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsenz J., Schulz T. F., Lambris J. D., Sim R. B., Dierich M. P. Structural and functional analysis of the complement component factor H with the use of different enzymes and monoclonal antibodies to factor H. Biochem J. 1985 Dec 15;232(3):841–850. doi: 10.1042/bj2320841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow P. N., Baron M., Norman D. G., Day A. J., Willis A. C., Sim R. B., Campbell I. D. Secondary structure of a complement control protein module by two-dimensional 1H NMR. Biochemistry. 1991 Jan 29;30(4):997–1004. doi: 10.1021/bi00218a016. [DOI] [PubMed] [Google Scholar]
- Barlow P. N., Steinkasserer A., Norman D. G., Kieffer B., Wiles A. P., Sim R. B., Campbell I. D. Solution structure of a pair of complement modules by nuclear magnetic resonance. J Mol Biol. 1993 Jul 5;232(1):268–284. doi: 10.1006/jmbi.1993.1381. [DOI] [PubMed] [Google Scholar]
- Conrad D. H., Carlo J. R., Ruddy S. Interaction of beta1H globulin with cell-bound C3b: quantitative analysis of binding and influence of alternative pathway components on binding. J Exp Med. 1978 Jun 1;147(6):1792–1805. doi: 10.1084/jem.147.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B., Smith C. A., Müller-Eberhard H. J. Visualization of human C4b-binding protein and its complexes with vitamin K-dependent protein S and complement protein C4b. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3461–3465. doi: 10.1073/pnas.80.11.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiScipio R. G. The binding of human complement proteins C5, factor B, beta 1H and properdin to complement fragment C3b on zymosan. Biochem J. 1981 Dec 1;199(3):485–496. doi: 10.1042/bj1990485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiScipio R. G. Ultrastructures and interactions of complement factors H and I. J Immunol. 1992 Oct 15;149(8):2592–2599. [PubMed] [Google Scholar]
- Estaller C., Schwaeble W., Dierich M., Weiss E. H. Human complement factor H: two factor H proteins are derived from alternatively spliced transcripts. Eur J Immunol. 1991 Mar;21(3):799–802. doi: 10.1002/eji.1830210337. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1683–1687. doi: 10.1073/pnas.74.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishelson Z., Müller-Eberhard H. J. C3 convertase of human complement: enhanced formation and stability of the enzyme generated with nickel instead of magnesium. J Immunol. 1982 Dec;129(6):2603–2607. [PubMed] [Google Scholar]
- Gordon D. L., Kaufman R. M., Blackmore T. K., Kwong J., Lublin D. M. Identification of complement regulatory domains in human factor H. J Immunol. 1995 Jul 1;155(1):348–356. [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hong K., Kinoshita T., Dohi Y., Inoue K. Effect of trypsinization on the activity of human factor H. J Immunol. 1982 Aug;129(2):647–652. [PubMed] [Google Scholar]
- Horstmann R. D., Pangburn M. K., Müller-Eberhard H. J. Species specificity of recognition by the alternative pathway of complement. J Immunol. 1985 Feb;134(2):1101–1104. [PubMed] [Google Scholar]
- Kalli K. R., Hsu P. H., Bartow T. J., Ahearn J. M., Matsumoto A. K., Klickstein L. B., Fearon D. T. Mapping of the C3b-binding site of CR1 and construction of a (CR1)2-F(ab')2 chimeric complement inhibitor. J Exp Med. 1991 Dec 1;174(6):1451–1460. doi: 10.1084/jem.174.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klickstein L. B., Bartow T. J., Miletic V., Rabson L. D., Smith J. A., Fearon D. T. Identification of distinct C3b and C4b recognition sites in the human C3b/C4b receptor (CR1, CD35) by deletion mutagenesis. J Exp Med. 1988 Nov 1;168(5):1699–1717. doi: 10.1084/jem.168.5.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Skerka C., Zipfel P. F. Mapping of the complement regulatory domains in the human factor H-like protein 1 and in factor H1. J Immunol. 1995 Dec 15;155(12):5663–5670. [PubMed] [Google Scholar]
- Makrides S. C., Scesney S. M., Ford P. J., Evans K. S., Carson G. R., Marsh H. C., Jr Cell surface expression of the C3b/C4b receptor (CR1) protects Chinese hamster ovary cells from lysis by human complement. J Biol Chem. 1992 Dec 5;267(34):24754–24761. [PubMed] [Google Scholar]
- Meri S., Pangburn M. K. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc Natl Acad Sci U S A. 1990 May;87(10):3982–3986. doi: 10.1073/pnas.87.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. D., DiScipio R. G., Cooper N. R., Nemerow G. R. Hydrodynamic, electron microscopic, and ligand-binding analysis of the Epstein-Barr virus/C3dg receptor (CR2). J Biol Chem. 1989 Dec 5;264(34):20576–20582. [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K. A fluorimetric assay for native C3. The hemolytically active form of the third component of human complement. J Immunol Methods. 1987 Aug 24;102(1):7–14. doi: 10.1016/s0022-1759(87)80003-0. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Atkinson M. A., Meri S. Localization of the heparin-binding site on complement factor H. J Biol Chem. 1991 Sep 5;266(25):16847–16853. [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Kinetic and thermodynamic analysis of the control of C3b by the complement regulatory proteins factors H and I. Biochemistry. 1983 Jan 4;22(1):178–185. doi: 10.1021/bi00270a026. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. J., Baker P. J., Rosse W. F. Comparison of binding characteristics of factors B and H to C3b on normal and paroxysmal nocturnal hemoglobinuria erythrocytes. J Immunol. 1983 Nov;131(5):2484–2489. [PubMed] [Google Scholar]
- Reid K. B., Day A. J. Structure-function relationships of the complement components. Immunol Today. 1989 Jun;10(6):177–180. doi: 10.1016/0167-5699(89)90317-4. [DOI] [PubMed] [Google Scholar]
- Ripoche J., Day A. J., Harris T. J., Sim R. B. The complete amino acid sequence of human complement factor H. Biochem J. 1988 Jan 15;249(2):593–602. doi: 10.1042/bj2490593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A. K., Pangburn M. K. Biologically active recombinant human complement factor H: synthesis and secretion by the baculovirus system. Gene. 1994 Jun 10;143(2):301–302. doi: 10.1016/0378-1119(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Sim R. B., DiScipio R. G. Purification and structural studies on the complement-system control protein beta 1H (Factor H). Biochem J. 1982 Aug 1;205(2):285–293. doi: 10.1042/bj2050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman H. F., Bartow T., Leppo M. K., Marsh H. C., Jr, Carson G. R., Concino M. F., Boyle M. P., Roux K. H., Weisfeldt M. L., Fearon D. T. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990 Jul 13;249(4965):146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- Zipfel P. F., Skerka C. Complement factor H and related proteins: an expanding family of complement-regulatory proteins? Immunol Today. 1994 Mar;15(3):121–126. doi: 10.1016/0167-5699(94)90155-4. [DOI] [PubMed] [Google Scholar]