Abstract
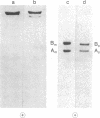
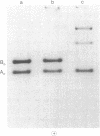
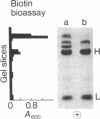
3-Methylcrotonyl-CoA carboxylase (MCase; EC 6.4.1.4) and propionyl-CoA carboxylase (PCase; EC 6.4.1.3) have been obtained in highly purified form from bovine kidney mitochondria. Polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate revealed that each enzyme is composed of nonidentical subunits, including a smaller biotin-free subunit (Mr 62,000 and 58,000 for MCase and PCase, respectively), and a larger biotin-containing subunit (Mr 80,000 and 74,000 for MCase and PCase, respectively). The possibility that these subunits were derived from a single, larger precursor polypeptide via proteolysis was explored by purification and electrophoresis of each enzyme in the presence of protease inhibitors, but no evidence for proteolysis was obtained. Specific antisera directed towards each enzyme were prepared. The anti-PCase preparation was used to precipitate crossreacting PCase from a pig heart extract. Analysis of the immunoprecipitate obtained revealed a biotin-containing polypeptide (Mr 78,000) and a biotin-free polypeptide (Mr 55,000), suggesting that pig heart PCase also contains nonidentical subunits analogous to those seen in the kidney mitochondrial MCase and PCase. A bipartite subunit structure may be a common feature in mammalian MCase and PCase.
Keywords: mitochondria, immunoprecipitate, pig heart propionyl-CoA carboxylase
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad F., Ahmad P. M., Pieretti L., Watters G. T. Purification and subunit structure of rat mammary gland acetyl coenzyme A carboxylase. J Biol Chem. 1978 Mar 10;253(5):1733–1737. [PubMed] [Google Scholar]
- Barden R. E., Taylor B. L., Isoashi F., Frey W. H., Zander G., Lee J. C., Utter M. F. Structural properties of pyruvate carboxylases from chicken liver and other sources. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4308–4312. doi: 10.1073/pnas.72.11.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce C. F., Crichton R. R. The subunit structure of horse spleen apoferritin. I. The molecular weight of the subunit. J Biol Chem. 1971 Jul 10;246(13):4198–4205. [PubMed] [Google Scholar]
- Clinkenbeard K. D., Reed W. D., Mooney R. A., Lane M. D. Intracellular localization of the 3-hydroxy-3-methylglutaryl coenzme A cycle enzymes in liver. Separate cytoplasmic and mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A generating systems for cholesterogenesis and ketogenesis. J Biol Chem. 1975 Apr 25;250(8):3108–3116. [PubMed] [Google Scholar]
- Fall R. R., Hector M. L. Acyl-coenzyme A carboxylases. Homologous 3-methylcrotonyl-CoA and geranyl-CoA carboxylases from Pseudomonas citronellolis. Biochemistry. 1977 Sep 6;16(18):4000–4005. doi: 10.1021/bi00637a010. [DOI] [PubMed] [Google Scholar]
- Giorgio A. J., Plaut G. W. The effect of univalent cations on activities catalyzed by bovine-liver propionyl-CoA carboxylase. Biochim Biophys Acta. 1967 Jul 11;139(2):487–501. doi: 10.1016/0005-2744(67)90052-6. [DOI] [PubMed] [Google Scholar]
- Gravel R. A., Lam K. F., Scully K. J., Hsia Y. Genetic complementation of propionyl-CoA carboxylase deficiency in cultured human fibroblasts. Am J Hum Genet. 1977 Jul;29(4):378–388. [PMC free article] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- HALENZ D. R., FENG J. Y., HEGRE C. S., LANE M. D. Some enzymic properties of mitochondrial propionyl carboxylase. J Biol Chem. 1962 Jul;237:2140–2147. [PubMed] [Google Scholar]
- Hector M. L., Fall R. R. Multiple acyl-coenzyme A carboxylases in Pseudomonas citronellolis. Biochemistry. 1976 Aug 10;15(16):3465–3472. doi: 10.1021/bi00661a011. [DOI] [PubMed] [Google Scholar]
- Inoue H., Lowenstein J. M. Acetyl coenzyme A carboxylase from rat liver. Purification and demonstration of different subunits. J Biol Chem. 1972 Aug 10;247(15):4825–4832. [PubMed] [Google Scholar]
- KAZIRO Y., OCHOA S., WARNER R. C., CHEN J. Y. Metabolism of propionic acid in animal tissues. VIII. Crystalline propionyl carboxylase. J Biol Chem. 1961 Jul;236:1917–1923. [PubMed] [Google Scholar]
- Koch A. L., Putnam S. L. Sensitive biuret method for determination of protein in an impure system such as whole bacteria. Anal Biochem. 1971 Nov;44(1):239–245. doi: 10.1016/0003-2697(71)90366-6. [DOI] [PubMed] [Google Scholar]
- LICHSTEIN H. C. The presence of bound biotin in purified preparations of oxalacetic carboxylase. J Biol Chem. 1955 Jan;212(1):217–222. [PubMed] [Google Scholar]
- Mackall J. C., Lane M. D. Changes in mammary-gland acetyl-coenzyme A carboxylase associated with lactogenic differentiation. Biochem J. 1977 Mar 15;162(3):635–642. doi: 10.1042/bj1620635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Carabott M. J., Keech D. B., Wallace J. C. Properties of the isocitrate synthase system from rat liver. Biochem Soc Trans. 1976;4(6):1058–1060. doi: 10.1042/bst0041058. [DOI] [PubMed] [Google Scholar]
- Meyer H., Nevaldine B., Meyer F. Acyl-coenzyme A carboxylase of the free-living nematode Turbatrix aceti. 1. Its isolation and molecular characteristics. Biochemistry. 1978 May 16;17(10):1822–1827. doi: 10.1021/bi00603a003. [DOI] [PubMed] [Google Scholar]
- Schiele U., Niedermeier R., Stürzer M., Lynen F. Investigations of the structure of 3-methylcrotonyl-CoA carboxylase from Achromobacter. Eur J Biochem. 1975 Dec 1;60(1):259–266. doi: 10.1111/j.1432-1033.1975.tb20998.x. [DOI] [PubMed] [Google Scholar]
- Swack J. A., Zander G. L., Utter M. F. Use of avidin-sepharose to isolate and idenify biotin polypeptides from crude extracts. Anal Biochem. 1978 Jun 15;87(1):114–126. doi: 10.1016/0003-2697(78)90575-4. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Wada K., Okazaki T., Numa S. Acetyl-coenzyme-A carboxylase from rat liver. Subunit structure and proteolytic modification. Eur J Biochem. 1975 Sep 1;57(1):15–24. doi: 10.1111/j.1432-1033.1975.tb02272.x. [DOI] [PubMed] [Google Scholar]
- Utter M. F., Barden R. E., Taylor B. L. Pyruvate carboxylase: an evaluation of the relationships between structure and mechanism and between structure and catalytic activity. Adv Enzymol Relat Areas Mol Biol. 1975;42:1–72. doi: 10.1002/9780470122877.ch1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weyler W., Sweetman L., Maggio D. C., Nyhan W. L. Deficiency of propionyl-Co A carboxylase and methylcrotonyl-Co A carboxylase in a patient with methylcrotonylglycinuria. Clin Chim Acta. 1977 May 2;76(3):321–328. doi: 10.1016/0009-8981(77)90158-9. [DOI] [PubMed] [Google Scholar]
- Wood H. G., Barden R. E. Biotin enzymes. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]