Abstract
Periodontally involved teeth have been implicated as ‘microbial reservoirs’ in the etiology of peri-implant diseases. Therefore, the purpose of this investigation was to use a deep-sequencing approach to identify the degree of congruence between adjacent peri-implant and periodontal microbiomes in states of health and disease. Subgingival and peri-implant biofilm samples were collected from 81 partially edentulous individuals with periodontal and peri-implant health and disease. Bacterial DNA was isolated, and the 16S rRNA gene was amplified and sequenced by pyrotag sequencing. Chimera-depleted sequences were compared against a locally hosted curated database for bacterial identification. Statistical significance was determined by paired Student’s t tests between tooth-implant pairs. The 1.9 million sequences identified represented 523 species. Sixty percent of individuals shared less than 50% of all species between their periodontal and peri-implant biofilms, and 85% of individuals shared less than 8% of abundant species between tooth and implant. Additionally, the periodontal microbiome demonstrated significantly higher diversity than the implant, and distinct bacterial lineages were associated with health and disease in each ecosystem. Analysis of our data suggests that simple geographic proximity is not a sufficient determinant of colonization of topographically distinct niches, and that the peri-implant and periodontal microbiomes represent microbiologically distinct ecosystems.
Keywords: dental implants, phylogenetic biogeography, peri-implantitis, periodontitis, computational biology, biofilms
Introduction
According to the National Institute of Dental and Craniofacial Research (NIDCR), 90% of Americans will have lost at least 3 functioning teeth in their dentition before 50 yrs of age (Dye et al., 2012) and require replacement to restore form and function. Dental implants have become a well-accepted treatment modality for replacing missing teeth, with over 400,000 implants being placed every year and an anticipated growth of 9.1% annually (Millennium Research Group, 2000).
Implants have a survival rate of 95% over a 10-year period; however, the past 3 decades have seen the emergence of 2 new oral diseases: peri-implantitis and peri-implant mucositis (Zitzmann and Berglundh, 2008). Evidence indicates that peri-implant mucositis occurs in 50% to 90% of implants, while 20% of implants with an average function time of 5 to 11 yrs develop peri-implantitis (Zitzmann and Berglundh, 2008). It is now established that these diseases are biofilm-induced (Lindhe et al., 2008), and current therapeutic interventions and prognostic algorithms are based on a paradigm of microbial similarity with periodontal diseases (Mombelli et al., 1995; Millennium Research Group, 2000; Rutar et al., 2001; Takanashi et al., 2004; Shibli et al., 2008; Tabanella et al., 2009). However, the outcomes of these therapies have been modest (Renvert et al., 2009), with disturbingly high rates of disease recurrence (Esposito et al., 2012), suggesting that teeth and implants may be microbiologically different.
Dental implants in partially edentulous patients are biologically unique entities, since the tooth and adjoining implant share an interproximal space. While it appears logical that bacteria can translocate from the tooth to the adjacent implant, and that inflammation induced in the gingival sulcus by periodontal disease would affect the whole interdental space and therefore result in inflammation around the implant, evidence is emerging to suggest that the peri-implant crevice may be immunologically, histologically, and microbiologically distinct from the subgingival sulcus (Berglundh et al., 2011; Kumar et al., 2012; Salvi et al., 2012). Therefore, to understand the etiology and pathogenesis of peri-implant diseases, it is important that one investigate the extent to which disease within the gingival sulcus affects the peri-implant crevice.
Using a deep-sequencing methodology, we have previously demonstrated that peri-implant microbial communities differ significantly from subgingival communities (Kumar et al., 2012). The purpose of the present investigation was to use the same methodology to characterize adjoining peri-implant and periodontal microbiomes in states of health and disease and to identify the degree of similarity (or dissimilarity) between the 2 ecosystems.
Materials & Methods
Participant Recruitment
Approval for this study was obtained from the Institutional Review Board of the Ohio State University (Protocol number: 2011H0023). Dentate adults with at least 1 tooth-bounded dental implant in function for at least 1 yr were recruited, and written consent was obtained. Exclusion criteria included: diabetes; pregnancy; HIV; use of immunosuppressant medications, bisphosphonates, or steroids; antibiotic therapy or oral prophylactic procedures within the preceding 3 mos; need for antibiotic coverage before dental treatment; and fewer than 20 teeth present in the dentition. A diagnosis of implant health and disease was made according to the criteria delineated by the Consensus Report of the Sixth European Workshop on Periodontology (Lindhe et al., 2008). Briefly, peri-implant health was diagnosed when the implant exhibited no bleeding on probing (BOP), suppuration, or mobility and radiographic bone loss of less than 2 mm after placement of a coronal restoration. Peri-implant disease was diagnosed when the implants presented with mucosal inflammation with or without 2 mm or more of bone loss following restoration. A diagnosis of periodontal health and disease was made based on the American Academy of Periodontology classifications (Armitage, 1999). Briefly, periodontal health was diagnosed when the teeth exhibited probing depths of less than 3 mm, attachment loss of less than 1 mm, and no BOP, redness, or swelling. Diagnosis of disease was made when these criteria were not satisfied.
Sample Collection and DNA Isolation
The selected sites were isolated by means of cotton rolls, and supragingival and marginal plaque was removed. Subgingival and peri-implant biofilm samples were collected by the insertion of 10 sterile endodontic paperpoints (DENTSPLY-Caulk, Milford, DE, USA) into each peri-implant crevice and subgingival sulcus for 10 sec. Samples were placed in 1.5-mL microcentrifuge tubes and frozen at -80oC until further analysis. Bacteria were separated from the paperpoints by the addition of 200 μL of phosphate-buffered saline to the tubes and vortexing. The points were then removed, and DNA was isolated with a Qiagen DNA MiniAmp kit (Qiagen, Valencia, CA, USA) by the tissue protocol according to the manufacturer’s instructions.
Sequencing and Data Analysis
Multiplexed bacterial tag-encoded FLX amplicon pyrosequencing was performed on the Titanium platform (Roche Applied Science, Indianapolis, IN, USA) as previously described (Dowd et al., 2008) in a commercial facility (MRDNALab, Shallowater, TX, USA). Briefly, a single-step PCR with broad-range universal primers and 22 cycles of amplification was used to amplify the 16S rRNA genes as well as to introduce adaptor sequences and sample-specific bar-code oligonucleotide tags into the DNA. Two regions of the 16S rRNA genes were sequenced: V1–V3 and V7–V9. The primers used for sequencing have been previously described (Kumar et al., 2011). Adaptor sequences were trimmed from raw data with 98% or more of bases demonstrating a quality control of 30, and sequences were binned into individual sample collections based on bar-code sequence tags, which were then trimmed. Sequences < 300 bp were discarded, and the rest were clustered into species-level operational taxonomic units (s-OTUs) at 97% sequence similarity and assigned a taxonomic identity by alignment to a locally hosted version of the Greengenes database (DeSantis et al., 2006) by the Blastn algorithm. Analyses were conducted in the QIIME pipeline (Caporaso et al., 2010), as well as our own internally developed analysis pipeline. Results were visualized with the Python library matplotlib. Phylogenetic tree data were visualized through the Interactive Tree Of Life Web server (itol.embl.de).
Statistical Analysis
Participants were grouped into the following 4 categories based on the health status of each site: periodontal and peri-implant health (HT/HI), healthy periodontium adjoining a diseased implant (HT/DI), diseased periodontium adjoining a healthy implant (DT/HI), and both sites diseased (DT/DI). Single and multiple comparisons of distributions were carried out with the statistical facilities provided by JMP (SAS Institute Inc.), as well as the Python libraries SciPy, pandas, and statsmodels. Statistical significances of sOTUs within the 4 groups were determined by paired Student’s t tests between tooth-implant pairs per individual.
Results
Eighty-one individuals were recruited; samples were collected from the peri-implant crevice and paired with a sample from the adjacent subgingival sulcus. Based on the health status of each site (as defined in “Materials & Methods”), the samples fell into the following groups: 33 HT/HI, 23 HT/DI, 8 DT/HI, and 17 DT/DI. Thirteen of the DT represented gingivitis and 12 represented periodontitis, while 20 DI represented peri-implant mucositis and 20 represented peri-implantitis. Other than site-specific health-related characteristics, there were no significant clinical or demographic differences among the 4 groups (Appendix Table).
After quality control and noise filtering, approximately 1.9 million sequences were identified to species-level operational taxonomic units (sOTU). Of the initial 2.7 million sequences, 300,000 sequences remained unclassified. Overall, the identified sequences represented 12 phyla, 20 classes, 34 orders, 63 families, 110 genera, and 523 species (Appendix Fig.).
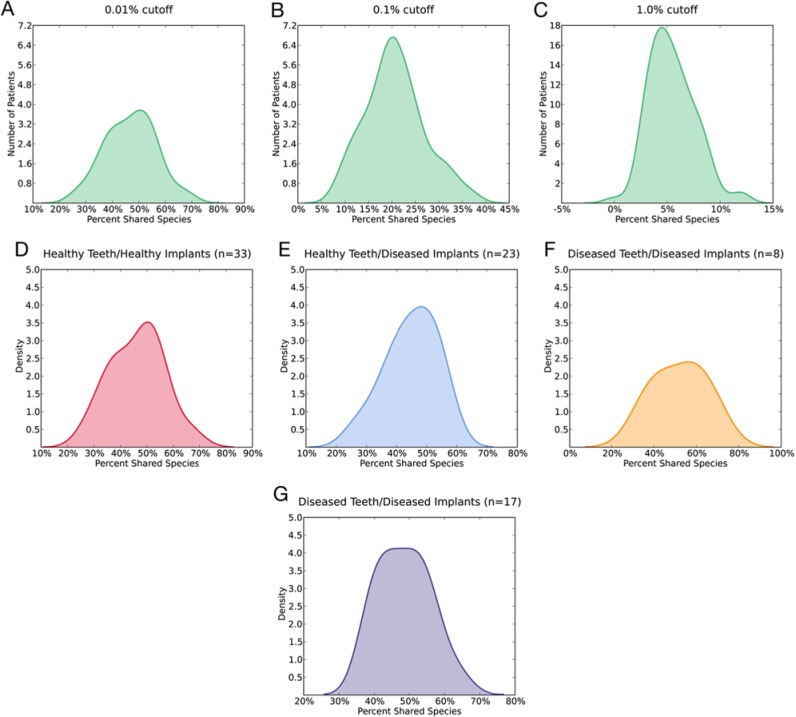
The percentage of species shared between each pair of tooth and implant was calculated for all participants across all groups. At an abundance level of 0.01%, 47.0 ± 9.5% (range, 26.0%-70.0%) of species were shared (Fig. 1A). At an abundance level of 0.1%, 20.7 ± 6.28% (range, 8.62%-37.1%) species were present in both peri-implant and periodontal crevices (Fig. 1B). At an abundance of 1.0%, 5.48 ± 2.18% (range, 0.0%-12.0%) species were shared (Fig. 1C). No statistically significant differences were found at the 0.01% abundance level in the mean number of shared species, based on periodontal or peri-implant health status (p = .25, one-way analysis of variance [ANOVA]) (Figs. 1D-1G).
Figure 1.
Percentage of shared microbial species between each pair of tooth and implant overall and across the health status groups. (A) Shared microbial species at 0.01% site-relative abundance cutoff value. (B) 0.1% cutoff value. (C) 1.0% cutoff value. (D-G) Shared species calculated at 0.01% abundance cutoff value for the 4 groups.
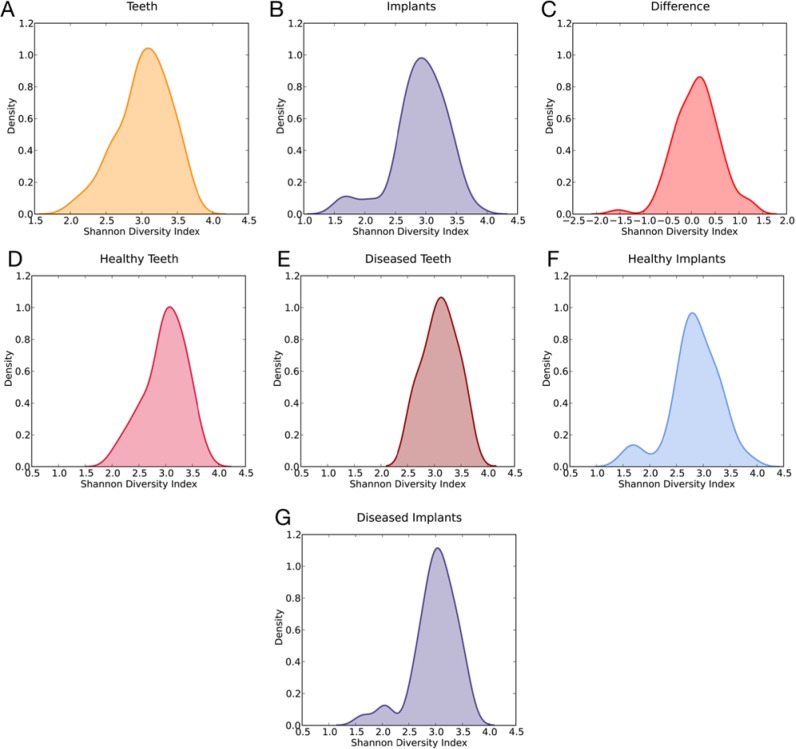
The Shannon Diversity index for the periodontal community was 3.03 (Fig. 2A), and 2.91 for the peri-implant community (Fig. 2B). The difference between the 2 distributions was significant (p = .023, Wilcoxon signed-rank test) in favor of the teeth (Fig. 2C). The breakouts by health status resulted in 4 distributions highly similar to the parent distributions, with a significant difference found only in the HT/HI group (p = .016, Wilcoxon signed-rank test).
Figure 2.
Shannon Diversity Index. (A) Teeth. (B) Implants. (C) Teeth minus implants. The difference between the 2 distributions was significant (p < .05, Wilcoxon signed-rank test) in favor of the teeth. (D-G) Shannon Diversity indices by health status. Statistically significant difference was found only in the HT/HI group (p < .05, Wilcoxon signed-rank test).
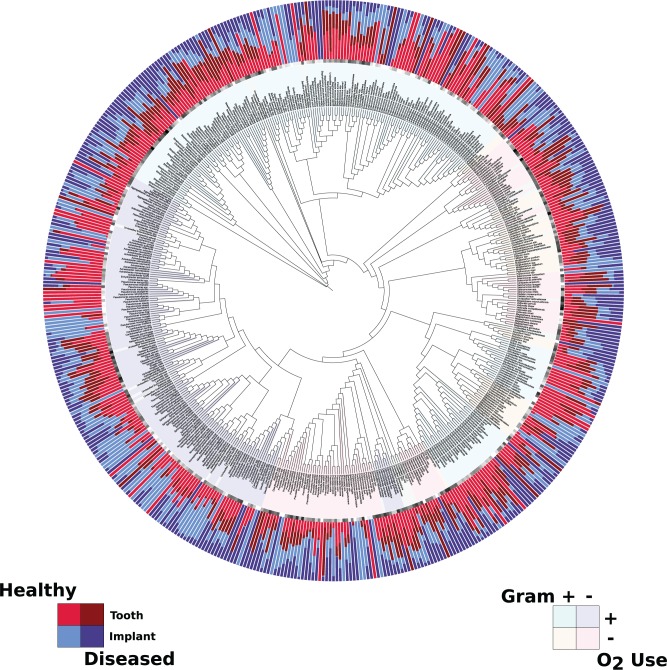
Fig. 3 shows the relative abundance of the sOTUs in each of the 4 groups, with the sOTU names color-coded by Gram status and oxygen use. The tree is composed mainly of aerobes: Gram-positive (194) and Gram-negative (148), with the balance split among Gram-positive anaerobes (47), Gram-negative anaerobes (99), and organisms of unknown status (34). The health-status-specific sOTU abundance differences exhibit many widely varying levels of many species, the significant (p < .05) of which appear in the Table.
Figure 3.
Relative abundance of the species-level Operational Taxonomic Units (sOTUs) for each of the 4 groups. The center of the Fig. is a circular phylogenetic tree representing the evolutionary relationships of the identified sOTUs. The sOTUs are color-coded by Gram status and oxygen use, represented in the inner ring of each Fig. The outer ring represents the normalized mean relative abundance of the identified sOTUs from the peri-implant and periodontal biofilm samples.
Table.
Species-level Operational Taxonomic Units (sOTUs) Demonstrating Significant Differences between Peri-implant and Periodontal Microbiomes
| Healthy Tooth/Healthy Implant |
Healthy Tooth/Diseased Implant |
Diseased Tooth/Healthy Implant |
Diseased Tooth/Diseased Implant |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species/Phylotypes | Location | p value | Species/Phylotypes | Location | p value | Species/Phylotypes | Location | p value | Species/Phylotypes | Location | p value |
| Actinomyces gerencseriae | Implant | .002 | Streptococcus castoreus | Implant | .015 | Streptococcus castoreus | Implant | .030 | Staphylococcus pettenkoferi | Implant | .017 |
| Actinomyces bovis | Implant | .008 | Treponema amylovorum | Implant | .024 | Fusobacterium spp. | Implant | .034 | Hylemonella spp. | Implant | .022 |
| Veillonella dispar | Implant | .017 | Lactobacillus psittaci | Implant | .029 | Prevotella nanceiensis | Implant | .044 | Staphylococcus hominis | Implant | .023 |
| Haemophilus influenza | Implant | .020 | Thermomonas spp. | Implant | .039 | Fusobacterium nucleatum | Implant | .048 | Prevotella baroniae | Implant | .023 |
| Streptococcus minor | Implant | .026 | Streptococcus equi | Implant | .040 | Unclassified Bifidobacteriales | Tooth | .010 | Streptococcus agalactiae | Implant | .029 |
| Mycoplasma faucium | Implant | .031 | Mogibacterium spp. | Implant | .040 | Streptococcus gallinaceus | Tooth | .014 | Atopobium rimae | Implant | .032 |
| Streptococcus macedonicus | Implant | .032 | Prevotella marshii | Implant | .045 | Streptococcus oligofermentans | Tooth | .023 | Prevotella oralis | Implant | .032 |
| Streptococcus pseudoporcinus | Implant | .037 | Neisseria lactamica | Tooth | .015 | Streptococcus didelphis | Tooth | .025 | Megasphaera elsdenii | Implant | .033 |
| Unclassified Bacillales | Implant | .038 | Unclassified Lachnospiraceae | Tooth | .015 | Kingella kingae | Tooth | .027 | Prevotella loescheii | Implant | .034 |
| Actinomyces radicidentis | Implant | .039 | Rothia spp. | Tooth | .015 | Neisseria subflava | Tooth | .028 | Aggregatibacter aphrophilus | Implant | .039 |
| Streptococcus infantis | Implant | .040 | Unclassified Gemellales | Tooth | .027 | Streptococcus phocae | Tooth | .029 | Arthrobacter spp. | Implant | .039 |
| Actinomyces meyeri | Implant | .043 | Selenomonas spp. | Tooth | .030 | Streptococcus pseudoporcinus | Tooth | .030 | Campylobacter sputorum | Implant | .039 |
| Streptococcus ursoris | Implant | .045 | Granulicatella adiacens | Tooth | .032 | Renibacterium spp. | Tooth | .032 | Streptococcus parasanguinis | Implant | .041 |
| Veillonella spp. | Implant | .050 | Selenomonas noxia | Tooth | .035 | Asticcacaulis biprosthecium | Tooth | .038 | Clostridium botulinum | Implant | .043 |
| Caulobacter spp. | Tooth | .019 | Leptotrichia spp. | Tooth | .036 | Pseudomonas putida | Tooth | .040 | Unclassified Methylobacteriaceae | Implant | .046 |
| Peptostreptococcus anaerobius | Tooth | .031 | Oribacterium spp. | Tooth | .040 | Veillonella dispar | Tooth | .040 | Neisseria elongata | Implant | .047 |
| Unclassified Rs-045 | Tooth | .038 | Unclassified Actinomycetaceae | Tooth | .042 | Enterococcus spp. | Tooth | .040 | Veillonella parvula | Implant | .052 |
| Desulfobulbus spp. | Tooth | .040 | Hylemonella spp. | Tooth | .042 | Actinobacillus spp. | Tooth | .040 | Actinomyces meyeri | Tooth | .045 |
| Bulleidia spp. | Tooth | .048 | Actinomyces gerencseriae | Tooth | .045 | Capnocytophaga granulosa | Tooth | .041 | |||
| Cardiobacterium spp. | Tooth | .046 | Veillonella spp. | Tooth | .041 | ||||||
| Sphingomonas spp. | Tooth | .041 | |||||||||
| Cardiobacterium spp. | Tooth | .043 | |||||||||
| Methylobacterium mesophilicum | Tooth | .046 | |||||||||
| Caulobacter spp. | Tooth | .048 | |||||||||
| Neisseria meningitidis | Tooth | .048 | |||||||||
Significant differences in abundance of the species in this Table were observed between teeth and implants in all 4 groups (p < .05, paired Student’s t test). Putative periodontal pathogens are shown in light shading and putative health-compatible periodontal bacteria in dark shading.
Discussion
The presence of periodontal disease in the dentition is 1 of the 2 known risk factors for peri-implantitis (Lindhe et al., 2008). The currently accepted mechanism is that periodontally involved teeth act as reservoirs for periodontal pathogens that translocate to the implant and cause disease in this site. This line of thinking has been influenced by studies that have used targeted approaches to identify or to quantify selected periodontal pathogens around implants (Quirynen and Listgarten, 1990; Papaioannou et al., 1996; van Winkelhoff et al., 2000; Rutar et al., 2001; Takanashi et al., 2004; Renvert et al., 2007). However, evidence is emerging that the implant microenvironment differs from its subgingival counterpart in topography as well as in host response. Therefore, it is possible that organisms that cause periodontal disease may not necessarily be the same that cause peri-implant disease. Hence, the present investigation re-examined the microbial reservoir hypothesis by combining an open-ended molecular approach that characterized 99.9% of the community with a robust clinical study design that allowed for meaningful statistical comparisons, and revealed a surprising picture of these 2 geographically distinct ecosystems.
Since both peri-implant and periodontal diseases encompass 2 entities – disease limited to the mucosal tissue alone or disease extending to the bone and attachment apparatus – we included equal numbers of participants with peri-implant mucositis and peri-implantitis, as well as those with gingivitis and periodontitis, in the disease categories. The number of participants was insufficient to permit further breakdown of the samples in the disease groups; however, a power analysis based on our earlier publication (Kumar et al., 2012) indicated that data from seven participants in each group would give us 80% to 90% power with α = 0.05 to detect a difference of 1% between the groups (given an SD of 0.7%). Therefore, we are confident that the differences between the 2 ecosystems in health and disease are valid and accurate representations of the population.
We investigated the idea that neighboring teeth highly influence the microflora surrounding osseointegrated dental implants by computing the species shared between the tooth-implant pairs from each participant. To capture meaningful relationships, we defined a species as shared if it was found in both sites at an abundance of 0.01% or greater. This cutoff value was chosen since it represented the overall abundances of 2 well-known periodontal pathogens (Porphyromonas gingivalis and Treponema denticola) in the present investigation, but it also captured 97% of the sequence data. At this level, it is clear that there is a wide variation in the number of species shared between a tooth and an implant, regardless of their health status (Fig. 1A). At the 0.1% abundance cutoff (Fig. 1B), set to preserve another known periodontal pathogen, Tannerella forsythia, no individual shared more than a third of the flora between tooth and implant. This metric dropped even more as the abundance increased to 1%, such that nearly 85% of participants shared less than 8% of species between tooth and implant (Fig. 1C). When red complex bacteria were identified in the subgingival sulcus, they were found in the peri-implant sulcus in only 37% of the cases. Analysis of these data corroborates results of previous studies to the extent that certain periodontal pathogens may be shared between tooth and implants in certain individuals (Leonhardt et al., 1993; Mombelli et al., 1995; Rutar et al., 2001; Takanashi et al., 2004; Tabanella et al., 2009); however, the majority of the flora, especially the abundant species, remain distinct between the 2 ecosystems. Thus, it appears that simple geographic proximity is not sufficient to fully determine the inhabitants of a microenvironment. Indeed, even subdivision of the data from Fig. 1A into groups by health status (Figs. 1D-1G) merely results in essentially identical graphs on a smaller scale.
Further pursuing location-specific differences, we turned to population diversity. The Shannon Index quantifies this metric through a count of the number of species (richness) scaled by abundance to determine the evenness of spread across the dataset. The periodontal microbiome is more diverse than the peri-implant microbiome (Figs. 2A-2C), especially in health (Figs. 2D-2G). Disease appears to shape the populations by increasing the diversity in the diseased ecosystem. These findings are in line with those from our previous investigations on both periodontal and peri-implant health and disease (Kumar et al., 2006, 2012). Furthermore, analysis of our data suggests that not all species present in the subgingival sulcus are capable of surviving and thriving in the peri-implant sulcus, and there are several lines of previous evidence to support this finding: First, bacteria that translocate from diseased teeth to healthy teeth do not necessarily colonize the niche (Christersson et al., 1985); and, second, the architecture, surface energy, and surface characteristics of abiotic structures (for example, implants) dictate the composition of the ecosystem around them (Yoshinari et al., 2000; Grossner-Schreiber et al., 2009).
Analysis of the data in Fig. 3 and the Table reveals that different bacterial lineages contribute to the composition of the 2 ecosystems. For example, members of the genera Staphylococcus and Treponema are significantly associated with diseased implants, but not teeth. Staphylococci have previously been associated with peri-implant disease (Salvi et al., 2008), and analysis of our data suggests that they may be important in certain individuals but are not universally associated with peri-implant infections. Our previous study suggested that a mutualistic relationship might exist between members of the genus Treponema and several butyrate producers (Kumar et al., 2012). The present study demonstrates a similar relationship in diseased implants, but not in teeth. Analysis of our data, taken together, demonstrates that, at both the population and participant levels, the implant microbiome is distinct from the periodontal microbiome.
Baas-Becking and Beijerinck famously said, “Everything is everywhere, but the environment selects”(de Wit and Bouvier, 2006). Based on our findings with respect to shared species, diversity, and species-specific content in health and disease, we submit that osseointegrated implants create truly unique microenvironments that force microbial adaptation and selection. Analysis of our data strongly suggests that the ‘microbial reservoir’ hypothesis should be revisited to identify species that play an etiologic role in peri-implant disease and to examine their transmission from the tooth to the implant surface, rather than searching for specific periodontal pathogens in the peri-implant sulcus.
Supplementary Material
Footnotes
The study was funded through internal funding sources at the College of Dentistry. Shareef Dabdoub is supported by the NIDCR (grant R01-DE022579).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Armitage GC. (1999). Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4:1-6. [DOI] [PubMed] [Google Scholar]
- Berglundh T, Zitzmann NU, Donati M. (2011). Are peri-implantitis lesions different from periodontitis lesions? J Clin Periodontol 38(Suppl 11):188-202. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christersson LA, Slots J, Rosling BG, Genco RJ. (1985). Microbiological and clinical effects of surgical treatment of localized juvenile periodontitis. J Clin Periodontol 12:465-476. [DOI] [PubMed] [Google Scholar]
- de Wit R, Bouvier T. (2006). ‘Everything is everywhere, but, the environment selects’; what did Baas Becking and Beijerinck really say? Environ Microbiol 8:755-758. [DOI] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. (2006). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd SE, Sun Y, Wolcott RD, Domingo A, Carroll JA. (2008). Bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) for microbiome studies: bacterial diversity in the ileum of newly weaned Salmonella-infected pigs. Foodborne Pathog Dis 5:459-472. [DOI] [PubMed] [Google Scholar]
- Dye BA, Li X, Beltrán-Aguilar ED. (2012). Selected oral health indicators in the United States, 2005–2008, NCHS data brief, no.96. In: NCfH Statistics editor; Hyattsville, MD: URL accessed on 8/1/2013 at: http://www.cdc.gov/nchs/data/databriefs/db96.htm. [PubMed] [Google Scholar]
- Esposito M, Grusovin MG, Worthington HV. (2012). Treatment of peri-implantitis: what interventions are effective? A Cochrane systematic review. Eur J Oral Implantol 5(Suppl):21-41. [PubMed] [Google Scholar]
- Grossner-Schreiber B, Teichmann J, Hannig M, Dorfer C, Wenderoth DF, Ott SJ. (2009). Modified implant surfaces show different biofilm compositions under in vivo conditions. Clin Oral Implants Res 20:817-826. [DOI] [PubMed] [Google Scholar]
- Kumar PS, Leys EJ, Bryk JM, Martinez FJ, Moeschberger ML, Griffen AL. (2006). Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol 44:3665-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PS, Brooker MR, Dowd SE, Camerlengo T. (2011). Target region selection is a critical determinant of community fingerprints generated by 16S pyrosequencing. PLoS One 6:e20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PS, Mason MR, Brooker MR, O’Brien K. (2012). Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J Clin Periodontol 39:425-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt A, Adolfsson B, Lekholm U, Wikström M, Dahlén G. (1993). A longitudinal microbiological study on osseointegrated titanium implants in partially edentulous patients. Clin Oral Implants Res 4:113-120. [DOI] [PubMed] [Google Scholar]
- Lindhe J, Meyle J; Group D of European Workshop on Periodontology (2008). Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol 35(8 Suppl):282-285. [DOI] [PubMed] [Google Scholar]
- Millennium Research Group (2000). Annual Industry Report. Implant Dentistry 9:192-194. [Google Scholar]
- Mombelli A, Marxer M, Gaberthüel T, Grunder U, Lang NP. (1995). The microbiota of osseointegrated implants in patients with a history of periodontal disease. J Clin Periodontol 22:124-130. [DOI] [PubMed] [Google Scholar]
- Papaioannou W, Quirynen M, Van Steenberghe D. (1996). The influence of periodontitis on the subgingival flora around implants in partially edentulous patients. Clin Oral Implants Res 7:405-409. [DOI] [PubMed] [Google Scholar]
- Quirynen M, Listgarten MA. (1990). Distribution of bacterial morphotypes around natural teeth and titanium implants ad modum Brånemark. Clin Oral Implants Res 1:8-12. [DOI] [PubMed] [Google Scholar]
- Renvert S, Roos-Jansaker AM, Lindahl C, Renvert H, Rutger Persson G. (2007). Infection at titanium implants with or without a clinical diagnosis of inflammation. Clin Oral Implants Res 18:509-516. [DOI] [PubMed] [Google Scholar]
- Renvert S, Samuelsson E, Lindahl C, Persson GR. (2009). Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: Clinical results. J Clin Periodontol 36:604-609. [DOI] [PubMed] [Google Scholar]
- Rutar A, Lang NP, Buser D, Burgin W, Mombelli A. (2001). Retrospective assessment of clinical and microbiological factors affecting periimplant tissue conditions. Clin Oral Implants Res 12:189-195. [DOI] [PubMed] [Google Scholar]
- Salvi GE, Furst MM, Lang NP, Persson GR. (2008). One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin Oral Implants Res 19:242-248. [DOI] [PubMed] [Google Scholar]
- Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. (2012). Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res 23:182-190. [DOI] [PubMed] [Google Scholar]
- Shibli JA, Melo L, Ferrari DS, Figueiredo LC, Faveri M, Feres M. (2008). Composition of supra- and subgingival biofilm of subjects with healthy and diseased implants. Clin Oral Implants Res 19:975-982. [DOI] [PubMed] [Google Scholar]
- Tabanella G, Nowzari H, Slots J. (2009). Clinical and microbiological determinants of ailing dental implants. Clin Implant Dent Relat Res 11:24-36. [DOI] [PubMed] [Google Scholar]
- Takanashi K, Kishi M, Okuda K, Ishihara K. (2004). Colonization by Porphyromonas gingivalis and Prevotella intermedia from teeth to osseointegrated implant regions. Bull Tokyo Dent Coll 45:77-85. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff AJ, Goené RJ, Benschop C, Folmer T. (2000). Early colonization of dental implants by putative periodontal pathogens in partially edentulous patients. Clin Oral Implants Res 11:511-520. [DOI] [PubMed] [Google Scholar]
- Yoshinari M, Oda Y, Kato T, Okuda K, Hirayama A. (2000). Influence of surface modifications to titanium on oral bacterial adhesion in vitro. J Biomed Mater Res 52:388-394. [DOI] [PubMed] [Google Scholar]
- Zitzmann NU, Berglundh T. (2008). Definition and prevalence of peri-implant diseases. J Clin Periodontol 35(8 Suppl):286-291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.