Abstract
Myocardial infarction (MI) remains a major cause of morbidity and mortality worldwide. Rapid advances in the treatment of acute MI have significantly improved short-term outcomes in patient, due in large part to successes in preventing myocardial cell death and limiting infarct area during the time of ischemia and subsequent reperfusion. Matrix metalloproteases (MMPs) play key roles in post-MI cardiac remodeling and in the development of adverse outcomes. This review highlights the importance of MMPs in the injury and remodeling response of the left ventricle and also discusses their potential as therapeutic targets Additional pre-clinical and clinical research is needed to further investigate and understand the cardioprotective effects of MMPs inhibitors.
Keywords: myocardial infarction, left ventricle, extracellular matrix, matrix metalloproteinases, left ventricle remodeling
1 Introduction
Acute myocardial infarction (MI) occurs as a result of myocardial cell death due to prolonged ischemia. Ischemia occurs when the blood supply to the myocardium stops, often due to the formation of a thrombus in the lumen of the artery supplying oxygen. Ischemic heart disease remains a primary contributor to morbidity and mortality, highlighting the need for new drug targets. Following MI, the necrotic myocytes in the myocardium activate an inflammatory response. This response is beneficial and necessary for wound healing, but at the same time can be detrimental because it further damages the left ventricle (LV) to expand the initial region of injury. Tissue remodeling post-MI involves both a cellular component as well as an extracellular matrix (ECM) remodeling component.
The ECM represents an important cardiac element that adapts to coordinate the functional requirements of the myocardium. In addition to providing structural support, cardiac ECM serves as a reservoir to house a variety of cytokines and growth factors, which are surrounded by a hydrated proteoglycan and glycosaminoglycan-rich environment [1]. The ECM possesses a number of physiological properties and functions, but is primarily directed at preserving cardiac integrity and architecture to facilitate and govern cellular activity [2]. The myocardial ECM directs contractile force generated by cardiomyocytes and sustains shear stress generated by cardiomyocytes. In addition, the myocardial ECM produces and maintains certain levels of a variety of growth factors, as well as modulates cell proliferation and differentiation, ECM synthesis and remodeling [3–5].
Following MI, the ECM is degraded and synthesized through a process that is modulated by the balance between matrix metalloproteinase (MMP) activity and the function of the main source of most of the ECM, the fibroblast [6]. Presently, 23 human MMPs have been identified, and although they share a common homology, their functions vary tremendously [2]. MMP activity is regulated by two major types of endogenous inhibitors: α2-macroglobulin (a plasma protein that acts as a general proteinase inhibitor) and the tissue inhibitors of metalloproteinases (TIMPs) [2]. Four TIMPs have been identified: TIMP-1, -2, -3 and -4. TIMPs form high-affinity complexes with activated MMPs and neutralize substrate degradation by blocking the MMP catalytic domain [7]. TIMP-1 is known to inhibit most MMPs, with the exception of MMP-14 (MT1-MMP), but has a higher affinity for MMP-9 than MMP-2. TIMP-2 potentially inhibits all MMPs but has a higher affinity to MMP-2 than MMP-9. TIMP-3 has been shown to bind to MMP-1, -2, -3, -9, and -13 and TIMP-4 inhibits MMP-1, -3, -7, and -9 [8].
To better understand the significance of MMPs as drug targets for MI, this review article will summarize the latest developments in the MMP inhibitor arena, focusing on potential applications of MMP inhibitors for post-MI remodeling.
2 LV remodeling following MI
The composite of tissue changes occurring to the LV in response to MI are collectively termed as LV remodeling. LV remodeling modifies LV size, shape, and function, and these changes start immediately after MI and can continue for years [9]. MMPs are involved in all aspects of remodeling, including the cellular and extracellular responses.
2.1 Cellular responses to MI (Table 1)
Table 1.
| Cell type | Functions |
|---|---|
| Neutrophils |
|
| Macrophages |
|
| Fibroblasts |
|
Prolonged ischemia leads to myocyte death, which occurs by apoptosis and necrosis [10]. Myocyte death stimulates the release of a variety of bioactive substances, including complement components. Complement is an essential component of the humoral defense mechanism and also mediates the inflammatory process [11]. The activation of the complement system is organized via interaction between various specific protein components. Certain complexes of complement system are involved in coating of damaged tissue fragments, thereby facilitating their ingestion by phagocytic cells. The other mediate chemotaxis associated recruitment of leukocytes into the infarcted area via receptor-mediated mechanisms [12]. The late-acting complement complexes form macromolecular complexes, which express cytotoxic properties to the local cells [13]. Leukocytes produce a variety of biologically active substances, which in turn initiate signaling pathways locally and systemically to provide a robust inflammatory response at the site of injury [14].
2.1.1 Polymorphonuclear leukocyte infiltration
Polymorphonuclear leukocytes (PMNs) are the first line of defense against foreign bodies and are the first to infiltrate into the infarct region, in the absence of reperfusion. PMNs produce pro-inflammatory cytokines (e.g. tumor necrosis factor-alpha (TNF-α) and interleukin 1-beta (IL-1β)), a number of chemokines (e.g. IL-8 and macrophage inducible protein 1-alpha (MIP-1α)), and several growth factors (e.g. vascular endothelial growth factor (VEGF) and transforming growth factor (TGF) β [15].
The recruitment of PMNs into the infarcted region begins with their adhesion to the endothelial cells in the vessel wall. PMNs migrate to the site of injury and secrete superoxide anions; myeloperoxidase; MMPs -8, -9, and other proteolytic enzymes (e.g. serine elastase); and TIMP-1. These factors are secreted as a protective measure but actually results in extending the tissue damage [15–17]. MMP-9, in particular, is rapidly released within the early hours post-MI and positively correlates with PMN numbers [16, 18]. Serine elastase, stored within secretory granules of the PMNs, serves as a local MMP-9 activator [19]. By day 5 post-MI, PMNs begin to undergo apoptosis and are phagocytized by macrophages.
2.1.2 Macrophage infiltration
Macrophages originate from circulating blood monocytes, which are derived from CD34+ bone marrow progenitors [20]. The conversion of monocytes into macrophages begins with their adhesion to the vessel wall. This process is followed by the induction of several cytokines, including macrophage colony stimulating factor, TNFα, platelet derived endothelial cell growth factor, TGFα and β, IL-1, and insulin-like growth factor [21]. A predominant function of the macrophage post-MI is to facilitate wound healing and scar formation by phagocytosis of necrotic or apoptotic cells and by secretion of angiogenic molecules and growth factors. Macrophage migration into the injured myocardium is mediated by locally released chemoattractants [22]. Subsequently, activated macrophages produce cytokines, chemokines, and proteases. For example, macrophages are a source of MMPs -1, -2, -3, -7, -8, -9, -12, -14, and -28 as well as TIMPs -1, -2, -3, and -4 [20, 23].
Phagocytosis triggers TGFβ production in macrophages, which in turn downregulates MMP-9 activity by inducing TIMP-1 expression [20]. In addition to phagocytic roles, macrophages also contribute to angiogenesis by secreting factors that directly stimulate endothelial cell proliferation and by releasing MMPs. Moldovan and colleagues reported macrophage-derived MMPs degrade the ECM necessary for the formation of new vessels, which subsequently are inhabited by endothelial cells [24]. ECM degradation and chemoattractant production both stimulate and inhibit angiogenesis in the post-MI setting [25]. Several ECM proteins have angiogenic activities after being degraded into smaller fragments. For example, hyaluronic acid displays neovascularization properties in vitro and in vivo [26, 27]. Mathematical models show that ECM density influences the velocity of formation and branching of the vessels, while the alignment of ECM fibers dictate the orientation and shape of endothelial cells [28]. Degradation of matrix components at low ECM densities inhibits angiogenesis, whereas matrix degradation at high densities of ECM stimulates angiogenesis [29].
2.1.3 Myofibroblast activation
A major outcome of the inflammatory response is fibroblast activation, which ultimately coordinates scar formation. Activation involves the differentiation of fibroblasts into myofibroblasts (fibroblasts that express contractile proteins) [30]. Myofibroblasts activation can be observed by day 3 post-MI which corresponds to the timing of macrophage infiltration. Moreover, the positive correlation between macrophage number and collagen mRNA levels in the infarcted area was observed in a rat occlusion and reperfusion model 5 days post-reperfusion [31]. At later stages, the maintenance of the scar is coordinated by the continued presence of myofibroblasts [32, 33]. Among the factors that stimulate myofibroblast activation, macrophages are a primary source of activators, such as TGFβ, MMPs and TIMPs [20]. TIMPs stimulate fibroblast proliferation as well as phenotypic differentiation of fibrocytes into myofibroblasts, and this stimulation is independent of its MMP blocking activity [34].
In addition to ECM, fibroblasts and myofibroblasts synthesize many other factors relevant to LV remodeling, including interleukin (IL) -1, IL-6, TGFβ, connective tissue growth factor, tumor necrosis factor α (TNFα), angiotensin II, endothelin I, MMPs, and TIMPs [33, 35–38]. Myofibroblasts, under certain stimuli, activate or express a number of MMP family subtypes, including MMP-1, -2, -9, -13, and membrane type MMP-14 [33]. MMP-2 secreted by myofbroblasts in vitro, and could be further induced by IL-1β, TNFα, TGFβ, mechanical load and oxidative stress [33]. Increased TIMP-2 is associated with increased fibroblast collagen synthesis, while TIMP-4 inhibits cell growth and triggers apoptosis of differentiated myofibroblasts [39]. TIMP-3 facilitates programmed cell death of both normal and injured myofibroblasts to prevent excessive myocardial fibrosis [34].
2.2 Extracellular response to MI
A number of ECM proteins are expressed in normal myocardium, including collagens, laminin, fibronectin, and low levels of matricellular proteins (e.g. thrombospondin-1 (TSP-1)) [40]. These ECM proteins play a central role in the physiological performance of the heart during various stage of development and in response to pathophysiological signals [41–43].
2.2.1 Collagen
The collagen found in normal LV includes types I, III, IV, V and VI [44]. The most abundant collagen type present in the LV is type I, which represents 70% of the total cardiac collagen [45]. Collagen fibers form a complex network to provide strength sufficient to support the three-dimensional structure surrounding cardiac muscle fibers and neighboring vascular tissues [46]. These fibers prevent excessive cardiomyocyte stretching due to the elastic energy that is saved during myocardial contraction. Additionally, collagen fibers allow the LV walls to resist the intracavitary pressure. Collagen is synthetized and secreted by fibroblasts in the form of a collagen precursor, which later is converted to a matured collagen under the effect of specific collagen proteinases [47].
Elevated amounts of collagen III are observed two days post-MI in rats [48]. This increase in collagen III is consistent with an early wound healing response, where collagen III is quickly laid down to provide structural support. However, the production of the main collagen component, collagen I, occurs more slowly. The increase in collagen III continues through the first 21 days post-MI, while collagen I production continues to remain high for more than 90 days post-MI [49, 50]. An increase in collagen contents is observed in both infarcted and distant region of the myocardium, although levels are a magnitude higher in the infarct [48].
The major causes that lead to cardiac rupture after acute MI are the misbalance between ECM degradation and synthesis, and alterations in the cross-linking of collagen.
Post-MI, there is a balance between ECM synthesis and degradation, and the fibroblast contributes to the synthesis part of the equation. If ECM degradation exceeds synthesis, LV aneurysm or rupture can develop. The later usually occurs after transmural MI. It may develop in the early period post-MI as well as in the later stages within the first 7 to 10 days when the infarct region is the most vulnerable [51]. Among many in vivo studies directed to determine the possible causes for cardiac rupture, the first place belongs to imbalanced system of collagen production and cross-linking [52]. If ECM synthesis exceeds degradation, increased LV stiffness can lead to heart failure. MMPs and TIMPs regulate both parts of the equation [53]. While many studies suggest that deletion of MMP-9 is beneficial in the early post-MI setting, therapeutic approaches of mediating direct effects on collagen have not been explored [54].
2.2.2 Fibronectin
In the normal myocardium, fibronectin is localized to the basement membrane of endothelial cells, myocytes, and smooth muscle cells [55]. Fibronectin binds growth factors, fibrin, heparin, collagens, and integrins [1]. In the MI setting, increased fibronectin deposition has been observed from 12 hours to 14 days after the infarction [55, 56]. In the MI setting, fibronectin is produced by ischemic cardiomyocytes and local fibroblasts [55, 57–59]. Fibronectin acts as a chemoattractant for a variety of cell types involved in wound healing [57]. Fibronectin is also degraded by MMPs, and fibronectin fragments have been shown to have biological activity [60]. In particular, fibronectin has been shown an in vivo substrate for MMP-7 and the MMP-9 in post-MI LV [61, 62].
2.2.3 Laminins
Like fibronectin, laminin is localized to the basement membrane of endothelial cells, myocytes, and smooth muscle cells in the normal myocardium. Laminin links the extracellular space and the intracellular milieu by binding several cell-surface receptors including the integrins α6β1, α1β1, α2β1, and α3β1 [63]. In addition, laminin binds to sarcoglycans through the α-dystroglycan to form connections with intracellular dystrophin.
Laminin is a well-known MMP substrate associated with early remodeling post-MI [64]. Dinh et al. showed that laminin levels are higher in patients with severely reduced LV function and proposed laminin as a surrogate marker of ECM remodeling post-MI [65]. Horstmann and colleagues demonstrated a negative correlation between MMP-9 levels and intact laminin levels in patients with acute stroke, suggesting that laminin processing may be a key event in remodeling [66]. Laminin may help early survival after MI by preventing cardiac rupture but may also inhibit macrophage infiltration, which could delay wound healing [67]. Laminin improved the differentiation of adipose-derived stem cells in vitro to become cardiomyocytes, suggesting a role in stem cell biology [68].
2.2.4 Matricellular proteins
Secreted protein acidic and rich in cysteine (SPARC), also known as osteonectin or BM-40, is restricted in adult to tissues that undergo consistent turnover or to sites of injury and disease [69, 70]. The capacity of SPARC to bind ECM, modulate growth factor efficacy, increase MMP expression, and alter cell shape as a counter-adhesive factor highlights the importance of SPARC in the response to injury [71]. SPARC binds to a number of ECM components, including collagen types I, II, III, IV, and V. SPARC binds to growth factors such as platelet-derived growth factor and vascular endothelial growth factor and modulates TGFβ activity to stimulate cell proliferation, migration, and differentiation [72].
SPARC levels increase significantly in the post-MI LV, to organize the formation of granulation tissue in the scar. The role of SPARC in LV remodeling follows the TGFβ signaling pathway, indicating a strong interaction with TGFβ [40]. SPARC deletion has beneficial effects in early post-MI LV function [70]. Impaired fibroblast activation in SPARC-deleted mice, however, suggested that long-term deletion would be detrimental. This turned out to be correct, as there was an increase in LV rupture rates in the longer post-MI period [73]. SPARC up-regulates MT1-MMP expression and MMP-2 activation, indicating that its post-MI roles are complex [74].
Thrombospondin-1 (TSP-1) is another matricellular protein with multiple biological functions associated with early remodeling post-MI. TSP-1 is expressed in α-granules of platelets and by endothelial cells and macrophages in a highly regulated manner [75]. TSP-1 possesses protective properties post-MI, however the exact mechanisms remain unclear [76].
TSP-1 increases at days 7–28 after ischemia and reperfusion, and TSP-1 clearly demarcates the infarcted area from the non-infarcted myocardium. TSP-1 deletion led to expansion of the post-MI inflammatory reaction and extension of granulation tissue formation into the non-infarcted remote region [76]. Moreover, TSP-1 inhibited MMP activity in the border region to control excessive ECM degradation [40].
2.2.5 Proteoglycan
Proteoglycans maintain the tensile strength of ECM and provide a reservoir for a number of growth factors. Biglycan, the major small chondroitin sulphate/dermatan sulphate, is involved in the interaction with other matrix components, especially type I collagen, and TGFβ [77, 78]. During MI, the expression of biglycans positively correlates with collagen mRNA expression during the fibrotic process in the LV [79]. Biglycan null mice showed alteration in scar formation in the mouse model of permanent coronary artery ligation 30 days post-MI [79].
Decorin, another proteoglycan base component, is widely distributed in the ECM and is associated with collagen fibrils. Decorin is upregulated during later fibrotic stages. The mRNA expression of decorin positively correlated with type I collagen mRNA levels in the infarct area in the rat model of permanent coronary artery ligation 7, 14 and 28 days post-MI [80]. Decorin was reported to mediate the fibrotic processes via suppression of TGFβ1 mRNA expression in vivo [81].
Syndecans belong to a family of cell-surface heparan sulphate proteoglycans. Its level is upregulated in the infarcted tissue and is associated with macrophage infiltration [82]. Syndecan null mice exhibited impaired cardiac function and showed increased mortality rate after MI in the model of permanent coronary artery ligation in acute stage post-MI. Although TGFβ1-dependant cell signaling was preserved in these mice, the cell migration, fibronectin-induced cell signaling, and differentiation in cardiac fibroblasts were defective [83].
3 MMPs as therapeutic targets
The generation of the smallest possible scar that does not affect the biomechanics of the heart requires a carefully balanced activity of MMPs and TIMPs in the infarcted heart. Multiple MMPs are elevated post-MI, and roles for several of these MMPs have been elucidated. Well-healed infarcts contain large amounts of ECM, which can occupy up to 80% of the infarct area [84]. MMP-3 is an upstream activator of other MMPs and may contribute to aneurysm formation in the infarcted LV [85, 86]. MMP-7 increases the potential for post-MI arrhythmias, through cleaving connexin-43 [87]. MMP-9 levels increase in multiple animal models of MI and positively correlate with infarct rupture rates in humans [88]. MMP-12 is highly expressed in macrophages and may also play a role in post-MI remodeling [85]. Therefore, there remains a strong rationale to study MMPs as possible therapeutic targets in the MI setting.
3.1 Direct MMPs inhibitors and selectivity
The search for the first orally bioavailable small molecule MMP inhibitor began in the late 1970s and was directed at treating arthritis [89]. At that time, only a few MMPs had been identified, and the first generation of MMP inhibitors (e.g., batimastat and galardin) had poor bioavailability that substantially limited their development [90]. Later, second generation of MMP inhibitors was also reported to possess numerous side effects [91]. In the past decade, both macromolecular MMPs inhibitors (monoclonal antibodies and natural TIMPs) and small molecules (synthetic and natural products) have been tested as potential therapeutic agents [92, 93].
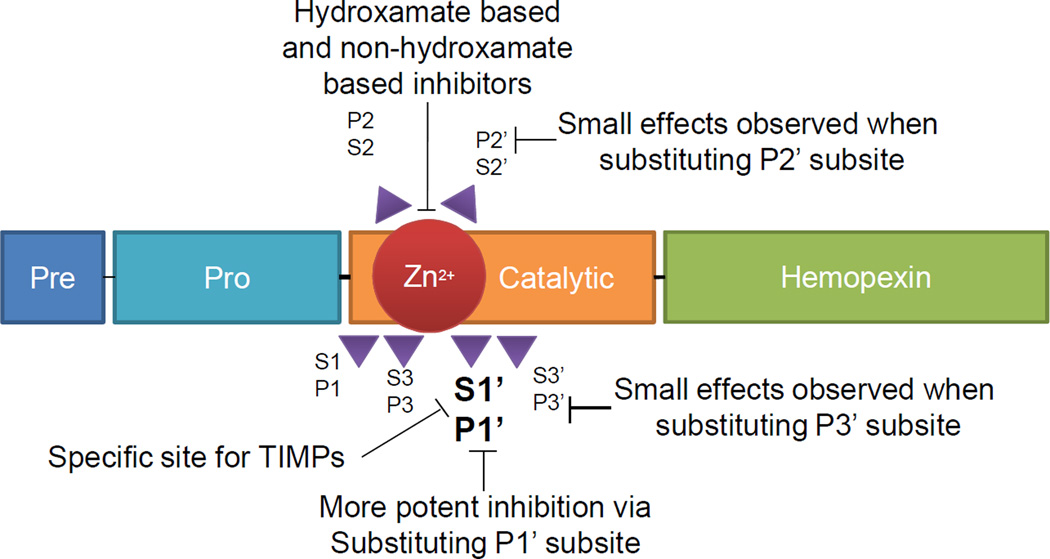
A large body of literature now supports a key role for the two motifs (S1’ subsite and P1’ residue) in the interactions of MMPs with their substrates (or inhibitors). X-ray crystallographic studies have classified MMPs into two broad structural classes dependent on the depth of S1’ pocket, which was suggested as the selectivity pocket for MMP inhibitors [94]. This selectivity pocket was later shown to be relatively deep for some MMPs, but partially or completely blocked for others due to differences in amino acid chains forming the pocket. Therefore, developing selective inhibitor of MMPs was rather limited in the application towards deep pocket enzymes over short pocket enzymes, via incorporation of an extended P1’ group. In this case, the presence of the smaller P1’ group led to wide spectrum selectivity (Figure 1).
Figure 1.
MMP subsites as targets for different classes of MMP inhibitors. Pre – a signal sequence pre-domain, Pro – pro-catalytic domain, Catalytic – catalytic domain, Zn2+ - metal center of the catalytic domain, Hemopexin – hemopexin-like domain (absent in MMP-7 and MMP-26) [142, 143].
Two major classes of inhibitors were identified from these structural assessments: hydroxamate-based inhibitors and non-hydroxamate-based inhibitors. The main idea was based on a creation of a compound that would substitute the active site of the active MMP with a peptide-like structure [92]. In hydroxamate-based inhibitors, most of the small molecules use hydroxamate as their zinc-binding site. Hydrogen bonds between hydroxamate-NH group and carbonyl oxygen Ala128 this interaction is also known to contribute to the binding. The interactions observed were reported to be productive without any adverse effect [95]. Hydroxamate inhibitors are further grouped into substrate-analogue peptides, such as succinyl, sulphonamide, phosphinamide hydroxamates [96]. Non-hydroxamate inhibitors have limited use due to a general lack of specificity.
Another step in the MMP inhibitor field has been to develop inhibitors based on mechanism. Mobashery et al. demonstrated a novel approach to highly selective gelatinase inhibition through coordination of the thiirane group of the inhibitor with the active zinc-site of the MMP [97]. This coordination caused conformational change of the MMP and the covalent attachment of inhibitor to the active-site Glu of the MMP [98]. From the many MMP inhibitors tested to date, only doxycycline (Periostat) has been approved by the FDA [99].
3.2 The MMP inhibitory properties of currently used post-MI medications
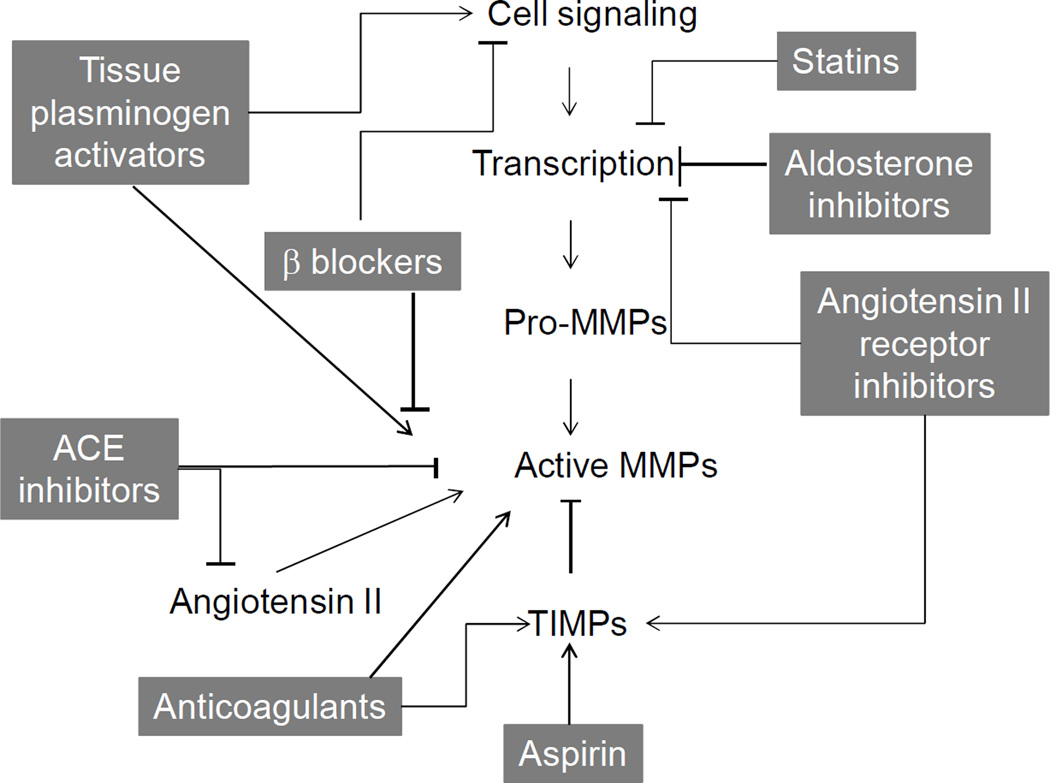
While selective and specific MMP inhibitors have not been successfully tested and applied to the post-MI setting, many of the drug therapies currently used to treat MI have indirect MMP inhibition effects (Figure 2). These effects are summarized below in Table 2.
Figure 2.
Medications used post-MI that directly or indirectly affect MMP production or activity.
Table 2.
Medications used in post-MI patient management, their applications, and effects on MMPs and TIMPs [23, 58, 116, 92, 94, 95, 100–102, 105, 107, 109, 110, 117, 120, 143–150, 153–160].
| Medication | Applications | MMP Effects |
|---|---|---|
| Thrombolysis- tissue plasminogen activators | Catalyze the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown | ↑ MMP-1, -2, -3, -9, -12, -14 ↑ TIMP-1, -2 |
| Anticoagulants | Bind to and activate anti-thrombin III. Activated anti-thrombin III inactivates thrombin and other proteases involved in blood clotting, most notably factor Xa. | ↑ MMP-2 and -9 release in blood ↑ TIMP-2 ↓ TIMP-1 |
| Angiotensin converting enzyme Inhibitors | Inhibit the angiotensin-converting enzyme to lower blood pressure | ↓ MMP-1, -2, -3, -9 ↑ TIMP-1 |
| Angiotensin II receptor inhibitors | Modulate the renin-angiotensin-aldosterone system to lower blood pressure | ↓ MMP-2, -3, -9 ↑ TIMP-1 |
| Aldosterone antagonists | Antagonize aldosterone at the mineralocorticoid receptor level | ↓ MMP-1, -2, -9 ↑ TIMP-2 |
| Beta-blockers | Block endogenous catecholamines, including epinephrine (adrenaline) and norepinephrine (noradrenaline) | ↓ MMP-2, -9 ↑ TIMP-1, -2, -3 |
| Statins | Inhibit HMG-CoA reductase to lower cholesterol levels; anti-inflammatory effects | ↓ MMP-1, -2, -9, -12 ↑ TIMP-1 |
| Non-steroidal anti-inflammatory drugs (aspirin) | Irreversibly inhibit COX-1-mediated platelet aggregation | ↓ MMP-1 ↑ TIMP-1 |
3.2.1 Thrombolytic agents and anticoagulants
In clinical guidelines, acute vascular events such as acute MI and acute ischemic stroke share similar treatment approaches, namely the timely use of thrombolytic agents. MMP-3, -9, and -14 levels increase in the serum or plasma following treatment with tissue plasminogen activator after acute ischemic stroke in both humans and a rat model [66, 100]. Alteplase treatment increases MMP-2 and MMP-9 concentrations in patients with ST segment elevation acute MI [101]. The rise in MMP-9 levels suggested that MMP activity may be responsible for the failure or thrombolytic agents [102].
Thrombolysis is often accompanied with anticoagulant therapy. Three main classes of anticoagulants are used for MI therapy: heparins, synthetic heparins, and direct thrombin inhibitors [103, 104]. Anticoagulant therapy increases MMP-9 in blood samples of stroke patients [105, 106]. At the same time, anticoagulant therapy increases TIMPs levels in the plasma. Manello et al. reported that heparin directly activates MMPs and also increases the release of TIMP-2 to block their activity [107].
3.2.2 ACE inhibitors and angiotensin II receptor inhibitors
Angiotensin converting enzyme (ACE) inhibition is an effective post-MI treatment [108]. Because the regions close to the zinc in ACE are very similar to analogous regions in other metalloproteases, several ACE inhibitors have high affinities for MMPs, suggesting a direct inhibitor role. Yamamoto et al. demonstrated strong MMP-9 inhibition by ACE inhibitors in a post-MI animal model [109, 110]. ACE inhibitors and angiotensin II receptor inhibitors: ACE inhibitors directly bind to MMP-9 at the S1’ subsite, which forms a deep hydrophobic pocket compatible with hydrophobic moieties present in ACE inhibitors. The Yamamoto lab has also shown that different ACE inhibitors have varying levels of inhibitor activity depending on their affinities. Lisinopril is stabilized in the active site of MMP-9 by specific hydrogen bonds and hydrophobic interactions, while imidapril possesses a higher affinity to MMP-9, probably due to a stronger interaction with the S1 site [110]. This team has shown that captopril directly binds to the MMP-9 active site [111]. Perindopril decreases MMP-9 activity and cytokine production in peripheral blood in the acute period of ischemic stroke and MI, while trandopril and valsartan supresses MMP-9 activity and cardiac remodeling post-MI [112].
In addition to ACE inhibition, blocking the angiotensin II receptor has also been shown to attenuate remodeling. Harada et al. demonstrated that angiotensin II type 1A receptor null mice displayed less LV remodeling and improved survival post-MI [113]. Irbesartan, an angiotensin II receptor antagonist, inhibits MMP activity in patients with symptomatic carotid artery stenosis [114]. Inhibiting both angiotensin II type 1 and type 2 receptors with Sar1-Ile8-Ang II reduces collagen type I and elastin deposition but also increases vascular stiffness, fibronectin, and MMP-2 activity [115]. Olmesartan improves LV remodeling in apo E null mice by inhibiting nuclear factor κ-B (NF-κB) and MMP-9 activities [116]. Yang and colleagues reported that valsartan decreased levels of MMP-2, -3, and -9 post-MI [117]. More studies are needed, however, to further explore the long-term effects of these inhibition strategies.
3.2.3 Aldosterone antagonists
Aldosterone antagonists are recommended for acute MI patients who have symptoms and/or signs of heart failure and left ventricular systolic dysfunction. According to the National Institutes for Health and Clinical Excellence recommendations, treatment with an aldosterone antagonist should be initiated within 3–14 days post-MI, preferably after ACE inhibitor therapy has been initiated [118].
Aldosterone induces MMPs activity through the activation of mineralocorticoid receptor, protein kinase, and reactive oxygen species dependent activation of mitogen/extracellular signal-regulated kinase and extracellular signal-regulated kinase pathway [119]. Aldosterone antagonists demonstrate indirect effects on MMP activity and collagen deposition post-MI. Spironolactone prevents cardiac collagen deposition post-MI in rodents [120]. Spironolactone and hydrochlorothiazide both demonstrated an ability to reduce vascular MMP-2 activity and expression in a model of renovascular hypertension [121]. Moreover, prolonged treatment with spironolactone for 24 weeks in patients with heart failure decreased the level of MMPs and improved cardiac function [122].
3.2.4 Beta-blockers
Beta-adrenergic antagonists are another class of medications commonly used in post-MI patients with developing heart failure. Beta-blockers possess antioxidant, anti-proliferative, and free radical scavenging effects, which may alter MMP abundance [123]. Carvedilol reduces plasma MMP-9 levels in younger patients with idiopathic dilated cardiomyopathy and improves LV remodeling in a mouse model of acute myocarditis [124, 125]. While carvedilol has been shown to inhibit MMP-2 and -9, it also increases MMP-8 and -13, indicating that the connection between β blockers and MMP activity is complex [126, 127]. Atenolol in supra-therapeutic doses reduces MMP activity and prevents ventricular stiffening in a dog model of pacing-induced cardiac dysfunction [128]. Short-term beta-adrenergic blockade decreases MMP-9 promoter activity in the human ECV304 endothelial cell line and plasma gelatinase activity in patients with heart failure [129].
3.2.5 Statins
Hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are broadly used to treat patients with high cholesterol levels [130]. Statins also block the synthesis of isoprenoid intermediates, which serve as lipid attachments for a variety of intracellular signaling molecules. In particular, the rho-family small GTP-binding proteins, whose proper membrane localization and function are dependent on isoprenylation, are blocked by statins. [131]. Statins improve endothelial cell function, inhibit proliferation and migration of smooth muscle cells, and decrease vascular inflammation. Statins inhibit MMPs by suppressing macrophage infiltration [132]. Luan et al. demonstrated that statins stabilize the atherosclerotic plaque by inhibiting several MMPs, including MMP-1, -2, -3 and -9 [133]. Statins, however, can also trigger the upregulation of macrophage elastase (MMP-12) [134]. Pravastatin reduces serum soluble CD40L, C-reactive protein, MMP-2, and MMP-9 levels in post-MI patients [135–137]. Turner et al. demonstrated that simvastatin suppresses TNF-α-induced invasion of human cardiac myofibroblasts by both MMP-9-dependent and -independent mechanisms, indicating that statins likely have effects on MMPs through multiple pathways [138].
3.2.6 Non-steroid anti-inflammatory drugs (NSAIDs)
NSAIDs occupy an important place in treatment of patients presenting with acute MI. NSAIDs suppress gene expression of MMPs SP1 transcription factor binding site [139]. Experimentally, however, no studies have been performed to investigate the potential use of NSAIDs as MMPs inhibitors in the MI setting. Aspirin, the most commonly used NSAID among patients with myocardial infarction and heart failure, suppresses MMP-1 in isolated human coronary endothelial cells but did not affect MMP-2 or -9 levels [140, 141].
4 Conclusion
LV remodeling post-MI involves MMP activity at every step. MMPs coordinate key biological activities, including inflammation and scar formation. The alteration in MMP and TIMP expression may lead to undesired consequences, resulting in the development of a variety of possible complications, including sudden cardiac death, LV rupture, or the development of congestive heart failure. The potential roles of MMPs as therapeutic targets in the MI setting, therefore, are warranted. Many direct inhibitors of MMP transcription and activity have been tested; however, none of these inhibitors have translated to clinical relevance for the post-MI patient. At the same time, many of the currently used medications to treat MI influence MMP and TIMP activity. Identifying selective MMP inhibition strategies for the post-MI patient, particularly therapies that limit the progression to heart failure remain a highly desired goal.
Acknowledgements
We acknowledge support from NIH/NHLBI HHSN 268201000036C (N01-HV-00244) for the San Antonio Cardiovascular Proteomics Center and R01 HL075360, the Max and Minnie Tomerlin Voelcker Fund, and the Veteran’s Administration (Merit) to MLL.
References
- 1.Hein S, Schaper J. The extracellular matrix in normal and diseased myocardium. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2001;8(2):188–196. doi: 10.1067/mnc.2001.113331. Epub 2001/04/11. [DOI] [PubMed] [Google Scholar]
- 2.Hobeika MJ, Thompson RW, Muhs BE, Brooks PC, Gagne PJ. Matrix metalloproteinases in peripheral vascular disease. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2007;45(4):849–857. doi: 10.1016/j.jvs.2006.09.066. Epub 2007/04/03. [DOI] [PubMed] [Google Scholar]
- 3.Pelouch V, Dixon IM, Golfman L, Beamish RE, Dhalla NS. Role of extracellular matrix proteins in heart function. Mol Cell Biochem. 1993;129(2):101–120. doi: 10.1007/BF00926359. [DOI] [PubMed] [Google Scholar]
- 4.Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. Faseb J. 1997;11(1):51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 5.Lindsey ML, Borg TK. Understanding the role of the extracellular matrix in cardiovascular development and disease: where do we go from here? Journal of molecular and cellular cardiology. 2010;48(3):431–432. doi: 10.1016/j.yjmcc.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu P, Sun M, Sader S. Matrix metalloproteinases in cardiovascular disease. The Canadian journal of cardiology. 2006;22(Suppl B):25B–30B. doi: 10.1016/s0828-282x(06)70983-7. Epub 2006/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. Epub 2000/06/28. [DOI] [PubMed] [Google Scholar]
- 8.Phatharajaree W, Phrommintikul A, Chattipakorn N. Matrix metalloproteinases and myocardial infarction. The Canadian journal of cardiology. 2007;23(9):727–733. doi: 10.1016/s0828-282x(07)70818-8. Epub 2007/07/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vu DT, Kofidis T. Myocardial restoration: is it the cell or the architecture or both? Cardiology research and practice. 2012;2012:240497. doi: 10.1155/2012/240497. Epub 2012/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, et al. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Laboratory investigation; a journal of technical methods and pathology. 1996;74(1):86–107. Epub 1996/01/01. [PubMed] [Google Scholar]
- 11.Homeister JW, Lucchesi BR. Complement activation and inhibition in myocardial ischemia and reperfusion injury. Annual review of pharmacology and toxicology. 1994;34:17–40. doi: 10.1146/annurev.pa.34.040194.000313. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 12.Distelmaier K, Adlbrecht C, Jakowitsch J, Winkler S, Dunkler D, Gerner C, et al. Local complement activation triggers neutrophil recruitment to the site of thrombus formation in acute myocardial infarction. Thrombosis and haemostasis. 2009;102(3):564–572. doi: 10.1160/TH09-02-0103. Epub 2009/09/01. [DOI] [PubMed] [Google Scholar]
- 13.Zuidema MY, Zhang C. Ischemia/reperfusion injury: The role of immune cells. World journal of cardiology. 2010;2(10):325–332. doi: 10.4330/wjc.v2.i10.325. Epub 2010/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kempf T, Zarbock A, Vestweber D, Wollert KC. Anti-inflammatory mechanisms and therapeutic opportunities in myocardial infarct healing. Journal of molecular medicine. 2012;90(4):361–369. doi: 10.1007/s00109-011-0847-y. Epub 2012/01/10. [DOI] [PubMed] [Google Scholar]
- 15.Di Filippo C, Rossi F, D'Amico M. Targeting polymorphonuclear leukocytes in acute myocardial infarction. The Scientific World Journal. 2007;7:121–134. doi: 10.1100/tsw.2007.45. Epub 2007/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsey ML, Zamilpa R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovascular therapeutics. 2012;30(1):31–41. doi: 10.1111/j.1755-5922.2010.00207.x. Epub 2010/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romanic AM, Harrison SM, Bao W, Burns-Kurtis CL, Pickering S, Gu J, et al. Myocardial protection from ischemia/reperfusion injury by targeted deletion of matrix metalloproteinase-9. Cardiovascular research. 2002;54(3):549–558. doi: 10.1016/s0008-6363(02)00254-7. Epub 2002/05/29. [DOI] [PubMed] [Google Scholar]
- 18.Deten A, Volz HC, Holzl A, Briest W, Zimmer H-G. Effect of propranolol on cardiac cytokine expression after myocardial infarction in rats. Mol Cell Biochem. 2003;251(1–2):127–137. [PubMed] [Google Scholar]
- 19.Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, et al. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation. 2001;103(17):2181–2187. doi: 10.1161/01.cir.103.17.2181. Epub 2001/05/23. [DOI] [PubMed] [Google Scholar]
- 20.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. International journal of cardiology. 2008;130(2):147–158. doi: 10.1016/j.ijcard.2008.04.059. Epub 2008/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer AJ, Clark RA. Cutaneous wound healing. The New England journal of medicine. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. Epub 1999/09/02. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111(5):635–646. doi: 10.1016/s0092-8674(02)01079-6. Epub 2002/12/05. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Chiao YA, Zhang J, Manicone AM, Jin YF, Lindsey ML. Matrix metalloproteinase-28 deletion amplifies inflammatory and extracellular matrix responses to cardiac aging. Microscopy and microanalysis : the official journal of Microscopy Society of America, Microbeam Analysis Society, Microscopical Society of Canada. 2012;18(1):81–90. doi: 10.1017/S1431927611012220. Epub 2011/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE. Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circulation research. 2000;87(5):378–384. doi: 10.1161/01.res.87.5.378. Epub 2000/09/02. [DOI] [PubMed] [Google Scholar]
- 25.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. Journal of leukocyte biology. 1994;55(3):410–422. doi: 10.1002/jlb.55.3.410. Epub 1994/03/01. [DOI] [PubMed] [Google Scholar]
- 26.West DC, Kumar S. The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Experimental cell research. 1989;183(1):179–196. doi: 10.1016/0014-4827(89)90428-x. Epub 1989/07/01. [DOI] [PubMed] [Google Scholar]
- 27.Demirdogen B, Elcin AE, Elcin YM. Neovascularization by bFGF releasing hyaluronic acid-gelatin microspheres: in vitro and in vivo studies. Growth Factors. 2010;28(6):426–436. doi: 10.3109/08977194.2010.508456. Epub 2010/09/22. [DOI] [PubMed] [Google Scholar]
- 28.Bauer AL, Jackson TL, Jiang Y. Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS computational biology. 2009;5(7):e1000445. doi: 10.1371/journal.pcbi.1000445. Epub 2009/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovascular research. 2010;86(2):226–235. doi: 10.1093/cvr/cvq049. Epub 2010/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. The American journal of pathology. 2007;170(6):1807–1816. doi: 10.2353/ajpath.2007.070112. Epub 2007/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yano T, Miura T, Whittaker P, Miki T, Sakamoto J, Nakamura Y, et al. Macrophage colony-stimulating factor treatment after myocardial infarction attenuates left ventricular dysfunction by accelerating infarct repair. Journal of the American College of Cardiology. 2006;47(3):626–634. doi: 10.1016/j.jacc.2005.09.037. Epub 2006/02/07. [DOI] [PubMed] [Google Scholar]
- 32.Riches K, Morley ME, Turner NA, O'Regan DJ, Ball SG, Peers C, et al. Chronic hypoxia inhibits MMP-2 activation and cellular invasion in human cardiac myofibroblasts. Journal of molecular and cellular cardiology. 2009;47(3):391–399. doi: 10.1016/j.yjmcc.2009.06.002. Epub 2009/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacology & therapeutics. 2009;123(2):255–278. doi: 10.1016/j.pharmthera.2009.05.002. Epub 2009/05/23. [DOI] [PubMed] [Google Scholar]
- 34.Lovelock JD, Baker AH, Gao F, Dong JF, Bergeron AL, McPheat W, et al. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. American journal of physiology Heart and circulatory physiology. 2005;288(2):H461–H468. doi: 10.1152/ajpheart.00402.2004. Epub 2005/01/15. [DOI] [PubMed] [Google Scholar]
- 35.Lijnen P, Petrov V, Fagard R. Transforming growth factor-beta 1-mediated collagen gel contraction by cardiac fibroblasts. Journal of the renin-angiotensin-aldosterone system : JRAAS. 2003;4(2):113–118. doi: 10.3317/jraas.2003.011. Epub 2003/06/14. [DOI] [PubMed] [Google Scholar]
- 36.Ma F, Li Y, Jia L, Han Y, Cheng J, Li H, et al. Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF beta/Smad activation and cardiac fibrosis induced by angiotensin II. PloS one. 2012;7(5):e35144. doi: 10.1371/journal.pone.0035144. Epub 2012/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner NA, Das A, Warburton P, O'Regan DJ, Ball SG, Porter KE. Interleukin-1alpha stimulates proinflammatory cytokine expression in human cardiac myofibroblasts. American journal of physiology Heart and circulatory physiology. 2009;297(3):H1117–H1127. doi: 10.1152/ajpheart.00372.2009. Epub 2009/08/04. [DOI] [PubMed] [Google Scholar]
- 38.Zhan S, Chan CC, Serdar B, Rockey DC. Fibronectin stimulates endothelin-1 synthesis in rat hepatic myofibroblasts via a Src/ERK-regulated signaling pathway. Gastroenterology. 2009;136(7):2345–2355. e1–e4. doi: 10.1053/j.gastro.2009.01.062. Epub 2009/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tummalapalli CM, Heath BJ, Tyagi SC. Tissue inhibitor of metalloproteinase-4 instigates apoptosis in transformed cardiac fibroblasts. Journal of cellular biochemistry. 2001;80(4):512–521. doi: 10.1002/1097-4644(20010315)80:4<512::aid-jcb1005>3.0.co;2-n. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 40.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiological reviews. 2012;92(2):635–688. doi: 10.1152/physrev.00008.2011. Epub 2012/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circulation research. 2012;110(8):1125–1138. doi: 10.1161/CIRCRESAHA.111.246108. Epub 2012/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsey ML, Mann DL, Entman ML, Spinale FG. Extracellular matrix remodeling following myocardial injury. Ann Med. 2003;35(5):316–326. doi: 10.1080/07853890310001285. [DOI] [PubMed] [Google Scholar]
- 43.Spinale FG. Myocardial Matrix Remodeling and the Matrix Metalloproteinases: Influence on Cardiac Form and Function. Physiological reviews. 2007;87(4):1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 44.Mieke MHvM. Matrix Metalloproteinases and Collagen Remodeling. BMTE. 2006 [Google Scholar]
- 45.Medugorac I. Characterization of intramuscular collagen in mammalian left ventricle. Basic ResCardiol. 1982;77(6):589–598. doi: 10.1007/BF01908312. [DOI] [PubMed] [Google Scholar]
- 46.Kanzaki Y, Yamauchi Y, Okabe M, Terasaki F, Ishizaka N. Three-dimensional architecture of cardiomyocytes and connective tissues in hypertrophic cardiomyopathy: a scanning electron microscopic observation. Circulation. 2012;125(5):738–739. doi: 10.1161/CIRCULATIONAHA.111.054668. Epub 2012/02/09. [DOI] [PubMed] [Google Scholar]
- 47.Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 48.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147(2):325–338. [PMC free article] [PubMed] [Google Scholar]
- 49.Wei S, Chow LT, Shum IO, Qin L, Sanderson JE. Left and right ventricular collagen type I/III ratios and remodeling post-myocardial infarction. Journal of cardiac failure. 1999;5(2):117–126. doi: 10.1016/s1071-9164(99)90034-9. Epub 1999/07/15. [DOI] [PubMed] [Google Scholar]
- 50.Zimmerman SD, Thomas DP, Velleman SG, Li X, Hansen TR, McCormick RJ. Time course of collagen and decorin changes in rat cardiac and skeletal muscle post-MI. American journal of physiology Heart and circulatory physiology. 2001;281(4):H1816–H1822. doi: 10.1152/ajpheart.2001.281.4.H1816. Epub 2001/09/15. [DOI] [PubMed] [Google Scholar]
- 51.Solomon SD, Pfeffer MA. Renin-angiotensin system and cardiac rupture after myocardial infarction. Circulation. 2002;106(17):2167–2169. doi: 10.1161/01.cir.0000034039.01213.39. Epub 2002/10/23. [DOI] [PubMed] [Google Scholar]
- 52.Sane DC, Mozingo WS, Becker RC. Cardiac rupture after myocardial infarction: new insights from murine models. Cardiology in review. 2009;17(6):293–299. doi: 10.1097/CRD.0b013e3181bf4ab4. Epub 2009/10/16. [DOI] [PubMed] [Google Scholar]
- 53.Fraccarollo D, Galuppo P, Bauersachs J, Ertl G. Collagen accumulation after myocardial infarction: effects of ETA receptor blockade and implications for early remodeling. Cardiovascular research. 2002;54(3):559–567. doi: 10.1016/s0008-6363(02)00256-0. Epub 2002/05/29. [DOI] [PubMed] [Google Scholar]
- 54.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. The Journal of clinical investigation. 2000;106(1):55–62. doi: 10.1172/JCI8768. Epub 2000/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willems IE, Arends JW, Daemen MJ. Tenascin and fibronectin expression in healing human myocardial scars. The Journal of pathology. 1996;179(3):321–325. doi: 10.1002/(SICI)1096-9896(199607)179:3<321::AID-PATH555>3.0.CO;2-8. Epub 1996/07/01. [DOI] [PubMed] [Google Scholar]
- 56.van Dijk A, Niessen HW, Ursem W, Twisk JW, Visser FC, van Milligen FJ. Accumulation of fibronectin in the heart after myocardial infarction: a putative stimulator of adhesion and proliferation of adipose-derived stem cells. Cell and tissue research. 2008;332(2):289–298. doi: 10.1007/s00441-008-0573-0. Epub 2008/02/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulrich MM, Janssen AM, Daemen MJ, Rappaport L, Samuel JL, Contard F, et al. Increased expression of fibronectin isoforms after myocardial infarction in rats. Journal of molecular and cellular cardiology. 1997;29(9):2533–2543. doi: 10.1006/jmcc.1997.0486. Epub 1997/09/23. [DOI] [PubMed] [Google Scholar]
- 58.Mosher DF. A role for fibronectin in self-repair after ischemic injury. Nature medicine. 2001;7(3):290–292. doi: 10.1038/85426. Epub 2001/03/07. [DOI] [PubMed] [Google Scholar]
- 59.Santiago JJ, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, et al. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239(6):1573–1584. doi: 10.1002/dvdy.22280. Epub 2010/05/27. [DOI] [PubMed] [Google Scholar]
- 60.Trial J, Rossen RD, Rubio J, Knowlton AA. Inflammation and ischemia: macrophages activated by fibronectin fragments enhance the survival of injured cardiac myocytes. Exp Biol Med (Maywood) 2004;229(6):538–545. doi: 10.1177/153537020422900612. Epub 2004/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zamilpa R, Lopez EF, Chiao YA, Dai Q, Escobar GP, Hakala K, et al. Proteomic analysis identifies in vivo candidate matrix metalloproteinase-9 substrates in the left ventricle post-myocardial infarction. Proteomics. 2010;10(11):2214–2223. doi: 10.1002/pmic.200900587. Epub 2010/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiao YA, Zamilpa R, Lopez EF, Dai Q, Escobar GP, Hakala K, et al. In vivo matrix metalloproteinase-7 substrates identified in the left ventricle post-myocardial infarction using proteomics. Journal of proteome research. 2010;9(5):2649–2657. doi: 10.1021/pr100147r. Epub 2010/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gronthos S, Simmons PJ, Graves SE, Robey PG. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001;28(2):174–181. doi: 10.1016/s8756-3282(00)00424-5. Epub 2001/02/22. [DOI] [PubMed] [Google Scholar]
- 64.Gu Z, Cui J, Brown S, Fridman R, Mobashery S, Strongin AY, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(27):6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. Epub 2005/07/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dinh W, Bansemir L, Futh R, Nickl W, Stasch JP, Coll-Barroso M, et al. Increased levels of laminin and collagen type VI may reflect early remodelling in patients with acute myocardial infarction. Acta cardiologica. 2009;64(3):329–334. doi: 10.2143/AC.64.3.2038017. Epub 2009/07/15. [DOI] [PubMed] [Google Scholar]
- 66.Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke; a journal of cerebral circulation. 2003;34(9):2165–2170. doi: 10.1161/01.STR.0000088062.86084.F2. Epub 2003/08/09. [DOI] [PubMed] [Google Scholar]
- 67.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. The Journal of clinical investigation. 2005;115(3):599–609. doi: 10.1172/JCI22304. Epub 2005/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Dijk A, Niessen HW, Zandieh Doulabi B, Visser FC, van Milligen FJ. Differentiation of human adipose-derived stem cells towards cardiomyocytes is facilitated by laminin. Cell and tissue research. 2008;334(3):457–467. doi: 10.1007/s00441-008-0713-6. Epub 2008/11/08. [DOI] [PubMed] [Google Scholar]
- 69.Harris BS, Zhang Y, Card L, Rivera LB, Brekken RA, Bradshaw AD. SPARC regulates collagen interaction with cardiac fibroblast cell surfaces. American journal of physiology Heart and circulatory physiology. 2011;301(3):H841–H877. doi: 10.1152/ajpheart.01247.2010. Epub 2011/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCurdy SM, Dai Q, Zhang J, Zamilpa R, Ramirez TA, Dayah T, et al. SPARC mediates early extracellular matrix remodeling following myocardial infarction. American journal of physiology Heart and circulatory physiology. 2011;301(2):H497–H505. doi: 10.1152/ajpheart.01070.2010. Epub 2011/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix biology : journal of the International Society for Matrix Biology. 2000;19(7):569–580. doi: 10.1016/s0945-053x(00)00105-0. Epub 2000/12/05. [DOI] [PubMed] [Google Scholar]
- 72.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. The Journal of clinical investigation. 2001;107(9):1049–1054. doi: 10.1172/JCI12939. Epub 2001/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. The Journal of experimental medicine. 2009;206(1):113–123. doi: 10.1084/jem.20081244. Epub 2008/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McClung HM, Thomas SL, Osenkowski P, Toth M, Menon P, Raz A, et al. SPARC upregulates MT1-MMP expression, MMP-2 activation, and the secretion and cleavage of galectin-3 in U87MG glioma cells. Neuroscience letters. 2007;419(2):172–177. doi: 10.1016/j.neulet.2007.04.037. Epub 2007/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sezaki S, Hirohata S, Iwabu A, Nakamura K, Toeda K, Miyoshi T, et al. Thrombospondin-1 is induced in rat myocardial infarction and its induction is accelerated by ischemia/reperfusion. Exp Biol Med (Maywood) 2005;230(9):621–630. doi: 10.1177/153537020523000904. Epub 2005/09/24. [DOI] [PubMed] [Google Scholar]
- 76.Chatila K, Ren G, Xia Y, Huebener P, Bujak M, Frangogiannis NG. The role of the thrombospondins in healing myocardial infarcts. Cardiovascular & hematological agents in medicinal chemistry. 2007;5(1):21–27. doi: 10.2174/187152507779315813. Epub 2007/02/03. [DOI] [PubMed] [Google Scholar]
- 77.Scott JE. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cavalcante FSA, Ito S, Brewer K, Sakai H, Alencar AM, Almeida MP, et al. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol. 2005;98(2):672–679. doi: 10.1152/japplphysiol.00619.2004. [DOI] [PubMed] [Google Scholar]
- 79.Campbell PH, Hunt DL, Jones Y, Harwood F, Amiel D, Omens JH, et al. Effects of biglycan deficiency on myocardial infarct structure and mechanics. Mol Cell Biomech. 2008;5(1):27–35. [PMC free article] [PubMed] [Google Scholar]
- 80.Takemoto S, Murakami T, Kusachi S, Iwabu A, Hirohata S, Nakamura K, et al. Increased expression of dermatopontin mRNA in the infarct zone of experimentally induced myocardial infarction in rats: comparison with decorin and type I collagen mRNAs. Basic Res Cardiol. 2002;97(6):461–468. doi: 10.1007/s00395-002-0371-x. [DOI] [PubMed] [Google Scholar]
- 81.Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nature medicine. 1996;2(4):418–423. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Brown LF, Laham RJ, Volk R, Simons M. Macrophage-dependent regulation of syndecan gene expression. Circulation research. 1997;81(5):785–796. doi: 10.1161/01.res.81.5.785. [DOI] [PubMed] [Google Scholar]
- 83.Matsui Y, Ikesue M, Danzaki K, Morimoto J, Sato M, Tanaka S, et al. Syndecan-4 prevents cardiac rupture and dysfunction after myocardial infarction. Circulation research. 2011;108(11):1328–1339. doi: 10.1161/CIRCRESAHA.110.235689. [DOI] [PubMed] [Google Scholar]
- 84.Cleutjens JPM, Blankesteijn WM, Daemen MJAP, Smits JFM. The infarcted myocardium: Simply dead tissue, or a lively target for therapeutic interventions. Cardiovascular research. 1999;44(2):232–241. doi: 10.1016/s0008-6363(99)00212-6. [DOI] [PubMed] [Google Scholar]
- 85.Lamblin N, Bauters C, Hermant X, Lablanche JM, Helbecque N, Amouyel P. Polymorphisms in the promoter regions of MMP-2, MMP-3, MMP-9 and MMP-12 genes as determinants of aneurysmal coronary artery disease. Journal of the American College of Cardiology. 2002;40(1):43–48. doi: 10.1016/s0735-1097(02)01909-5. Epub 2002/07/10. [DOI] [PubMed] [Google Scholar]
- 86.Kelly D, Khan S, Cockerill G, Ng LL, Thompson M, Samani NJ, et al. Circulating stromelysin-1 (MMP-3): a novel predictor of LV dysfunction, remodelling and all-cause mortality after acute myocardial infarction. European journal of heart failure. 2008;10(2):133–139. doi: 10.1016/j.ejheart.2007.12.009. Epub 2008/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, et al. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006;113(25):2919–2928. doi: 10.1161/CIRCULATIONAHA.106.612960. Epub 2006/06/14. [DOI] [PubMed] [Google Scholar]
- 88.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nature reviews Cardiology. 2010;7(1):30–37. doi: 10.1038/nrcardio.2009.199. Epub 2009/12/02. [DOI] [PubMed] [Google Scholar]
- 89.Hodgson J. Remodeling MMPIs. Biotechnology (N Y) 1995;13(6):554–557. doi: 10.1038/nbt0695-554. Epub 1995/06/01. [DOI] [PubMed] [Google Scholar]
- 90.Bachmann LH, Stephens J, Richey CM, Hook EW., 3rd Measured versus self-reported compliance with doxycycline therapy for chlamydia-associated syndromes: high therapeutic success rates despite poor compliance. Sexually transmitted diseases. 1999;26(5):272–278. doi: 10.1097/00007435-199905000-00006. Epub 1999/05/20. [DOI] [PubMed] [Google Scholar]
- 91.Renkiewicz R, Qiu L, Lesch C, Sun X, Devalaraja R, Cody T, et al. Broad-spectrum matrix metalloproteinase inhibitor marimastat-induced musculoskeletal side effects in rats. Arthritis and rheumatism. 2003;48(6):1742–1749. doi: 10.1002/art.11030. Epub 2003/06/10. [DOI] [PubMed] [Google Scholar]
- 92.Skiles JW, Gonnella NC, Jeng AY. The design, structure, and therapeutic application of matrix metalloproteinase inhibitors. Current medicinal chemistry. 2001;8(4):425–474. doi: 10.2174/0929867013373417. Epub 2001/02/15. [DOI] [PubMed] [Google Scholar]
- 93.Whittaker M, Floyd CD, Brown P, Gearing AJ. Design and therapeutic application of matrix metalloproteinase inhibitors. Chemical reviews. 2001;101(7):2205–2206. doi: 10.1021/cr0100345. (Chem. Rev. 1999, 99, 2735–2776. Published on the web september 8, 1999). Epub 2001/12/26. [DOI] [PubMed] [Google Scholar]
- 94.Kontogiorgis CA, Papaioannou P, Hadjipavlou-Litina DJ. Matrix metalloproteinase inhibitors: a review on pharmacophore mapping and (Q)SARs results. Current medicinal chemistry. 2005;12(3):339–355. doi: 10.2174/0929867053363243. Epub 2005/02/23. [DOI] [PubMed] [Google Scholar]
- 95.Sarkhel S, Desiraju GR. N-H...O, O-H...O, and C-H...O hydrogen bonds in protein-ligand complexes: strong and weak interactions in molecular recognition. Proteins. 2004;54(2):247–259. doi: 10.1002/prot.10567. Epub 2003/12/30. [DOI] [PubMed] [Google Scholar]
- 96.Makitalo L, Kolho KL, Karikoski R, Anthoni H, Saarialho-Kere U. Expression profiles of matrix metalloproteinases and their inhibitors in colonic inflammation related to pediatric inflammatory bowel disease. Scandinavian journal of gastroenterology. 2010;45(78):862–871. doi: 10.3109/00365520903583863. Epub 2010/04/07. [DOI] [PubMed] [Google Scholar]
- 97.Kim DH, Mobashery S. Mechanism-based inhibition of zinc proteases. Current medicinal chemistry. 2001;8(8):959–965. doi: 10.2174/0929867013372805. Epub 2001/05/29. [DOI] [PubMed] [Google Scholar]
- 98.Kleifeld O, Kotra LP, Gervasi DC, Brown S, Bernardo MM, Fridman R, et al. X-ray absorption studies of human matrix metalloproteinase-2 (MMP-2) bound to a highly selective mechanism-based inhibitor. comparison with the latent and active forms of the enzyme. The Journal of biological chemistry. 2001;276(20):17125–17131. doi: 10.1074/jbc.M011604200. Epub 2001/03/30. [DOI] [PubMed] [Google Scholar]
- 99.Peterson JT. Matrix metalloproteinase inhibitor development and the remodeling of drug discovery. Heart failure reviews. 2004;9(1):63–79. doi: 10.1023/B:HREV.0000011395.11179.af. Epub 2004/01/24. [DOI] [PubMed] [Google Scholar]
- 100.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke; a journal of cerebral circulation. 2002;33(3):831–836. doi: 10.1161/hs0302.104542. Epub 2002/03/02. [DOI] [PubMed] [Google Scholar]
- 101.Tziakas DN, Chalikias GK, Parissis JT, Hatzinikolaou EI, Papadopoulos ED, Tripsiannis GA, et al. Serum profiles of matrix metalloproteinases and their tissue inhibitor in patients with acute coronary syndromes. The effects of short-term atorvastatin administration. International journal of cardiology. 2004;94(23):269–277. doi: 10.1016/j.ijcard.2003.05.013. Epub 2004/04/20. [DOI] [PubMed] [Google Scholar]
- 102.Heo JH, Kim SH, Lee KY, Kim EH, Chu CK, Nam JM. Increase in plasma matrix metalloproteinase-9 in acute stroke patients with thrombolysis failure. Stroke; a journal of cerebral circulation. 2003;34(6):e48–e50. doi: 10.1161/01.STR.0000073788.81170.1C. Epub 2003/05/17. [DOI] [PubMed] [Google Scholar]
- 103.Nutescu EA, Shapiro NL, Chevalier A, Amin AN. A pharmacologic overview of current and emerging anticoagulants. Cleveland Clinic journal of medicine. 2005;72(Suppl 1):S2–S6. doi: 10.3949/ccjm.72.suppl_1.s2. Epub 2005/04/28. [DOI] [PubMed] [Google Scholar]
- 104.Nutescu EA, Shapiro NL, Chevalier A. New anticoagulant agents: direct thrombin inhibitors. Cardiology clinics. 2008;26(2):169–187. v–vi. doi: 10.1016/j.ccl.2007.12.005. Epub 2008/04/15. [DOI] [PubMed] [Google Scholar]
- 105.Rababah M, Worthmann H, Deb M, Tryc AB, Ma YT, El Bendary OM, et al. Anticoagulants affect matrix metalloproteinase 9 levels in blood samples of stroke patients and healthy controls. Clinical biochemistry. 2012;45(6):483–489. doi: 10.1016/j.clinbiochem.2012.01.028. Epub 2012/02/22. [DOI] [PubMed] [Google Scholar]
- 106.Castellazzi M, Tamborino C, Fainardi E, Manfrinato MC, Granieri E, Dallocchio F, et al. Effects of anticoagulants on the activity of gelatinases. Clinical biochemistry. 2007;40(16–17):1272–1276. doi: 10.1016/j.clinbiochem.2007.08.011. Epub 2007/10/02. [DOI] [PubMed] [Google Scholar]
- 107.Mannello F, Jung K, Tonti GA, Canestrari F. Heparin affects matrix metalloproteinases and tissue inhibitors of metalloproteinases circulating in peripheral blood. Clinical biochemistry. 2008;41(18):1466–1473. doi: 10.1016/j.clinbiochem.2008.09.104. Epub 2008/10/18. [DOI] [PubMed] [Google Scholar]
- 108.Pfeffer MA. ACE inhibitors in acute myocardial infarction: patient selection and timing. Circulation. 1998;97(22):2192–2194. doi: 10.1161/01.cir.97.22.2192. Epub 1998/06/19. [DOI] [PubMed] [Google Scholar]
- 109.Yamamoto D, Takai S. Pharmacological implications of MMP-9 inhibition by ACE inhibitors. Current medicinal chemistry. 2009;16(11):1349–1354. doi: 10.2174/092986709787846514. Epub 2009/04/10. [DOI] [PubMed] [Google Scholar]
- 110.Yamamoto D, Takai S, Miyazaki M. Prediction of interaction mode between a typical ACE inhibitor and MMP-9 active site. Biochemical and biophysical research communications. 2007;354(4):981–984. doi: 10.1016/j.bbrc.2007.01.088. Epub 2007/02/06. [DOI] [PubMed] [Google Scholar]
- 111.Yamamoto D, Takai S, Miyazaki M. Inhibitory profiles of captopril on matrix metalloproteinase-9 activity. European journal of pharmacology. 2008;588(23):277–279. doi: 10.1016/j.ejphar.2008.04.031. Epub 2008/05/27. [DOI] [PubMed] [Google Scholar]
- 112.Miyazaki S, Kasai T, Miyauchi K, Miyazaki T, Akimoto Y, Takagi A, et al. Changes of matrix metalloproteinase-9 level is associated with left ventricular remodeling following acute myocardial infarction among patients treated with trandolapril, valsartan or both. Circulation journal : official journal of the Japanese Circulation Society. 2010;74(6):1158–1164. doi: 10.1253/circj.cj-09-0412. Epub 2010/04/10. [DOI] [PubMed] [Google Scholar]
- 113.Harada K, Sugaya T, Murakami K, Yazaki Y, Komuro I. Angiotensin II type 1A receptor knockout mice display less left ventricular remodeling and improved survival after myocardial infarction. Circulation. 1999;100(20):2093–2039. doi: 10.1161/01.cir.100.20.2093. Epub 1999/11/16. [DOI] [PubMed] [Google Scholar]
- 114.Cipollone F, Fazia M, Iezzi A, Pini B, Cuccurullo C, Zucchelli M, et al. Blockade of the angiotensin II type 1 receptor stabilizes atherosclerotic plaques in humans by inhibiting prostaglandin E2-dependent matrix metalloproteinase activity. Circulation. 2004;109(12):1482–1488. doi: 10.1161/01.CIR.0000121735.52471.AC. Epub 2004/03/24. [DOI] [PubMed] [Google Scholar]
- 115.Brassard P, Amiri F, Schiffrin EL. Combined angiotensin II type 1 and type 2 receptor blockade on vascular remodeling and matrix metalloproteinases in resistance arteries. Hypertension. 2005;46(3):598–606. doi: 10.1161/01.HYP.0000176744.15592.7d. Epub 2005/07/27. [DOI] [PubMed] [Google Scholar]
- 116.Yamashita C, Hayashi T, Mori T, Tazawa N, Kwak CJ, Nakano D, et al. Angiotensin II receptor blocker reduces oxidative stress and attenuates hypoxia-induced left ventricular remodeling in apolipoprotein E-knockout mice. Hypertension research : official journal of the Japanese Society of Hypertension. 2007;30(12):1219–1230. doi: 10.1291/hypres.30.1219. Epub 2008/03/18. [DOI] [PubMed] [Google Scholar]
- 117.Yang D, Ma S, Li D, Tang B, Yang Y. Angiotensin II receptor blockade improves matrix metalloproteinases/tissue inhibitor of matrix metalloproteinase-1 balance and restores fibronectin expression in rat infarcted myocardium. Biochemical and biophysical research communications. 2009;388(3):606–611. doi: 10.1016/j.bbrc.2009.08.073. Epub 2009/08/22. [DOI] [PubMed] [Google Scholar]
- 118.Excellence NIfHaC. Prophylaxis for patients who have experienced a myocardial infarction. 2011 [Google Scholar]
- 119.Rude MK, Duhaney T-AS, Kuster GM, Judge S, Heo J, Colucci WS, et al. Aldosterone stimulates matrix metalloproteinases and reactive oxygen species in adult rat ventricular cardiomyocytes. Hypertension. 2005;46(3):555–561. doi: 10.1161/01.HYP.0000176236.55322.18. [DOI] [PubMed] [Google Scholar]
- 120.Mill JG, Milanez Mda C, de Resende MM, Gomes Mda G, Leite CM. Spironolactone prevents cardiac collagen proliferation after myocardial infarction in rats. Clinical and experimental pharmacology & physiology. 2003;30(10):739–744. doi: 10.1046/j.1440-1681.2003.03906.x. Epub 2003/10/01. [DOI] [PubMed] [Google Scholar]
- 121.Ceron CS, Castro MM, Rizzi E, Montenegro MF, Fontana V, Salgado MC, et al. Spironolactone and hydrochlorothiazide exert antioxidant effects and reduce vascular matrix metalloproteinase-2 activity and expression in a model of renovascular hypertension. British journal of pharmacology. 2010;160(1):77–87. doi: 10.1111/j.1476-5381.2010.00678.x. Epub 2010/03/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li MJ, Huang CX, Okello E, Yanhong T, Mohamed S. Treatment with spironolactone for 24 weeks decreases the level of matrix metalloproteinases and improves cardiac function in patients with chronic heart failure of ischemic etiology. The Canadian journal of cardiology. 2009;25(9):523–526. doi: 10.1016/s0828-282x(09)70138-2. Epub 2009/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bernstein M, Tyagi SC. b-Blocker Improves Cardiac Function by Reducing Oxidative Stress and Metalloproteinase Activity After Myocardial Infarction. J Appl Res. 2001;1(2) Epub 2001. [Google Scholar]
- 124.Ohtsuka T, Hamada M, Saeki H, Ogimoto A, Hara Y, Shigematsu Y, et al. Serum levels of matrix metalloproteinases and tumor necrosis factor-alpha in patients with idiopathic dilated cardiomyopathy and effect of carvedilol on these levels. The American journal of cardiology. 2003;91(8):1024–1047. A8. doi: 10.1016/s0002-9149(03)00133-4. Epub 2003/04/11. [DOI] [PubMed] [Google Scholar]
- 125.Pauschinger M, Rutschow S, Chandrasekharan K, Westermann D, Weitz A, Peter Schwimmbeck L, et al. Carvedilol improves left ventricular function in murine coxsackievirus-induced acute myocarditis association with reduced myocardial interleukin-1beta and MMP-8 expression and a modulated immune response. European journal of heart failure. 2005;7(4):444–452. doi: 10.1016/j.ejheart.2004.07.002. Epub 2005/06/01. [DOI] [PubMed] [Google Scholar]
- 126.Wu TC, Chen YH, Leu HB, Chen YL, Lin FY, Lin SJ, et al. Carvedilol, a pharmacological antioxidant, inhibits neointimal matrix metalloproteinase-2 and-9 in experimental atherosclerosis. Free radical biology & medicine. 2007;43(11):1508–1522. doi: 10.1016/j.freeradbiomed.2007.08.010. Epub 2007/10/30. [DOI] [PubMed] [Google Scholar]
- 127.Cimmino G, Ibanez B, Giannarelli C, Prat-Gonzalez S, Hutter R, Garcia M, et al. Carvedilol administration in acute myocardial infarction results in stronger inhibition of early markers of left ventricular remodeling than metoprolol. International journal of cardiology. 2011;153(3):256–261. doi: 10.1016/j.ijcard.2010.08.018. Epub 2010/09/25. [DOI] [PubMed] [Google Scholar]
- 128.Senzaki H, Paolocci N, Gluzband YA, Lindsey ML, Janicki JS, Crow MT, et al. beta-blockade prevents sustained metalloproteinase activation and diastolic stiffening induced by angiotensin II combined with evolving cardiac dysfunction. Circulation research. 2000;86(7):807–815. doi: 10.1161/01.res.86.7.807. Epub 2000/04/14. [DOI] [PubMed] [Google Scholar]
- 129.Song G, Hennessy M, Zhao YL, Li Q, Han WD, Qi Y, et al. Adrenoceptor blockade alters plasma gelatinase activity in patients with heart failure and MMP-9 promoter activity in a human cell line (ECV304) Pharmacological research : the official journal of the Italian Pharmacological Society. 2006;54(1):57–64. doi: 10.1016/j.phrs.2006.02.006. Epub 2006/04/01. [DOI] [PubMed] [Google Scholar]
- 130.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nature medicine. 2002;8(11):1257–1262. doi: 10.1038/nm1102-1257. Epub 2002/11/02. [DOI] [PubMed] [Google Scholar]
- 131.Koh KK. Effects of statins on vascular wall: vasomotor function, inflammation, and plaque stability. Cardiovascular research. 2000;47(4):648–657. doi: 10.1016/s0008-6363(00)00146-2. Epub 2000/09/07. [DOI] [PubMed] [Google Scholar]
- 132.Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103(2):276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 133.Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2003;23(5):769–775. doi: 10.1161/01.ATV.0000068646.76823.AE. Epub 2003/03/29. [DOI] [PubMed] [Google Scholar]
- 134.Arikan MC, Shapiro SD, Mariani TJ. Induction of macrophage elastase (MMP-12) gene expression by statins. Journal of cellular physiology. 2005;204(1):139–145. doi: 10.1002/jcp.20271. Epub 2004/12/18. [DOI] [PubMed] [Google Scholar]
- 135.Yasuda S, Miyazaki S, Kinoshita H, Nagaya N, Kanda M, Goto Y, et al. Enhanced cardiac production of matrix metalloproteinase-2 and-9 and its attenuation associated with pravastatin treatment in patients with acute myocardial infarction. Clin Sci (Lond) 2007;112(1):43–49. doi: 10.1042/CS20060110. Epub 2006/08/31. [DOI] [PubMed] [Google Scholar]
- 136.Li J, Zhao SP, Peng DQ, Xu ZM, Zhou HN. Early effect of pravastatin on serum soluble CD40L, matrix metalloproteinase-9, and C-reactive protein in patients with acute myocardial infarction. Clinical chemistry. 2004;50(9):1696–1699. doi: 10.1373/clinchem.2003.030940. Epub 2004/07/22. [DOI] [PubMed] [Google Scholar]
- 137.Nakaya R, Uzui H, Shimizu H, Nakano A, Mitsuke Y, Yamazaki T, et al. Pravastatin suppresses the increase in matrix metalloproteinase-2 levels after acute myocardial infarction. International journal of cardiology. 2005;105(1):67–73. doi: 10.1016/j.ijcard.2004.12.024. Epub 2005/10/07. [DOI] [PubMed] [Google Scholar]
- 138.Turner NA, Aley PK, Hall KT, Warburton P, Galloway S, Midgley L, et al. Simvastatin inhibits TNFalpha-induced invasion of human cardiac myofibroblasts via both MMP-9-dependent and -independent mechanisms. Journal of molecular and cellular cardiology. 2007;43(2):168–176. doi: 10.1016/j.yjmcc.2007.05.006. Epub 2007/06/15. [DOI] [PubMed] [Google Scholar]
- 139.Pan M-R, Hung W-C. Nonsteroidal anti-inflammatory drugs inhibit matrix metalloproteinase-2 via suppression of the ERK/Sp1-mediated transcription. The Journal of biological chemistry. 2002;277(36):32775–32780. doi: 10.1074/jbc.M202334200. [DOI] [PubMed] [Google Scholar]
- 140.Mehta JL, Chen J, Yu F, Li DY. Aspirin inhibits ox-LDL-mediated LOX-1 expression and metalloproteinase-1 in human coronary endothelial cells. Cardiovascular research. 2004;64(2):243–249. doi: 10.1016/j.cardiores.2004.07.002. Epub 2004/10/16. [DOI] [PubMed] [Google Scholar]
- 141.Kiran MS, Sameer Kumar VB, Viji RI, Sudhakaran PR. Temporal relationship between MMP production and angiogenic process in HUVECs. Cell biology international. 2006;30(9):704–713. doi: 10.1016/j.cellbi.2006.05.001. Epub 2006/07/11. [DOI] [PubMed] [Google Scholar]
- 142.Babine RE, Bender SL. Molecular Recognition of Proteinminus signLigand Complexes: Applications to Drug Design. Chemical reviews. 1997;97(5):1359–1472. doi: 10.1021/cr960370z. Epub 1997/08/05. [DOI] [PubMed] [Google Scholar]
- 143.Hu J, Van den Steen PE, Sang Q-XA, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6(6):480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 144.Shen XH, Chen Q, Shi Y, Li HW. Association of neutrophil/lymphocyte ratio with long-term mortality after ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Chinese medical journal. 2010;123(23):3438–3443. Epub 2011/12/15. [PubMed] [Google Scholar]
- 145.Nenan S, Boichot E, Lagente V, Bertrand CP. Macrophage elastase (MMP-12): a pro-inflammatory mediator? Memorias do Instituto Oswaldo Cruz. 2005;100(Suppl 1):167–172. doi: 10.1590/s0074-02762005000900028. Epub 2005/06/18. [DOI] [PubMed] [Google Scholar]
- 146.Klingenberg R, Luscher TF. Inflammation in Coronary Artery Disease and Acute Myocardial Infarction - is the Stage Set for Novel Therapies? Current pharmaceutical design. 2012 doi: 10.2174/138161212802481219. Epub 2012/03/07. [DOI] [PubMed] [Google Scholar]
- 147.Khandoga A, Kessler JS, Hanschen M, Khandoga AG, Burggraf D, Reichel C, et al. Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. Journal of leukocyte biology. 2006;79(6):1295–1305. doi: 10.1189/jlb.0805468. Epub 2006/03/23. [DOI] [PubMed] [Google Scholar]
- 148.Khan HA, Alhomida AS, Sobki SH, Moghairi AA, Koronki HE. Blood cell counts and their correlation with creatine kinase and C-reactive protein in patients with acute myocardial infarction. International journal of clinical and experimental medicine. 2012;5(1):50–55. Epub 2012/02/14. [PMC free article] [PubMed] [Google Scholar]
- 149.Granfeldt A, Jiang R, Wang NP, Mykytenko J, Eldaif S, Deneve J, et al. Neutrophil inhibition contributes to cardioprotection by postconditioning. Acta anaesthesiologica Scandinavica. 2012;56(1):48–56. doi: 10.1111/j.1399-6576.2011.02577.x. Epub 2011/11/23. [DOI] [PubMed] [Google Scholar]
- 150.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. American Journal of Physiology-Cell Physiology. 1999;277(1):C1–C19. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 151.Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. CurrOpinBiotechnol. 2003;14(5):538–546. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 152.Hinz B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. European journal of cell biology. 2006;85(34):175–181. doi: 10.1016/j.ejcb.2005.09.004. Epub 2006/03/21. [DOI] [PubMed] [Google Scholar]