Review on the role of cellular stress proteins in innate immune signaling responses during infection and inflammation.
Keywords: PRR signaling, innate immune response, oxidative stress, heat shock, ER stress, DNA damage
Abstract
Extensive research in the past decade has identified innate immune recognition receptors and intracellular signaling pathways that culminate in inflammatory responses. Besides its role in cytoprotection, the importance of cell stress in inflammation and host defense against pathogens is emerging. Recent studies have shown that proteins in cellular stress responses, including the heat shock response, ER stress response, and DNA damage response, interact with and regulate signaling intermediates involved in the activation of innate and adaptive immune responses. The effect of such regulation by cell stress proteins may dictate the inflammatory profile of the immune response during infection and disease. In this review, we describe the regulation of innate immune cell activation by cell stress pathways, present detailed descriptions of the types of stress response proteins and their crosstalk with immune signaling intermediates that are essential in host defense, and illustrate the relevance of these interactions in diseases characteristic of aberrant immune responses, such as chronic inflammatory diseases, autoimmune disorders, and cancer. Understanding the crosstalk between cellular stress proteins and immune signaling may have translational implications for designing more effective regimens to treat immune disorders.
The innate and adaptive immune system defends the body against a multitude of pathogens and infections. The first line of the host defense is provided by the innate immune system and comprises cell types such as macrophages, DCs, and NK cells that express PRRs, including TLRs, NLRs, and RLRs, on their cell surface, which can recognize PAMPs on bacterial and viral pathogens [1, 2]. Upon recognition of PAMPs by PRRs, distinct signaling pathways are initiated, culminating in induction of inflammatory mediators including proinflammatory cytokines and chemokines and type I and II IFNs, essential in host defense. Recruitment and activation of adaptive immune cells including T and B cells by these chemokines and cytokines, results in inflammation, elimination of pathogens, and clearance of infection [3, 4]. Increasing evidence suggests a link between the cellular redox state, stress proteins, and immune activation. The fundamental role of PRRs in host defense and their regulation by cellular stress pathways, often activated in infections and chronic diseases, raise interest in the crosstalk between these pathways as targets for clinical applications.
Cellular stress responses, originally identified as survival mechanisms activated by cells in response to stressful stimuli, can be classified as the oxidative stress response, heat shock response, UPR, and DDR, depending on the type of stress [5]. Although key proteins in these stress pathways have been extensively characterized in cytoprotection, their crosstalk with innate immune signaling pathways and their role in the regulation of host defense are less clear. Understanding the function of these stress proteins in innate immune defenses, particularly in a setting of disease or infection, may provide a better understanding of disease pathogenesis and present novel targets for manipulation of immune responses in therapeutic treatments. The goal of this review is to illustrate the crosstalk between the major players in innate immune signaling pathways and cellular stress response pathways and to describe the clinical relevance of this crosstalk in translational therapeutic strategies.
We will first describe the TLR, NLR, and RLR signaling pathways and the cytokine and chemokine mediators involved in host innate immune defenses. Then, we will enumerate the major proteins involved in each of the stress responses and their crosstalk with the immune signaling pathways and describe the importance of this crosstalk in the context of infection and immune and inflammatory disorders.
INNATE IMMUNE RESPONSES DURING INFECTION AND INFLAMMATION
The innate immune system provides the first line of host defense against bacterial or viral infection and disease. The recognition of pathogens by PRRs induces activation of specific signaling pathways in innate immune cells. Downstream signaling causes the secretion of microbicidal effectors and inflammatory mediators, resulting in a protective response against the pathogen. This innate immune response, comprising the various cell types, PRR signaling pathways, and inflammatory cytokines, is reviewed below.
Cells involved in innate immune responses
Mast cells, neutrophils, monocytes, macrophages, DCs, and NK cells [6] are all involved in innate immune responses. Each of these cell subsets mounts specific responses and performs distinct functions for the induction of inflammation during the clearance of an infection. One of the mechanisms used by the immune cells for the clearance of pathogens is oxidative stress caused by production of ROS and RNS [7]. Neutrophils are the most abundant innate immune effector cells and are recruited rapidly to the site of infection and inflammation. They eliminate pathogens by phagocytosis or release of granules containing oxidizing agents such as NADPH oxidase, which can generate superoxide and other free oxygen radicals, resulting in oxidative stress. ROS and RNS produced by neutrophils serve dual functions: they act as potent antimicrobial agents that facilitate microbial killing by activating signaling pathways involved in inflammatory and immune responses, and they disrupt homeostasis. Disruption of the equilibrium between microbial pathogens and neutrophils results in a proinflammatory cascade induced by ROS-mediated TLR activation [8]. Thus, the redox state of neutrophils can shift the balance and generate a hyperinflammatory state. Nonphagocytic NK cells kill infected cells by the release of pore-forming proteins called perforins and proteolytic enzymes called granzymes. The NK cells monitor and recognize infected host cells by their low surface expression of the self protein MHC I. Host cells expressing peptide derived from the stress protein Hsp60, which is bound to MHC I, have also been shown to activate NK cells, suggesting a mechanism for recognition and killing of stressed cells by NK cells mediated by Hsps [9].
The most prominent cells of the innate immune system are the monocytes, macrophages, and DCs, which recognize PAMPs through PRRs, such as TLRs, NLRs, and RLRs, and can engulf and phagocytose pathogens directly to degrade them intracellularly. Similar to neutrophils, these phagocytes subject the endocytosed pathogen to lysosomal digestion by enzymes and ROS and RNS production by NADPH oxidase and NO synthase [7]. The combination of ROS superoxide and RNS NO forms peroxynitrite, which is extremely bactericidal and toxic, thus enabling pathogen killing. Macrophages also work though TLR-induced mitochondrial ROS for clearance of bacteria [10]. In addition, macrophages and dendritic cells play an important role as APCs, wherein they present antigens or peptides from the intracellularly degraded pathogenic proteins on their cell surfaces to adaptive immune cells, such as T and B cells. Although ROS mediates pathogen killing, oxidative stress can impair intracellular events, such as antigen processing and generation of peptides [11, 12]. Experimental data on the effect of ER stress on antigen presentation by macrophages has been contradictory. Increased degradation of cytosolic proteins has been shown to increase MHC I-bound antigen presentation, whereas in other cases, ER stress seems to impair antigen presentation by macrophages affecting subsequent T-cell activation, suggesting a mechanism of immune evasion via the ER stress response [13–15]. In the setting of an infection, recognition of pathogenic antigen on APCs in the presence of costimulatory molecules activates T and B cells and thus initiates an adaptive immune response to maintain the host defense. Thus, the APCs bridge the gap between innate and adaptive immunity by initial recognition of the pathogen, which is critical for appropriate immune responses.
PRR signaling pathways in innate immune cells
Innate immune cells recognize broad groups of pathogens, such as bacteria and viruses, through components that are conserved within each group. The different groups of PRRs, including the TLRs, the cytoplasmic NLRs and RLRs, recognize a large variety of PAMPs and thus enable responses to a broad spectrum of pathogens.
Toll-like receptors.
Of these PPRs, the TLRs and their downstream signaling pathways have been the most extensively studied [16–21] (Figs. 1 and 2). The TLR is a membrane-spanning receptor consisting of an extracellular domain containing leucine-rich repeats and a cytoplasmic signaling Toll/IL-1R (TIR) domain. To date, 11 TLRs have been identified, and TLR1 to -10 have been characterized in humans. TLR1, -2, -4, -5, and -6, which recognize bacterial components, such as lipopeptides, LPS, and flagellin, are expressed on the cell surface, and TLR3, -7/8, and -9, which recognize viral double-stranded DNA, single-stranded RNA, and CpG DNA, respectively, are endosomal. In addition to bacterial and viral ligands, TLRs are activated by DAMPs, which are endogenous or exogenous molecules released by cells during stress [22, 23]. For example, Hsps are DAMPs that can bind to TLR2 and -4 to upregulate the expression of costimulatory molecules and induce release of cytokines [24]. TLR engagement by ligands can result in the activation of NADPH oxidase and production of ROS in neutrophils and macrophages [25–27]. ROS, in turn, mediate release of other DAMPs and promote sterile inflammation [28]. For example, ROS-induced oxidation of extracellular matrix proteins also generates DAMPs such as heparan sulfate [29]. TLR4-induced ROS-mediated release of the DAMP HMGB1 protein has been found in liver [30].
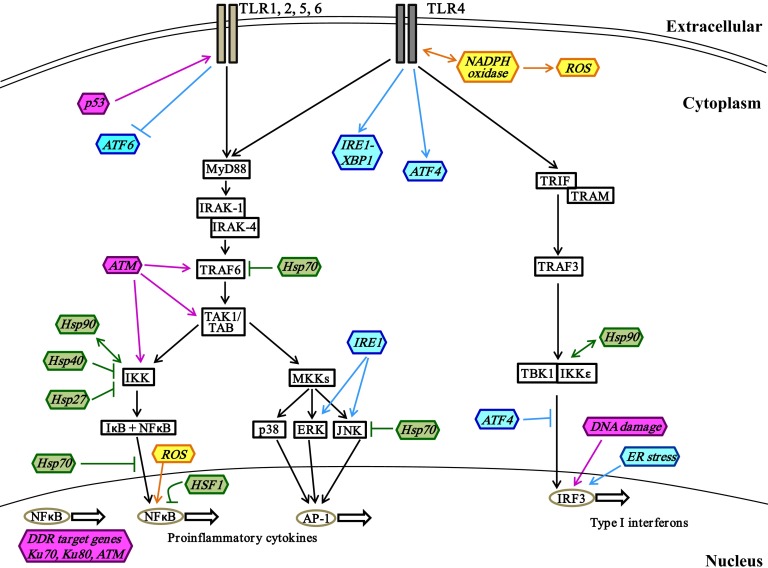
Figure 1. Interactions of stress proteins and cell surface TLR signaling pathways.
Engagement of cell surface TLR1, -2, -4, -5, or -6 initiates the MyD88-dependent signaling pathway, which results in activation of the downstream IKK and MKK signaling branches. Each of these branches induces expression of proinflammatory cytokines such as TNFα, IL-6, and IL-1β by the transcription factors NF-κB and AP-1. The TRIF-dependent signaling branch downstream of TLR4 results in expression of type I IFNs mediated by IRF3. The regulatory roles of stress proteins important in oxidative stress (yellow), heat shock response (green), UPR (blue), and DDR (pink) in these TLR signaling pathways are illustrated. →, stimulation; ↔, direct interaction; ⫞, inhibition.
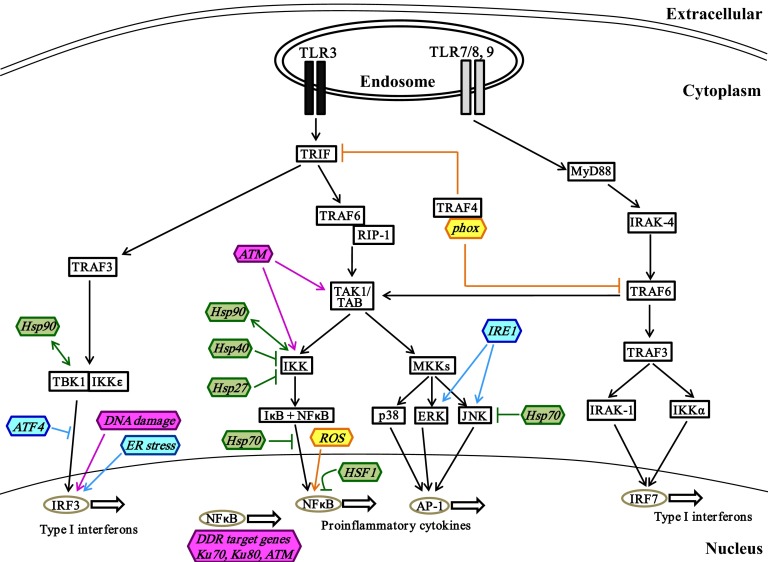
Figure 2. Interactions of stress proteins and endosomal TLR signaling pathways.
Endosomal TLR7/8 or -9 activation induces the expression of type I IFNs such as IFNα and -β via MyD88-dependent activation of IRF7, in addition to NF-κB- and AP-1-induced proinflammatory cytokines. The TRIF-dependent signaling branch downstream of TLR3 results in expression of type I IFNs mediated by IRF3. The regulatory roles of stress proteins important in oxidative stress (yellow), heat shock response (green), UPR (blue), and DDR (pink) in these TLR signaling pathways are illustrated. →, stimulation; ↔, direct interaction; ⫞, inhibition.
TLRs can be classified based on cellular localization, which correlates with the ligands recognized, as cell surface (membrane-bound) (Fig. 1) and endosomal (Fig. 2). TLR signaling is divided into two distinct intracellular pathways: the MyD88 dependent pathway, which is used by all TLRs except TLR3, and the MyD88-independent pathway, which is downstream of TLR3 and -4 [16, 19].
Membrane-bound TLRs.
Upon recognition of the appropriate PAMP or DAMP, cell surface TLR dimerization occurs, and downstream signaling pathways are activated (Fig. 1). In the MyD88-dependent pathway, TLR activation results in recruitment of MyD88 to the receptor via the TIR domain. MyD88 in turn recruits and activates IRAK-4 and -1 through its death domain. The IRAKs can then phosphorylate and activate TRAF6, which in turn activates a complex containing TAK1 and TAB-1, -2, and -3 [31–33]. This complex triggers the activation of two signaling branches: the NF-κB pathway and the MAPK pathway [34]. The TAK1 complex phosphorylates and activates the IKK complex, which comprises IKKα, IKKβ, and NEMO [31]. The transcription factor NF-κB, sequestered in the cytoplasm by inhibitory IκB, is released and translocated to the nucleus for subsequent activation of gene transcription as a result of phosphorylation of IκB by IKK [35–38]. The MAPK signaling pathway is triggered by the activation of MKK3/6 and -4/7 (by TAK1) and of MEK1/2 (by Tpl2 kinase downstream of IKK), which phosphorylate downstream molecules such as p38, JNK, and ERK—MAPKs that activate the transcription factor AP-1 [39, 40]. The transcription factors NF-κB and AP-1 promote expression of proinflammatory cytokine genes, such as TNFα, IL-6, and IL-1β, and chemokines, such as IL-8, MCP-1, and MIP-1α [41–43]. TLR4 activation can also induce signaling via the MyD88-independent pathway by recruitment of the adaptor molecule TRIF to the TIR domain (Fig. 1) [44]. TLR4 must have the additional adaptor molecule TRAM for the activation of MyD88-independent signaling, which results in induction of the cytokines and chemokines responsible for viral clearance, such as type I IFNs and RANTES [45]. The MyD88-independent signaling pathway, which is also downstream of endosomal TLR3, is more fully described in the following section. Of note, TLR signaling intermediates have been shown to interact with oxidative-stress–mediated pathways. For instance, in phagocytic cells, TLR4 directly interacts with Nox4, an NADPH oxidase family member, and results in induction of ROS production and oxidative stress, culminating in NF-κB activation [46–49]. NADPH oxidase subunit p47phox can also promote TLR4 expression and trafficking in vascular muscle cells and macrophages [50–52]. ROS also contribute to TLR4-mediated calcium mobilization and calmodulin kinase in monocytic cells [30, 53]. Engagement of TLR2 and -4 activates the ER stress response proteins IRE1α and XBP1 (detailed in the unfolded protein response section), which is necessary for optimal production of inflammatory cytokines by macrophages [54]. Furthermore, MAPKs, including JNK and p38 (also termed SAPKs), are activated downstream of physiological stress including oxidative stress, heat shock, and ER stress in macrophages and fibroblasts [55–58].
Endosomal TLRs.
Activation of viral endosomal TLRs such as TLR7/8 and -9 leads to induction of proinflammatory cytokines via MyD88-dependent NF-κB activation and also type I IFNs via IRF-7 activation, (Fig. 2) [59, 60]. Recruitment of the adaptor MyD88 to the TLR results in activation of IRAK-4 and TRAF6 [45, 60, 61], leading to activation of TRAF3 and subsequent phosphorylation and activation of IKKα and IRAK-1. The transcription factor IRF-7 is phosphorylated by IRAK1 and IKKα, leading to its translocation to the nucleus and induction of type I IFNs, such as IFNα and -β which are necessary for elimination of viral infection [45, 60, 61]. Finally, the MyD88-independent pathway downstream of another endosomal receptor, TLR3, involves the adaptor molecule TRIF, which is recruited to the TIR domain (Fig. 2) [44]. TRIF induces activation of TRAF6 and subsequent downstream NF-κB DNA binding activity [62]. TRIF also mediates activation of TRAF3 and leads to the activation of the noncanonical IKKs TBK1 and IKKε, which phosphorylate and activate IRF3, leading to nuclear translocation and transcription of chemokines such as RANTES and type I IFNs, such as IFNβ [63, 64]. These inflammatory mediators play an important role in the clearance of viral infection. It should be noted that viral infections induce rapid generation of ROS which potentiate activation of downstream TLR signaling in macrophages [65]. ER stress induced by viruses also augments IRF3-mediated production of type I IFN downstream of TLR activation [66, 67]. Cellular oxidative stress can also enhance TLR3 mediated NF-κB in airway epithelial cells, probably by enhancement of TLR3 expression [68]. Modulation of these stress-mediated effects on TLR signaling may be a therapeutic target in viral-induced exacerbations of diseases, including chronic obstructive pulmonary disease.
Cytoplasmic PRRs.
In addition to TLRs, cytoplasmic PRRs play a central role in pathogen recognition and activation of immune responses (Fig. 3). These include the RLRs RIG-I, MDA5, and LGP2, which are RNA helicases activated by viruses [69]. Upon recognition of double-stranded viral RNA, the RLRs bind to the adaptor protein IPS1 (also known as MAVS and Cardif), which activates IRF3 and -7 through TRAF3, NAP1, and IKKε/TBK1 [70, 71]. NADPH oxidase and ROS are essential for this RLR-mediated induction of type I IFNs [72]. Interaction of IPS-1 with FADD and RIP1 also results in NF-κB activation [70, 71].
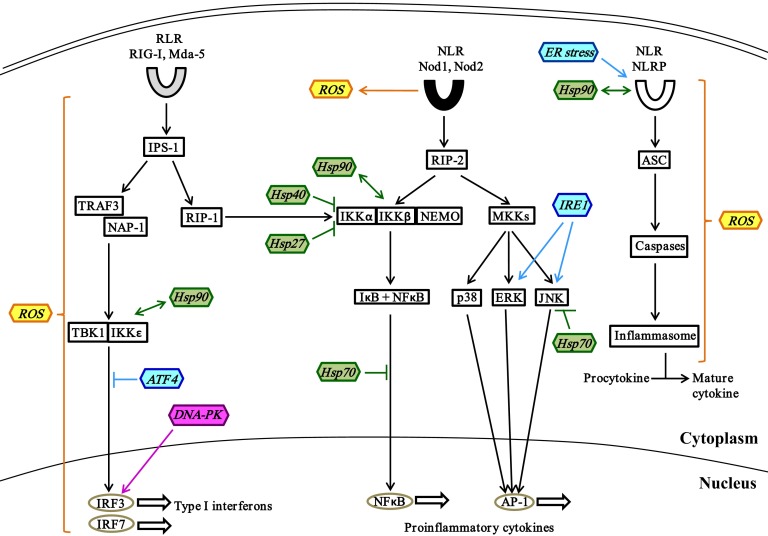
Figure 3. Stress proteins in crosstalk with cytoplasmic PRR signaling.
Activation of cytoplasmic RLRs by viral RNA leads to IRF3- and -7-mediated induction of type I IFNs via the IPS-1–TRAF3–TBK1 pathway. DNA-PK, a DDR protein, can also act as a cytoplasmic PRR to induce expression of type I IFNs. IPS-1-activated RIP-1 also induces the expression of proinflammatory cytokines by activating the NF-κB pathway. The recognition of bacterial peptidoglycans by the NLRs Nod1 and -2 upregulates the expression of proinflammatory cytokines via activation of NF-κB and MKK pathways. Cytoplasmic NLRPs induce activation of inflammasomes via ASC and caspases and is essential for processing of procytokines into their active forms. The crosstalk of stress proteins generated by oxidative stress (yellow), heat shock response (green), UPR (blue), DDR (pink) and cytoplasmic PRR signaling is illustrated. →, stimulation; ↔, direct interaction; ⫞, inhibition.
The other group of cytoplasmic PRRs are the NLRs, including Nod1 and -2, which recognize bacterial peptidoglycan constituents and activate the MAPK and NF-κB signaling pathways via interacting protein RIP2 [73]. NADPH oxidase-induced ROS are effectors of Nod2-mediated antibacterial responses [74]. NLRPs are another subfamily of NLRs that recognize bacterial DNA, toxins, and double-stranded RNA [75]. Upon ligand recognition, NLRPs associate with adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (i.e., ASC) resulting in activation of caspases that can cleave procytokines into the active form. Mitochondrial ROS are crucial for activation of the NLRP inflammasome, which in turn plays a major role in the production by monocytes of the proinflammatory cytokine IL-1β [76, 77]. This ROS-mediated activation of the NLRP inflammasome is facilitated by thioredoxin-interacting protein [78]. ER stress also induces NLRP inflammasome activation independent of the UPR pathway in monocytes [79]. Certain NLRPs interact with stress-mediated Hsp90 chaperones, to maintain their correctly folded, signal-receptive state [80]. Thus, it is likely that cellular stress proteins and the PRR signaling molecules interact directly and are necessary for host defense. Activation of PRR signaling pathways ultimately results in the expression of different cytokines that play specific roles in pathogen clearance and host defense.
Cytokines involved in inflammation and clearance of infections
Proinflammatory cytokines, such as TNFα, IL-6, and IL-1β, are induced downstream of PRR activation during bacterial and viral infections. These effector molecules facilitate an inflammatory response to clear infections. Cytokines can induce the production of oxidants, prostaglandins, and mitochondrial ROS by macrophages, thus contributing to inflammatory responses [81]. TNFα stimulates ROS-mediated phagocytosis in macrophages, allowing internalization and destruction of bacteria [82]. TNFα and IL-6 also promote the acute-phase response in the liver, leading to upregulation of proteins such as C-reactive protein, which can promote opsonization of microbes and activate the complement system to clear pathogens. On the other hand, TNFα promotes a proapoptotic profile in macrophages by blocking the heat shock stress response, thus increasing the cell's susceptibility to heat-induced apoptosis [83]. Similarly, IL-1β induces inflammatory and proapoptotic responses mediated by factors such as prostaglandins, ROS, inducible NO synthase, and NO [84–86]. IL-1β also promotes T-cell activation and proliferation, B-cell maturation, and T-cell-dependent antibody production by B cells, thereby initiating an adaptive immune response to the pathogens [87–90]. The heterodimeric proinflammatory cytokine IL-12, produced by stimulation of TLR in innate immune cells, induces production of IFNγ by NK and T cells and also enhances their cytolytic activity [91]. IL-12 plays a role in Th cell differentiation into Th1 cells, which promotes cell-mediated immunity [91]. Production of IL-12 by monocyte-derived DCs upon stimulation of TLR is enhanced by induction of the heat shock stress response [92]. Notably, proinflammatory cytokines can directly cooperate with stress-mediated proteins and contribute to the clearance of bacterial infection by both innate and adaptive immune cells.
Besides proinflammatory cytokines, PRR recognition of viral components induces the type I interferons IFNα and -β. The release of interferons such as IFNβ alerts the neighboring cells to the presence of viral particles in the environment, inducing cell-intrinsic mechanisms to inhibit, or interfere with, viral replication at every stage [93, 94]. For example, induction of 2′–5′ oligoadenylate synthase by IFN activates the nuclease RNase L which can degrade viral RNA [95]. Furthermore Mx GTPase, induced by IFNs, captures viral nucleocapsid proteins in the cytoplasm, thereby blocking viral assembly [96]. Another effect of IFNs is the upregulation of MHC molecules on cells, making them an easy target for recognition by T cells [97]. Expression of IFNβ downstream of TLR activation is enhanced by induction of ER stress [67]. IFNs also induce production of ROS and sensitize the fibroblasts to apoptosis upon subsequent viral exposure, to prevent spread of infection [98, 99]. Stress-mediated regulation of cytokine responses can play a complementary role in host defense to bacterial or viral infections. Additional studies are needed to further understand the precise interactions between stress proteins and PRRs and their signaling intermediates and their clinical relevance.
CELL STRESS RESPONSES IN IMMUNE SIGNALING
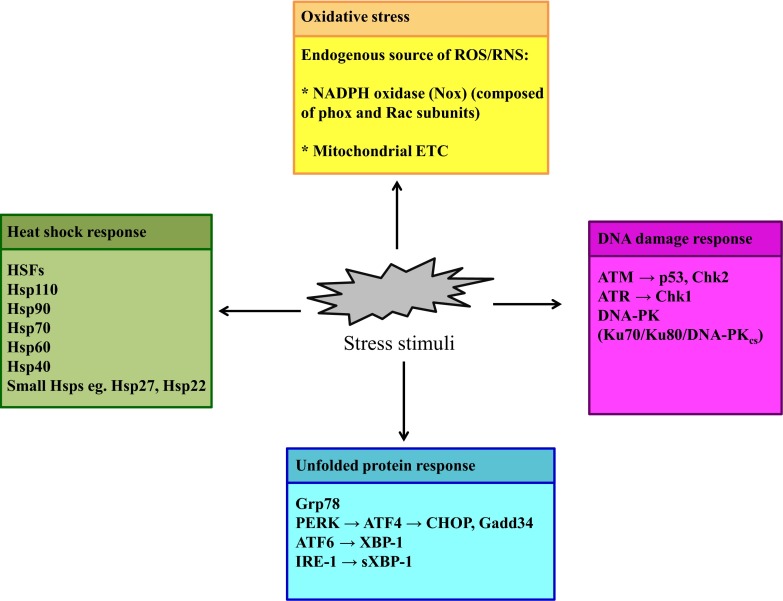
Sudden and substantial changes in the environment of an organism (stress) cause the organism to mount specific cytoprotective responses for survival, including those at the cellular level [5]. For example, via increased ROS/RNS, infectious agents, radiation, and environmental toxins can induce oxidative stress that is countered by antioxidant defense mechanisms. The heat shock response confers thermotolerance during exposure to high heat by preventing denaturation of proteins and facilitating the refolding of already denatured proteins in the heat-exposed cells, thus preventing cellular apoptosis. In this section, we elaborate on the major intracellular stress response pathways, such as the oxidative stress response, the heat shock response, UPR, and DDR, which are critical for crosstalk with the immune PRR signaling pathways (Fig. 4). The stress protein interactions with PRR pathways regulate downstream immune responses and play an important role in host defense and inflammation.
Figure 4. Cellular stress responses.
Exposure to stress stimuli (e.g., bacteria, viruses, radiation, and environmental toxins) induces cytoprotective responses that restore cellular homeostasis. Oxidative stress (yellow) is an example of stress that is a result of an oxidant–antioxidant imbalance in cells. The stress responses are categorized into heat shock response (green), UPR (blue), and DDR (pink). The major proteins or molecules responsible for mediating these stresses and stress responses and that play a pivotal role in innate immune signaling are enumerated.
ROS and oxidative stress response
Oxidative stress results from an imbalance between the systemic level of oxidants such as ROS/RNS and the body's ability to counter these levels with antioxidants [5, 100]. The ROS, which include superoxide radicals, hydrogen peroxide, and the hydroxyl radical, mediate oxidative stress in addition to RNS such as NO and nitrite. The oxidants are produced endogenously as byproducts or metabolites of various metabolic processes, including aerobic metabolism in mitochondria or reactions mediated by cyclooxygenases, NADPH oxidase, and xanthine oxidase [101]. The endogenous source of cellular ROS is mainly two organelles: the ER and mitochondria. In the ER, the oxidative formation of disulfide bonds during protein folding can result in the accumulation of ROS [102]. The major source of cellular ROS is the mitochondria, as a result of the reduction of molecular oxygen into superoxide ion at the end of the ETC [103, 104]. Chemical agents such as hydrogen peroxide or physical factors such as UV light can also induce production of ROS. When oxidants accumulate, cells mount a stress response for protection against oxidative stress–mediated damage. This includes upregulation of antioxidant proteins, such as glutathione reductase and glutathione peroxidase [103]. In the event of irreparable damage, caspase-mediated apoptotic cell death is induced. The roles of oxidative stress and ROS as mediators of PRR signaling and immune response to bacterial and viral infections and their contribution to inflammation have been described in previous sections (Figs. 1–3).
Heat shock response
The Hsp-mediated cellular defense mechanism in response to heat shock was one of the earliest reported stress responses [105–107]. Hsps, expressed in the cytoplasm, nucleus, ER, and mitochondria of eukaryotic cells, are a class of ATP-dependent molecular chaperone proteins involved in protein folding in response to cellular stress. Hsps are induced by various environmental insults in addition to heat, such as oxidative stress, nutritional deprivation, exposure to toxins, and infectious agents. These cellular insults can cause protein denaturation and unfolding within the cells, leading to formation of unwanted protein aggregates that can eventually kill the cells. Hsps facilitate protein refolding, target misfolded proteins to the proteasome, and stabilize and transport partially folded proteins to different cellular compartments. Thus, Hsps maintain normal cellular homeostasis in response to stress. Heat exposure was originally identified as beneficial to animals by rendering protection from mortality associated with the systemic inflammation induced by sepsis [108, 109]. Subsequent studies have shown that, depending on the extra- or intracellular location and the type of immune cells, Hsps can exert an inflammatory immune activating signal for host defense against infection or an anti-inflammatory immunosuppressive signal to prevent excessive inflammation [110]. The family of Hsps includes the large Hsps (e.g., Hsp110, -90, -70, -60, and -40) and the small Hsps (e.g., Hsp25 and -27) based on their molecular size.
Hsp90.
Among the Hsps identified with an immunomodulatory function, Hsp90, -70, and -60 have been extensively studied. The chaperone functions of cell-compartment–specific Hsp90 proteins are predominantly to prevent formation of aggregates of unfolded proteins and to restore stability and function by binding specific client–substrate proteins that are in the late stages of folding. Some client proteins of cytoplasmic Hsp90 that are involved in immune signaling pathways include transcription factors and protein kinases, such as IRF3, IKK, and TBK1 (Figs. 1–3) [111]. Hsp90 plays a regulatory role in the activation or repression of these specific substrate client proteins via direct association or indirect regulation. For instance, activation of the IKK complex requires interaction with Hsp90, whereas noncanonical NF-κB is inhibited by Hsp90-mediated stabilization of the protein Monarch-1, which promotes degradation of NF-κB-inducing kinase in macrophages [112–114]. ER-specific Hsp90, also called gp96, is important for the processing and membrane expression of TLRs, including TLR4, -7, and -9 in macrophages [115]. Hsp90 is also essential for optimal function and activation of NK and T cells [116]. The association of extracellular Hsp90β with peptides leads to receptor-mediated endocytosis of the Hsp–peptide complex and enhances presentation of the peptide by MHC I on DCs [117]. These observations point to a complex pro- or anti-inflammatory role of Hsp90 in immune signaling.
Hsp70.
Hsp70 proteins are also implicated in immune responses, and these proteins are also involved in the folding of newly synthesized or stress-denatured proteins, the dissociation of unfolded protein aggregates, and the transport of proteins into different cellular compartments [118, 119]. Of note is the role of extracellular Hsp70 as DAMPs, released after cell death or by active secretion, to activate cell surface receptors on immune cells and induce downstream signaling [120]. Exogenous Hsp70 has been shown to elicit rapid calcium flux, activate NF-κB, and induce the production of inflammatory cytokines and chemokines by monocytes and DCs via the TLR2 and -4 pathways [121]. Extracellular Hsp70 can also associate with peptides and bind to cell surface receptors, such as CD91 and Lox-1, allowing the Hsp–peptide complex to be internalized by endocytosis and thus enhance the cross-presentation of the Hsp-bound peptide by MHC I on DCs [117]. In contrast to these proinflammatory functions, Hsp70 can also dampen the inflammatory response to prevent tissue damage. Heat-shocked macrophages show reduced TNF expression at specific time points that correlate with maximum upregulation of Hsp70 [122]. Overexpression of Hsp70 has been shown to inhibit the LPS-induced production of cytokines such as TNFα and IL-1β by human macrophages [123]. Hsp70 treatment reduces the capacity of DCs to stimulate T cells [124]. The effect of Hsp70 on the NF-κB signaling pathway has been extensively studied over the years: The expression of Hsp70 in macrophages blocks LPS-induced NF-κB activation by binding to TRAF6 and inhibiting its ubiquitination, thus preventing activation of the inflammatory signaling cascade (Figs. 1–3) [125]. Binding of Hsp70 to the NEMO protein impedes formation of the active IKK complex, which in turn inhibits NF-κB activation [126, 127]. Hsp70 expression prevents degradation of IkB and inhibits nuclear translocation of the NF-κB complex, suggesting inactivation of the NF-κB pathway as the mechanism of Hsp70-induced LPS tolerance in macrophages [128, 129]. With regard to the MAPK signaling pathways, Hsp70 can bind to and inhibit JNK activation in fibroblasts [130].
Hsp60.
Hsp60, another immunomodulatory stress protein, is expressed in the mitochondria and predominantly plays a role in intracellular protein trafficking, although there is increasing evidence of its expression in the cytoplasm as well. Studies have indicated a possible role for Hsp60 in immune response signaling and have also linked Hsp60 to cancer [131, 132]. On the one hand, Hsp60 can induce a proinflammatory profile by increasing the release of TNFα, IL-12, and other inflammatory cytokines from macrophages mediated by CD14 signaling [133]. On the other hand, treatment of effector T cells with recombinant Hsp60 inhibits activation of NF-κB and production of TNFα in a TLR2-dependent manner, indicating an immunosuppressive role [134]. Stimulation of Tregs with exogenous Hsp60 enhances their immunosuppressive activity on effector T cells in terms of cytokine production and proliferation, and this effect is mediated by TLR2 signaling [135].
Hsp110, Hsp40, and sHsps.
Among the other members of the Hsp family, the Hsp110 protein localizes to the nuclear and cytoplasmic compartments in response to hyperthermic exposure [136, 137]. Hsps have adjuvant-like properties, whereby their association with the antigenic peptide elicits a rapid and efficient TLR-mediated immune response to the respective antigen [138]. This phenomenon has been illustrated by the immunization of mice with Hsp110 complexed to melanoma antigen in a model of targeted immunotherapy [139, 140]. Members of the Hsp40 protein family serve as co-chaperones and associate with and activate the ATPase activity of Hsp70 [141, 142]. The DNAJA3 Hsp40 protein family member exerts an anti-inflammatory role via association with IKK and inhibition of its activity, resulting in stabilization of cytoplasmic IκB and inhibition of NF-κB activation, as demonstrated in tumor cells [143]. Among the sHsps, Hsp22 acts as an agonist of TLR4 inducing TNFα and IL-6 by DCs [144]. Hsp27 mediates the interaction between p38 and NF-κB for the induction of proinflammatory mediators during viral infection, and it is also necessary for activation of the TAK1, MKK, and IKK signaling pathways downstream of cytokine stimulation in fibroblasts, illustrating its proinflammatory role [145, 146]. Hsp27 exerts its anti-inflammatory effect by binding to the IKK β subunit and inactivating the IKK complex [147]. Exogenous Hsp27 induces production of the anti-inflammatory cytokine IL-10 from monocytes and promotes differentiation of monocytes into inhibitory DCs [148, 149].
HSFs.
The expression of Hsps is regulated by stress-activated transcription factors known as HSFs [150]. There have been reports that illustrate the anti-inflammatory role of HSFs in certain conditions. The expression of the transcription factor HSF1 inhibits transcription of IL-1β in LPS-stimulated monocytes [151]. HSF1 also represses TNFα transcription in macrophages [152]. HSF-1 deficient mice are unable to upregulate expression of Hsp70 and to produce excessive TNF after a single stimulation of LPS, compared with wild-type mice [153]. Heat shock–induced HSF1 has been shown to competitively inhibit binding of NF-κB to target genes in macrophages (Figs. 1 and 2) [154]. These studies illustrate the anti-inflammatory role of HSFs in immune responses. It should also be noted that heat shock stress can directly activate SAPKs such as JNK, which are involved in PRR signaling. Like UV radiation, heat shock response is a potent inducer of JNK activation in fibroblasts, resulting in increased AP-1 activity via different mechanisms [155].
The different inflammatory and anti-inflammatory roles played by the heat shock stress proteins and the crosstalk between Hsps and immune signaling molecules are among the most studied, suggesting that they have a complex immunomodulatory role in host defense (Figs. 1–3). Further studies are needed to highlight the multifaceted role of Hsps in immune signaling pathways. Do Hsps dictate proinflammatory vs. anti-inflammatory responses during bacterial infection, cancer, and autoimmunity? What is the mechanism of action of these Hsps in the immune response? Are their chaperone activity and direct interaction essential? Do they crosstalk with signaling pathways downstream of other immune receptors, such as T-cell receptors? Interaction between Hsp90 and the TCR signaling kinase Zap-70 has been found in T cells, which suggests a role for these stress proteins in adaptive immune signaling pathways, as well [156]. These results could provide information about novel mechanisms of regulation of immune responses and stress-mediated Hsps during infection or disease.
Unfolded protein response
The ER is an important cellular compartment wherein secreted, membrane-bound, or organelle-specific proteins undergo translational modifications and folding. Stress stimuli, such as nutrient deprivation, hypoxia, or viral infection, can perturb this folding process, leading to accumulation of misfolded or unfolded proteins in the ER and activation of the UPR [157, 158]. The UPR is characterized by an increase in the expression of the chaperone proteins Grp78 (also known as BiP) and -94, to aid in protein folding and restore homeostasis. ER stress also mediates leakage of calcium, contributing to ER stress–mediated NF-κB activation [159, 160]. On the other hand, inflammatory cytokines such as TNFα can upregulate ER stress in a ROS-dependent manner [161]. UPR, induced by proinflammatory cytokines such as IL-6 and -1β and also by LPS, can promote further inflammation by cleavage of CREBH in the liver [162]. This cleavage releases an amino terminal fragment that can translocate to the nucleus and activate the transcription of genes such as C-reactive protein and serum amyloid P component, which play important roles in acute-phase response and in opsonization of microbes for detection by innate immunity. In mammalian cells, UPR is mediated by 3 stress sensors, IRE1, ATF6, and PERK, which sense the protein-folding conditions in the ER and transduce the appropriate signals to initiate transcription of UPR target genes [157, 158]. Upon initiation of UPR, Grp78 is sequestered by unfolded and misfolded proteins, releasing ATF6, PERK, and IRE1 from its effects. The signaling from these 3 proteins converges in the nucleus to activate transcription of target genes, which can be mediated via the ERSE present in the promoter regions of many UPR target genes in mammals.
PERK.
Upon ER stress, activated PERK inhibits eIF2α by phosphorylation at serine 51, which blocks the exchange of GDP for GTP that is necessary for eIF2α activation, resulting in overall inhibition of protein translation. Of note, this global inhibition of protein translation initiated by the PERK-eIF2α pathway can result in activation of NF-κB, thus promoting a proinflammatory immune profile, as is seen in fibroblasts [163]. Phosphorylation of eIF2α leads to increased translation of ATF4, which in turn induces expression of genes such as CHOP and Gadd34 [164]. It has been shown that stimulation of TLR2 or -4 inhibits ER stress-mediated activation of PERK and expression of CHOP in macrophages [54]. ER-stress–induced expression of CHOP in peritoneal macrophages is also inhibited by TLR3 or -4 activation in a TRIF-dependent manner [165]. In contrast, a recent study showed that stimulation of human monocytes by TLR4 directly upregulates expression and nuclear translocation of ATF4, which is dependent on MyD88 and JNK, resulting in production of IL-1β, -6 and -8 and RANTES [166]. However, ATF4 inhibits TBK1/IKKε-mediated phosphorylation and activation of IRF7, thereby suppressing expression of type I IFNs (Figs. 1 and 2) [167]. These observations indicate complex crosstalk in the PERK–eIF2α–ATF4 pathway and innate immune signaling, which merits further study.
ATF6.
ATF6, another ER stress sensor, activates transcription of target genes including XBP1; chaperone proteins, such as Grp78; and components of the ERAD. The ERAD targets misfolded proteins from the ER into the cytosol for proteasomal degradation. The induction of acute-phase response proteins by UPR is mediated by interaction of ATF6 with CREBH, to induce transcription of the target genes [162]. On the one hand, ER-stress–induced ATF6 mediates transient Akt phosphorylation and NF-κB activation in a short timeframe but subsequently blunts NF-κB activation in the later phase in an Akt-mTOR- and C/EBPβ-dependent manner [168, 169]. On the other hand, TLR2 or -4 inhibits ER-stress–induced ATF6 processing in macrophages (Fig. 1) [54]. Viruses such as human cytomegalovirus and hepatitis C induce and regulate the 3 branches of UPR to potentiate viral replication and viral protein folding and prevent early cellular apoptosis [170–172]. Virus-induced ER stress allows the pathogen to evade antiviral type I IFN responses by ATF6/IRE1-mediated inhibition of signaling downstream of interferons in fibroblasts [173].
IRE1.
There are two identified isoforms of IRE1 in mammals: IRE1α and -β [174, 175]. Upon activation, IRE1 endoribonuclease excises the intron from XBP1 mRNA, generating the sXBP1 transcription factor. The role of IRE1/XBP1 in immune responses is evident from several observations. XBP1 and IRE1α are necessary for the development of adaptive immune B cells, specifically antibody-producing, terminally differentiated plasma cells [176, 177]. XBP1-deficient mice exhibit a reduced number of conventional and plasmacytoid DCs, and these cells show reduced survival in response to TLR activation, indicating that XBP1 is necessary for the survival and function of DCs [178]. Macrophages undergoing UPR respond to TLR3 and -4 activation with enhanced IFNβ production, which is dependent on XBP1 splicing (Figs. 1 and 2) [179]. LPS also induces increased production of the inflammatory cytokine IL-23 in macrophages undergoing UPR [180]. Of note, TLR4 by itself has been shown to activate this IRE1/XBP1 axis to produce the inflammatory cytokines TNFα and IL-6 in a TRAF6/NADPH oxidase–dependent manner [54]. Cytosolic IRE1 interacts with adaptor proteins, including TRAF2, resulting in activation of SAPKs/MAPKs, JNK, and p38 in fibroblasts [181, 182]. Therefore IRE1 induces activation of TRAF adaptors, MAPKs, and NF-κB, all components of the inflammatory signaling pathway [183].
The mechanism by which TLR mediates inhibition of PERK and ATF6 branches while activating the IRE1 pathway is unclear and warrants further investigation. Based on the current observations, we speculate that an ER-stressed immune cell retains its inflammatory function when encountering PAMPs, permitting activation of inflammatory signaling molecules via XBP1 and blocking protein degradation and inhibition of protein translation mediated by PERK and ATF6. It should also be noted that TLR directly induces IRE1/XBP1 and ATF4 in monocytes in the absence of UPR, indicating that these stress proteins play a UPR-independent role in TLR-induced inflammation [54, 166]. The crosstalk between the UPR pathways and immune responses is further corroborated by observations that abnormalities in the UPR can adversely affect the immune responses, leading to autoimmunity [184].
DNA damage response
The DDR, another stress-mediated pathway, is triggered to repair DNA lesions induced by environmental genotoxins and chemical agents. The DDR, also referred to as the DNA damage checkpoint pathway, consists of sensors that identify DNA lesions, such as SSBs and DSBs; transducers that convey the signal from the sensors downstream; and effectors that induce cell cycle arrest and initiate DNA repair pathways, such as HR or NHEJ [5, 185, 186]. It should be noted that early studies have reported the induction of immunomodulatory cytokines and altered antigen presentation in response to DNA damage, indicating crosstalk between DDR and immune signaling [187, 188]. This stress pathway plays a role in immune cell detection and killing of damaged cells that may be virally infected as illustrated by the following observations. Activation of the DDR by the arrest of DNA replication or ionizing radiation induces upregulation of cell surface expression of the ligand for activating the NK cell receptor NKG2D, thereby making the stressed cell a target for NK cell–mediated lysis [189]. Induction of DNA damage is also one of the means of ROS-mediated cytotoxicity used by immune cells for the elimination of infected cells [190]. Furthermore, the DDR proteins are necessary for the genetic recombination of antigen receptors and antibodies in adaptive immune T and B cells (called V(D)J recombination). This V(D)J recombination allows wide diversity in these receptors in recognizing antigens of a broad spectrum of pathogens and is mediated by the NHEJ DNA repair pathway [191]. In the event of irreparable and extensive DNA damage, cellular apoptotic pathways are activated by the DDR pathway. The major regulators of the DDR are the primary transducer proteins, ATM, ATR, and DNA-PK, which belong to a family of PI3K-related serine-threonine kinases [185]. Each of these proteins is activated by different kinds of DNA damage and stimulates specific signaling pathways in the DDR.
ATM.
Upon detection of DSBs by the sensor MRN complex, ATM is recruited to the site of DNA damage, resulting in its autophosphorylation and activation [192, 193]. ATM-mediated phosphorylation of different substrate proteins such as Chk2 and p53 potentiates DNA repair. Components of the ATM pathway play an important role in adaptive immune cell development and function. ATM-deficient animals display defects in T-cell development, indicating the requirement of this kinase in V(D)J recombination [194]. Nbs-1, the histone protein γ-H2AX, and ATM have been observed to form foci that colocalize to the DSBs introduced during V(D)J recombination [195, 196]. ATM-phosphorylated γ-H2AX serves as a platform for the recruitment of chromatin remodeling complexes, such as SWI/SNF and KAP-1 [192]. Upregulation of SWI/SNF increases expression of transcription factor AP-1, which enhances cytokine production and proliferation of adaptive immune T cells [197]. Treatment of cells with DNA damaging agents also results in the upregulation of expression of TLRs which is directly mediated by p53 [198, 199]. Stress-activated JNK has in turn been shown to play a role in regulating p53 expression in fibroblasts [200, 201]. Induction of DNA damage by genotoxic stress also induces expression of the IFN transcription factor IRF1, but this reaction is inhibited in cells deficient in ATM signaling [202]. IRF1 regulates DNA damage–induced apoptosis, indicating that it plays a functional role in DNA-damaged T cells [203]. Similar observations have been made with IRF3. Treatment of cells with DNA-damaging agents stimulates activation of IRF3 in terms of phosphorylation, nuclear translocation, and transcriptional activity (Figs. 1 and 2) [204]. Thus, DNA damage–induced ATM regulates expression and promotes activation of key transcription factors in immune signaling, such as IRFs and NF-κB. ATM has been shown to bind and phosphorylate NEMO, the regulatory subunit of IKK, to activate NF-κB downstream of DNA damage [205]. More recently, studies have shown that, in fibroblasts, ATM activates TAK1, which is upstream of IKK in the PRR signaling pathways [206]. ATM has been shown to bind to TRAF6 to induce polyubiquitin synthesis, leading to activation of TAK1 and NEMO by ubiquitination in a cIAP-1–dependent manner [207]. This process requires the activity of another DNA repair protein, PARP-1, and ultimately results in NF-κB activation. Conversely, activation of NF-κB enhances the repair of DNA damage–induced DSBs by homologous recombination, which is mediated by ATM and BRCA1 [208]. DDR genes such as Ku-70 and 80 (DNA-PK subunits), ATM, and BRCA2 have also been identified as targets for transcription by NF-κB in T cells and tumor cells [209–211]. These observations indicate crosstalk between the ATM DNA damage stress pathway and the inflammatory signaling pathways in innate immune responses.
ATR.
In contrast to ATM, ATR is primarily activated and recruited to ssDNA coated with sensor RPA via ATRIP [185, 212]. Stress caused by blocked DNA replication also results in activation of SAPKs, p38, and JNK in fibroblasts that collaborate with Chk1, which is essential in cell cycle progression [213]. Recent data suggest a certain degree of redundancy between the ATM and ATR pathways. Components of the ATR pathway, ATR and Chk1, are necessary for DNA damage–induced upregulation of the NKG2D ligand [189]. A global phosphoproteome analysis of the kinases regulated downstream of the TLR4 ligand also revealed activation of proteins involved in the DDR pathway, including ATM/ATR kinases and Chk1 in LPS-stimulated macrophages [214]. Inhibition of ATM in these TLR-stimulated macrophages enhanced expression of genes, including CXCL-10 and IL-10, suggesting that TLR-induced ATM is a negative regulator of these genes. These findings suggest that ATM/ATR and Chk kinases play a functional role downstream of TLR activation during immune responses.
DNA-PK.
DNA-PK, activated by the sensors Ku70 and -80, which bind DSBs, is also linked to immune responses and host defense [215, 216]. Mutations in TLR4 cause a decrease in expression of Ku70 and -80, indicating that PRRs can regulate the expression of these DNA damage stress proteins [217]. Conversely, DNA-PKcs has been found to interact with the transcription factor Aire, to upregulate the expression of TLR-1, -3, and -8 in macrophages [218]. Of particular importance, DNA-PK itself has recently been characterized as a cytoplasmic PRR that activates IRF3 in response to bacterial and viral infections in fibroblasts (Fig. 3) [219, 220]. Recognition of DNA viruses by DNA-PK results in induction of cytokines, including IL-6 and IFNβ, and chemokines, such as CXCL10, in an IRF3- and TBK1-dependent manner [220]. Stimulation of macrophages with CpG DNA (a TLR9 ligand) results in nuclear translocation of Akt in a DNA-PKcs-dependent manner [221]. DNA damage stress-induced DNA-PKcs is implicated in the activation of NF-κB via MEK–ERK–IKK signaling in fibroblasts [222]. In contrast, in an earlier report, DNA-PK phosphorylation of IκB was observed, which increased its association with NF-κB and resulted in the inhibition of NF-κB DNA binding activity in fibroblasts [223].
Overall, components of the DNA damage response and repair clearly play a role in immune signaling, whether it is nuclear proteins, such as ATM and ATR, or DNA-PK, which can colocalize to both nucleus and cytoplasm. The regulation of expression and activation of the DDR proteins by immune activation and vice versa indicates crosstalk between these two pathways. In addition to improving our understanding of the crosstalk between DDR and immunity, these observations identify DDR proteins as likely translational targets that are useful in regulating host immune activation in viral and bacterial infections and in inflammatory diseases.
CLINICAL SIGNIFICANCE OF STRESS RESPONSE IN INFECTION AND IN IMMUNE AND INFLAMMATORY DISORDERS
The functional significance of stress proteins in immune responses and host defense is evident at various levels, ranging from interactions of stress-induced Hsps, which serve as molecular chaperones, with the PRR signaling pathways, to the role of ER stress proteins and DNA damage response mediators in inflammation and signaling. The crosstalk between these stress and immune signaling pathways becomes clinically relevant in host defense against bacterial and viral infections and diseases associated with an abnormal or hyperactive immune response in autoreactive or autoimmune diseases, chronic inflammatory diseases, and cancer (Fig. 5). Research pertaining to the analysis of precise interactions between cellular stress pathways and innate immune signaling in the setting of infections, inflammatory diseases, and cancer has been sparse. Findings in recent studies suggest that oxidative stress generated by mitochondrial and cellular ROS regulates bactericidal activity and inflammatory responses in autoinflammatory diseases [10, 77]. Systematic analysis of stress and immune signaling interactions will not only provide a better understanding of the pathophysiology of immune disorders but will aid in identifying targets for drug discovery.
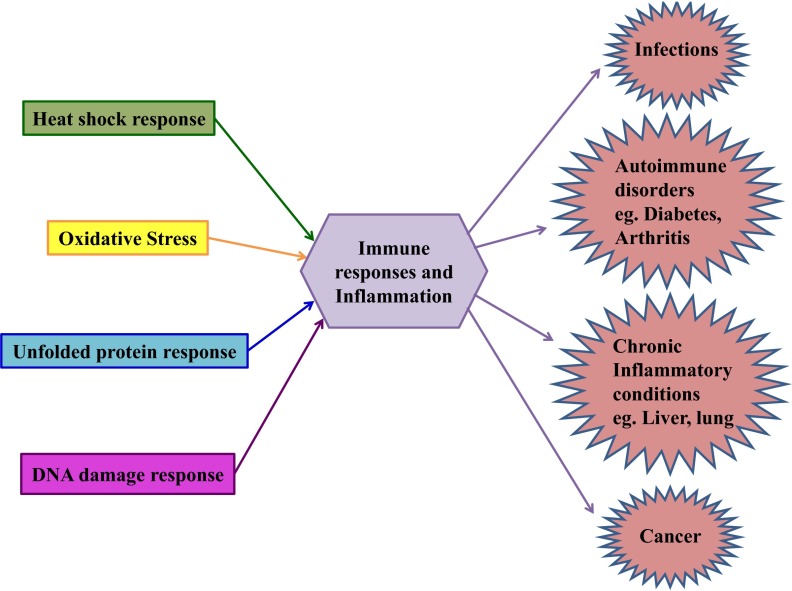
Figure 5. Clinical consequences of crosstalk between stress proteins and immune signaling molecules.
The clinical consequences of crosstalk between immune signaling and the individual stress responses in the context of infection, autoimmunity, chronic inflammation, and cancer are illustrated. The cellular stresses are categorized into oxidative stress (yellow), heat shock response (green), UPR(blue), and DDR (pink).
Infections
The diverse effects of pathogen-induced oxidative stress on upstream immune signaling intermediates and downstream transcription factors determine host resistance and susceptibility to infections. The highly regulated NADPH oxidase is a potent contributor to host resistance against infections, and an oxidative burst of ROS production is used by immune cells such as neutrophils for clearance of microbes [224]. Although intracellular ROS induces bactericidal activity and pathogen elimination, mitochondrial ROS can serve as signaling molecules to induce inflammatory responses. NLRX1-induced ROS enhance NF-κB and JNK signaling, and PRR-induced ROS can activate the NLRP3 inflammasome [76, 225]. NADPH oxidase could be an attractive target for therapeutic intervention during infections, in that microbes can subvert ROS-mediated effects via inhibition of the recruitment of NADPH oxidase subunits (phox and Rac) to the phagosome, resulting in increased host susceptibility to infections [226–228]. Recent studies have also shown increased production of Hsps in pathologic conditions, indicating that cellular stress caused by infection or immune assault upregulates the production of both host and pathogenic Hsps [229–232]. These Hsps serve as molecular chaperones for signaling kinases that are essential in host defense or survival and contribute to either elimination of infections or progression of disease.
Autoimmune Disorders
In addition to their roles in host defense against infection, stress proteins have been implicated in autoimmune disorders. Host Hsps appear to modulate immune responses directly and to serve as circulating DAMPs to induce uncontrolled immune activation. For example, immune reactivity to Hsp70 and -90 has been shown in autoimmune and chronic inflammatory diseases, such as rheumatoid arthritis, SLE, and multiple sclerosis [233, 234]. Pharmacologic inhibition of these Hsps in animal models of SLE and rheumatoid arthritis has resulted in ameliorated disease, indicating that these Hsps play a significant role in the pathogenesis of autoimmune disease [235, 236]. Since Hsps are phylogenetically highly conserved between mammals and pathogens, the leading hypothesis is that immune activation against pathogenic Hsps leads to cross-reactivity with sequence-homologous host Hsps, resulting in various autoreactive disorders [237]. Hsp22, which is known to induce DC maturation and cytokine production in a TLR4-dependent manner, is another example of Hsps in autoimmune disease, as illustrated by its abundant expression in the synovial tissue of patients with rheumatoid arthritis [144]. ER and oxidative stresses are also involved in autoimmunity and a broad range of inflammatory diseases. These pathways have been implicated in development of diabetes, wherein proinflammatory cytokines induce stress-mediated death of insulin-producing β cells [86, 238]. Activation of stress-mediated SAPK-JNK is also associated with insulin resistance and type 2 diabetes [239]. Activation of JNK downstream of ER stress or ATM-mediated DDR responses in obesity can result in insulin resistance. In the case of arthritis, deficiency of the NADPH oxidase complex, which lowers ROS production, increases susceptibility to disease in rodents [240]. The ER stress protein Grp78 associates with the autoantigen Ro52, which leads to Grp78's becoming a bystander target for subsequent immune reactivity in an animal model of arthritis [241]. Expression of the ER stress genes Grp78, CHOP, and Gadd45 is upregulated in the muscle tissue of patients with autoimmune myositis, indicating a role for these UPR proteins in the muscle damage that is characteristic of this disease [242]. In contrast, a study involving EAE, the inducible animal model of multiple sclerosis, showed that induction of ER stress by IFNγ in oligodendrocytes protects mice from subsequent disease in a PERK-dependent manner [243].
Chronic inflammatory diseases
The role of the stress pathways in disease can be extended to chronic inflammatory conditions. Oxidative stress, specifically that induced by ROS, plays a central role in lung and liver inflammatory disorders. Neutrophil-produced ROS, which diffuse into the liver cells, cause oxidative stress, leading to the efficient killing of these cells and causing inflammatory liver injury [244]. TLR4 interaction with Nox4 is necessary for LPS-induced ROS generation and NF-κB activation [46]. This TLR-stimulated production of oxidants by liver resident macrophages (Kupffer cells) has been shown to contribute to alcohol-induced inflammation and injury in the liver [245]. Persistent inhalation of pathogens or toxic agents can cause an oxidant–antioxidant imbalance in the lungs, resulting in excessive production of ROS. ROS-mediated activation of signaling pathways such as NF-κB and AP-1 produces proinflammatory mediators, such as TNFα, IL-8, and IL-1β, and promotes tissue injury and inflammation in the airways, as observed in cystic fibrosis, asthma, and chronic obstructive pulmonary disease [246]. The inflammatory responses in the lung that are observed in influenza infection and cigarette smoking are reduced in ROS-deficient mice [247, 248]. The UPR stress pathway has been implicated in intestinal inflammation. Induction of ER stress in the intestinal cells results in multicytokine-mediated colitis, similar to human ulcerative colitis [249]. Polymorphisms in the UPR gene XBP-1 has been associated with inflammatory bowel disease [250]. The significant role of these stress pathways in autoimmune and inflammatory disorders suggests possible targets for manipulation in the treatment of these diseases.
Cancer
In the context of cancer, the role of stress pathways in immune responses to tumors has been elucidated. The frequency of mutations and loss of p53 correlating with cancer suggests a critical role for this DDR protein in cancer [251]. The induction of NKG2D ligand expression by the DDR also facilitates tumor surveillance by the immune system. Inhibition of ATM or Chk1 blocks expression of Rae, an NKG2D ligand, in tumor cell lines [189]. In contrast, the anti-inflammatory role of Hsps can be exploited by tumor cells for immune evasion. Tumors cells express and secret Hsp70, which in turn promotes immunosuppression in a TLR2/MyD88-dependent manner [252–254]. Tumor-produced Hsp10 exerts a similar suppressive effect on T-cell activation [255]. Elevated expression of Hsps such as Hsp10 and -60 can also serve as biomarkers for diagnosis and prognosis of cancer [256, 257]. Targeting Hsp70 and -90 has shown promising results in the treatment of different cancers. Several chemical Hsp90 inhibitors such as 17-DMAG and BIIB021 are undergoing clinical trials for treatment of multiple myeloma, breast cancer, and leukemia. The first Hsp90 inhibitor tested in vivo was 17-AAG (tanespimycin), which was trialed in 1999 for treatment of solid and hematologic malignancies [258–260]. Targeting the transcription factor HSF1, a pivotal regulator of Hsp induction, by a triazole nucleoside analogue elicited anticancer activity in pancreatic cancer [261]. Neutralization of Hsp70 with an AIF-derived peptide and treatment with Hsp70 inhibitors such as PES and VER-155008 have also shown potent antitumor activity [262]. The complex role of stress-mediated JNK in cancer is established. Detection of high levels of JNK activity in cancer cell lines supports the observation that JNK promotes tumorigenesis, but tumor suppression may also be mediated by its pro-apoptotic properties [239]. Furthermore, in cancer, upregulation of UPR pathways, such as Grp78, ATF6, and XBP1 splicing in tumor cells in response to ER stressors, such as hypoxia, glucose deprivation, and low pH in the tumor microenvironment, is essential for tumorigenesis [263–266]. The induction of proinflammatory mediators such as TNFα and IL-6 by UPR proteins such as IRE1α and PERK in cancer cells facilitates tumor growth and regulates immune function [267]. Inflammation induced by oxidative stress potentiates tumorigenesis in a similar manner [268]. In addition to this cell-intrinsic regulation, recent studies have indicated a cell-extrinsic role for UPR in tumor cells via immunomodulation. Macrophages treated with conditioned medium of such ER-stressed tumor cells upregulated UPR signaling and production of inflammatory cytokines in a phenomenon termed transmissible ER stress (TERS), mediated by TLR4 [269]. Thus, the tumor microenvironment uses ER stress to initiate a tumorigenic inflammatory phenotype in APCs. Overall, several interactions between stress and immune signaling proteins in infections, autoimmunity, inflammatory diseases, and cancer suggest a significant role for stress proteins in disease pathogenesis, making them attractive therapeutic targets.
CONCLUDING REMARKS
Cellular stress and inflammation can activate or inhibit each other, depending on the immune cell type and the stress-inducing signals, such as pathogens and chemical or physical agents. Although understanding the pathophysiological role of cellular stress proteins in host defense and inflammatory diseases has progressed in recent years, future research should address critical questions. What are the key interacting mechanisms between stress-regulated cellular proteins and immune mediators crucial to host defense and disease pathogenesis? Can we target stress proteins to regulate inflammatory responses? An improved understanding of the underlying molecular mechanisms of stress proteins and their crosstalk with inflammatory pathways will provide new targets for therapeutic intervention.
ACKNOWLEDGMENTS
This study was supported by Public Health Service grant AA017986 (to P.M.) from the National Institute of Alcohol Abuse and Alcoholism, U.S. National Institutes of Health, Bethesda, MD, USA, and by the of Defense grant W81XWH-11-1-0420 (to P.M.).
Footnotes
- Aire
- autoimmune regulator
- ASC
- apoptosis-associated speck-like protein containing a caspase recruitment domain
- ATF
- activating transcription factor
- ATM
- ataxia telangiectasia-mutated
- ATR
- ATM and Rad-3-related
- ATRIP
- ATR-interacting protein
- CHOP
- C/EBP homologous protein
- cIAP
- complementary inhibitor of apoptotic protein
- CREBH
- hepatocyte specific CREB
- DAMPs
- damage associated molecular patterns
- DDR
- DNA damage response
- DNA-PKcs
- catalytic subunit of DNA-PK
- DNA-PK
- DNA-dependent protein kinase
- DSB
- double-strand breaks
- EAE
- experimental autoimmune encephalitis
- eIF2α
- eukaryotic translation initiation factor
- ER
- endoplasmic reticulum
- ERAD
- ER-associated degradation pathway
- ERSE
- ER stress element
- ETC
- electron transport chain
- FADD
- Fas-associated protein with death domain
- Gr
- granzyme
- HMGB1
- high-mobility group box 1
- HR
- homologous recombination
- HSF
- heat shock factor
- Hsp
- heat shock protein
- IKK
- IκB kinase
- IPS
- IFNβ promoter stimulator
- IRAK
- IL-1R associated kinase
- IRE1
- inositol-requiring kinase 1
- IRF
- IFN regulatory factor
- KAP-1
- Kruppel-associated box (KRAB)-associated protein 1
- LGP2
- laboratory of genetics and physiology 2
- MAVS
- mitochondrial antiviral signaling adaptor
- MDA5
- melanoma differentiation associated gene 5
- MKK
- MAP kinase kinase
- MRN
- Mre11 nuclease, Rad50 coiled-coil protein, and Nbs1 regulator protein
- mTOR
- mammalian target of rapamycin
- NAP
- NAK-associated protein 1
- Nbs
- nucleotide-binding site
- NEMO
- NF-kappa-B essential modulator
- NHEJ
- nonhomologous end-joining
- NKG2D
- natural-killer group 2, member D
- NLR
- nod-like receptor
- NLRP
- nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain—containing polymerase
- PERK
- PKR-like ER kinase
- PES
- pifithrin-μ
- PRRs
- pattern recognition receptors
- RIP1
- receptor-interacting protein 1
- RLR
- RIG-I-like receptors
- ROS/RNS
- reactive oxygen and nitrogen species
- Ro52
- ribonucleoprotein 52 kDa
- RPA
- replication protein A
- SAPK
- stress-activated protein kinase
- sHsp
- small Hsp
- SLE
- systemic lupus erythematosus
- SSB
- single-strand breaks
- SWI/SNF
- switch/sucrose nonfermentable
- sXBP
- spliced X-box binding protein
- TAB
- TAK-1 binding proteins
- TAK1
- TGF-β activated kinase 1
- TANK
- TRAF-associated NF-κB activator
- TBK1
- TANK-binding kinase 1
- TERS
- transmissible ER stress
- TIR
- Toll/IL-1R
- Tpl2
- tumor progression locus 2 kinase
- TRAF6
- TNF receptor-associated factor 6
- TRAM
- TRIF-related adaptor molecule
- Tregs
- regulatory T cells
- TRIF
- Toll/IL-1 receptor domain-containing adaptor inducing IFN-β
- UPR
- unfolded protein response
- XBP1
- X-box binding protein 1
AUTHORSHIP
S.M. composed, revised, and edited the manuscript. P.M. generated the overall concepts and critically reviewed the manuscript.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1. Kumar H., Kawai T., Akira S. (2011) Pathogen recognition by the innate immune system. Int. Rev. Immunol. 30, 16–34 [DOI] [PubMed] [Google Scholar]
- 2. Kawai T., Akira S. (2011) Regulation of innate immune signalling pathways by the tripartite motif (TRIM) family proteins. EMBO Mol. Med. 3, 513–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akira S., Takeda K., Kaisho T. (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2, 675–680 [DOI] [PubMed] [Google Scholar]
- 4. Medzhitov R., Janeway C. A., Jr. (1997) Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9, 4–9 [DOI] [PubMed] [Google Scholar]
- 5. Fulda S., Gorman A. M., Hori O., Samali A. (2010) Cellular stress responses: cell survival and cell death. Int. J. Cell Biol. 2010, 214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Medzhitov R., Janeway C., Jr. (2000) Innate immunity. N. Engl. J. Med. 343, 338–344 [DOI] [PubMed] [Google Scholar]
- 7. Nathan C., Shiloh M. U. (2000) Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. U. S. A. 97, 8841–8848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prince L. R., Whyte M. K., Sabroe I., Parker L. C. (2011) The role of TLRs in neutrophil activation. Curr. Opin. Pharmacol. 11, 397–403 [DOI] [PubMed] [Google Scholar]
- 9. Michaelsson J., Teixeira de Matos C., Achour A., Lanier L. L., Karre K., Soderstrom K. (2002) A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J. Exp. Med. 196, 1403–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. West A. P., Brodsky I. E., Rahner C., Woo D. K., Erdjument-Bromage H., Tempst P., Walsh M. C., Choi Y., Shadel G. S., Ghosh S. (2011) TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Preynat-Seauve O., Coudurier S., Favier A., Marche P. N., Villiers C. (2003) Oxidative stress impairs intracellular events involved in antigen processing and presentation to T cells. Cell Stress Chaperones 8, 162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Osna N. A., White R. L., Todero S., McVicker B. L., Thiele G. M., Clemens D. L., Tuma D. J., Donohue T. M., Jr. (2007) Ethanol-induced oxidative stress suppresses generation of peptides for antigen presentation by hepatoma cells. Hepatology 45, 53–61 [DOI] [PubMed] [Google Scholar]
- 13. Granados D. P., Tanguay P. L., Hardy M. P., Caron E., de Verteuil D., Meloche S., Perreault C. (2009) ER stress affects processing of MHC class I-associated peptides. BMC Immunol. 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mahadevan N. R., Zanetti M. (2011) Tumor stress inside out: cell-extrinsic effects of the unfolded protein response in tumor cells modulate the immunological landscape of the tumor microenvironment. J. Immunol. 187, 4403–4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ostankovitch M., Robila V., Engelhard V. H. (2005) Regulated folding of tyrosinase in the endoplasmic reticulum demonstrates that misfolded full-length proteins are efficient substrates for class I processing and presentation. J. Immunol. 174, 2544–2551 [DOI] [PubMed] [Google Scholar]
- 16. Barton G. M., Medzhitov R. (2003) Toll-like receptor signaling pathways. Science 300, 1524–1525 [DOI] [PubMed] [Google Scholar]
- 17. Akira S., Takeda K. (2004) Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 [DOI] [PubMed] [Google Scholar]
- 18. Kawai T., Akira S. (2006) TLR. signaling. Cell Death Differ. 13, 816–825 [DOI] [PubMed] [Google Scholar]
- 19. Janssens S., Beyaert R. (2003) Role of Toll-like receptors in pathogen recognition. Clin. Microbiol. Rev. 16, 637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Applequist S. E., Wallin R. P., Ljunggren H. G. (2002) Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int. Immunol. 14, 1065–1074 [DOI] [PubMed] [Google Scholar]
- 21. Ghosh T. K., Mickelson D. J., Fink J., Solberg J. C., Inglefield J. R., Hook D., Gupta S. K., Gibson S., Alkan S. S. (2006) Toll-like receptor (TLR) 2–9 agonists-induced cytokines and chemokines: I, comparison with T cell receptor-induced responses. Cell Immunol. 243, 48–57 [DOI] [PubMed] [Google Scholar]
- 22. Kubes P., Mehal W. Z. (2012) Sterile inflammation in the liver. Gastroenterology 143, 1158–1172 [DOI] [PubMed] [Google Scholar]
- 23. Mollen K. P., Anand R. J., Tsung A., Prince J. M., Levy R. M., Billiar T. R. (2006) Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock 26, 430–437 [DOI] [PubMed] [Google Scholar]
- 24. Asea A. (2008) Heat shock proteins and toll-like receptors. Handb Exp Pharmacol. 183, 111–127 [DOI] [PubMed] [Google Scholar]
- 25. Janke M., Poth J., Wimmenauer V., Giese T., Coch C., Barchet W., Schlee M., Hartmann G. (2009) Selective and direct activation of human neutrophils but not eosinophils by Toll-like receptor 8. J. Allergy Clin. Immunol. 123, 1026–1033 [DOI] [PubMed] [Google Scholar]
- 26. El Kebir D., Jozsef L., Filep J. G. (2008) Neutrophil recognition of bacterial DNA and Toll-like receptor 9-dependent and -independent regulation of neutrophil function. Arch. Immunol. Ther. Exp. (Warsz.) 56, 41–53 [DOI] [PubMed] [Google Scholar]
- 27. Bae Y. S., Lee J. H., Choi S. H., Kim S., Almazan F., Witztum J. L., Miller Y. I. (2009) Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ. Res. 104, 210–218, 21p following 218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen G. Y., Nunez G. (2010) Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kliment C. R., Tobolewski J. M., Manni M. L., Tan R. J., Enghild J., Oury T. D. (2008) Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung. Antioxid. Redox Signal. 10, 261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsung A., Klune J. R., Zhang X., Jeyabalan G., Cao Z., Peng X., Stolz D. B., Geller D. A., Rosengart M. R., Billiar T. R. (2007) HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 204, 2913–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang C., Deng L., Hong M., Akkaraju G. R., Inoue J., Chen Z. J. (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 [DOI] [PubMed] [Google Scholar]
- 32. Lomaga M. A., Yeh W. C., Sarosi I., Duncan G. S., Furlonger C., Ho A., Morony S., Capparelli C., Van G., Kaufman S., van der Heiden A., Itie A., Wakeham A., Khoo W., Sasaki T., Cao Z., Penninger J. M., Paige C. J., Lacey D. L., Dunstan C. R., Boyle W. J., Goeddel D. V., Mak T. W. (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13, 1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takaesu G., Kishida S., Hiyama A., Yamaguchi K., Shibuya H., Irie K., Ninomiya-Tsuji J., Matsumoto K. (2000) TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol. Cell 5, 649–658 [DOI] [PubMed] [Google Scholar]
- 34. Takeda K., Akira S. (2004) TLR signaling pathways. Semin. Immunol. 16, 3–9 [DOI] [PubMed] [Google Scholar]
- 35. Zandi E., Rothwarf D. M., Delhase M., Hayakawa M., Karin M. (1997) The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell 91, 243–252 [DOI] [PubMed] [Google Scholar]
- 36. Woronicz J. D., Gao X., Cao Z., Rothe M., Goeddel D. V. (1997) IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science 278, 866–869 [DOI] [PubMed] [Google Scholar]
- 37. Lee F. S., Hagler J., Chen Z. J., Maniatis T. (1997) Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 88, 213–222 [DOI] [PubMed] [Google Scholar]
- 38. Rothwarf D. M., Zandi E., Natoli G., Karin M. (1998) IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature 395, 297–300 [DOI] [PubMed] [Google Scholar]
- 39. Dong C., Davis R. J., Flavell R. A. (2002) MAP kinases in the immune response. Annu. Rev. Immunol. 20, 55–72 [DOI] [PubMed] [Google Scholar]
- 40. Newton K., Dixit V. M. (2012) Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 4, pii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hayden M. S., West A. P., Ghosh S. (2006) NF-kappaB and the immune response. Oncogene 25, 6758–6780 [DOI] [PubMed] [Google Scholar]
- 42. Pahl H. L. (1999) Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18, 6853–6866 [DOI] [PubMed] [Google Scholar]
- 43. Zenz R., Eferl R., Scheinecker C., Redlich K., Smolen J., Schonthaler H. B., Kenner L., Tschachler E., Wagner E. F. (2008) Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res. Ther. 10, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fitzgerald K. A., Rowe D. C., Barnes B. J., Caffrey D. R., Visintin A., Latz E., Monks B., Pitha P. M., Golenbock D. T. (2003) LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J. Exp. Med. 198, 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 46. Park H. S., Jung H. Y., Park E. Y., Kim J., Lee W. J., Bae Y. S. (2004) Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. 173, 3589–3593 [DOI] [PubMed] [Google Scholar]
- 47. Gill R., Tsung A., Billiar T. (2010) Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 48, 1121–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Asehnoune K., Strassheim D., Mitra S., Kim J. Y., Abraham E. (2004) Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J. Immunol. 172, 2522–2529 [DOI] [PubMed] [Google Scholar]
- 49. Bedard K., Krause K. H. (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313 [DOI] [PubMed] [Google Scholar]
- 50. Nakahira K., Kim H. P., Geng X. H., Nakao A., Wang X., Murase N., Drain P. F., Wang X., Sasidhar M., Nabel E. G., Takahashi T., Lukacs N. W., Ryter S. W., Morita K., Choi A. M. (2006) Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J. Exp. Med. 203, 2377–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lin F. Y., Chen Y. H., Tasi J. S., Chen J. W., Yang T. L., Wang H. J., Li C. Y., Chen Y. L., Lin S. J. (2006) Endotoxin induces toll-like receptor 4 expression in vascular smooth muscle cells via NADPH oxidase activation and mitogen-activated protein kinase signaling pathways. Arterioscler. Thromb. Vasc. Biol. 26, 2630–2637 [DOI] [PubMed] [Google Scholar]
- 52. Chen J. X., Stinnett A. (2008) Critical role of the NADPH oxidase subunit p47phox on vascular TLR expression and neointimal lesion formation in high-fat diet-induced obesity. Lab. Invest. 88, 1316–1328 [DOI] [PubMed] [Google Scholar]
- 53. Cuschieri J., Bulger E., Garcia I., Maier R. V. (2005) Oxidative-induced calcium mobilization is dependent on annexin VI release from lipid rafts. Surgery 138, 158–164 [DOI] [PubMed] [Google Scholar]
- 54. Martinon F., Chen X., Lee A. H., Glimcher L. H. (2010) TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11, 411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Han J., Lee J. D., Bibbs L., Ulevitch R. J. (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265, 808–811 [DOI] [PubMed] [Google Scholar]
- 56. Kyriakis J. M., Banerjee P., Nikolakaki E., Dai T., Rubie E. A., Ahmad M. F., Avruch J., Woodgett J. R. (1994) The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369, 156–160 [DOI] [PubMed] [Google Scholar]
- 57. Wilhelm D., Bender K., Knebel A., Angel P. (1997) The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol. Cell Biol. 17, 4792–4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ip Y. T., Davis R. J. (1998) Signal transduction by the c-Jun N-terminal kinase (JNK): from inflammation to development. Curr. Opin. Cell Biol. 10, 205–219 [DOI] [PubMed] [Google Scholar]
- 59. Cavlar T., Ablasser A., Hornung V. (2012) Induction of type I IFNs by intracellular DNA-sensing pathways. Immunol. Cell Biol. 90, 474–482 [DOI] [PubMed] [Google Scholar]
- 60. Uematsu S., Akira S. (2007) Toll-like receptors and type I interferons. J. Biol. Chem. 282, 15319–15323 [DOI] [PubMed] [Google Scholar]
- 61. Blasius A. L., Beutler B. (2010) Intracellular toll-like receptors. Immunity. 32, 305–315 [DOI] [PubMed] [Google Scholar]
- 62. Seya T., Oshiumi H., Sasai M., Akazawa T., Matsumoto M. (2005) TICAM-1 and TICAM-2: toll-like receptor adapters that participate in induction of type I interferons. Int. J. Biochem. Cell Biol. 37, 524–529 [DOI] [PubMed] [Google Scholar]
- 63. Fitzgerald K. A., McWhirter S. M., Faia K. L., Rowe D. C., Latz E., Golenbock D. T., Coyle A. J., Liao S. M., Maniatis T. (2003) IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 [DOI] [PubMed] [Google Scholar]
- 64. Genin P., Algarte M., Roof P., Lin R., Hiscott J. (2000) Regulation of RANTES chemokine gene expression requires cooperativity between NF-kappa B and IFN-regulatory factor transcription factors. J. Immunol. 164, 5352–5361 [DOI] [PubMed] [Google Scholar]
- 65. Gonzalez-Dosal R., Horan K. A., Rahbek S. H., Ichijo H., Chen Z. J., Mieyal J. J., Hartmann R., Paludan S. R. (2011) HSV infection induces production of ROS, which potentiate signaling from pattern recognition receptors: role for S-glutathionylation of TRAF3 and 6. PLoS Pathog. 7, e1002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu Y. P., Zeng L., Tian A., Bomkamp A., Rivera D., Gutman D., Barber G. N., Olson J. K., Smith J. A. (2012) Endoplasmic reticulum stress regulates the innate immunity critical transcription factor IRF3. J. Immunol. 189, 4630–4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zeng L., Liu Y. P., Sha H., Chen H., Qi L., Smith J. A. (2010) XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J. Immunol. 185, 2324–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Koarai A., Sugiura H., Yanagisawa S., Ichikawa T., Minakata Y., Matsunaga K., Hirano T., Akamatsu K., Ichinose M. (2010) Oxidative stress enhances toll-like receptor 3 response to double-stranded RNA in airway epithelial cells. Am. J. Respir. Cell. Mol. Biol. 42, 651–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nakhaei P., Genin P., Civas A., Hiscott J. (2009) RIG-I-like receptors: sensing and responding to RNA virus infection. Semin. Immunol. 21, 215–222 [DOI] [PubMed] [Google Scholar]
- 70. Arnoult D., Carneiro L., Tattoli I., Girardin S. E. (2009) The role of mitochondria in cellular defense against microbial infection. Semin. Immunol. 21, 223–232 [DOI] [PubMed] [Google Scholar]
- 71. Barber G. N. (2011) Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr. Opin. Immunol. 23, 10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Soucy-Faulkner A., Mukawera E., Fink K., Martel A., Jouan L., Nzengue Y., Lamarre D., Vande Velde C., Grandvaux N. (2010) Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 6, e1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kanneganti T. D. (2010) Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10, 688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lipinski S., Till A., Sina C., Arlt A., Grasberger H., Schreiber S., Rosenstiel P. (2009) DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J. Cell Sci. 122, 3522–3530 [DOI] [PubMed] [Google Scholar]
- 75. Zambetti L. P., Laudisi F., Licandro G., Ricciardi-Castagnoli P., Mortellaro A. (2012) The rhapsody of NLRPs: master players of inflammation … and a lot more. Immunol. Res. 53, 78–90 [DOI] [PubMed] [Google Scholar]
- 76. Zhou R., Yazdi A. S., Menu P., Tschopp J. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 [DOI] [PubMed] [Google Scholar]
- 77. Naik E., Dixit V. M. (2011) Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 208, 417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. (2009) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 [DOI] [PubMed] [Google Scholar]
- 79. Menu P., Mayor A., Zhou R., Tardivel A., Ichijo H., Mori K., Tschopp J. (2012) ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death. Dis. 3, e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shirasu K. (2009) The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu. Rev. Plant Biol. 60, 139–164 [DOI] [PubMed] [Google Scholar]
- 81. Kim J. J., Lee S. B., Park J. K., Yoo Y. D. (2010) TNF-alpha-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-X(L). Cell Death Differ. 17, 1420–1434 [DOI] [PubMed] [Google Scholar]
- 82. Pfeffer K. (2003) Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 14, 185–191 [DOI] [PubMed] [Google Scholar]
- 83. Schett G., Steiner C. W., Xu Q., Smolen J. S., Steiner G. (2003) TNFalpha mediates susceptibility to heat-induced apoptosis by protein phosphatase-mediated inhibition of the HSF1/hsp70 stress response. Cell Death Differ. 10, 1126–1136 [DOI] [PubMed] [Google Scholar]
- 84. Yang D., Elner S. G., Bian Z. M., Till G. O., Petty H. R., Elner V. M. (2007) Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye. Res. 85, 462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Banerjee M., Saxena M. (2012) Interleukin-1 (IL-1) family of cytokines: role in type 2 diabetes. Clin. Chim. Acta 413, 1163–1170 [DOI] [PubMed] [Google Scholar]
- 86. Holohan C., Szegezdi E., Ritter T., O'Brien T., Samali A. (2008) Cytokine-induced beta-cell apoptosis is NO-dependent, mitochondria-mediated and inhibited by BCL-XL. J. Cell Mol. Med. 12, 591–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pike B. L., Nossal G. J. (1985) Interleukin 1 can act as a B-cell growth and differentiation factor. Proc. Natl. Acad. Sci. U. S. A. 82, 8153–8157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ben-Sasson S. Z., Hu-Li J., Quiel J., Cauchetaux S., Ratner M., Shapira I., Dinarello C. A., Paul W. E. (2009) IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. U. S. A. 106, 7119–7124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nambu A., Nakae S., Iwakura Y. (2006) IL-1beta, but not IL-1alpha, is required for antigen-specific T cell activation and the induction of local inflammation in the delayed-type hypersensitivity responses. Int. Immunol. 18, 701–712 [DOI] [PubMed] [Google Scholar]
- 90. Nakae S., Asano M., Horai R., Iwakura Y. (2001) Interleukin-1 beta, but not interleukin-1 alpha, is required for T-cell-dependent antibody production. Immunology 104, 402–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Watford W. T., Moriguchi M., Morinobu A., O'Shea J. J. (2003) The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 14, 361–368 [DOI] [PubMed] [Google Scholar]
- 92. Peng J. C., Hyde C., Pai S., O'Sullivan B. J., Nielsen L. K., Thomas R. (2006) Monocyte-derived DC primed with TLR agonists secrete IL-12p70 in a CD40-dependent manner under hyperthermic conditions. J. Immunother. 29, 606–615 [DOI] [PubMed] [Google Scholar]
- 93. Stetson D. B., Medzhitov R. (2006) Type I interferons in host defense. Immunity 25, 373–381 [DOI] [PubMed] [Google Scholar]
- 94. Boo K. H., Yang J. S. (2010) Intrinsic cellular defenses against virus infection by antiviral type I interferon. Yonsei Med. J. 51, 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stark G. R., Kerr I. M., Williams B. R., Silverman R. H., Schreiber R. D. (1998) How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 [DOI] [PubMed] [Google Scholar]
- 96. Kochs G., Haller O. (1999) Interferon-induced human MxA GTPase blocks nuclear import of Thogoto virus nucleocapsids. Proc. Natl. Acad. Sci. U. S. A. 96, 2082–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Inacio R. F., Zanon R. G., Verinaud L., de Oliveira A. L. (2012) Interferon beta modulates major histocompatibility complex class I (MHC I) and CD3-zeta expression in PC12 cells. Neurosci. Lett. 513, 223–228 [DOI] [PubMed] [Google Scholar]
- 98. Seifert U., Bialy L. P., Ebstein F., Bech-Otschir D., Voigt A., Schroter F., Prozorovski T., Lange N., Steffen J., Rieger M., Kuckelkorn U., Aktas O., Kloetzel P. M., Kruger E. (2010) Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell. 142, 613–624 [DOI] [PubMed] [Google Scholar]
- 99. Moiseeva O., Mallette F. A., Mukhopadhyay U. K., Moores A., Ferbeyre G. (2006) DNA damage signaling and p53-dependent senescence after prolonged beta-interferon stimulation. Mol. Biol. Cell 17, 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chen B. P., Li M., Asaithamby A. (2012) New insights into the roles of ATM and DNA-PKcs in the cellular response to oxidative stress. Cancer Lett. 327, 103–110 [DOI] [PubMed] [Google Scholar]
- 101. Alfadda A. A., Sallam R. M. (2012) Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tu B. P., Weissman J. S. (2004) Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 164, 341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Droge W. (2002) Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95 [DOI] [PubMed] [Google Scholar]
- 104. Le Bras M., Clement M. V., Pervaiz S., Brenner C. (2005) Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol. Histopathol. 20, 205–219 [DOI] [PubMed] [Google Scholar]
- 105. Hightower L. E. (1991) Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 66, 191–197 [DOI] [PubMed] [Google Scholar]
- 106. Velazquez J. M., DiDomenico B. J., Lindquist S. (1980) Intracellular localization of heat shock proteins in Drosophila. Cell 20, 679–689 [DOI] [PubMed] [Google Scholar]
- 107. Pardue M. L., Scott M. P., Storti R. V., Lengyel J. A. (1980) The heat shock response: a model system for the study of gene regulation in Drosophila. Basic Life Sci. 16, 41–55 [DOI] [PubMed] [Google Scholar]
- 108. Hotchkiss R., Nunnally I., Lindquist S., Taulien J., Perdrizet G., Karl I. (1993) Hyperthermia protects mice against the lethal effects of endotoxin. Am. J. Physiol. 265, R1447–R1457 [DOI] [PubMed] [Google Scholar]
- 109. Yang R. C., Wang C. I., Chen H. W., Chou F. P., Lue S. I., Hwang K. P. (1998) Heat shock treatment decreases the mortality of sepsis in rats. Kaohsiung J. Med. Sci. 14, 664–672 [PubMed] [Google Scholar]
- 110. Kaul G., Thippeswamy H. (2011) Role of heat shock proteins in diseases and their therapeutic potential. Indian J. Microbiol. 51, 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yang K., Shi H., Qi R., Sun S., Tang Y., Zhang B., Wang C. (2006) Hsp90 regulates activation of interferon regulatory factor 3 and TBK-1 stabilization in Sendai virus-infected cells. Mol. Biol. Cell 17, 1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chen G., Cao P., Goeddel D. V. (2002) TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol. Cell 9, 401–410 [DOI] [PubMed] [Google Scholar]
- 113. Broemer M., Krappmann D., Scheidereit C. (2004) Requirement of Hsp90 activity for IkappaB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-kappaB activation. Oncogene 23, 5378–5386 [DOI] [PubMed] [Google Scholar]
- 114. Arthur J. C., Lich J. D., Aziz R. K., Kotb M., Ting J. P. (2007) Heat shock protein 90 associates with monarch-1 and regulates its ability to promote degradation of NF-kappaB-inducing kinase. J. Immunol. 179, 6291–6296 [DOI] [PubMed] [Google Scholar]
- 115. Yang Y., Liu B., Dai J., Srivastava P. K., Zammit D. J., Lefrancois L., Li Z. (2007) Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity 26, 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bae J., Munshi A., Li C., Samur M., Prabhala R., Mitsiades C., Anderson K. C., Munshi N. C. (2013) Heat shock protein 90 is critical for regulation of phenotype and functional activity of human T lymphocytes and NK cells. J. Immunol. 190, 1360–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Srivastava P. (2002) Roles of heat-shock proteins in innate and adaptive immunity. Nat. Rev. Immunol. 2, 185–194 [DOI] [PubMed] [Google Scholar]
- 118. Young J. C. (2010) Mechanisms of the Hsp70 chaperone system. Biochem. Cell Biol. 88, 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Daugaard M., Rohde M., Jaattela M. (2007) The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 581, 3702–3710 [DOI] [PubMed] [Google Scholar]
- 120. Torigoe T., Tamura Y., Sato N. (2009) Heat shock proteins and immunity: application of hyperthermia for immunomodulation. Int. J. Hyperthermia 25, 610–616 [DOI] [PubMed] [Google Scholar]
- 121. Asea A., Rehli M., Kabingu E., Boch J. A., Bare O., Auron P. E., Stevenson M. A., Calderwood S. K. (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277, 15028–15034 [DOI] [PubMed] [Google Scholar]
- 122. Snyder Y. M., Guthrie L., Evans G. F., Zuckerman S. H. (1992) Transcriptional inhibition of endotoxin-induced monokine synthesis following heat shock in murine peritoneal macrophages. J. Leukoc. Biol. 51, 181–187 [DOI] [PubMed] [Google Scholar]
- 123. Ding X. Z., Fernandez-Prada C. M., Bhattacharjee A. K., Hoover D. L. (2001) Over-expression of hsp-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine 16, 210–219 [DOI] [PubMed] [Google Scholar]
- 124. Stocki P., Wang X. N., Dickinson A. M. Inducible heat shock protein 70 reduces T cell responses and stimulatory capacity of monocyte-derived dendritic cells. J. Biol. Chem. 287, 12387–12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chen H., Wu Y., Zhang Y., Jin L., Luo L., Xue B., Lu C., Zhang X., Yin Z. (2006) Hsp70 inhibits lipopolysaccharide-induced NF-kappaB activation by interacting with TRAF6 and inhibiting its ubiquitination. FEBS Lett. 580, 3145–3152 [DOI] [PubMed] [Google Scholar]
- 126. Yoo C. G., Lee S., Lee C. T., Kim Y. W., Han S. K., Shim Y. S. (2000) Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J. Immunol. 164, 5416–5423 [DOI] [PubMed] [Google Scholar]
- 127. Ran R., Lu A., Zhang L., Tang Y., Zhu H., Xu H., Feng Y., Han C., Zhou G., Rigby A. C., Sharp F. R. (2004) Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 18, 1466–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dokladny K., Lobb R., Wharton W., Ma T. Y., Moseley P. L. (2010) LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: possible role of NF-kappaB. Cell Stress Chaperones 15, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Shi Y., Tu Z., Tang D., Zhang H., Liu M., Wang K., Calderwood S. K., Xiao X. (2006) The inhibition of LPS-induced production of inflammatory cytokines by HSP70 involves inactivation of the NF-kappaB pathway but not the MAPK pathways. Shock 26, 277–284 [DOI] [PubMed] [Google Scholar]
- 130. Park H. S., Lee J. S., Huh S. H., Seo J. S., Choi E. J. (2001) Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 20, 446–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cappello F., Conway de Macario E., Marasa L., Zummo G., Macario A. J. (2008) Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol. Ther. 7, 801–809 [DOI] [PubMed] [Google Scholar]
- 132. Habich C., Burkart V. (2007) Heat shock protein 60: regulatory role on innate immune cells. Cell. Mol. Life Sci. 64, 742–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kol A., Lichtman A. H., Finberg R. W., Libby P., Kurt-Jones E. A. (2000) Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol. 164, 13–17 [DOI] [PubMed] [Google Scholar]
- 134. Zanin-Zhorov A., Bruck R., Tal G., Oren S., Aeed H., Hershkoviz R., Cohen I. R., Lider O. (2005) Heat shock protein 60 inhibits Th1-mediated hepatitis model via innate regulation of Th1/Th2 transcription factors and cytokines. J. Immunol. 174, 3227–3236 [DOI] [PubMed] [Google Scholar]
- 135. Zanin-Zhorov A., Cahalon L., Tal G., Margalit R., Lider O., Cohen I. R. (2006) Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J. Clin. Invest. 116, 2022–2032 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136. Easton D. P., Kaneko Y., Subjeck J. R. (2000) The hsp110 and Grp1 70 stress proteins: newly recognized relatives of the Hsp70s. Cell Stress Chaperones 5, 276–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Facciponte J. G., Wang X. Y., Subjeck J. R. (2007) Hsp110 and Grp170, members of the Hsp70 superfamily, bind to scavenger receptor-A and scavenger receptor expressed by endothelial cells-I. Eur. J. Immunol. 37, 2268–2279 [DOI] [PubMed] [Google Scholar]
- 138. Segal B. H., Wang X. Y., Dennis C. G., Youn R., Repasky E. A., Manjili M. H., Subjeck J. R. (2006) Heat shock proteins as vaccine adjuvants in infections and Cancer Drug Discov. Today 11, 534–540 [DOI] [PubMed] [Google Scholar]
- 139. Han D., Xu H., Yan W. Q. (2012) Cytotoxic activity of spleen lymphocytes in Bal B/c mice immunized by HSP110-HER2/neu ICD [in Chinese]. Zhonghua Zhong Liu Za Zhi 34, 11–14 [PubMed] [Google Scholar]
- 140. Wang X. Y., Chen X., Manjili M. H., Repasky E., Henderson R., Subjeck J. R. (2003) Targeted immunotherapy using reconstituted chaperone complexes of heat shock protein 110 and melanoma-associated antigen gp100. Cancer Res. 63, 2553–2560 [PubMed] [Google Scholar]
- 141. Mitra A., Shevde L. A., Samant R. S. (2009) Multi-faceted role of HSP40 in Cancer Clin. Exp. Metastasis 26, 559–567 [DOI] [PubMed] [Google Scholar]
- 142. Fan C. Y., Lee S., Cyr D. M. (2003) Mechanisms for regulation of Hsp70 function by Hsp40. Cell Stress Chaperones 8, 309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cheng H., Cenciarelli C., Nelkin G., Tsan R., Fan D., Cheng-Mayer C., Fidler I. J. (2005) Molecular mechanism of hTid-1, the human homolog of Drosophila tumor suppressor l(2)Tid, in the regulation of NF-kappaB activity and suppression of tumor growth. Mol. Cell Biol. 25, 44–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Roelofs M. F., Boelens W. C., Joosten L. A., Abdollahi-Roodsaz S., Geurts J., Wunderink L. U., Schreurs B. W., van den Berg W. B., Radstake T. R. (2006) Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J. Immunol. 176, 7021–7027 [DOI] [PubMed] [Google Scholar]
- 145. Rajaiya J., Yousuf M. A., Singh G., Stanish H., Chodosh J. (2012) Heat shock protein 27 mediated signaling in viral infection. Biochemistry 51, 5695–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Alford K. A., Glennie S., Turrell B. R., Rawlinson L., Saklatvala J., Dean J. L. (2007) Heat shock protein 27 functions in inflammatory gene expression and transforming growth factor-beta-activated kinase-1 (TAK1)-mediated signaling. J. Biol. Chem. 282, 6232–6241 [DOI] [PubMed] [Google Scholar]
- 147. Park K. J., Gaynor R. B., Kwak Y. T. (2003) Heat shock protein 27 association with the I kappa B kinase complex regulates tumor necrosis factor alpha-induced NF-kappa B activation. J. Biol. Chem. 278, 35272–35278 [DOI] [PubMed] [Google Scholar]
- 148. De A. K., Kodys K. M., Yeh B. S., Miller-Graziano C. (2000) Exaggerated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J. Immunol. 165, 3951–3958 [DOI] [PubMed] [Google Scholar]
- 149. Miller-Graziano C. L., De A., Laudanski K., Herrmann T., Bandyopadhyay S. (2008) HSP27: an anti-inflammatory and immunomodulatory stress protein acting to dampen immune function. Novartis Found. Symp. 291, 196–208; discussion. 208–211, 221–224 [DOI] [PubMed] [Google Scholar]
- 150. Pirkkala L., Nykanen P., Sistonen L. (2001) Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 15, 1118–1131 [DOI] [PubMed] [Google Scholar]
- 151. Xie Y., Chen C., Stevenson M. A., Auron P. E., Calderwood S. K. (2002) Heat shock factor 1 represses transcription of the IL-1beta gene through physical interaction with the nuclear factor of interleukin 6. J. Biol. Chem. 277, 11802–11810 [DOI] [PubMed] [Google Scholar]
- 152. Singh I. S., He J. R., Hester L., Fenton M. J., Hasday J. D. (2004) Bacterial endotoxin modifies heat shock factor-1 activity in RAW 264.7 cells: implications for TNF-alpha regulation during exposure to febrile range temperatures. J. Endotoxin. Res. 10, 175–184 [DOI] [PubMed] [Google Scholar]
- 153. Xiao X., Zuo X., Davis A. A., McMillan D. R., Curry B. B., Richardson J. A., Benjamin I. J. (1999) HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 18, 5943–5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Song M., Pinsky M. R., Kellum J. A. (2008) Heat shock factor 1 inhibits nuclear factor-kappaB nuclear binding activity during endotoxin tolerance and heat shock. J. Crit. Care 23, 406–415 [DOI] [PubMed] [Google Scholar]
- 155. Adler V., Schaffer A., Kim J., Dolan L., Ronai Z. (1995) UV irradiation and heat shock mediate JNK activation via alternate pathways. J. Biol. Chem. 270, 26071–26077 [DOI] [PubMed] [Google Scholar]
- 156. Bartis D., Boldizsar F., Kvell K., Szabo M., Palinkas L., Nemeth P., Monostori E., Berki T. (2007) Intermolecular relations between the glucocorticoid receptor, ZAP-70 kinase, and Hsp-90. Biochem. Biophys. Res. Commun. 354, 253–258 [DOI] [PubMed] [Google Scholar]
- 157. Chakrabarti A., Chen A. W., Varner J. D. (2011) A review of the mammalian unfolded protein response. Biotechnol. Bioeng. 108, 2777–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Diehl J. A., Fuchs S. Y., Koumenis C. (2011) The cell biology of the unfolded protein response. Gastroenterology 141, 38–41, 41 e1-e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Deniaud A., Sharaf el dein O., Maillier E., Poncet D., Kroemer G., Lemaire C., Brenner C. (2008) Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 27, 285–299 [DOI] [PubMed] [Google Scholar]
- 160. Pahl H. L., Baeuerle P. A. (1996) Activation of NF-kappa B by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 392, 129–136 [DOI] [PubMed] [Google Scholar]
- 161. Xue X., Piao J. H., Nakajima A., Sakon-Komazawa S., Kojima Y., Mori K., Yagita H., Okumura K., Harding H., Nakano H. (2005) Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J. Biol. Chem. 280, 33917–33925 [DOI] [PubMed] [Google Scholar]
- 162. Zhang K., Shen X., Wu J., Sakaki K., Saunders T., Rutkowski D. T., Back S. H., Kaufman R. J. (2006) Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124, 587–599 [DOI] [PubMed] [Google Scholar]
- 163. Deng J., Lu P. D., Zhang Y., Scheuner D., Kaufman R. J., Sonenberg N., Harding H. P., Ron D. (2004) Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol. Cell Biol. 24, 10161–10168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wek R. C., Jiang H. Y., Anthony T. G. (2006) Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 34, 7–11 [DOI] [PubMed] [Google Scholar]
- 165. Woo C. W., Cui D., Arellano J., Dorweiler B., Harding H., Fitzgerald K. A., Ron D., Tabas I. (2009) Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat. Cell Biol. 11, 1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Zhang C., Bai N., Chang A., Zhang Z., Yin J., Shen W., Tian Y., Xiang R., Liu C. (2013) ATF4 is directly recruited by TLR4 signaling and positively regulates TLR4-trigged cytokine production in human monocytes. Cell. Mol. Immunol. 10, 84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Liang Q., Deng H., Sun C. W., Townes T. M., Zhu F. (2011) Negative regulation of IRF7 activation by activating transcription factor 4 suggests a cross-regulation between the IFN responses and the cellular integrated stress responses. J. Immunol. 186, 1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Nakajima S., Hiramatsu N., Hayakawa K., Saito Y., Kato H., Huang T., Yao J., Paton A. W., Paton J. C., Kitamura M. (2011) Selective abrogation of BiP/GRP78 blunts activation of NF-kappaB through the ATF6 branch of the UPR: involvement of C/EBPbeta and mTOR-dependent dephosphorylation of Akt. Mol. Cell Biol. 31, 1710–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Yamazaki H., Hiramatsu N., Hayakawa K., Tagawa Y., Okamura M., Ogata R., Huang T., Nakajima S., Yao J., Paton A. W., Paton J. C., Kitamura M. (2009) Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J. Immunol. 183, 1480–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Isler J. A., Skalet A. H., Alwine J. C. (2005) Human cytomegalovirus infection activates and regulates the unfolded protein response. J. Virol. 79, 6890–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Tardif K. D., Mori K., Siddiqui A. (2002) Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J. Virol. 76, 7453–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Qian Z., Xuan B., Chapa T. J., Gualberto N., Yu D. (2012) Murine cytomegalovirus targets transcription factor ATF4 to exploit the unfolded-protein response. J. Virol. 86, 6712–6723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ambrose R. L., Mackenzie J. M. (2013) ATF6 signalling is required for efficient West Nile Virus replication by promoting cell survival and inhibition of innate immune responses. J. Virol. 87, 2206–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hetz C., Martinon F., Rodriguez D., Glimcher L. H. (2011) The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol. Rev. 91, 1219–1243 [DOI] [PubMed] [Google Scholar]
- 175. Urano F., Bertolotti A., Ron D. (2000) IRE1 and efferent signaling from the endoplasmic reticulum. J. Cell Sci. 113, 3697–3702 [DOI] [PubMed] [Google Scholar]
- 176. Iwakoshi N. N., Lee A. H., Vallabhajosyula P., Otipoby K. L., Rajewsky K., Glimcher L. H. (2003) Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat. Immunol. 4, 321–329 [DOI] [PubMed] [Google Scholar]
- 177. Zhang K., Wong H. N., Song B., Miller C. N., Scheuner D., Kaufman R. J. (2005) The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J. Clin. Invest. 115, 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Iwakoshi N. N., Pypaert M., Glimcher L. H. (2007) The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J. Exp. Med. 204, 2267–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Smith J. A., Turner M. J., DeLay M. L., Klenk E. I., Sowders D. P., Colbert R. A. (2008) Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-beta induction via X-box binding protein 1. Eur. J. Immunol. 38, 1194–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. DeLay M. L., Turner M. J., Klenk E. I., Smith J. A., Sowders D. P., Colbert R. A. (2009) HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 60, 2633–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H. P., Ron D. (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 [DOI] [PubMed] [Google Scholar]
- 182. Hu P., Han Z., Couvillon A. D., Kaufman R. J., Exton J. H. (2006) Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol. Cell Biol. 26, 3071–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Kaneko M., Niinuma Y., Nomura Y. (2003) Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol. Pharm. Bull. 26, 931–935 [DOI] [PubMed] [Google Scholar]
- 184. Yoshida H. (2007) ER stress and diseases. FEBS J. 274, 630–658 [DOI] [PubMed] [Google Scholar]
- 185. Turnell A. S., Grand R. J. (2012) DNA viruses and the cellular DNA-damage response. J. Gen. Virol. 93, 2076–2097 [DOI] [PubMed] [Google Scholar]
- 186. Barzilai A., Yamamoto K. (2004) DNA damage responses to oxidative stress. DNA Repair (Amst.) 3, 1109–1115 [DOI] [PubMed] [Google Scholar]
- 187. Vink A. A., Strickland F. M., Bucana C., Cox P. A., Roza L., Yarosh D. B., Kripke M. L. (1996) Localization of DNA damage and its role in altered antigen-presenting cell function in ultraviolet-irradiated mice. J. Exp. Med. 183, 1491–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Nishigori C., Yarosh D. B., Ullrich S. E., Vink A. A., Bucana C. D., Roza L., Kripke M. L. (1996) Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes. Proc. Natl. Acad. Sci. U. S. A. 93, 10354–10359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Gasser S., Orsulic S., Brown E. J., Raulet D. H. (2005) The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436, 1186–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lieberman J. (2010) Granzyme A activates another way to die. Immunol. Rev. 235, 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Xu Y. (2006) DNA damage: a trigger of innate immunity but a requirement for adaptive immune homeostasis. Nat. Rev. Immunol. 6, 261–270 [DOI] [PubMed] [Google Scholar]
- 192. Bhatti S., Kozlov S., Farooqi A. A., Naqi A., Lavin M., Khanna K. K. (2011) ATM protein kinase: the linchpin of cellular defenses to stress. Cell. Mol. Life. Sci. 68, 2977–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Rupnik A., Lowndes N. F., Grenon M. (2010) MRN and the race to the break. Chromosoma 119, 115–135 [DOI] [PubMed] [Google Scholar]
- 194. Xu Y., Ashley T., Brainerd E. E., Bronson R. T., Meyn M. S., Baltimore D. (1996) Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 10, 2411–2422 [DOI] [PubMed] [Google Scholar]
- 195. Perkins E. J., Nair A., Cowley D. O., Van Dyke T., Chang Y., Ramsden D. A. (2002) Sensing of intermediates in V(D)J recombination by ATM. Genes Dev. 16, 159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Chen H. T., Bhandoola A., Difilippantonio M. J., Zhu J., Brown M. J., Tai X., Rogakou E. P., Brotz T. M., Bonner W. M., Ried T., Nussenzweig A. (2000) Response to RAG-mediated VDJ cleavage by NBS1 and gamma-H2AX. Science 290, 1962–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Jeong S. M., Lee C., Lee S. K., Kim J., Seong R. H. (2010) The SWI/SNF chromatin-remodeling complex modulates peripheral T cell activation and proliferation by controlling AP-1 expression. J. Biol. Chem. 285, 2340–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Menendez D., Shatz M., Azzam K., Garantziotis S., Fessler M. B., Resnick M. A. (2011) The Toll-like receptor gene family is integrated into human DNA damage and p53 networks. PLoS Genet. 7, e1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Hurley P. J., Bunz F. (2007) ATM and ATR: components of an integrated circuit. Cell Cycle 6, 414–417 [DOI] [PubMed] [Google Scholar]
- 200. Fuchs S. Y., Adler V., Pincus M. R., Ronai Z. (1998) MEKK1/JNK signaling stabilizes and activates p53. Proc. Natl. Acad. Sci. U. S. A. 95, 10541–10546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Fuchs S. Y., Adler V., Buschmann T., Yin Z., Wu X., Jones S. N., Ronai Z. (1998) JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev. 12, 2658–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Pamment J., Ramsay E., Kelleher M., Dornan D., Ball K. L. (2002) Regulation of the IRF-1 tumour modifier during the response to genotoxic stress involves an ATM-dependent signalling pathway. Oncogene 21, 7776–7785 [DOI] [PubMed] [Google Scholar]
- 203. Tamura T., Ishihara M., Lamphier M. S., Tanaka N., Oishi I., Aizawa S., Matsuyama T., Mak T. W., Taki S., Taniguchi T. (1995) An IRF-1-dependent pathway of DNA damage-induced apoptosis in mitogen-activated T lymphocytes. Nature 376, 596–599 [DOI] [PubMed] [Google Scholar]
- 204. Kim T., Kim T. Y., Song Y. H., Min I. M., Yim J., Kim T. K. (1999) Activation of interferon regulatory factor 3 in response to DNA-damaging agents. J. Biol. Chem. 274, 30686–30689 [DOI] [PubMed] [Google Scholar]
- 205. Wu Z. H., Shi Y., Tibbetts R. S., Miyamoto S. (2006) Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science 311, 1141–1146 [DOI] [PubMed] [Google Scholar]
- 206. Wu Z. H., Wong E. T., Shi Y., Niu J., Chen Z., Miyamoto S., Tergaonkar V. (2010) ATM- and NEMO-dependent ELKS ubiquitination coordinates TAK1-mediated IKK activation in response to genotoxic stress. Mol. Cell 40, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Hinz M., Stilmann M., Arslan S. C., Khanna K. K., Dittmar G., Scheidereit C. (2010) A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-kappaB activation. Mol. Cell 40, 63–74 [DOI] [PubMed] [Google Scholar]
- 208. Volcic M., Karl S., Baumann B., Salles D., Daniel P., Fulda S., Wiesmuller L. (2012) NF-kappaB regulates DNA double-strand break repair in conjunction with BRCA1-CtIP complexes. Nucleic Acids Res. 40, 181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Lim J. W., Kim H., Kim K. H. (2002) Expression of Ku70 and Ku80 mediated by NF-kappa B and cyclooxygenase-2 is related to proliferation of human gastric cancer cells. J. Biol. Chem. 277, 46093–46100 [DOI] [PubMed] [Google Scholar]
- 210. De Siervi A., De Luca P., Moiola C., Gueron G., Tongbai R., Chandramouli G. V., Haggerty C., Dzekunova I., Petersen D., Kawasaki E., Kil W. J., Camphausen K., Longo D., Gardner K. (2009) Identification of new Rel/NFkappaB regulatory networks by focused genome location analysis. Cell Cycle 8, 2093–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Wu K., Jiang S. W., Thangaraju M., Wu G., Couch F. J. (2000) Induction of the BRCA2 promoter by nuclear factor-kappa B. J. Biol. Chem. 275, 35548–35556 [DOI] [PubMed] [Google Scholar]
- 212. Cimprich K. A., Cortez D. (2008) ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 9, 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Llopis A., Salvador N., Ercilla A., Guaita-Esteruelas S., Barrantes Idel B., Gupta J., Gaestel M., Davis R. J., Nebreda A. R., Agell N. (2012) The stress-activated protein kinases p38alpha/beta and JNK1/2 cooperate with Chk1 to inhibit mitotic entry upon DNA replication arrest. Cell Cycle 11, 3627–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Weintz G., Olsen J. V., Fruhauf K., Niedzielska M., Amit I., Jantsch J., Mages J., Frech C., Dolken L., Mann M., Lang R. (2010) The phosphoproteome of toll-like receptor-activated macrophages. Mol. Syst. Biol. 6, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Hill R., Lee P. W. (2010) The DNA-dependent protein kinase (DNA-PK): More than just a case of making ends meet? Cell Cycle 9, 3460–3469 [DOI] [PubMed] [Google Scholar]
- 216. Neal J. A., Meek K. (2011) Choosing the right path: does DNA-PK help make the decision? Mutat. Res. 711, 73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Wang Z., Yan J., Lin H., Hua F., Wang X., Liu H., Lv X., Yu J., Mi S., Wang J., Hu Z. W. (2013) TLR4 activity protects against hepatocellular tumorigenesis and progression via regulating the expression of DNA repair protein Ku70(1). Hepatology 57, 1869–1881 [DOI] [PubMed] [Google Scholar]
- 218. Wu J., Zhu W., Fu H., Zhang Y., Sun J., Yang W., Li Y. (2012) DNA-PKcs interacts with Aire and regulates the expression of toll-like receptors in RAW264.7 cells. Scand. J. Immunol. 75, 479–488 [DOI] [PubMed] [Google Scholar]
- 219. Karpova A. Y., Trost M., Murray J. M., Cantley L. C., Howley P. M. (2002) Interferon regulatory factor-3 is an in vivo target of DNA-PK. Proc. Natl. Acad. Sci. U. S. A. 99, 2818–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ferguson B. J., Mansur D. S., Peters N. E., Ren H., Smith G. L. (2012) DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. Elife. 1, e00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Dragoi A. M., Fu X., Ivanov S., Zhang P., Sheng L., Wu D., Li G. C., Chu W. M. (2005) DNA-PKcs, but not TLR9, is required for activation of Akt by CpG-DNA. EMBO J. 24, 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Panta G. R., Kaur S., Cavin L. G., Cortes M. L., Mercurio F., Lothstein L., Sweatman T. W., Israel M., Arsura M. (2004) ATM and the catalytic subunit of DNA-dependent protein kinase activate NF-kappaB through a common MEK/extracellular signal-regulated kinase/p90(rsk) signaling pathway in response to distinct forms of DNA damage. Mol. Cell Biol. 24, 1823–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Liu L., Kwak Y. T., Bex F., Garcia-Martinez L. F., Li X. H., Meek K., Lane W. S., Gaynor R. B. (1998) DNA-dependent protein kinase phosphorylation of IkappaB alpha and IkappaB beta regulates NF-kappaB DNA binding properties. Mol. Cell Biol. 18, 4221–4234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Segal B. H., Grimm M. J., Khan A. N., Han W., Blackwell T. S. (2012) Regulation of innate immunity by NADPH oxidase. Free Radic. Biol. Med. 53, 72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Tattoli I., Carneiro L. A., Jehanno M., Magalhaes J. G., Shu Y., Philpott D. J., Arnoult D., Girardin S. E. (2008) NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 9, 293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. McCaffrey R. L., Allen L. A. (2006) Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J. Leukoc. Biol. 80, 1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Allen L. A., Beecher B. R., Lynch J. T., Rohner O. V., Wittine L. M. (2005) Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J. Immunol. 174, 3658–3667 [DOI] [PubMed] [Google Scholar]
- 228. Carlyon J. A., Abdel-Latif D., Pypaert M., Lacy P., Fikrig E. (2004) Anaplasma phagocytophilum utilizes multiple host evasion mechanisms to thwart NADPH oxidase-mediated killing during neutrophil infection. Infect. Immun. 72, 4772–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Pockley A. G., Bulmer J., Hanks B. M., Wright B. H. (1999) Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones 4, 29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Ripley B. J., Isenberg D. A., Latchman D. S. (2001) Elevated levels of the 90 kDa heat shock protein (hsp90) in SLE correlate with levels of IL-6 and autoantibodies to hsp90. J. Autoimmun. 17, 341–346 [DOI] [PubMed] [Google Scholar]
- 231. Njemini R., Bautmans I., Onyema O. O., Van Puyvelde K., Demanet C., Mets T. (2011) Circulating heat shock protein 70 in health, aging and disease. BMC Immunol. 12, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Ciocca D. R., Calderwood S. K. (2005) Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10, 86–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Panchapakesan J., Daglis M., Gatenby P. (1992) Antibodies to 65 kDa and 70 kDa heat shock proteins in rheumatoid arthritis and systemic lupus erythematosus. Immunol. Cell Biol. 70, 295–300 [DOI] [PubMed] [Google Scholar]
- 234. Georgopoulos C., McFarland H. (1993) Heat shock proteins in multiple sclerosis and other autoimmune diseases. Immunol. Today 14, 373–375 [DOI] [PubMed] [Google Scholar]
- 235. Rice J. W., Veal J. M., Fadden R. P., Barabasz A. F., Partridge J. M., Barta T. E., Dubois L. G., Huang K. H., Mabbett S. R., Silinski M. A., Steed P. M., Hall S. E. (2008) Small molecule inhibitors of Hsp90 potently affect inflammatory disease pathways and exhibit activity in models of rheumatoid arthritis. Arthritis Rheum. 58, 3765–3775 [DOI] [PubMed] [Google Scholar]
- 236. Han J. M., Kwon N. H., Lee J. Y., Jeong S. J., Jung H. J., Kim H. R., Li Z., Kim S. (2010) Identification of gp96 as a novel target for treatment of autoimmune disease in mice. PLoS One 5, e9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Zugel U., Kaufmann S. H. (1999) Immune response against heat shock proteins in infectious diseases. Immunobiology 201, 22–35 [DOI] [PubMed] [Google Scholar]
- 238. Pirot P., Eizirik D. L., Cardozo A. K. (2006) Interferon-gamma potentiates endoplasmic reticulum stress-induced death by reducing pancreatic beta cell defence mechanisms. Diabetologia 49, 1229–1236 [DOI] [PubMed] [Google Scholar]
- 239. Weston C. R., Davis R. J. (2007) The JNK signal transduction pathway. Curr. Opin. Cell Biol. 19, 142–149 [DOI] [PubMed] [Google Scholar]
- 240. Hultqvist M., Olsson L. M., Gelderman K. A., Holmdahl R. (2009) The protective role of ROS in autoimmune disease. Trends Immunol. 30, 201–208 [DOI] [PubMed] [Google Scholar]
- 241. Purcell A. W., Todd A., Kinoshita G., Lynch T. A., Keech C. L., Gething M. J., Gordon T. P. (2003) Association of stress proteins with autoantigens: a possible mechanism for triggering autoimmunity? Clin. Exp. Immunol. 132, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Nagaraju K., Casciola-Rosen L., Lundberg I., Rawat R., Cutting S., Thapliyal R., Chang J., Dwivedi S., Mitsak M., Chen Y. W., Plotz P., Rosen A., Hoffman E., Raben N. (2005) Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 52, 1824–1835 [DOI] [PubMed] [Google Scholar]
- 243. Lin W., Bailey S. L., Ho H., Harding H. P., Ron D., Miller S. D., Popko B. (2007) The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J. Clin. Invest. 117, 448–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Jaeschke H. (2011) Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol. 26(Suppl. 1), 173–179 [DOI] [PubMed] [Google Scholar]
- 245. Wheeler M. D., Kono H., Yin M., Nakagami M., Uesugi T., Arteel G. E., Gabele E., Rusyn I., Yamashina S., Froh M., Adachi Y., Iimuro Y., Bradford B. U., Smutney O. M., Connor H. D., Mason R. P., Goyert S. M., Peters J. M., Gonzalez F. J., Samulski R. J., Thurman R. G. (2001) The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic. Biol. Med. 31, 1544–1549 [DOI] [PubMed] [Google Scholar]
- 246. Lee I. T., Yang C. M. (2012) Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 84, 581–590 [DOI] [PubMed] [Google Scholar]
- 247. Vlahos R., Stambas J., Bozinovski S., Broughton B. R., Drummond G. R., Selemidis S. (2011) Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathog. 7, e1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Duong C., Seow H. J., Bozinovski S., Crack P. J., Anderson G. P., Vlahos R. (2010) Glutathione peroxidase-1 protects against cigarette smoke-induced lung inflammation in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 299, L425–L433 [DOI] [PubMed] [Google Scholar]
- 249. McGuckin M. A., Eri R. D., Das I., Lourie R., Florin T. H. (2011) Intestinal secretory cell ER stress and inflammation. Biochem. Soc. Trans. 39, 1081–1085 [DOI] [PubMed] [Google Scholar]
- 250. Kaser A., Lee A. H., Franke A., Glickman J. N., Zeissig S., Tilg H., Nieuwenhuis E. E., Higgins D. E., Schreiber S., Glimcher L. H., Blumberg R. S. (2008) XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Mirzayans R., Rais B., Scott A., Murray D. (2012) New insights into p53 signaling and cancer cell response to DNA damage: implications for cancer therapy. J. Biomed. Biotechnol. 2012, 170325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Mambula S. S., Calderwood S. K. (2006) Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J. Immunol. 177, 7849–7857 [DOI] [PubMed] [Google Scholar]
- 253. Multhoff G., Botzler C., Wiesnet M., Muller E., Meier T., Wilmanns W., Issels R. D. (1995) A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int. J. Cancer 61, 272–279 [DOI] [PubMed] [Google Scholar]
- 254. Chalmin F., Ladoire S., Mignot G., Vincent J., Bruchard M., Remy-Martin J. P., Boireau W., Rouleau A., Simon B., Lanneau D., De Thonel A., Multhoff G., Hamman A., Martin F., Chauffert B., Solary E., Zitvogel L., Garrido C., Ryffel B., Borg C., Apetoh L., Rebe C., Ghiringhelli F. (2010) Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest. 120, 457–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Akyol S., Gercel-Taylor C., Reynolds L. C., Taylor D. D. (2006) HSP-10 in ovarian cancer: expression and suppression of T-cell signaling. Gynecol. Oncol. 101, 481–486 [DOI] [PubMed] [Google Scholar]
- 256. Nakamura H., Minegishi H. (2012) HSP60 as a drug target. Curr. Pharm. Des. [PubMed] [Google Scholar]
- 257. Jia H., Halilou A. I., Hu L., Cai W., Liu J., Huang B. (2011) Heat shock protein 10 (Hsp10) in immune-related diseases: one coin, two sides. Int. J. Biochem. Mol. Biol. 2, 47–57 [PMC free article] [PubMed] [Google Scholar]
- 258. Trepel J., Mollapour M., Giaccone G., Neckers L. (2010) Targeting the dynamic HSP90 complex in Cancer Nat. Rev. Cancer 10, 537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Kummar S., Gutierrez M. E., Gardner E. R., Chen X., Figg W. D., Zajac-Kaye M., Chen M., Steinberg S. M., Muir C. A., Yancey M. A., Horneffer Y. R., Juwara L., Melillo G., Ivy S. P., Merino M., Neckers L., Steeg P. S., Conley B. A., Giaccone G., Doroshow J. H., Murgo A. J. (2010) Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a heat shock protein inhibitor, administered twice weekly in patients with advanced malignancies. Eur. J. Cancer 46, 340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Dickson M. A., Okuno S. H., Keohan M. L., Maki R. G., D'Adamo D. R., Akhurst T. J., Antonescu C. R., Schwartz G. K. (2012) Phase II study of the HSP90-inhibitor BIIB021 in gastrointestinal stromal tumors. Ann. Oncol. 24, 252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Xia Y., Liu Y., Rocchi P., Wang M., Fan Y., Qu F., Iovanna J. L., Peng L. (2012) Targeting heat shock factor 1 with a triazole nucleoside analog to elicit potent anticancer activity on drug-resistant pancreatic cancer. Cancer Lett. 318, 145–153 [DOI] [PubMed] [Google Scholar]
- 262. Goloudina A. R., Demidov O. N., Garrido C. (2012) Inhibition of HSP70: a challenging anti-cancer strategy. Cancer Lett. 325, 117–124 [DOI] [PubMed] [Google Scholar]
- 263. Feldman D. E., Chauhan V., Koong A. C. (2005) The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol. Cancer Res. 3, 597–605 [DOI] [PubMed] [Google Scholar]
- 264. Fernandez P. M., Tabbara S. O., Jacobs L. K., Manning F. C., Tsangaris T. N., Schwartz A. M., Kennedy K. A., Patierno S. R. (2000) Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res. Treat. 59, 15–26 [DOI] [PubMed] [Google Scholar]
- 265. Shuda M., Kondoh N., Imazeki N., Tanaka K., Okada T., Mori K., Hada A., Arai M., Wakatsuki T., Matsubara O., Yamamoto N., Yamamoto M. (2003) Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J. Hepatol. 38, 605–614 [DOI] [PubMed] [Google Scholar]
- 266. Blais J. D., Addison C. L., Edge R., Falls T., Zhao H., Wary K., Koumenis C., Harding H. P., Ron D., Holcik M., Bell J. C. (2006) Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol. Cell Biol. 26, 9517–9532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Mumm J. B., Oft M. (2008) Cytokine-based transformation of immune surveillance into tumor-promoting inflammation. Oncogene 27, 5913–5919 [DOI] [PubMed] [Google Scholar]
- 268. Reuter S., Gupta S. C., Chaturvedi M. M., Aggarwal B. B. (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 49, 1603–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Mahadevan N. R., Rodvold J., Sepulveda H., Rossi S., Drew A. F., Zanetti M. (2011) Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc. Natl. Acad. Sci. U. S. A. 108, 6561–6566 [DOI] [PMC free article] [PubMed] [Google Scholar]