Abstract
Acute cellular rejection (ACR) is a common and important clinical complication following lung transplantation. While there is a clinical need for the development of novel therapies to prevent ACR, the regulation of allospecific effector T cells in this process remains incompletely understood. Using the MHC-mismatched mouse orthotopic lung transplant model, we investigated the short-term role of anti-CD154 mAb therapy alone on allograft pathology and alloimmune T cell effector responses. Untreated C57BL/6 recipients of BALB/c left lung allografts had high-grade rejection and diminished CD4+:CD8+ graft ratios, marked by predominantly CD8+>CD4+ IFN-γ+ allospecific effector responses at day 10, compared to isograft controls. Anti-CD154 mAb therapy strikingly abrogated both CD8+ and CD4+ alloeffector responses and significantly increased lung allograft CD4+:CD8+ ratios. Examination of graft CD4+ T cells revealed significantly increased frequencies of CD4+CD25+Foxp3+ regulatory T cells in the lung allografts of anti-CD154-treated mice and was associated with significant attenuation of ACR compared to untreated controls. Together, these data show that CD154/CD40 costimulation blockade alone is sufficient to abrogate allospecific effector T cell responses and significantly shifts the lung allograft toward an environment predominated by CD4+ T regulatory cells in association with an attenuation of ACR.
Keywords: CD154, Mouse Orthotopic Lung Transplantation, Effector T Cell, Allograft Rejection, Regulatory T Cell
INTRODUCTION
Lung transplantation is the final therapeutic option for select patients with various end-stage lung diseases, though its long-term success is limited by the bronchiolitis obliterans syndrome (BOS) or chronic allograft rejection (1–3). Despite the use of broad immunosuppression, at least one episode of acute cellular rejection (ACR) of the lung allograft occurs in over 50% of lung transplant recipients, and is recognized as a major risk factor for the development of BOS (4). While evidence supports an important role for T cells in ACR, T cell effector mechanisms and their regulation in this process remain incompletely understood. The recent development of the orthotopic mouse lung transplantation model has been a significant advancement in the field, and enables studies in the immuno-pathogenesis of ACR, as well as mechanistic studies in lung allograft tolerance (5).
The CD154/CD40 costimulation pathway has been a focus of investigation since early studies demonstrated the potent efficacy of its blockade in establishing long-term allograft acceptance across multiple experimental transplant models (6–9). Activated CD4+ T cells are a major source of CD154 (CD40L), and their interaction with CD40 on antigen presenting cells is important in the development of Th1 responses (10, 11). While several studies have shown that CD154/CD40 blockade impairs CD4+ T cell-dependent rejection, the role of this pathway in CD8+ T cell regulation is more variable with several reports of costimulation blockade-resistant CD8+ T cell-mediated rejection (12–16). Early seminal studies by Wells et al. (17) and Li et al. (18) revealed an important role for activation-induced cell death in prolonged graft survival following costimulation blockade using anti-CD154 and cytotoxic T lymphocyte antigen 4-fusion protein (CTLA4-Ig). Subsequent work by Graca et al., showed that anti-CD154-induced tolerance could not be broken by the adoptive transfer of non-tolerant naïve cells, and provided evidence for infectious transplant tolerance through an unknown regulatory population (19). Studies by several groups later described a critical role for CD4+CD25+ regulatory T cells in the induction of tolerance to alloantigen (20, 21). Recently, costimulation blockade with anti-CD154 and CTLA4-Ig has been shown to attenuate acute cellular rejection pathology following MHC-mismatched mouse orthotopic lung transplant (22). In this study, we evaluated the effects of CD154 blockade alone on allospecific T cell responses following mouse orthotopic lung transplant. Herein, we report that CD154 blockade abrogates both CD8+ and CD4+ allospecific effector T cell responses and strikingly enhances intragraft CD4+CD25+Foxp3+ regulatory T cells.
MATERIALS AND METHODS
Mice
The Johns Hopkins University Institutional Animal Care and Use Committee approved all animal protocols. Pathogen-free male C57BL/6 (I-ab, H-2b) and BALB/c (I-ad, H-2d) mice 25–30g were purchased from Charles River (Wilmington, MA). All mice were housed in the Johns Hopkins University animal facilities under specific pathogen-free conditions, and experiments conducted under a protocol approved by the Johns Hopkins Animal Care and Use Committee.
Orthotopic Lung Transplant
Syngeneic transplantations were performed in the C57BL/6 → C57BL/6 strain combination and allogeneic transplantations were performed in the BALB/c → C57BL/6 strain combinations. Donor mice were sedated with etomidate (1 mg, intraperitoneal), intubated, and maintained on inhaled isoflurane until sacrifice. Recipients were both initially sedated and maintained on inhaled isoflurane. Transplantation was performed using a cuffed technique as previously described (23). Mice received subcutaneous buprenorphine (0.03–0.05 mg/kg) prior to extubation and every 6 hrs thereafter as needed. Unless otherwise specificed, animals were sacrificed for analysis at 10 days posttransplant.
Medium and Reagents
Cell culture medium RPMI 1640 (Biofluids, Rockville, MD) was supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO), 2mM glutamine, 1mM Sodium Pyruvate, 1% NEAA, 100 U/ml Penicillin, 100 MGC/ml Streptomycin, 50µM β-mercaptoethanol, and 25mM HEPES (Biofluids, Rockville, MD).
Cell Preparations, Stimulation, and Cytokine Detection
Spleen, draining lymph nodes (LN), and lungs were harvested from mice on day 10 after transplant. Lungs were minced and incubated at 37°C in an enzyme cocktail of RPMI containing 2.4 mg/ml of Collagenase I and 20µg/ml DNAse (Invitrogen, Carlsbad, CA), then mashed through a 70µm nylon cell strainer (BD Falcon). A 23% and 70% bilayer Percoll (Amersham Biosciences, Uppsala, Sweden) gradient was performed, and the interface collected. Isolated responder cells from allografts, LN, spleen, or lung mononuclear cells (LMNC) were cultured for 5h in medium alone, with BALB/c splenocytes (1:1), or in the presence of phorbol myristate acetate/ionomycin (PMA/ionomycin) (50ng/ml/500ng/ml) with brefeldin A (10 µg/ml; Sigma) present for the final 2h of stimulation. Following stimulation, cells were harvested for surface and intracellular staining.
Flow Cytometry
The following antibodies were purchased from BD PharMingen (San Diego, CA) or Ebioscience: Phycoerythrin (PE)-labeled anti-IL-17A; Allophycocyanin (APC)-labeled anti-IFN-γ; FITC-labeled anti-TNF-α; Peridinin-chlorophyll-protein complex (PerCP) Cy5.5-labeled anti-CD8; Alexa-700-labeled anti-CD4; Biotinylated anti-H2Dd; Pacific Blue-labeled streptavidin; APC-labeled Foxp3; PE-labeled anti-CD25; and respective isotype Abs. Surface antibody staining and intracellular cytokine staining (ICCS) was performed as previously described (36, 37). Flow cytometry analysis was performed using a FACSAria instrument and Flowjo software for analysis (Tree Star Inc, San Carlos, CA).
Histopathology and Acute Rejection Pathology Scoring
Grafts were fixed in 10% formalin, embedded in paraffin, sectioned and stained using Hematoxylin & Eosin. Hematoxylin and Eosin stained sections of grafts were scored by 3 blinded observers using a 5 point system developed to grade the severity of rejection. One point was given for the presence of each of the following characteristics: (1) Perivascular mononuclear cell infiltrate, (2) Peribronchial mononuclear cell infiltrate, (3) Interstitial inflammatory infiltrate, (4) Alveolar inflammatory infiltrate, (5) Hemorrhage/parenchymal necrosis. The acute rejection score is the sum of the points given (range 0–5).
Statistical analysis
Data were compared with two-tailed student’s t-test using Graphpad software. A p value of less than 0.05 was considered statistically significant.
RESULTS
Acute rejection in MHC-mismatched murine orthotopic lung allografts is associated with a decreased CD4:CD8 ratio in infiltrating lymphocytes
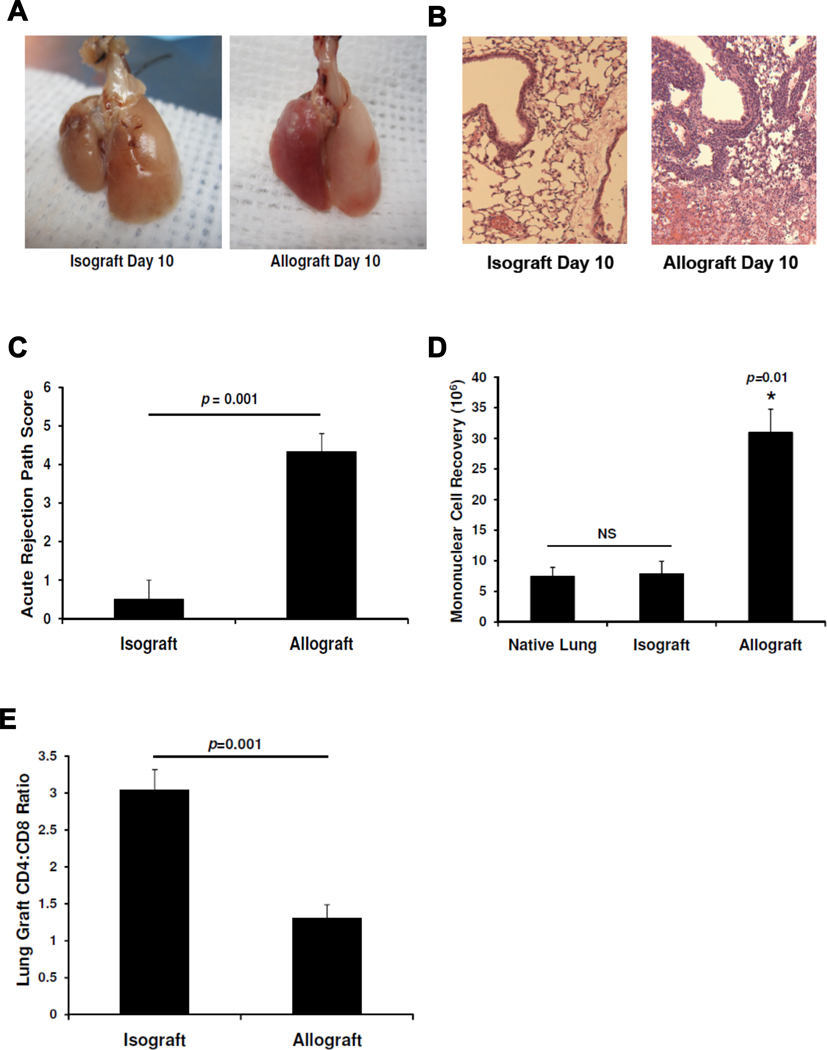
To evaluate the adaptive T cell response during acute rejection of murine orthotopic lung allografts, we compared graft pathology and T cell infiltration in C57BL/6 recipients of C57BL/6 [H-2b] isografts and BALB/c [H-2d] allografts. At day 10, allogeneic lung allografts demonstrated severe lung injury on gross pathology in striking contrast to syngeneic lung isografts (Fig 1A). Allogeneic allografts had massive mononuclear cell infiltration surrounding vessels and airways with inflammation extending into the interstitium and alveolar spaces and evidence of hemorrhage and necrosis often present, in striking contrast to isografts (Fig 1B). There was a significant difference in acute rejection scores at day 10 (Fig 1C). We isolated lung mononuclear cells and found a significant four-fold increase in the mean recovery of mononuclear cells from day 10 allografts compared to isografts or the native lungs of allograft recipients (Fig 1D). We next characterized the T cell subsets in lung grafts using flow cytometry and found a significant reduction in the CD4:CD8 ratio in allografts compared to isografts (Fig 1E). Together, these data show quantitative and qualitative differences in the T cell populations between lung allografts and isografts 10 days following transplantation.
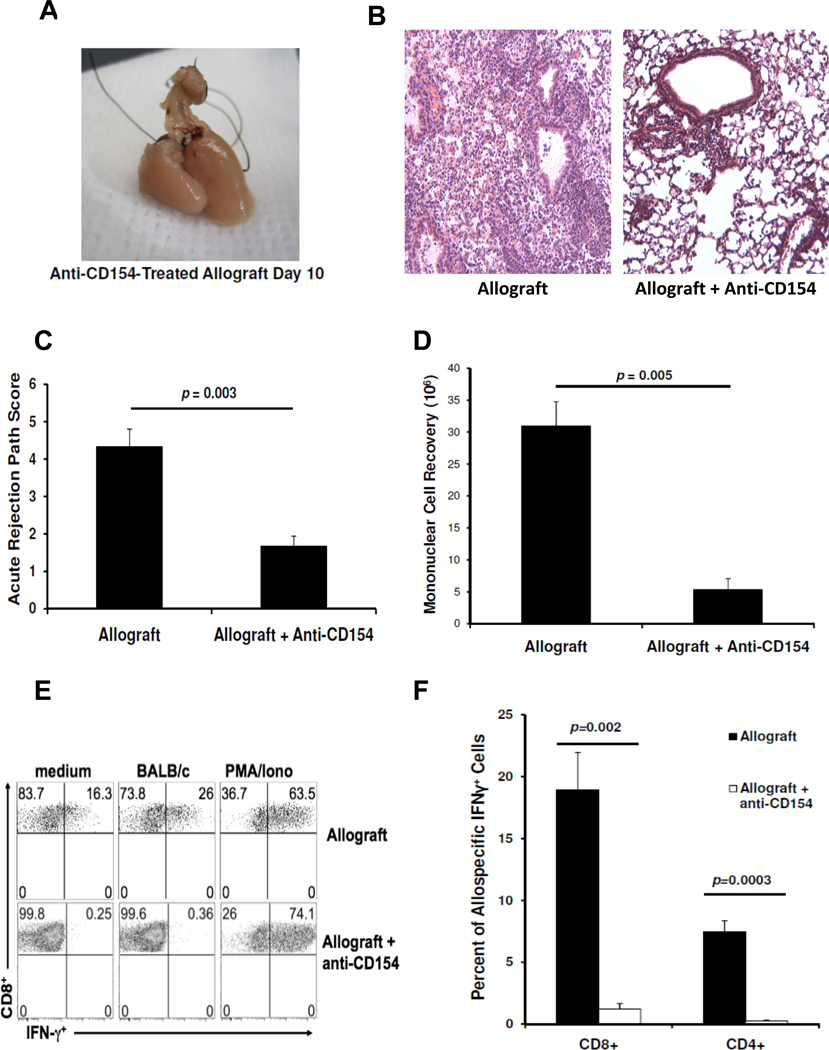
Figure 1. Acute cellular rejection following MHC-mismatched orthotopic lung transplant is associated with a decreased graft CD4:CD8 ratio.
(A) Gross pathology of BALB/c → B6 allograft and B6 →B6 isograft (graft left in photos) and (B) lung histology with H&E staining (10×) at day 10. (C) Acute rejection pathology score means at day 10 in isografts versus allografts (n=4 mice per group). (D) Comparison of mean mononuclear cell recovery at day 10 in isografts versus allografts (n=3–8 mice per group). (E) Mean lung graft CD4:CD8 ratio determined by flow cytometry in isografts versus allografts (n=3–8 mice per group).
Allospecific CD8+IFN-γ+ effector T cell responses predominate during acute cellular rejection in MHC-mismatched murine orthotopic lung allografts
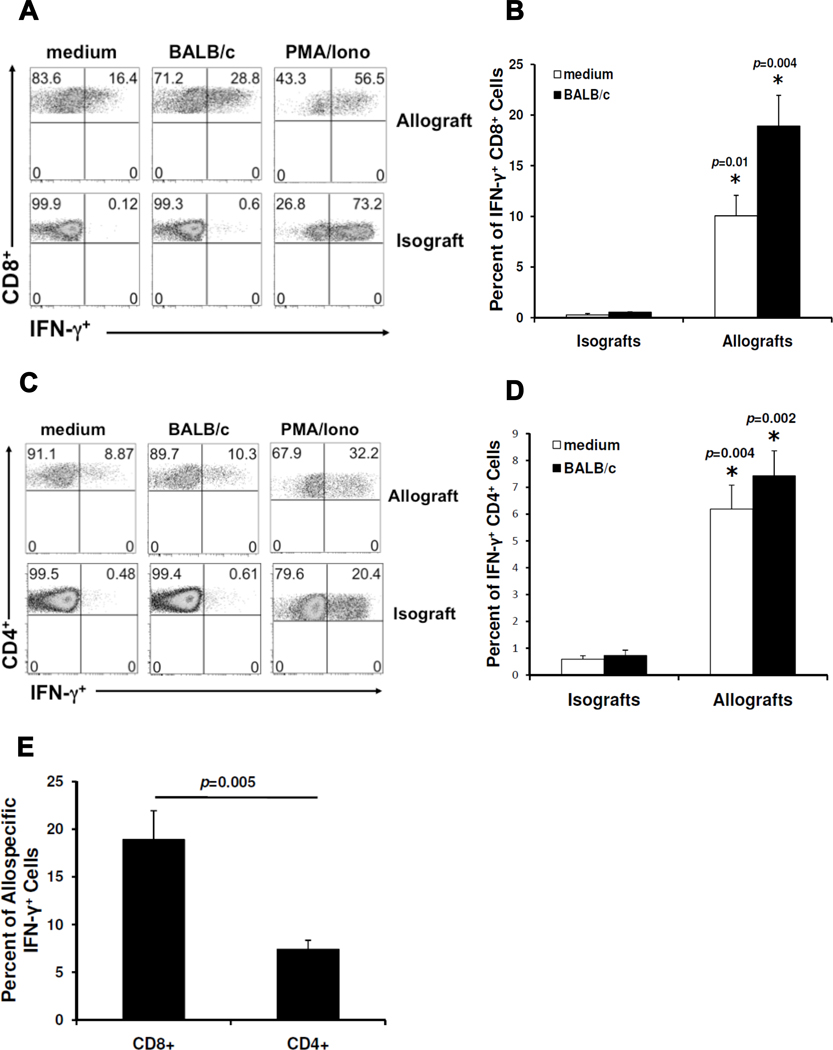
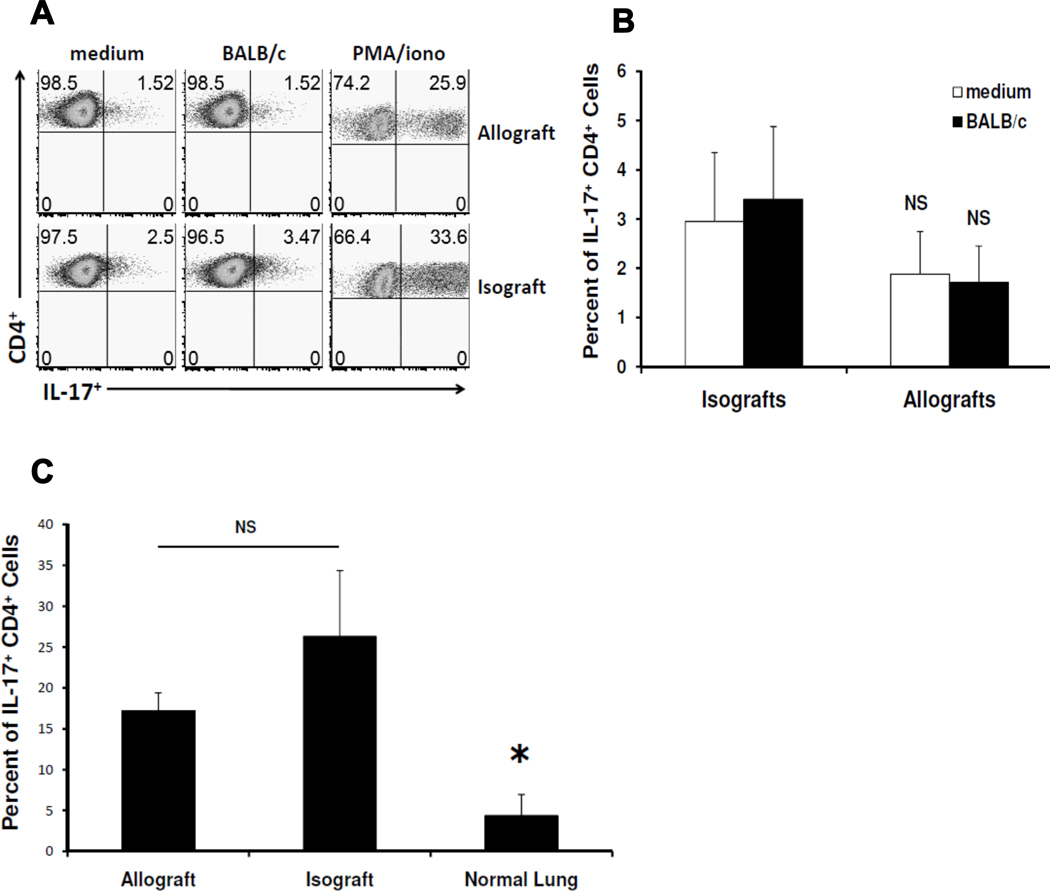
Next, we evaluated lung allograft T cells for allospecific cytokine responses. CD8+ T cells spontaneously secreting the type 1 effector cytokine IFN-γ were detectable in lung allografts. In vitro re-stimulation with BALB/c splenocytes dramatically increased the percentage of CD8+ T cells from lung allografts secreting IFN-γ. These findings are in striking contrast to CD8+ T cells from isografts, which rarely produced IFN-γ spontaneously or after in vitro re-stimulation with BALB/c alloantigen (Fig 2A, B), but had similar percentages of IFN-γ+ cells in response to PMA/ionomycin re-stimulation (Fig 2A). Constitutive production of IFN-γ could also be detected in CD4+ T cells from lung allografts but only modestly increased with alloantigen re-stimulation (Fig 2C, D). IFN-γ production from CD4+ T cells was nevertheless significantly increased in allografts compared to isografts, both constitutively and following re-stimulation (Fig 2D). Comparison of CD8+ and CD4+ allospecific responses (after restimulation with alloantigen) demonstrated that CD8+IFN-γ+ responses predominated during acute cellular rejection of lung allografts (Fig 2E). We also detected low frequencies of allospecific CD8+TNF-α+ cells in lung allografts, but were unable to detect allospecific IL-2 production in CD4+ or CD8+ T cells (data not shown). Finally, we were unable to detect allospecific IL-17 production by CD4+ T cells (Fig 3A, B) or CD8+ T cells (data not shown). Lung mononuclear cell cultures from allografts and isografts stimulated with PMA/Ionomycin had similar frequencies of polyclonal CD4+IL-17+ cells, but these were significantly higher than age-matched littermate controls who did not undergo lung transplant surgery (Fig 3C). Together, these data indicate that CD8+IFN-γ+ T cells are the predominant allospecific effector responses during acute cellular rejection in MHC-mismatched murine orthotopic lung allografts, though CD4+ T cells also contribute allospecific effector responses.
Figure 2. Allospecific CD8+IFN-γ+ effector responses predominate over CD4+ responses in MHC-mismatched orthotopic lung allografts.
(A) Representative day 10 flow plots of lung allograft or isograft mononuclear cells cultured for 5h in medium alone, in the presence of BALB/c splenocytes (1:1 ratio), or with PMA/ionomycin; gating on H-2d negative, CD8+ T cells in mixed cultures. (B) Cumulative day 10 mean CD8+ allospecific IFN-γ responses (expressed as percent of CD8+ cells positive for IFN-γ) in allografts versus isografts (n=3–6 mice per group). (C) Representative day 10 flow plots of lung allograft or isograft mononuclear cells with the same culture conditions and analysis as in (A); gating on H-2d negative, CD4+ T cells in mixed cultures. (D) Cumulative day 10 mean CD4+ allospecific IFN-γ responses (expressed as percent of CD4+ cells positive for IFN-γ) in allografts versus isografts (n=3–6 mice per group). (E) Comparison of day 10 mean CD8+ and CD4+ allospecific IFN-γ responses in allografts after 5h culture of lung mononuclear cells with BALB/c splenocytes (n=6 mice).
Figure 3. Lung transplant surgery is sufficient for the induction of polyclonal CD4+IL-17+ T cells.
(A) Representative flow plots of CD4+ IL-17 responses at day 10 in lung graft mononuclear cells cultured for 5h in medium alone, in the presence of BALB/c splenocytes (1:1 ratio), or with PMA/ionomycin; gating performed on H-2d negative, CD4+ T cells in mixed cultures. (B) Cumulative day 10 mean CD4+IL-17+ responses (expressed as percent of CD4+ cells positive for IL-17) from isografts versus allografts after culture of lung mononuclear cells in the presence or absence of BALB/c splenocytes (n=3–5 mice per group). (C) Cumulative day 10 mean polyclonal CD4+IL-17+ responses (expressed as percent of CD4+ cells positive for IL-17) from isografts, allografts, and left lungs of littermates receiving no transplant after culture of lung mononuclear cells with PMA/ionomycin.
Allospecific effector T cell responses are detectable in secondary lymphoid tissue and the native lung following murine orthotopic lung transplantation
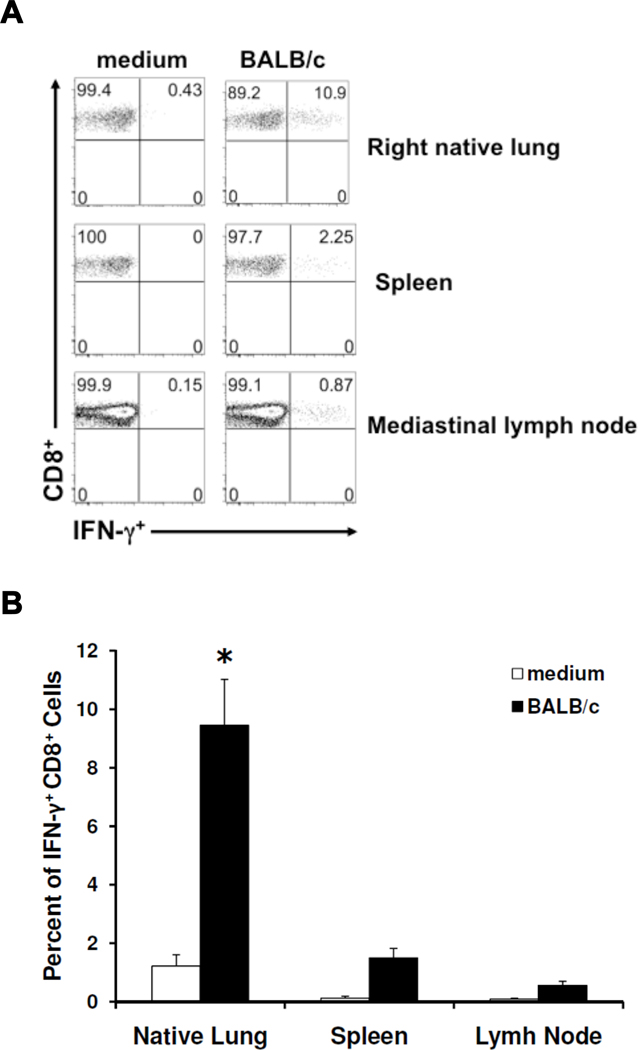
In the same experiments, we determined whether allospecific effector T cell responses were detectable in other systemic tissues at day 10. The predominant CD8+IFN-γ+ allospecific response was consistently detectable in the mediastinal lymph node and spleen, as well as the native lung, shown in Fig 4A. Moreover, allospecific responses in the native lung were detectable at significantly higher frequencies (Fig 4B). In contrast, CD4+IFN-γ+ alloresponses were not consistently detectable in these tissues (data not shown).
Figure 4. Systemic allospecific effector responses are detectable following orthotopic lung transplant.
(A) Representative flow plots of CD8+ IFN-γ responses at day 10 in mononuclear cells isolated from the native lung, spleen, and mediastinal lymph node of transplanted mice, cultured for 5h in the presence or absence of BALB/c splenocytes (1:1 ratio); gating performed on H-2d negative, CD8+ T cells in mixed cultures. (B) Cumulative day 10 mean allospecific CD8+ IFN-γ responses (expressed as percent of CD8+ cells positive for IFN-γ) from the native lung, spleen, and mediastinal lymph node of mice receiving allografts (n=4–6 mice per group). (p=0.0006 when comparing means of native lung and spleen, and p=0.002 when comparing means of native lung and lymph node.)
Anti-CD154 costimulation blockade attenuates acute rejection pathology and abrogates CD8+ and CD4+ allospecific effector responses
Because the CD154/CD40 costimulation pathway has been shown to play an important role in allograft rejection, we evaluated orthotopic lung allografts following treatment with anti-CD154 Ab therapy (500 µg i.p. at days 0 and 2). At day 10, the gross appearance of lung allografts from anti-CD154 Ab-treated recipients had significantly less hemorrhagic injury (Fig 5A). Histology revealed remarkable preservation of lung architecture in the allografts of anti-CD154 Ab-treated mice compared to untreated allografts, though some mononuclear cell infiltration primarily surrounding vessels persisted (Fig 5B). There was a significant decrease in the acute rejection scores and mean mononuclear cell recovery in allografts from anti-CD154 Ab-treated mice compared to untreated allografts (Fig 5C, 5D). Next, we evaluated whether these histologic changes were associated with differences in allospecific T cell responses at day 10. The predominant lung allograft CD8+IFN-γ+ allospecific responses were abrogated in anti-CD154 Ab-treated recipients compared to untreated allograft recipients, as well as allograft CD4+IFN-γ+ allospecific T cell responses (Fig 5E, F). In contrast, anti-CD154 Ab therapy had no effect on PMA/ionomycin-induced CD8+IFN-γ+ responses. Together, these data indicate a critical role for the CD154/CD40 pathway in the regulation of T cell allospecific effector responses following MHC-mismatched mouse orthotopic lung transplantation.
Figure 5. Anti-CD154 Ab treatment attenuates acute cellular rejection and abrogates CD8+ and CD4+ allospecific effector responses following mouse orthotopic lung transplant.
(A) Representative day 10 gross pathology of an allograft from an anti-CD154 Ab-treated mouse and (B) lung allograft histology of untreated and anti-CD154 Ab-treated mice. (C) Acute rejection pathology score means in allografts of untreated versus anti-CD154 treated mice (n=4 mice per group). (D) Comparison of mean mononuclear cell recoveries at day 10 in untreated versus anti-CD154 Ab-treated mice (n=4–6 per group). (E) Representative flow plots of day 10 CD8+ IFN-γ responses in lung mononuclear cells from allograft of untreated mouse versus anti-CD154 Ab -treated mouse after 5h culture conditions of medium alone, in the presence of BALB/c splenocytes (1:1 ratio), or with PMA/ionomycin; gating on H-2d negative, CD8+ T cells in mixed cultures. (F) Cumulative mean allospecific CD8+ and CD4+ IFN-γ responses (expressed as percent of cells positive for IFN-γ) from lung allografts of both groups after 5h culture of lung mononuclear cells with BALB/c splenocytes (n=4–6 mice per group).
Anti-CD154 costimulation blockade increases the CD4:CD8 ratio and the frequency of CD4+CD25+Foxp3+ T cells in murine orthotopic lung allografts
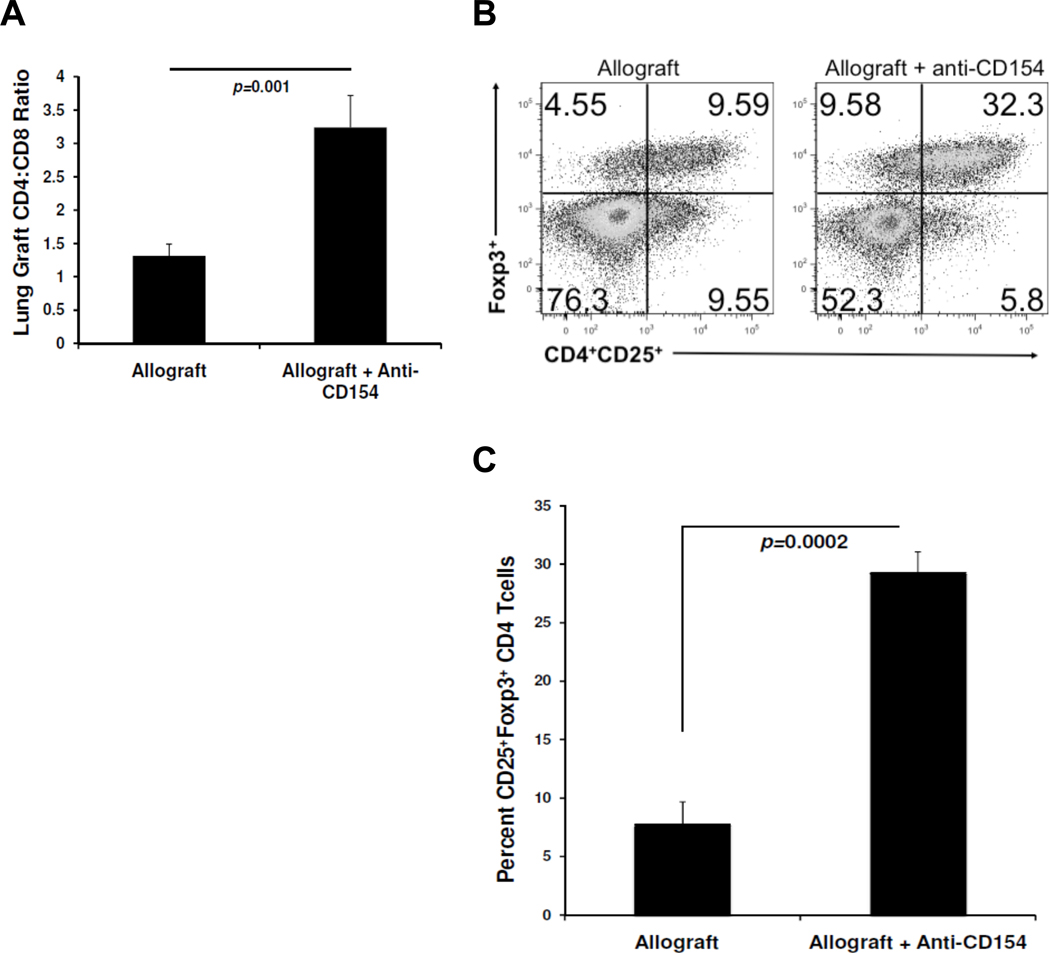
We next evaluated the lung CD4:CD8 ratio in anti-CD154 Ab-treated allograft recipients compared to untreated lung allograft recipients. As shown in Fig 6A, we observed significantly increased CD4:CD8 ratios in lung allografts from anti-CD154-treated recipients compared to recipients receiving no treatment. We then examined whether there were differences in the frequencies of CD4+ T cells with a regulatory phenotype, i.e. CD4+CD25+Foxp3+ T cells between these groups. We found that lung allografts from anti-CD154-treated mice contained significantly increased frequencies of CD25+Foxp3+ cells as a percent of total CD4+ T cells compared to control mice (Fig 6B, 6C).
Figure 6. Anti-CD154 Ab treatment increases the lung allograft CD4:CD8 ratio and enhances CD4+CD25+Foxp3+ T regulatory cells.
(A) Mean lung allograft CD4:CD8 ratio determined by flow cytometry in anti-CD154-treated mice versus untreated mice (n=4 mice per group). (B) Representative flow plot of CD4+CD25+Foxp3+ T cells in lung allografts from anti-CD154-treated mice versus untreated mice; gating on CD4+ cells (n=4 mice per group). (C) Cumulative mean frequency of CD25+Foxp3+cells as a percent of CD4+ T cells in lung allografts from anti-CD154-treated versus untreated mice (n=4 mice per group).
DISCUSSION
The regulation of allospecific T cell effector responses following solid organ transplant remains incompletely understood. Our studies show that CD8+ T cells are the predominant allospecific responders in MHC-mismatched (BALB/c → B6) mouse orthotopic lung allograft rejection, with these cells producing predominantly IFN-γ in response to alloantigen. Our results are consistent with previous studies in the mouse orthotopic lung transplant model showing a high percentage of CD8+ T cells have the capacity for IFN-γ following re-stimulation using the calcium ionophore, PMA/ionomycin (5, 24). It should be pointed out however, that PMA/ionomycin-induced responses are not specific to allograft recipients, given that we found CD8+ T cells from isografts also demonstrate a high capacity for IFN-γ production under these re-stimulation conditions. In contrast, we show that constitutive IFN-γ responses and increased IFN-γ responses in response to re-stimulation with BALB/c alloantigen were very specific to allograft recipients. It is not surprising, however, that CD8+ T cells (and CD4+ T cells) produced constitutive IFN-γ in allografts, as these allograft-derived cultures already contain some alloantigen (25). Our studies also demonstrate a decrease in the CD4:CD8 ratio in day 10 lung allografts compared to isografts due to increased numbers of CD8+ T cells. Our results are consistent with the report by Gelman et al. that showed CD8+ T cells outnumbering CD4+ T cells in mouse lung allografts despite differences in the timing (day 7) and mouse strains used (B6 → CBA) (24). Our current results are also similar to our previous report in the murine heterotopic tracheal transplant model of predominant CD8+IFN-γ+ allospecific effectors at the peak of inflammation (25). Together, our results suggest a predominant role for CD8+ allospecific effector T cells in the acute rejection response following MHC-mismatched mouse lung transplant.
We also found CD4+ allospecific IFN-γ responses in the lung allograft indicating these cells are capable of participating in the alloimmune response, albeit at lower frequencies compared to CD8+ T cells. While a report by Gelman et al. reported CD4+ T cells were not necessary for acute cellular lung rejection mediated by CD8+ T cells using CD4-depletion and CD4-deficient mice in this model (24), allospecific CD4+ responses were not evaluated in that study. As activated CD4+ T cells are a major source of CD154 costimulation in providing ‘help’, it is possible that CD8+ T cell-mediated rejection in the absence of CD4+ T cells may be facilitated by other sources of CD154, such as activated platelets (26). Alternatively, CD4+ T cell deficiency may actually promote acute cellular rejection in the absence of regulatory T cells. Thus, the role(s) of CD4+ T cells in allogeneic mouse othotopic lung transplant need to be more fully elucidated.
Because IL-17 production has been implicated in chronic lung allograft rejection in humans (27), we asked whether it was produced in response to alloantigen. While we were unable to detect allospecific IL-17 at day 10 (or day 21), approximately 17–35% of CD4+ T cells in isografts and allografts had the capacity to secrete IL-17 in response to PMA/Ionomycin stimulation. We found that the lung transplant surgery alone was sufficient to induce these polyclonal IL-17 responses, which were increased compared to lower responses found in control mice that had not undergone surgery. While the explanation for this finding remains unclear at this time, it is possible that surgery-related injury could unmask autoantigens capable of driving IL-17 induction in rat lung isografts (28). Together, these findings indicate that CD4+ T cells do not produce high levels of IL-17 in response to alloantigen in the acute rejection response under these conditions of MHC-mismatched mouse orthotopic lung transplant. However, it is conceivable that other antigens or exogenous stimuli may elicit IL-17 secretion, and therefore our studies do not necessarily exclude a potential role for this cytokine in chronic lung allograft rejection.
Several studies in the past few years have demonstrated the capacity for tertiary lymphoid organs (TLO), such as the lung, to establish aggregates of antigen-specific T and B cells during inflammation independent of secondary lymphoid organs (SLO) (29–31). Recently, work by Gelman et al. showed the capacity for acute cellular rejection in the mouse orthotopic lung transplant model to occur in the absence of secondary lymphoid organs (32). Our studies detected allospecific CD8+IFN-γ+ T cells in the mediastinal lymph node and spleen consistent with systemic priming in SLO following orthotopic lung transplant. Further, we found increased allospecific CD8+IFN-γ+ cells in the native lung indicating that effector memory cells can stochastically traffic to non-inflamed effector sites. This is reminiscent of our prior findings in the heterotopic tracheal transplant model where allospecific CD8+ effectors were detectable in the recipient lungs posttransplant (25). Thus, while T cell priming may occur in the lung allograft itself, our findings indicate that systemic T cell allosensitization following mouse orthotopic lung transplantation also occurs.
Previous studies have shown an important role for the CD154/CD40 costimulation pathway in allograft acceptance in experimental transplant models, including our studies in the heterotopic airway transplant model (6–9, 33). Our results demonstrate a critical role for the CD154/CD40 pathway in the regulation of both allospecific CD8+ and CD4+ type 1 T cell responses and attenuation of acute rejection following mouse orthotopic lung transplantation. While some studies have found CD8+ T cells to be resistant to costimulation blockade (12–16), our results are consistent with studies demonstrating abrogation of allospecific CD8+ T cell responses with CD154/CD40 pathway disruption (34–37). Prior work in skin and bone marrow transplant models suggest allospecific CD8+ T cells undergo clonal deletion (37, 38). Interestingly, a recent study using anti-CD154 MR1 Ab in conjunction with CTLA4-Ig showed that attenuation of acute rejection pathology in mouse orthotopic lung transplant was abrogated in Bcl-2 transgenic recipients resistant to T cell apoptosis, consistent with a role for deletion of allospecific T cells in costimulation blockade mediated tolerance (39). Previously, we reported activated airway allograft CD8+ T cells with uncoupled effector cytokine production and proliferation following heterotopic tracheal allotransplant in the setting of CD154 deficiency (33). While these are different experimental systems, our current findings are similar in that we find 1) significantly improved allograft acceptance and 2) significantly reduced allospecific CD8+ effector responses and diminished mononuclear infiltration with disruption of CD154/CD40 signaling. However, in contrast to our previous work in the airway transplant model, our current studies observed a CD4+ T cell predominance in lung allografts following disruption of CD154/CD40 signaling. The potential uncoupling of T cell expansion and effector function as a mechanism for allograft acceptance was not assessed in this current study, and it remains to be determined in future studies. This is of particular interest given our current findings of the persistence of graft mononuclear infiltrates and lung CD8+ T cells in anti-CD154-treated allografts, similar to earlier reports (9, 36).
Our results also show anti-CD154 therapy significantly increases the frequencies of CD4+CD25+Foxp3+ T cells in the lung allograft. This finding is consistent with prior in vitro and in vivo studies implicating an important role for regulatory T cells (Tregs) in the establishment of tolerance to alloantigens (20, 21, 40, 41). While it remains unclear the precise role or mechanism(s) by which Tregs contribute to allograft acceptance under conditions of CD154 blockade, growing evidence supports contributory roles for both deletion and regulation of allospecific T cell responses in transplant tolerance (42–44).
In conclusion, our studies find that anti-CD154 Ab therapy alone is sufficient to attenuate acute cellular rejection in an MHC-mismatched mouse orthotopic lung transplant model. Improved lung allograft acceptance in anti-CD154 Ab-treated recipients was associated with abrogated CD8+ and CD4+ allospecific effector responses and increased frequencies of CD4+CD25+Foxp3+ regulatory T cells in the lung allografts. Further studies in the mouse orthotopic lung transplant model are required to determine whether these cellular effects following CD154 costimulation blockade represent distinct mechanisms contributing to lung allograft acceptance or are interdependent. Finally, these data indicate that novel approaches to block the CD154/CD40 costimulation pathway may be beneficial in the clinical transplant setting if this can be achieved safely (45).
Acknowledgments
Funding source: This work was supported by USPHS grants R01 AI 079175 (JFM)
LIST OF ABBREVIATIONS
- BOS
Bronchiolitis obliterans syndrome
- ACR
Acute cellular rejection
- CTLA4-Ig
Cytotoxic T lymphocyte antigen 4-fusion protein immunoglobulin
- LN
Lymph node
- LMNC
Lung mononuclear cells
- PMA/ionomycin
Phorbol myrisate acetate /ionomycin
- PE
Phycoerythrin
- APC
Allophycocyanin
- IFN-γ
Interferon-gamma
- FITC
Fluorescein isothiocyanate
- TNF-α
Tumor necrosis factor-alpha
- IL-17
Interleukin 17
- PerCPCy 5.5
Peridinin -chlorophyll-protein complex cyanine 5.5 conjugate
- ICCS
Intracellular cytokine staining
- Foxp3
Forkhead box P3
- Tregs
Regulatory T cells
- SLO
Secondary lymphoid organs
- TLO
Tertiary lymphoid organs
Footnotes
DISCLOSURE STATEMENT
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Contributor Information
Jeffrey M. Dodd-o, Division of Pulmonary and Critical Care Medicine, Department of Anesthesiology, Johns Hopkins University, School of Medicine, Baltimore, MD, 21224.
Elizabeth A. Lendermon, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University, School of Medicine, Baltimore, MD, 21224.
Hannah L. Miller, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University, School of Medicine, Baltimore, MD, 21224
Qiong Zhong, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University, School of Medicine, Baltimore, MD, 21224.
Emily R. John, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University, School of Medicine, Baltimore, MD, 21224
Wolfgang M. Jungraithmayr, Division of Thoracic Surgery, University Hospital Zurich, Zurich, Switzerland
Franco R. D’Alessio, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University, School of Medicine, Baltimore, MD, 21224
John F. McDyer, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University, School of Medicine, Baltimore, MD, 21224
REFERENCES
- 1.Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report--2010. J Heart Lung Transplant. 29(10):1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Belperio JA, Weigt SS, Fishbein MC, Lynch JP., 3rd Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009;6(1):108–121. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- 3.Wilkes DS, Egan TM, Reynolds HY. Lung transplantation: opportunities for research and clinical advancement. Am J Respir Crit Care Med. 2005;172(8):944–955. doi: 10.1164/rccm.200501-098WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinu T, Howell DN, Palmer SM. Acute cellular rejection and humoral sensitization in lung transplant recipients. Semin Respir Crit Care Med. 31(2):179–188. doi: 10.1055/s-0030-1249113. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7(6):1672–1679. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 6.Elster EA, Xu H, Tadaki DK, Montgomery S, Burkly LC, Berning JD, et al. Treatment with the humanized CD154-specific monoclonal antibody, hu5C8, prevents acute rejection of primary skin allografts in nonhuman primates. Transplantation. 2001;72(9):1473–1478. doi: 10.1097/00007890-200111150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Hancock WW, Sayegh MH, Zheng XG, Peach R, Linsley PS, Turka LA. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci U S A. 1996;93(24):13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markees TG, Phillips NE, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, et al. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation. 1997;64(2):329–335. doi: 10.1097/00007890-199707270-00026. [DOI] [PubMed] [Google Scholar]
- 9.Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5(6):686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 10.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378(6557):617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 11.McDyer JF, Goletz TJ, Thomas E, June CH, Seder RA. CD40 ligand/CD40 stimulation regulates the production of IFN-gamma from human peripheral blood mononuclear cells in an IL-12- and/or CD28-dependent manner. J Immunol. 1998;160(4):1701–1707. [PubMed] [Google Scholar]
- 12.Ensminger SM, Witzke O, Spriewald BM, Morrison K, Morris PJ, Rose ML, et al. CD8+ T cells contribute to the development of transplant arteriosclerosis despite CD154 blockade. Transplantation. 2000;69(12):2609–2612. doi: 10.1097/00007890-200006270-00022. [DOI] [PubMed] [Google Scholar]
- 13.Guo Z, Meng L, Kim O, Wang J, Hart J, He G, et al. CD8 T cell-mediated rejection of intestinal allografts is resistant to inhibition of the CD40/CD154 costimulatory pathway. Transplantation. 2001;71(9):1351–1354. doi: 10.1097/00007890-200105150-00033. [DOI] [PubMed] [Google Scholar]
- 14.Jones ND, Van Maurik A, Hara M, Spriewald BM, Witzke O, Morris PJ, et al. CD40-CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J Immunol. 2000;165(2):1111–1118. doi: 10.4049/jimmunol.165.2.1111. [DOI] [PubMed] [Google Scholar]
- 15.Lunsford KE, Jayanshankar K, Eiring AM, Horne PH, Koester MA, Gao D, et al. Alloreactive (CD4-Independent) CD8+ T cells jeopardize long-term survival of intrahepatic islet allografts. Am J Transplant. 2008;8(6):1113–1128. doi: 10.1111/j.1600-6143.2008.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104(12):1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5(11):1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5(11):1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 19.Graca L, Honey K, Adams E, Cobbold SP, Waldmann H. Cutting edge: anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J Immunol. 2000;165(9):4783–4786. doi: 10.4049/jimmunol.165.9.4783. [DOI] [PubMed] [Google Scholar]
- 20.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193(11):1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Maurik A, Herber M, Wood KJ, Jones ND. Cutting edge: CD4+CD25+ alloantigen-specific immunoregulatory cells that can prevent CD8+ T cell-mediated graft rejection: implications for anti-CD154 immunotherapy. J Immunol. 2002;169(10):5401–5404. doi: 10.4049/jimmunol.169.10.5401. [DOI] [PubMed] [Google Scholar]
- 22.Okazaki M, Gelman AE, Tietjens JR, Ibricevic A, Kornfeld CG, Huang HJ, et al. Maintenance of airway epithelium in acutely rejected orthotopic vascularized mouse lung transplants. Am J Respir Cell Mol Biol. 2007;37(6):625–630. doi: 10.1165/rcmb.2007-0257RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jungraithmayr WM, Korom S, Hillinger S, Weder W. A mouse model of orthotopic, single-lung transplantation. J Thorac Cardiovasc Surg. 2009;137(2):486–491. doi: 10.1016/j.jtcvs.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Gelman AE, Okazaki M, Lai J, Kornfeld CG, Kreisel FH, Richardson SB, et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 2008;180(7):4754–4762. doi: 10.4049/jimmunol.180.7.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West EE, Lavoie TL, Orens JB, Chen ES, Ye SQ, Finkelman FD, et al. Pluripotent allospecific CD8+ effector T cells traffic to lung in murine obliterative airway disease. Am J Respir Cell Mol Biol. 2006;34(1):108–118. doi: 10.1165/rcmb.2005-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu S, Zhou Y, Yang S, Ren Y, Zhang C, Quan C, et al. Platelet-derived growth factor receptor alpha gene mutations in vitiligo vulgaris. Acta Derm Venereol. 90(2):131–135. doi: 10.2340/00015555-0820. [DOI] [PubMed] [Google Scholar]
- 27.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6(4):724–735. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 29.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10(9):927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 30.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25(4):643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 31.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206(11):2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelman AE, Li W, Richardson SB, Zinselmeyer BH, Lai J, Okazaki M, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182(7):3969–3973. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah PD, West EE, Whitlock AB, Orens JB, McDyer JF. CD154 deficiency uncouples allograft CD8+ T-cell effector function from proliferation and inhibits murine airway obliteration. Am J Transplant. 2009;9(12):2697–2706. doi: 10.1111/j.1600-6143.2009.02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fehr T, Takeuchi Y, Kurtz J, Wekerle T, Sykes M. Early regulation of CD8 T cell alloreactivity by CD4+CD25− T cells in recipients of anti-CD154 antibody and allogeneic BMT is followed by rapid peripheral deletion of donor-reactive CD8+ T cells, precluding a role for sustained regulation. Eur J Immunol. 2005;35(9):2679–2690. doi: 10.1002/eji.200526190. [DOI] [PubMed] [Google Scholar]
- 35.Zhai Y, Shen XD, Gao F, Coito AJ, Wasowska BA, Salama A, et al. The CD154-CD40 T cell costimulation pathway is required for host sensitization of CD8(+) T cells by skin grafts via direct antigen presentation. J Immunol. 2002;169(3):1270–1276. doi: 10.4049/jimmunol.169.3.1270. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z, Wang Y, Gao F, Shen X, Zhai Y, Kupiec-Weglinski JW. Critical role of CD4 help in CD154 blockade-resistant memory CD8 T cell activation and allograft rejection in sensitized recipients. J Immunol. 2008;181(2):1096–1102. doi: 10.4049/jimmunol.181.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol. 2000;164(1):512–521. doi: 10.4049/jimmunol.164.1.512. [DOI] [PubMed] [Google Scholar]
- 38.Haspot F, Fehr T, Gibbons C, Zhao G, Hogan T, Honjo T, et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008;112(5):2149–2155. doi: 10.1182/blood-2007-12-127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okazaki M, Sugimoto S, Lai J, Kornfeld CG, Hotchkiss RS, Richardson SB, et al. Costimulatory blockade-mediated lung allograft acceptance is abrogated by overexpression of Bcl-2 in the recipient. Transplant Proc. 2009;41(1):385–387. doi: 10.1016/j.transproceed.2008.10.068. [DOI] [PubMed] [Google Scholar]
- 40.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201(7):1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor PA, Friedman TM, Korngold R, Noelle RJ, Blazar BR. Tolerance induction of alloreactive T cells via ex vivo blockade of the CD40:CD40L costimulatory pathway results in the generation of a potent immune regulatory cell. Blood. 2002;99(12):4601–4609. doi: 10.1182/blood.v99.12.4601. [DOI] [PubMed] [Google Scholar]
- 42.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004;114(10):1398–1403. doi: 10.1172/JCI23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verbinnen B, Billiau AD, Vermeiren J, Galicia G, Bullens DM, Boon L, et al. Contribution of regulatory T cells and effector T cell deletion in tolerance induction by costimulation blockade. J Immunol. 2008;181(2):1034–1042. doi: 10.4049/jimmunol.181.2.1034. [DOI] [PubMed] [Google Scholar]
- 44.Meng L, Wu Z, Wang Y, Lassman C, Busuttil RW, Zhai Y, et al. Differential impact of CD154 costimulation blockade on alloreactive effector and regulatory T cells in murine renal transplant recipients. Transplantation. 2008;85(9):1332–1338. doi: 10.1097/TP.0b013e31816c4f2b. [DOI] [PubMed] [Google Scholar]
- 45.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6(2):114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]