Abstract
Obliterative bronchiolitis (OB) limits the long-term success of lung transplantation, while T-cell effector mechanisms in this process remain incompletely understood. Using the murine heterotopic tracheal transplant model of obliterative airway disease (OAD) to characterize airway allograft rejection, we previously reported an important role for CD8+ T cells in OAD. Herein, we studied the role of CD154/CD40 costimulation in the regulation of allospecific CD8+ T cells, as airway rejection has been reported to be CD154-dependent. Airway allografts from CD154−/− recipients had significantly lower day 28 OAD scores compared to wild-type (WT) recipients, and adoptive transfer of CD8+ T cells from WT recipients, but not CD154−/− recipients, were capable of airway rejection in fresh CD154−/− allograft recipients. Intragraft CD8+ T cells from CD154−/− mice showed similar expression of the surface markers CD69, CD62Llow CD44high and PD-1, but markedly impaired IFN-γ and TNF-α secretion and granzyme B expression versus WT controls. Unexpectedly, intragraft and systemic CD8+ T cells from CD154−/− recipients demonstrated robust in vivo expansion similar to WT recipients, consistent with an uncoupling of proliferation from effector function. Together, these data suggest that a lack of CD154/CD40 costimulation results in ineffective allospecific priming of CD8+ T cells required for murine OAD.
Keywords: Allograft rejection, CD8 T lymphocyte, lung transplantation, T-cell priming
Introduction
Lung transplantation is the final therapeutic option for select patients with end-stage pulmonary disease, yet its long-term success is significantly limited by obliterative bronchiolitis (OB) or chronic allograft rejection (1–3). Despite evidence that adaptive T-cell responses play an important role in OB, the T-cell effector mechanisms in this process remain poorly understood (4,5). To address this, we used the murine heterotopic allogeneic airway transplant model to study effector mechanisms during obliterative airway disease (OAD), a term used specifically to describe tissue rejection in this preclinical model (4,6,7). Studies by us and other investigators have shown an important role for CD8+ T cells in airway obliteration following major histocompatibility (MHC)-mismatched heterotopic airway transplant (4,7–10). Moreover, our recent study demonstrated that intragraft and systemic allospecific CD8+ T cells with effector polyfunction (IFN-γ +, TNF-α+) and phenotype (CD44high, granzyme B+) predominate over allospe-cific CD4+ responses prior to obliterative fibrosis of airway allografts (10). However, the factors regulating allospecific CD8+ effectors during OAD have not yet been elucidated.
The CD154/CD40 costimulation pathway has been a major focus in experimental transplantation since early studies demonstrated that CD154 blockade abrogated allograft rejection in select models (11–13). In this regard, several reports have shown significant protection from murine OAD following allogeneic airway transplantation into CD154−/− recipients up to 6 weeks and the establishment of long-term tolerance (90 days) following anti-CD154 Ab therapy in conjunction with donor-specific transfusion (DST) (14–16). Activated CD4+ T cells are a major source of CD154 (or CD40 ligand), and via CD40-mediated activation of antigen presenting cells, this pathway plays an important role in Type 1 T-cell responses (17–19). While several studies have reported that blockade of CD154/CD40 costimulation readily impairs CD4+ T-cell-dependent rejection, the role of this pathway in the regulation of CD8+ T-cell-mediated rejection has shown variable results among different transplant models. In certain intestinal, heterotopic heart and skin models allospecific CD8+ T cells have been shown to induce allograft rejection independent of CD154 costimulation (20–24); however in other cardiac and skin models, anti-CD154 Ab-blockade-induced tolerance is associated with impaired CD8+ T-cell-mediated rejection (25–28). Although the clonal deletion of allospecific CD8+ T cells during DST/anti-CD154 Ab tolerance protocols has been a major proposed mechanism, non-human primate studies by Kirk et al. described infiltrating CD8+ T cells in non-rejecting kidney allografts following CD154 blockade, raising questions as to whether alternative mechanisms of CD8+ T-cell impairment exist (25,28–30). Other studies suggest a role for CD4+CD25+ regulatory T cells during CD154 blockade-induced tolerance, though this mechanism might be less important in the regulation of CD8+ T cells than in CD4+ T cells (13,25,31–34).
Few studies have focused on the function of intragraft CD8+ T cells, particularly in CD154-deficient recipients in the absence of DST. Furthermore, the mechanisms by which disruption of CD154/CD40 costimulation prevents allograft rejection have not been rigorously evaluated in airway transplantation. Because we have shown an important role for CD8+ T cells in murine OAD, we hypothesized that CD154 deficiency alters allospecific CD8+ T-cell function. To test this, we compared allospecific CD8+ T-cell function in MHC-mismatched CD154−/− and wild-type (WT) airway allograft recipients. Herein, we report that disruption of the CD154/CD40 pathway surprisingly uncouples intragraft allospecific CD8+ T-cell effector function from activation and proliferation consistent with impaired priming and inhibits airway rejection.
Materials and Methods
Mice
Wild-type female C57BL/6 (I-ab, H-2b), and BALB/c (I-ad, H-2d) mice, 5–8 weeks old, were purchased from the National Cancer Institute (Frederick, MD). C57BL/6 congenic CD45.1 mice (B6.SJL-Ptprca Pep3b/ BoyJ) and CD154 −/− mice (C57BL/6J-Tnfsf5tm1Imx) were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed in the Bayview Laboratory Animal Resource Center at the Johns Hopkins University Asthma and Allergy Center, and experiments conducted under a protocol approved the Johns Hopkins Animal Care and Use Committee.
Heterotopic tracheal transplant
Donor BALB/c tracheae were excised from mice euthanized via CO2 ashyxiation, and tracheae placed in cold media. Recipient mice (C57BL/6, CD154−/−) were anesthetized and prepped as previously described (10). A small incision was made and the tracheal graft was placed in a subcutaneous pocket. Two tracheal grafts per mouse were used for experiments with functional assays (day 14) and single grafts for histopathology. The wound was closed with two clips (Samuel Perkins, Quincy, MA) and recipient mice were given an i.p. injection of cefazolin 25 mg/kg (Apothecon, Barneveld, the Netherlands). Mice were euthanized at day 14 for functional assays, and day 14 or 28 for graft histopathology.
Medium and reagents
Cell culture medium RPMI 1640 (Biofluids, Rockville, MD) was supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO), 2 mM glutamine, 1 mM Sodium Pyruvate, 1% NEAA, 100 U/mL Penicillin, 100 MGC/mL Streptomycin, 50 μM β-mercaptoethanol and 25 mM HEPES (Biofluids, Rockville, MD).
Cell preparations, stimulation and cytokine detection
Spleen, draining lymph nodes (LN), lungs and grafts were recovered from mice on days 14 and 28; and mononuclear cells isolated per previously described methods (10). Lungs and grafts were digested in RPMI with 2.4 mg/mL of Collagenase I and 20 μg/mL DNAse I, and filtered through a 70 μm cell strainer. A 23% and 70% bilayer Percoll (Amersham Biosciences, Uppsala, Sweden) gradient was performed on lung, and the interface collected. Isolated responder cells from allografts, LN, spleen or lung mononuclear cells were co-cultured for 4 h in medium alone, with BALB/c splenocytes (1:3), or phorbol myristate acetate/ionomycin (PMA/I) (1 μM). Brefeldin A (10 μg/mL; Sigma) was added for the final 3 h of stimulation. Following stimulation, cells were stained with surface and intracellular antibodies.
Flow cytometry
The following antibodies were purchased from BD PharMingen (San Diego, CA) or Ebioscience: Fluorescein Isothiocyanate (FITC), Phycoerythrin (PE)-labeled anti-CD4; Peridinin-chlorophyll-protein complex (PerCP) Cy5.5-labeled anti-mouse CD8; FITC, PE labeled H2Dd, FITC-labeled CD62L PE-labeled anti-CD45.1; PE-labeled anti-PD-1, PE-labeled anti-CD25, PECy-7 labeled CD69, Allophycocyanin (APC)-labeled CD44; APC-labeled anti-IFN-γ , APC- labeled Foxp3; FITC-labeled anti-TNF-α; PE-labeled anti-granzyme B and respective isotype Abs. Surface marker and intracellular cytokine staining (ICCS) was performed as previously described (35,36). Flow cytometry analysis was performed using a FACSCalibur, CellQuest for data acquisition (Becton Dickinson, San Jose, CA), and Flowjo software for analysis (Tree Star Inc, San Carlos, CA).
Cell proliferation assays
In select experiments, naïve CD45.1+ CD8+ T cells were isolated with greater than 95% purity using magnetic beads and separation columns (Miltenyi Biotec, GA) per manufacturer’s protocol. Cells were labeled with carboxyfluorescein ester dye (CFSE) at 650 nM, and 5 × 106 cells injected i.v. via tail vein into recipient mice. To measure endogenous proliferation, mice were injected i.p. with 1.0 mg of 5 bromo deoxyuridine (BrdU) (Sigma-Aldrich) on day 0 and subsequently fed 0.8 mg/mL BrdU in drinking water for 7 days before sacrifice. BrdU incorporation was assayed with BrdU FITC Flow Kit (2345KK) (BD Pharmingen) per manufacturer’s protocol.
Histopathology
Grafts were fixed in 10% formalin, embedded in paraffin, sectioned and stained using hematoxylin & eosin. OAD scores were calculated by two independent blinded reviewers using a 4-point scale to calculate the mean degree of injury (0 = no injury, 4 = very severe) based on four parameters: epithelial injury, airway obliteration, collagen deposition and lymphocytic infiltration, as previously described (37).
In vivo CD8+ T-cell depletion
In select experiments, C57/BL6 mice were given an i.v. injection of either 1 mg of anti-CD8 antibody (clone 2.43, a generous gift from F. Finkelman, Cincinnati, OH) or Rat IgG control immediately prior to heterotopic BALB/c transplant and repeated every 7 days for 4 doses.
Statistical analysis
Ordinal and continuous integral variables were compared by rank sum test for paired comparisons or Kruskal–Wallis test for multigroup comparisons using SPSS software. A p-value of less than 0.05 was considered statistically significant.
Results
CD8+ T cells predominate in both allogeneic airway grafts from rejecting WT recipients and CD154−/− recipients with OAD resistance
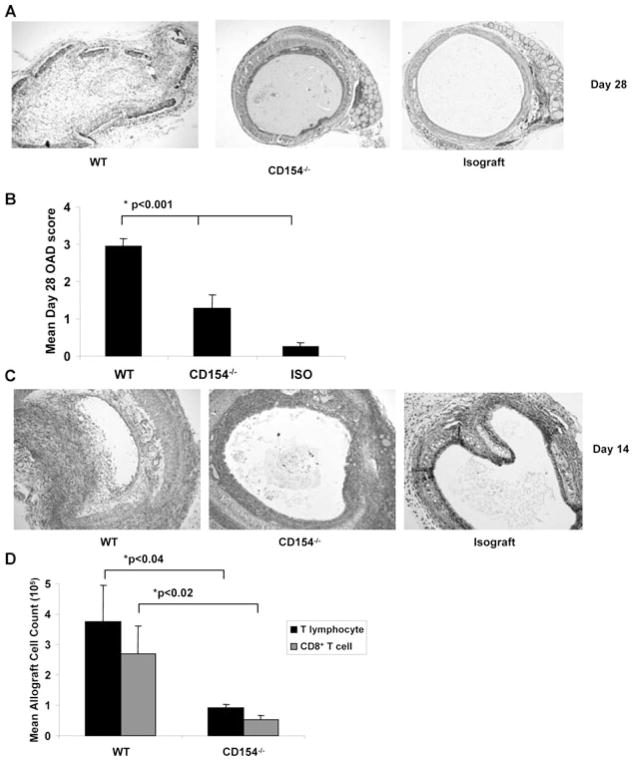
To evaluate the role of CD154/CD40 costimulation in the regulation of T cells following airway transplant, we compared graft histology and T-cell infiltration in WT [H-2b] or CD154−/− [H-2b] recipients of BALB/c [H-2d] tracheal allografts. Using a standardized scoring system for murine OAD analysis (see Materials and Methods), we found that CD154−/− recipients had significantly reduced day 28 OAD scores compared to WT allograft recipients, though higher than syngeneic C57BL/6 C57BL/6 WT isograft controls (1.28 ± 0.35 [SEM] vs. 2.96 ± 0.21 vs. 0.25 ± 0.12, p < 0.001) (Figure 1A and B). At the peak of graft cellular infiltration on day 14, we observed that CD154−/− recipients had increased infiltration compared to WT isograft recipients, though less than WT allograft controls (Figure 1C). Despite decreased absolute number of total intragraft T cells (mean 9.09 × 104 ± 0.14 vs. 3.74 × 105 ± 1.33, p < 0.04) and CD8+ T cells (mean 6.22 × 104 ± 0.12 vs. 2.68 × 105 ± 0.90, p < 0.02), CD8+ T cells comprised over two-thirds of the T-cell compartment in CD154−/− recipient mice, similar to WT recipients (Figure 1D).
Figure 1. CD154−/− recipient mice have significantly attenuated OAD, with CD8+ T-cell predominance similar to WT airway allografts.
C57BL/6 WT recipient mice or C57BL/6 CD154−/− mice were transplanted with trachea from BALB/c mice and compared to syngeneic isograft recipients of C57BL/6 tracheae. (A) Representative day 28 histopathology from each treatment group; tracheal grafts were fixed, stained with H/E as per materials and methods. (B) Mean OAD scores with bars representing mean values + SEM for treatment group with p-values determined by rank sum test. (C) Representative day 14 histopathology from each treatment group; tracheal grafts were fixed, stained as in (A). (D) Total lymphocyte counts were determined by determined by hemocytometer-based cell count, followed by fluorescent dye-conjugated anti-CD4 and anti-CD8 Ab staining and flow cytometric analysis. Total T lymphocyte count determined as sum of CD4+ and CD8+ fraction. Note that too few cells were recovered from isografts to quantitate T lymphocytes. Bars represent mean values ± SEM of cell counts for treatment group with p-values determined by rank sum test for paired samples and Kruskal–Wallis test for multiple groups. Results represent a minimum of three independent experiments with 8–10 mice per group.
Transferred CD8+ T cells from rejecting WT recipients, but not CD154−/− recipients, are sufficient to induce OAD in fresh CD154−/− allograft recipients
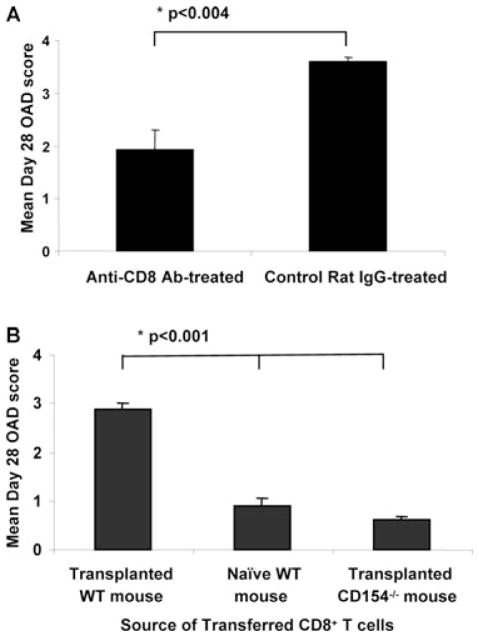
To evaluate the importance of CD8+ T cells in OAD pathogenesis, we treated WT allograft recipients with anti-CD8+ (mAb 2.43) for 3 weeks and found that CD8+-depleted mice had significantly lower day 28 mean OAD scores of WT MHC-mismatched airway allografts (1.94 ± 0.37 vs. 3.61 ± 0.08, p < 0.004), compared to control rat IgG treatment (Figure 2A), consistent with previous reports (7,10). Flow cytometric analysis of pooled splenocytes confirmed > 85% depletion with frequency of CD8 at 0.29% in anti-CD8- treated mice compared to 2.81% in rat IgG treated mice. We next asked whether previously alloprimed CD8+ T cells were sufficient to induce rejection in OAD-resistant CD154−/− hosts. To do this, we first isolated systemic CD8+ T cells (lung, spleen, LN) from three groups: (1) day 14 WT allograft recipients, in whom we have previously demonstrated alloeffector functional responses (10); (2) day 14 CD154−/− recipients or (3) naive untransplanted C57BL/6 mice. We then adoptively transferred 5 × 106 pooled CD8+ T cells from each respective group into day 0 CD154−/− airway allograft recipients intravenously, and assessed airway allograft rejection. As shown in Figure 2B, tracheal allografts from CD154−/− mice that received alloprimed WT CD8+ T cells demonstrated significantly higher day 28 mean OAD score compared to recipients that received cells from either CD154−/− allograft recipients or naïve WT CD8+ T cells (2.87 ± 0.19 vs. 0.625 ±.07 vs. 0.90 ± 0.15, p < 0.001). Together, these data support an important role for CD8+ T cells in OAD pathogenesis, with CD8+ T cells primed in the presence of CD154/CD40 costimulation sufficient to reject otherwise resistant CD154−/− hosts.
Figure 2. CD8+ T cells from transplanted WT mice, but not CD154−/− recipients, are sufficient to transfer OAD pathology.
(A) C57BL/6 allograft recipients received 1.0 mg of either anti-CD8 Ab (clone 2.43, rat IgG2b) or rat IgG control Ab i.v. at day 0, 7, 14 and 21 post-transplant. Tracheal grafts were recovered on day 28, fixed and stained for histopathology. Bars represent mean + SEM OAD scores with p-values determined by rank sum test from two separate experiments with eight mice per group. (B) CD8+ T cells were isolated and purified from day 14 spleen, LN and lung of WT allograft recipients, CD154−/− allograft recipients or naïve WT mice. 5 × 106 CD8+ T cells from these groups were then adoptively transferred into fresh CD154−/− allograft recipients at d0, and allografts were assessed for OAD histopathology at day 28. Bars represent mean OAD scores ± SEM with p-values determined by rank sum test for paired samples and Kruskal–Wallis test for multiple groups. Results represent two separate experiments with 8–10 individual mice per group.
Inhibition of OAD in CD154−/− recipients is associated with impaired intragraft allospecific CD8+ T-cell effector function and phenotype
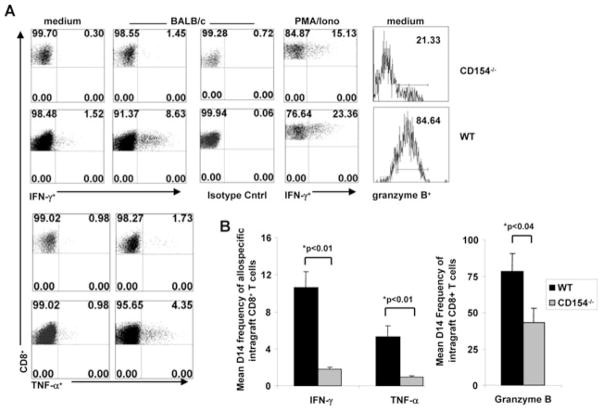
Next, we asked whether inhibition of OAD observed in CD154−/− allograft recipients was associated with altered allospecific CD8+ T-cell effector function or cytolytic phenotype compared to WT allograft recipients. To assess this, mononuclear cells were isolated from airway allografts at day 14 post-transplant and cultured in vitro in medium or restimulated with BALB/c splenocytes (1:3 stimulator to responder ratio), followed by surface staining and subsequent intracellular staining for IFN-γ , TNF-α and the cytolytic molecule granzyme B, the latter of which has been shown to correlate with cytolytic killing (38,39). As shown in a representative experiment in Figure 3A, both constitutive and induced allospecific effector cytokine production (IFN-γ and TNF-α) and granzyme B expression by intragraft CD8+ T cells from CD154−/− recipients was strikingly reduced in CD154−/− recipients compared to WT recipients. As a positive control, CD8+ IFN-γ secretion in response to the calcium ionophore, PMA/ionomycin, was also found to be modestly reduced in CD154−/− hosts. Analysis of cumulative data revealed more than a five-fold reduction in mean intragraft allospecific CD8+ IFN-γ + frequencies (9.87 ± 1.71% vs. 1.69 ± 0.21%, p < 0.01), a 10-fold decrease in mean allospecific CD8+TNF-α+ frequencies (5.33 ± 1.15% vs. 0.57 ± 0.19% p < 0.01), and a twofold reduction in granzyme B+ CD8+ frequencies (70.0 ± 11.2% vs. 34.6 ± 7.1%, p < 0.05) in CD154−/− allograft recipients compared to WT recipients (Figure 3B). Mean frequencies of constitutive CD8+IFN-γ + and CD8+TNF-α+ secretion were also reduced in CD154−/− hosts compared to WT hosts (1.51 ± 0.44 vs. 3.61 ± 0.64, p < 0.04, and 0.54 ± 0.22 vs. 2.36 ± 0.62, p < 0.02). These data suggest that CD154 deficiency results in a polyfunctional impairment in intragraft CD8+ T cells that is associated with attenuated OAD.
Figure 3. CD154 deficiency results in significantly impaired allospecific CD8+ T-cell effector function.
(A) Representative ICCS for IFN-γ , TNF-α, APC- isotype control, and granzyme B in CD154−/− and WT control recipients in d14 intragraft CD8+ T cells with medium, ex vivo BALB/c splenocyte restimulation or PMA/Ionomycin. Quadrant values represent detected frequencies of each population, gating on CD8+, H2Dd− T cells. (B) Mean CD8+ allospecific IFN-γ production, TNF-α production, , granzyme B expression at d14 from CD154−/− or WT recipients by ICCS. Bars represent mean frequencies ± SEM from each treatment group with p-values determined by rank sum test. Results are combined from minimum 4 separate experiments with pooled responses from 3 to 5 mice per group.
CD154 deficiency uncouples intragraft allospecific CD8+ T-cell effector function from proliferation
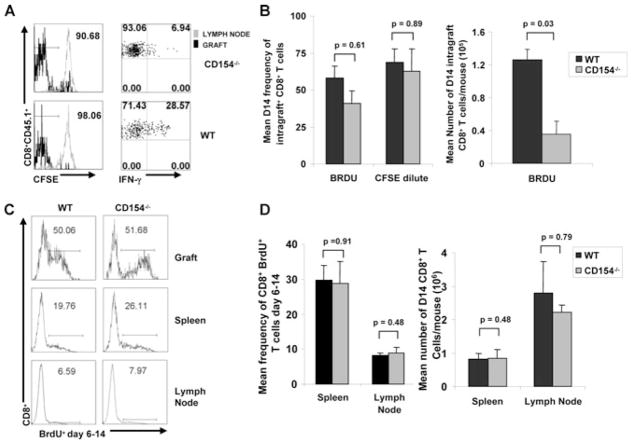
Because total T-cell numbers and effector function were diminished in CD154−/− mice, we hypothesized that intragraft CD8+ T cells have reduced proliferative expansion and effector differentiation. To assess proliferative expansion, we purified and labeled naive congenic CD45.1+CD8+ T cells (1:1 spleen: LN mix) with CFSE, and adoptively transferred 5 × 106 cells into WT and CD154−/− mice at time of airway transplant. At day 14, graft and LN mononuclear cells were isolated and assessed for CFSE dilution. Unexpectedly, CD8+CD45.1+ T cells recovered from airway allografts of CD154−/− recipients demonstrated massive in vivo proliferative expansion despite profound impairment in allospecific IFN-γ secretion upon in vitro restimulation as shown by representative flow plot in Figure 4A. Cumulative data confirmed that mean CFSE dilution of intragraft CD8+ T cells in CD154−/− recipients was comparable to that from WT recipients (68.31 ± 9.53% vs. 75.12 ± 15.03%, p = 0.89). To further determine whether proliferation of endogenous CD8+ T cells differed between these groups, we evaluated incorporation of the synthetic thymidine analog, Bromodeoxyuridine (BrdU), in CD154−/− and WT allograft recipients at various time intervals and determined that the period of maximal proliferation occurred from day 6 to 14 (data not shown). As shown in Figure 4B, endogenous CD8+ T cells from CD154−/− mice demonstrated substantial BrdU incorporation comparable to WT controls from day 6 to 14 (58.75 ± 5.92 vs. 52.33% ± 9.68, p = 0.61); however, the absolute number of dividing cells isolated from the allograft was decreased in CD154−/− hosts (0.56 × 105 ± 0.20 vs. 1.41 × 105 ± 0.59, p = 0.03). Given the decrease in intragraft T-cell numbers, we assessed cell proliferation in spleen and LN to determine whether a difference in systemic proliferation existed. As shown by a representative flow cytometry plot in Figure 4C, CD8+ T cells from both CD154−/− recipients and WT hosts demonstrated less BrdU incorporation in the spleen and LN compared to allografts. However, as shown in Figure 4D the mean frequencies of BrdU+ CD8+ T cells were similar between CD154−/− and WT recipients in both spleen (29.67 ± 4.32 vs. 28.75 ± 6.29, p = 0.91), and LN (8.11 ± 0.71 vs. 9.33 ± 1.61, p = 0.48), and were also associated with similar absolute numbers of CD8+ T cells in spleen (8.52 × 105 ± 2.66 × 105 vs. 8.38 × 105 ± 1.57 × 105, p = 0.48) and LN (2.29 × 106 ± 2.21 × 105 vs. 2.79 × 106 ± 0.95 × 105, p = 0.79). Collectively, our data show a significant uncoupling of proliferation from intragraft allospecific effector function in the absence of CD154/CD40 costimulation.
Figure 4. Intragraft CD8+ T-cell proliferation is uncoupled from effector function in CD154 deficiency.
(A) Tracer population of naïve congenic CD45.1+CD8+ T cells were labeled with CFSE and adoptively transferred into CD154−/− versus WT D0 airway allograft recipients. Day 14 graft mononuclear cells and LN were isolated; immediate CFSE dye dilution and IFN-γ secretion following 4-h ex vivo restimulation with BALB/c splenocytes was assessed on CD45.1+CD8+ T cells via surface and ICCS staining and flow cytometric analysis. Histograms display frequency of CFSE dilution with intragraft values displayed in top right corner; quadrant values represent detected frequencies of each population. (B) In separate experiments, recipient mice were administered BrdU from days 6 to 14 (see Materials and Methods). Day 14 allograft mononuclear cells were isolated and immediately surface stained for CD8 and BrdU incorporation followed by flow cytometric analysis. Bar graphs represent mean frequencies of CFSE dilution (transferred CD45.1+CD8+ cells), endogenous BrdU incorporation and absolute numbers of BRDU + CD8+ T cells ± SEM by day 14 graft CD8+ cells in WT versus CD154−/− allograft recipients, with p-values determined by rank sum test. (C) Histograms display frequency of BrdU incorporation expression by CD8+ T cells from allograft, splenocytes and LN for each treatment group, with percent of intragraft positive cells numerically displayed in upper right corner. (D) Bar graphs represent mean endogenous BrdU incorporation ± SEM by day 14 CD8+ T cells in spleen and LN in WT versus CD154−/− allograft recipients, with p-values determined by rank sum test. Results are from three separate experiments with pooled allograft cells from 3 to 4 mice per group.
Despite impaired effector function, CD8+ T cells from CD154−/− recipients display an activated, effector-memory phenotype
We next compared CD8+ T-cell activation, differentiation phenotypes in CD154-deficient and WT recipients to further examine alterations in CD8+ T-cell priming that could result in functional impairment. The reduction in allospecific CD8+ effector function in the absence of CD154/CD40 costimulation was not due to lack of early activation, as intragraft CD8+ T cells from CD154-deficient and WT allograft recipients expressed similarly high levels of CD69 (mean 62.30 ± 5.2% vs. 58.5 ± 3.77%, p = 0.49) (Figure 5A). Further phenotyping revealed that majority of intragraft CD8+ T cells in both groups expressed an effector memory phenotype with high expression of CD44hi (75.3 ± 4.35% vs. 81.52 ± 4.74%, p = 0.52), CD62Llow (77.15 ± 1.04% vs. 57.45 ± 10.81%, p = 0.10) and had similar upregulation of PD-1 (38.67 ± 6.14% vs. 45.67% ± 2.13%, p = 0.62). Having demonstrated similar activation, proliferative capacity and effector memory phenotypes, we next examined potential inhibitory cells by quantifying intragraft CD4+ regulatory T cells, which have been previously implicated in CD154 costimulatory blockade-mediated regulation of CD4+ T cells. As shown by a representative flow cytometry plot in 5c, the mean frequencies of CD4+CD25+Foxp3+ T cells were similar in both groups (mean 0.56% ± 0.21% vs. 0.58% ± 0.14% p = 0.48). Together, these data suggest that although intragraft CD8+ T cells from CD154−/− recipients undergo activation, proliferation and upregulation of effector-memory markers, there remains a critical defect in priming allospecific T-cell effector function sufficient to induce OAD.
Figure 5. Despite impaired effector function, CD8+ T cells from CD154−/− recipients display an activated, effector memory phenotype.
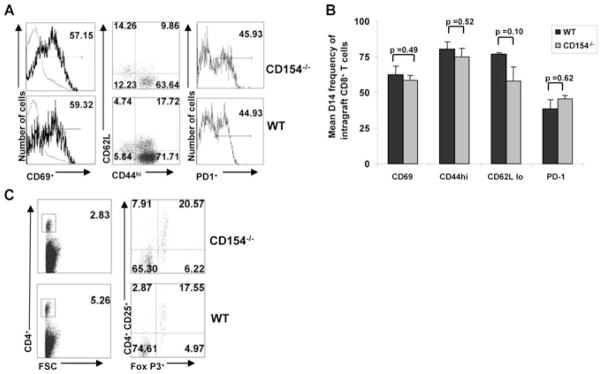
(A) Representative flow cytometry for CD69, CD44, CD62L and PD-1 surface expression in day 14 CD154−/− and WT control recipients in intragraft CD8+ T cells. Quadrant values represent detected frequencies of each population, gating on CD8+ T cells. In histograms, percent of intragraft positive CD8+ T cells are numerically displayed in upper right corner. (B) Mean CD69, CD44hi, CD62Llow and PD-1 expression in day 14 CD154−/− and WT control recipients. Bars represent mean frequencies ± SEM from each treatment group with p-values determined by rank sum test. Results are combined from minimum four separate experiments with pooled responses from 3 to 5 mice per group. (C) Representative flow cytometry for CD4, CD25, and intracellular Foxp3 expression, in day 14 CD154−/− and WT control recipients. Quadrant values represent detected frequencies of each population, gating on CD4+ T cells.
Discussion
Our studies demonstrate an important role for CD154/CD40 costimulation in the priming of allograft allospecific CD8+ T cells and murine OAD following airway transplant. While several prior studies have also reported an intact CD154/CD40 pathway to be critical for OAD development, our study is the first to show an uncoupling of allospecific CD8+ effector function from proliferation in graft-infiltrating T cells with disruption of the CD154/CD40 pathway (15,16). Moreover, these data show that transfer of CD8+ T cells primed in the presence of CD154/CD40 costimulation are sufficient to induce airway rejection in CD154−/− hosts, whereas CD8+ T cells primed in the absence of CD154/CD40 costimulation are incapable of inducing OAD pathology. Together, our findings underscore the important role of the CD154/CD40 pathway in CD8+ T-cell-mediated OAD, and are consistent with studies showing that while CD154/CD40 costimulation can readily impact other T-cell subsets, successful inhibition of allograft rejection does not appear to occur without impairment of allospecific CD8+ T cells (20–26,28).
The presence of CD8+ T cells in both WT and CD154−/− allografts is consistent with studies in a vascularized cardiac and non-human primate renal transplant model which found evidence of graft-infiltrating CD8+ T cells despite CD154/CD40 costimulation blockade, with the former study showing evidence of impaired CD8+ effector function (26,30). Our findings of high CD69 and PD-1 expression in intragraft CD154−/− CD8+ T cells suggest that these cells are highly activated and further express the CD44high and CD62Llow differentiation markers consistent with an ‘effector-memory’ phenotype, despite impairment in effector cytokine secretion and reduced expression of granzyme B. Thus, our data indicate that, although CD8+ T cells exposed to alloantigen in CD154 deficient environment undergo activation, CD154 deficiency impairs the development of polyfunctional allospecific effector responses.
Despite a reduction in the absolute number of airway allograft CD8+ T cells in CD154−/− hosts, we found similar proliferation between WT and CD154−/− recipients in intra-graft CD8+ T cells. To address these seemingly discordant findings, we examined systemic proliferation but did not detect differences between the two groups. One possibility is that systemic CD8+ T-cell proliferation is differentially reduced in the donor reactive subset of CD154−/− recipients; however, this subset is challenging to identify in a polyclonal model of airway allograft rejection. It is also possible that CD8+ T cells in CD154−/− hosts are being clonally deleted as has been implicated in studies in skin and bone marrow transplant models of CD154 costimulatory blockade (25,28,29). While we cannot fully exclude deletion, our findings of CD8 predominance in both WT and CD154−/− allografts suggest that a subset of alloreactive T cells is not deleted. However, given the reduced numbers of intragraft cells, it is possible that a subset of donor reactive T cells are deleted in systemic compartments and could reflect variability in the susceptibility to clonal deletion, as has been observed in a recent skin transplant model (40). An alternative explanation for reduced numbers is that fewer donor reactive T cells traffic to the allograft in the absence of CD154/CD40 interactions (41–43). Nonetheless, beyond a reduction in the number of CD8+ T cells, our data indicate that the uncoupling of CD8+ effector function from proliferation and activation results in a qualitative functional impairment associated with inhibition of OAD in CD154−/− recipients.
We found that transferred CD8+ T cells derived from WT allograft recipients, but not CD154−/− recipients or naïve WT CD8+ T cells, were sufficient to induce OAD in otherwise disease-resistant CD154−/− hosts. These data are consistent with studies in heterotopic and vascularized cardiac allografts in which CD8+ T-cell-mediated rejection from transferred cells was CD154-dependent (39,44). However, these findings also indicate there is not an ongoing requirement for the CD154/CD40 pathway in CD8+-mediated OAD development beyond a critical priming period and are consistent with previous reports that allospecific memory CD8+ T cells are resistant to CD154 costimulation-blockade (27,45). The similar numbers of regulatory T cells between CD154−/− and WT mice further suggest that the functional impairment in CD8+ T cells is due to ineffective priming rather than chronic regulation (25). This insufficient priming could be due to a combination of factors including inhibition of IL-12, costimulatory molecules (CD80/CD86) or other Type 1-polarizing molecules downstream of CD154/CD40 interactions (19,46). Finally, while our previous studies showed modest allospecific CD4+ T-cell responses in WT allografts, the inability of naïve WT CD8+ T cells to induce OAD upon transfer into CD154−/− recipients suggests that their priming is likely dependent on helper CD4+ T cells, the major source of CD154 (10).
There are several caveats to our studies. We conducted our studies to evaluate allospecific T-cell responses under polyclonal, fully MHC-mismatched conditions, as is typically the case in human lung transplantation. Although the heterotopic tracheal transplant model of murine OAD bears similarities to human OB pathology, these airway grafts are not directly vascularized lung tissue, yet they undergo rapid neovascularization (6,47). Furthermore, while vascularization of various allografts has not been predictive of CD8+ susceptibility to disruption of CD154/CD40 pathway, the mixed results seen among models might be due to other factors including graft tissue type, allospecific T-cell precursor frequencies and differing antigen presenting cell populations or cytokine milieus (21,26,39,48–50). Nevertheless, this well-established model is useful to study alloimmune mechanisms of airway obliteration in a controlled manner, in the absence of environmental exposures. Although one should exercise caution in extrapolating findings in the heterotopic tracheal transplant model to human OB, our studies do provide plausible cellular mechanisms that may be important in human disease (5,51).
In conclusion, our studies provide new insights into how CD154 deficiency disrupts CD8+ T-cell priming by uncoupling effector function from proliferation and activation, resulting in the inhibition of OAD. Our studies are consistent with previous reports showing that impairment of alloreactive CD8+ T cells is important in preventing allograft rejection across various models. Understanding the signals regulating allospecific CD8+ effector priming may improve future strategies aimed at allograft tolerance induction.
Acknowledgments
This work was supported by USPHS grants HL-068682 (JFM).
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest.
References
- 1.Hertz MI, Aurora P, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: Introduction to the 2007 annual reports–100 000 transplants and going strong. J Heart Lung Transplant. 2007;26:763–768. doi: 10.1016/j.healun.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Belperio JA, Weigt SS, Fishbein MC, Lynch JP., 3rd Chronic lung allograft rejection: Mechanisms and therapy. Proc Am Thorac Soc. 2009;6:108–121. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- 3.Wilkes DS, Egan TM, Reynolds HY. Lung transplantation: Opportunities for research and clinical advancement. Am J Respir Crit Care Med. 2005;172:944–955. doi: 10.1164/rccm.200501-098WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards DM, Dalheimer SL, Hertz MI, Mueller DL. Trachea allograft class I molecules directly activate and retain CD8+ T cells that cause obliterative airways disease. J Immunol. 2003;171:6919–6928. doi: 10.4049/jimmunol.171.12.6919. [DOI] [PubMed] [Google Scholar]
- 5.McDyer JF. Human and murine obliterative bronchiolitis in transplant. Proc Am Thorac Soc. 2007;4:37–43. doi: 10.1513/pats.200605-107JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hertz MI, Jessurun J, King MB, Savik SK, Murray JJ. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. Am J Pathol. 1993;142:1945–1951. [PMC free article] [PubMed] [Google Scholar]
- 7.Higuchi T, Maruyama T, Jaramillo A, Mohanakumar T. Induction of obliterative airway disease in murine tracheal allografts by CD8+ CTLs recognizing a single minor histocompatibility antigen. J Immunol. 2005;174:1871–1878. doi: 10.4049/jimmunol.174.4.1871. [DOI] [PubMed] [Google Scholar]
- 8.Higuchi T, Jaramillo A, Kaleem Z, Patterson GA, Mohanakumar T. Different kinetics of obliterative airway disease development in heterotopic murine tracheal allografts induced by CD4+ and CD8+ T cells. Transplantation. 2002;74:646–651. doi: 10.1097/00007890-200209150-00010. [DOI] [PubMed] [Google Scholar]
- 9.Kelly KE, Hertz MI, Mueller DL. T-cell and major histocompatibility complex requirements for obliterative airway disease in heterotopically transplanted murine tracheas. Transplantation. 1998;66:764–771. doi: 10.1097/00007890-199809270-00011. [DOI] [PubMed] [Google Scholar]
- 10.West EE, Lavoie TL, Orens JB, et al. Pluripotent allospecific CD8+ effector T cells traffic to lung in murine obliterative airway disease. Am J Respir Cell Mol Biol. 2006;34:108–118. doi: 10.1165/rcmb.2005-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elster EA, Xu H, Tadaki DK, et al. Treatment with the humanized CD154-specific monoclonal antibody, hu5C8, prevents acute rejection of primary skin allografts in nonhuman primates. Transplantation. 2001;72:1473–1478. doi: 10.1097/00007890-200111150-00001. [DOI] [PubMed] [Google Scholar]
- 12.Hancock WW, Sayegh MH, Zheng XG, Peach R, Linsley PS, Turka LA. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci U S A. 1996;93:13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markees TG, Phillips NE, Noelle RJ, et al. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation. 1997;64:329–335. doi: 10.1097/00007890-199707270-00026. [DOI] [PubMed] [Google Scholar]
- 14.Rumbley CA, Silver SJ, Phillips SM. Dependence of murine obstructive airway disease on CD40 ligand. Transplantation. 2001;72:1616–1625. doi: 10.1097/00007890-200111270-00007. [DOI] [PubMed] [Google Scholar]
- 15.Chalermskulrat W, McKinnon KP, Brickey WJ, et al. Combined donor specific transfusion and anti-CD154 therapy achieves airway allograft tolerance. Thorax. 2006;61:61–67. doi: 10.1136/thx.2005.047316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez FG, McKane B, Marshbank S, Patterson GA, Mohanakumar T. Inhibition of obliterative airway disease development following heterotopic murine tracheal transplantation by costimulatory molecule blockade using anti-CD40 ligand alone or in combination with donor bone marrow. J Heart Lung Transplant. 2005;24(7 Suppl):S232–S238. doi: 10.1016/j.healun.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 18.McDyer JF, Goletz TJ, Thomas E, June CH, Seder RA. CD40 ligand/CD40 stimulation regulates the production of IFN-gamma from human peripheral blood mononuclear cells in an IL-12- and/or CD28-dependent manner. J Immunol. 1998;160:1701–1707. [PubMed] [Google Scholar]
- 19.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ensminger SM, Witzke O, Spriewald BM, et al. CD8+ T cells contribute to the development of transplant arteriosclerosis despite CD154 blockade. Transplantation. 2000;69:2609–2612. doi: 10.1097/00007890-200006270-00022. [DOI] [PubMed] [Google Scholar]
- 21.Guo Z, Meng L, Kim O, et al. CD8 T cell-mediated rejection of intestinal allografts is resistant to inhibition of the CD40/CD154 costimulatory pathway. Transplantation. 2001;71:1351–1354. doi: 10.1097/00007890-200105150-00033. [DOI] [PubMed] [Google Scholar]
- 22.Jones ND, Van Maurik A, Hara M, et al. CD40-CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J Immunol. 2000;165:1111–1118. doi: 10.4049/jimmunol.165.2.1111. [DOI] [PubMed] [Google Scholar]
- 23.Lunsford KE, Jayanshankar K, Eiring AM, et al. Alloreactive (CD4-Independent) CD8+ T cells jeopardize long-term survival of intra-hepatic islet allografts. Am J Transplant. 2008;8:1113–1128. doi: 10.1111/j.1600-6143.2008.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trambley J, Bingaman AW, Lin A, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fehr T, Takeuchi Y, Kurtz J, Wekerle T, Sykes M. Early regulation of CD8 T cell alloreactivity by CD4+CD25− T cells in recipients of anti-CD154 antibody and allogeneic BMT is followed by rapid peripheral deletion of donor-reactive CD8+ T cells, precluding a role for sustained regulation. Eur J Immunol. 2005;35:2679–2690. doi: 10.1002/eji.200526190. [DOI] [PubMed] [Google Scholar]
- 26.Zhai Y, Shen XD, Gao F, et al. The CD154-CD40 T cell costimulation pathway is required for host sensitization of CD8(+) T cells by skin grafts via direct antigen presentation. J Immunol. 2002;169:1270–1276. doi: 10.4049/jimmunol.169.3.1270. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z, Wang Y, Gao F, Shen X, Zhai Y, Kupiec-Weglinski JW. Critical role of CD4 help in CD154 blockade-resistant memory CD8 T cell activation and allograft rejection in sensitized recipients. J Immunol. 2008;181:1096–1102. doi: 10.4049/jimmunol.181.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol. 2000;164:512–521. doi: 10.4049/jimmunol.164.1.512. [DOI] [PubMed] [Google Scholar]
- 29.Haspot F, Fehr T, Gibbons C, et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008;112:2149–2155. doi: 10.1182/blood-2007-12-127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 31.Jarvinen LZ, Blazar BR, Adeyi OA, Strom TB, Noelle RJ. CD154 on the surface of CD4+CD25+ regulatory T cells contributes to skin transplant tolerance. Transplantation. 2003;76:1375–1379. doi: 10.1097/01.TP.0000093462.16309.73. [DOI] [PubMed] [Google Scholar]
- 32.Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, Noelle RJ. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: The interplay of clonal anergy and immune regulation. J Immunol. 2005;175:771–779. doi: 10.4049/jimmunol.175.2.771. [DOI] [PubMed] [Google Scholar]
- 33.Banuelos SJ, Markees TG, Phillips NE, et al. Regulation of skin and islet allograft survival in mice treated with costimulation blockade is mediated by different CD4+ cell subsets and different mechanisms. Transplantation. 2004;78:660–667. doi: 10.1097/01.tp.0000130449.05412.96. [DOI] [PubMed] [Google Scholar]
- 34.van Maurik A, Herber M, Wood KJ, Jones ND. Cutting edge: CD4+CD25+ alloantigen-specific immunoregulatory cells that can prevent CD8+ T cell-mediated graft rejection: Implications for anti-CD154 immunotherapy. J Immunol. 2002;169:5401–5404. doi: 10.4049/jimmunol.169.10.5401. [DOI] [PubMed] [Google Scholar]
- 35.Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 36.Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc. 2006;1:1507–1516. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
- 37.Belperio JA, Keane MP, Burdick MD, et al. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–556. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 39.Filatenkov AA, Jacovetty EL, Fischer UB, Curtsinger JM, Mescher MF, Ingulli E. CD4 T cell-dependent conditioning of dendritic cells to produce IL-12 results in CD8-mediated graft rejection and avoidance of tolerance. J Immunol. 2005;174:6909–6917. doi: 10.4049/jimmunol.174.11.6909. [DOI] [PubMed] [Google Scholar]
- 40.Brehm MA, Mangada J, Markees TG, et al. Rapid quantification of naive alloreactive T cells by TNF-alpha production and correlation with allograft rejection in mice. Blood. 2007;109:819–826. doi: 10.1182/blood-2006-03-008219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thienel U, Loike J, Yellin MJ. CD154 (CD40L) induces human endothelial cell chemokine production and migration of leukocyte subsets. Cell Immunol. 1999;198:87–95. doi: 10.1006/cimm.1999.1583. [DOI] [PubMed] [Google Scholar]
- 42.Sitati E, McCandless EE, Klein RS, Diamond MS. CD40-CD40 ligand interactions promote trafficking of CD8+ T cells into the brain and protection against West Nile virus encephalitis. J Virol. 2007;81:9801–9811. doi: 10.1128/JVI.00941-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogel JD, West GA, Danese S, et al. CD40-mediated immune-nonimmune cell interactions induce mucosal fibroblast chemokines leading to T-cell transmigration. Gastroenterology. 2004;126:63–80. doi: 10.1053/j.gastro.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 44.Zhai Y, Meng L, Busuttil RW, Sayegh MH, Kupiec-Weglinski JW. Activation of alloreactive CD8+ T cells operates via CD4-dependent and CD4-independent mechanisms and is CD154 blockade sensitive. J Immunol. 2003;170:3024–3028. doi: 10.4049/jimmunol.170.6.3024. [DOI] [PubMed] [Google Scholar]
- 45.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: Therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169:4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 46.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: Dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166:440–444. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 48.Wilkes DS, Thompson LK, Cummings OW, Bragg S, Heidler KM. Instillation of allogeneic lung macrophages and dendritic cells cause differential effects on local IFN-gamma production, lymphocytic bronchitis, and vasculitis in recipient murine lungs. J Leukoc Biol. 1998;64:578–586. doi: 10.1002/jlb.64.5.578. [DOI] [PubMed] [Google Scholar]
- 49.Ford ML, Koehn BH, Wagener ME, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones ND, Turvey SE, Van Maurik A, et al. Differential susceptibility of heart, skin, and islet allografts to T cell-mediated rejection. J Immunol. 2001;166:2824–2830. doi: 10.4049/jimmunol.166.4.2824. [DOI] [PubMed] [Google Scholar]
- 51.Deuse T, Schrepfer S, Reichenspurner H, et al. Techniques for experimental heterotopic and orthotopic tracheal transplantations –When to use which model? Transpl Immunol. 2007;17:255–261. doi: 10.1016/j.trim.2007.01.009. [DOI] [PubMed] [Google Scholar]