SUMMARY
Studies support the importance of microRNAs in physiological and pathological processes. Here we describe the regulation and function of miR-29 in myogenesis and Rhabdomyosarcoma (RMS). Results demonstrate that in myoblasts miR-29 is repressed by NF-κB acting through YY1 and the Polycomb. During myogenesis, NF-κB and YY1 downregulation causes derepression of miR-29, which in turn accelerates differentiation by targeting its repressor YY1. However, in RMS cells and primary tumors that possess impaired differentiation, miR-29 is epigenetically silenced by an activated NF-κB-YY1 pathway. Reconstitution of miR-29 in RMS in mice inhibits tumor growth and stimulates differentiation, suggesting that miR-29 acts as a tumor suppressor through its pro-myogenic function. Together, results identify a NF-κB–YY1–miR-29 regulatory circuit whose disruption may contribute to RMS.
SIGNIFICANCE
MicroRNAs regulate skeletal myogenesis, but their impact in muscle diseases is not well understood. Here we describe miR-29 as an enhancer of myogenic differentiation and a suppressor of RMS. We find that miR-29 exists in a regulatory circuit involving NF-κB and YY1. In myoblasts NF-B acts through YY1 to epigenetically suppress miR-29, while during differentiation miR-29 is induced to facilitate myogenesis by a negative feedback on YY1. Significantly, RMS tumors lose miR-29 due to an elevation in NF-B and YY1, and readjustment of miR-29 levels in RMS stimulates differentiation. Thus, myogenesis is dependent on NF-κB–YY1–miR-29 circuitry whose dysfunction may contribute to RMS pathogenesis. Such findings offer potential avenues for the diagnosis and treatment of muscle relevant cancers.
Keywords: microRNA, differentiation, myogenesis, YY1, NF-kappaB, Rhabdomyosarcoma
INTRODUCTION
MicroRNAs (miRNAs) are small non-coding single-stranded RNAs that constitute a novel class of gene regulators. These RNAs are initially transcribed by RNA polymerase II as primary transcripts (Lee et al., 2004; Zeng et al., 2005) that are then processed in the nucleus by Drosha into a ~70 nucleotide precursor miRNA that forms hairpin structures (Gregory et al., 2005). Precursor miRNA are then exported into the cytoplasm and serve as substrates to generate mature miRNAs (He and Hannon, 2004). Approximately 50% of miRNAs are found in clusters, transcribed as polycistronic primary transcripts (Mourelatos et al., 2002).
MiRNAs negatively regulate gene expression at the posttranscriptional level by base pairing with the 3’ untranslated region (UTR) of their target mRNAs. It is believed that if this pairing is perfect or nearly perfect, as predominantly seen in plants (Gregory et al., 2005), the mRNA becomes cleaved and degraded (Pattanayak et al., 2005). However, with most mammalian miRNAs, the pairing is imperfect, resulting in translational repression (Hutvagner, 2005). Since their initial discovery, more than 3000 miRNAs have been identified in animals, plants, and viruses. With over 500 miRNAs in the human genome and a plethora of predicted mRNA targets, it is believed that these small RNAs have an enormous regulatory potential in gene expression programs (Bartel, 2004). Indeed, the functions of miRNAs have been found to extend to both physiological and pathological conditions, including cell proliferation, cell death, differentiation, development, metabolism, viral infection, and cancer (Miska, 2005; Shivdasani, 2006).
One cellular process under miRNA control is skeletal muscle differentiation. This process is orchestrated by transcription factors MyoD, Myf5, myogenin, MRF4, and Mef2. These factors activate muscle genes to coordinate myoblasts to terminally withdraw from the cell cycle and subsequently fuse into multinucleated myotubes (Sabourin and Rudnicki, 2000). Several muscle miRNAs are regulated by these myogenic transcription factors and are required for skeletal muscle formation. For instance, MyoD and Mef2 regulate expression of miR-1 that suppresses HDAC-4 resulting in augmented Mef2 activity (Rao et al., 2006; Zhao et al., 2005). MyoD induces miR-206 which targets DNA polymerase to facilitate cell cycle exit, as well as follistatin to enhance myogenesis (Anderson et al., 2006; Kim et al., 2006; Rosenberg et al., 2006). In addition, miR-133 synthesis is controlled by Mef2, but functionally this miRNA inhibits myogenesis by downregulating serum response factor (SRF), which maintains myoblast proliferation (Chen et al., 2006; Liu et al., 2007).
Analogous to muscle miRNAs, signal transduction pathways function within a hierarchical network to positively and negatively regulate myogenesis. Among these is NF-κB, which is active in myoblasts and functions to block differentiation in vitro and in vivo (Acharyya et al., 2007; Guttridge et al., 2000; Wang et al., 2007). NF-κB exists as a dimer with the p50/p65 heterodimer form being the most common (Hayden and Ghosh, 2004). In resting cells, NF-κB is retained in the cytoplasm through binding of its IκB inhibitor. Classical activation of NF-κB occurs by factors that stimulate the IκB kinase complex to phosphorylate and degrade IκB, leading to NF-κB nuclear translocation and subsequent target gene expression (Karin and Ben-Neriah, 2000).
Recent work demonstrates that NF-κB’s ability to repress myogenesis occurs through multiple mechanisms that depend on components of the NF-κB classical pathway (Bakkar et al., 2008). One such mechanism involves the target gene, Ying Yang 1 (YY1), which itself is capable of repressing muscle differentiation (Caretti et al., 2004; Lee et al., 1994; Wang et al., 2007). YY1 is also a member of the Polycomb Group (PcG) that functions to silence transcription of a selected set of genes by chromatin modification (Sparmann and van Lohuizen, 2006). In myoblasts NF-κB regulates YY1 that in turn binds and inhibits myofibrillar promoters by recruiting the PcG member, Ezh2, as well as the histone deacetylase protein, HDAC-1 (Caretti et al., 2004; Wang et al., 2007). Thus, activation of NF-κB ensures that YY1 levels and Polycomb activity are maintained in undifferentiated cells.
In addition to their underlying etiology in a variety of muscle related diseases including dystrophies and cachexia, a muscle lineage is also considered to be the origin of Rhabdomyosarcoma, an aggressive, malignant pediatric soft-tissue sarcoma (Breitfeld and Meyer, 2005; Mackall and Helman, 2001). These tumors are thought to arise as a consequence of a dysfunctional balance of the growth and terminal differentiation of muscle progenitor cells. Despite current modes of intensive chemotherapy, radiation, and surgery, the survival for children with advanced disease is dismal and prognosis has remained unchanged for decades. RMS is divided into two major histological subtypes, embryonal and alveolar. Whereas embryonal RMS (ERMS) recapitulates the phenotypical and biological features of embryonic muscle, alveolar RMS (ARMS) is a more loosely organized tumor displaying poor muscle differentiation (Qualman et al., 1998). Current knowledge of the molecular mechanisms that contribute to the failure in differentiation of RMS tumors is limited. Although miRNAs are considered to act as tumor suppressors or oncogenes in an assortment of cancers, notably, their potential role in RMS has not been explored. In this study we reveal the regulation of miR-29 by the NF-κB-YY1 regulatory pathway and elucidate its significance in normal skeletal muscle differentiation and RMS.
RESULTS
NF-κB and YY1 negatively regulate miR-29b/c in C2C12 myoblasts
Although miRNAs are involved in the complex network of skeletal myogenesis, their interactions with other myogenic regulatory factors are only beginning to be revealed. Of particular interest is the regulation of miRNA by transcription factors. Since we recently discovered that NF-κB functions to inhibit myogenesis through YY1 (Wang et al., 2007), we initiated a screen for miRNAs potentially regulated by these two transcription factors. The use of positional weight matrix from TRANSFAC (Wingender et al., 1997) identified numerous YY1 binding sites upstream of pre-miRNAs. To narrow our search, we utilized the UCSC genome browser (Kent et al., 2002) and rVISTA (Loots and Ovcharenko, 2004) to find YY1 sites conserved among species. This gave rise to a handful of miRNAs that represented potential targets of YY1 (Suppl. Table 1). Among these we singled out miR-29b2 and miR-29c that lie in close proximity to each other on mouse and human chromosome 1 (Suppl. Figure 1A). Four YY1 binding sites (referred to as A, B, C, and D) were identified in a highly conserved region approximately 20Kb upstream of miR-29b2 and miR29c ( Figure 1A and Suppl. Figure 1B). This same region also contains several conserved cis-elements for myogenic transcription factors MyoD, myogenin, Mef2, and SRF. In comparison, no conserved YY1 sites were identified upstream of miR-29a and miR-29b1, clustered on mouse chromosome 6 and human chromosome 7 (Suppl. Figure 1A).
Figure 1. YY1 represses miR-29b/c through binding to a conserved regulatory region.
(A) An rVISTA schematic showing the degree of sequence conservation between human and mouse chromosome (Chr) 1 in a region upstream of the miR-29b/c cluster. Predicted YY1, MyoD, myogenin, SRF, and Mef2 sites are displayed. (B) EMSA performed from C2C12 MB or MT with probes corresponding to YY1 sites A-D. With MB extracts, a supershift EMSA was performed using YY1 antisera. Arrows denote YY1/DNA bound complexes. (C) ChIP assays for YY1 was performed with chromatin from C2C12 MB or MT. Precipitated DNA was amplified with oligonucleotides spanning regions A-D. Total inputs are indicated. (D) ChIPs as in (C) were repeated for Ezh2, H3K27, HDAC-1, SRF or Mef2. (E) MB were transfected with either an YY1 expression plasmid (pCMV-YY1) or YY1 siRNA oligos and then induced to differentiate for 24h, at which time miR-29b and miR-29c were measured by qRT-PCR and normalized to U6. Fold changes are shown with respect to control siRNA transfected cells where miR-29 levels were set to a value of 1. (F) MB were transfected with a miR-29b/c-enhancer-Luc reporter and maintained as MB or differentiated in MT for 48h, at which time luciferase activities were determined. (G) MB were transfected with a miR-29b/c-enhancer-Luc reporter, along with plasmids for YY1, SRF or Mef2. Cells were then switched differentiation conditions and luciferase activity was subsequently measured. (H) MB were transfected with the miR-29b/cenhancer- Luc reporter (YY1 wt) or with an enhancer reporter containing a deletion mutation in the YY1 “D” site (YY1 mut), and subsequently differentiated for 48h (MT) at which time luciferase activity was determined (left graph). Separate transfections were performed with YY1 wt and YY1 mut reporters along with an YY1 expression plasmid (pCMV-YY1) or YY1 siRNA. Cells were subsequently differentiated for 48h at which time luciferase was determined. Vector siRNA oligo transfected cells were used as a control (right graph). All luciferase data were normalized to β-galactosidase protein and represent the average of three independent experiments ± S.D.
To test whether sites A through D upstream of the miR-29b2 (from here on referred to simply as miR29b) and miR-29c locus were competent for YY1 binding, EMSAs were performed with extracts prepared from C2C12 myoblasts and myotubes. Results showed that all four sites produced binding complexes in myoblasts (MB) (Figure 1B), but only complexes C and D were supershifted with YY1 antisera. Furthermore, in line with previous findings that YY1 activity decreases in myogenesis (Caretti et al., 2004; Wang et al., 2007), complex C and D were also reduced in myotubes (MT). However, by ChIP only site D was bound by YY1 (Figure 1C). Consistent with EMSA, binding was only detected in MB, suggesting that YY1 binds to a putative regulatory element of miR-29b/c.
Since YY1 represses myogenic genes through recruitment of the PcG and HDAC-1 (Caretti et al., 2004), we asked whether similar regulation occurred on the miR-29b/c locus. Indeed, in addition to YY1, binding for Ezh2 and its trimethylated activity on histone H3 lysine 27 (H3K27) was also detected, along with HDAC-1 (Figure 1D). In MT, these repressors were replaced with SRF and Mef2, whose presence is associated with activation of muscle specific genes. To further investigate the regulation of miR-29b/c by YY1, C2C12 myoblasts were transfected with an YY1 expression plasmid. By quantitative RT-PCR, miR-29b and miR-29c levels decreased two fold in the presence of YY1 (Figure 1E). In contrast, siRNA knockdown of YY1 significantly enhanced expression of both miRNAs (Suppl. Figure 2A and Figure 1E), suggestive that YY1 is a repressor of miR29b/c. Next we tested the activity of the miR29b/c upstream regulatory region by cloning a 4.5Kb fragment spanning YY1, SRF, and Mef2 binding sites into a luciferase reporter plasmid. Myogenesis caused the increase in reporter activity (Figure 1F). Furthermore, this activity was repressed by addition of YY1, but stimulated with either YY1 knockdown or addition of SRF or Mef2 (Figure 1G and 1H), suggesting that this region upstream of miR-29b/c functions as an enhancer. To examine the requirement of YY1 binding for enhancer activity, the D site was mutated and compared to its wild type counterpart. Loss of the YY1 site led to higher reporter activity, and compared to the wild type enhancer, was unresponsive to YY1 knockdown (Figure 1H). Collectively, these data demonstrate that the miR29b/c locus contains a regulatory region that in MB is under negative control by YY1 and the PcG, and in MT is positively regulated by myogenic transcription factors.
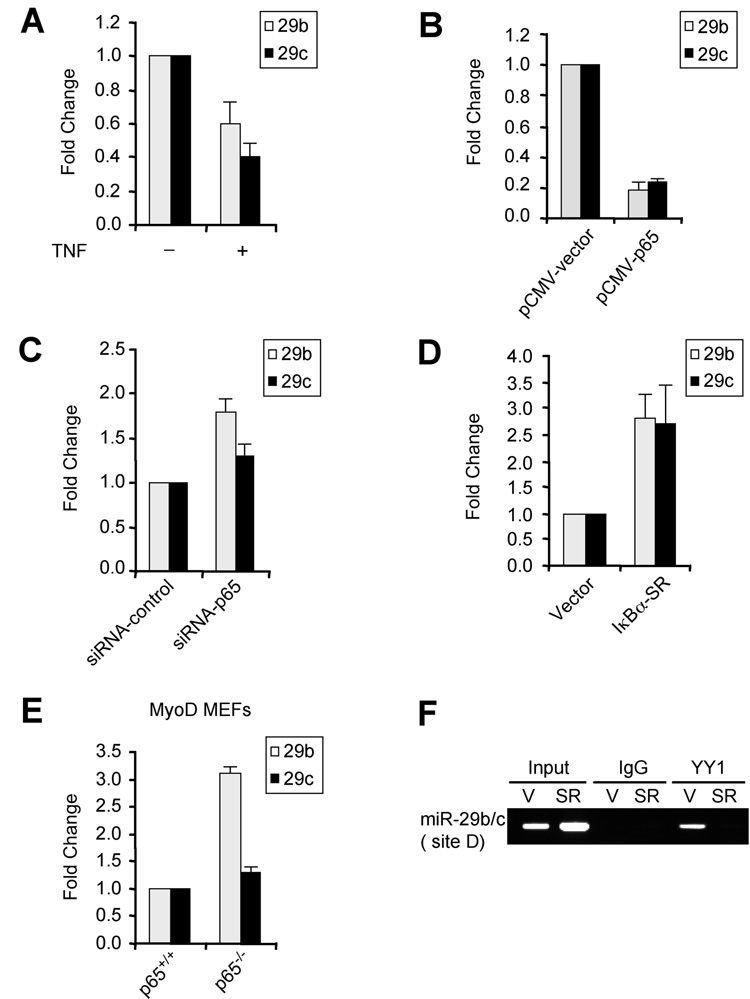
Since YY1 is a transcriptional target of NF-κB (Wang et al., 2007), we reasoned that miR-29b/c should also come under negative control of NF-κB. Consistent with this thinking, treatment of C2C12 myoblasts with TNFα as an activator of NF-κB reduced miR-29b/c expression (Figure 2A). Similar reduction of miR-29 was observed in C2C12 cells stably expressing the p65 subunit of NF-κB (Figure 2B). Conversely, MB containing siRNA against p65 or expressing the IκBα-SR inhibitor of NF-κB led to higher levels of miR-29b/c over that of control cells (Figure 2C, Figure 2D and Suppl. Figure 2B). Similar enhancement of miR-29 was observed in p65−/− fibroblasts expressing MyoD (Figure 2E). In addition, ChIP analysis showed that YY1 was bound to the miR-29b/c locus in vector MB, but absent in cells devoid of NF-κB (Figure 2F). These findings strongly suggest that NF-κB negatively regulates miR-29b/c via YY1.
Figure 2. NF-κB negatively regulates miR-29b/c.
(A) C2C12 cells were treated with TNFα and miR-29 was measured by qRT-PCR normalized to U6. Fold changes are shown with respect to vector cells where miR-29 levels were set to a value of 1. (B) MB were transfected with vector or a p65 plasmid and miR-29b/c levels were measured 48h post-transfection. (C) MB were transfected with either vector or p65 siRNA oligos and miR-29 expression was then measured as in (B). (D) MiR-29b/c were measured in MB stably expressing vector or IκBα-SR. Fold changes are shown with respect to vector cells, which were set to a value of 1. (E) MyoD was stably expressed in p65+/+ or p65−/− mouse embryonic fibroblasts (Bakkar et al., 2008) and qRT-PCR was performed for miR-29b and miR-29c. (F) ChIPs with YY1 or control IgG were performed on chromatins derived from either vector control (V) or Iκ Bα-SR (SR) expressing MB. Primers specific to site D were used for the PCR amplification. Total inputs are indicated.
miR-29 functions as a positive regulator of myogenesis
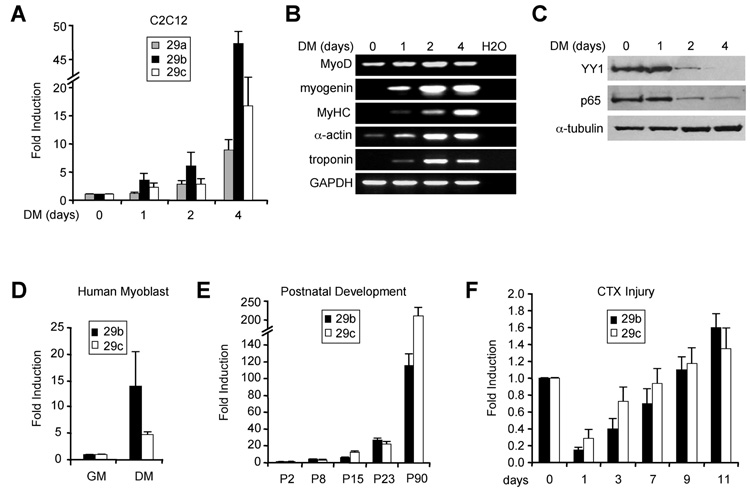
Results above led us to speculate that miR-29b/c might itself be regulated during muscle differentiation. To test this, we examined miR-29 in both in vitro and in vivo models of myogenesis. Results showed that levels of miR-29b/c steadily increased in differentiating C2C12 cells, correlating with induction of known differentiation markers and repression of its inhibitors, NF-κB and YY1 (Figure 3A, 3B, and 3C). Interestingly, miR-29a was also induced during myogenesis, albeit to lower levels, indicating that this miRNA might be subject to similar transcriptional silencing in MB. Regulation of miR-29b was not species specific since similar induction was seen in differentiating human muscle cells (Figure 3D). We also found that miR-29b/c was stimulated in developing post-natal muscle (Figure 3E), suggesting that this miRNA is relevant in skeletal muscle in vivo, and similar to cultured cells, there was an inverse relationship between miR-29b/c and NF-κB/YY1 expression (Suppl. Figure 3A). Furthermore, in a cardiotoxin (CTX) model of muscle injury, miR-29b/c levels initially dropped then steadily increased in accordance with the regenerative program. This regulation again correlated strongly with changes in YY1 (Figure 3F and Suppl. Figure 3B). Altogether, these results support that miR-29 is positively regulated during muscle differentiation.
Figure 3. miR-29 is induced during muscle differentiation in vitro and in vivo.
(A) C2C12 MB were induced to differentiate up to 4 days in differentiation medium (DM) and at indicated times miR-29a, miR-29b, and miR-29c were measured by qRT-PCR. (B) RT-PCR analysis of myogenic markers MyoD, myogenin, MyHC, skeletal actin (α-actin), troponin T performed at similar times to those in (A). (C) Westerns probing for YY1 and nuclear p65 in differentiating C2C12 cells. (D) Expression of miR-29b, and miR-29c in primary human myoblasts (GM) and myotubes (DM). (E) Measurement of miR-29 from either lower limb muscles at P2 and P8 or from TA muscles at P15, P23, and P90 in C57/BL6 mice. (F) Same measurement as in (E) from CTX injected TA muscles.
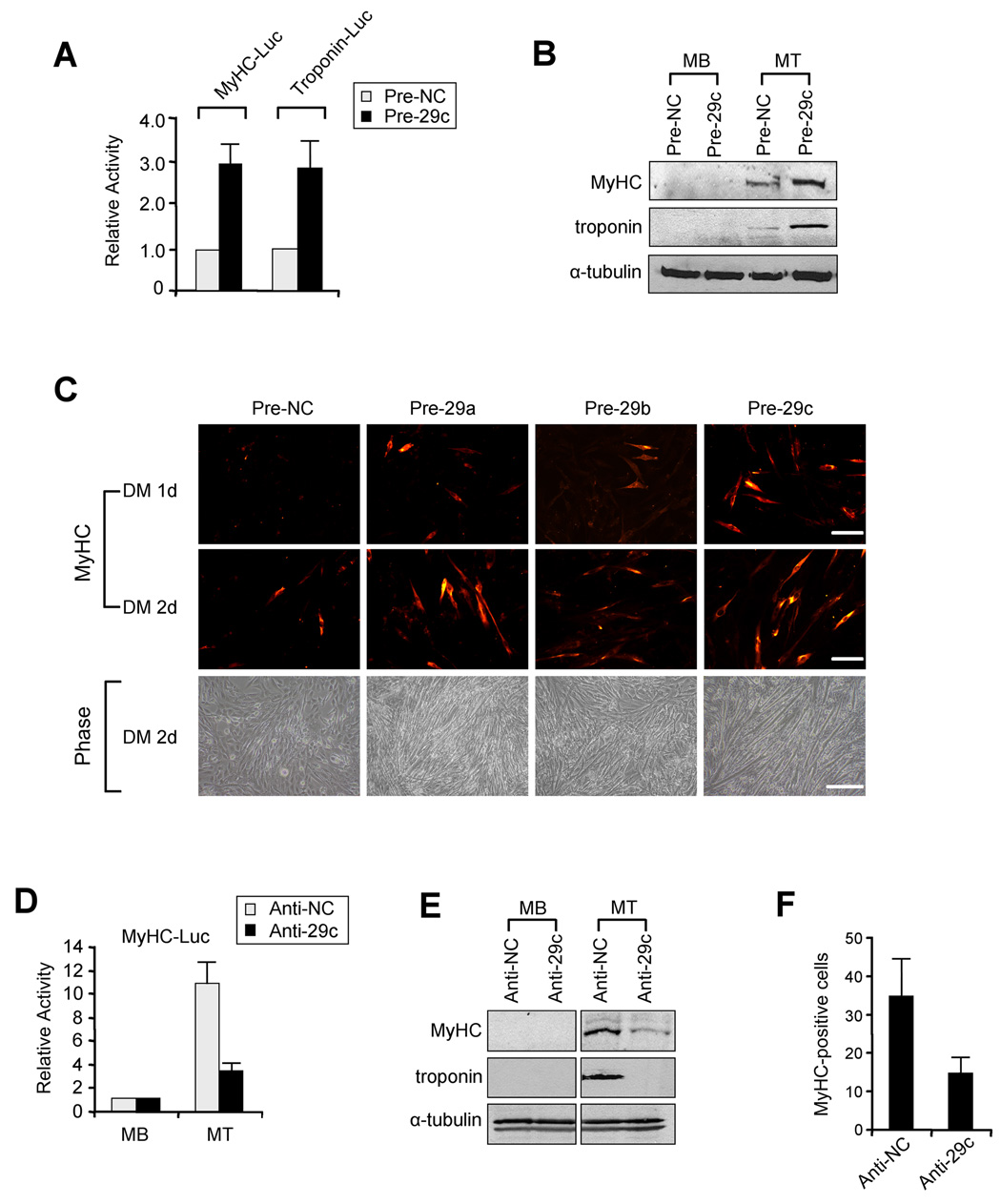
To test the functional relevance of miR-29 regulation during myogenesis, we employed a gain-of-function approach by ectopically expressing miR-29 in C2C12 MB with precursor miRNA oligos (Pre-29). Expression of miR-29 by this technique was verified by qRT-PCR (Suppl. Figure 4A). Compared to control (Pre-NC), Pre-29 stimulated promoter activities of myofibrillar genes (Figure 4A) and increased endogenous MyHC and troponin protein (Figure 4B) and RNA (Suppl. Figure 4B). Although Pre-29c was used in this analysis, we observed that all three miR-29 members were capable of strongly enhancing muscle differentiation (Figure 4C and Suppl. Figure 4C). To verify miR-29 specificity, transfections were repeated with a mutant form of Pre-29c lacking its seed region (Pre-29-mut). Results showed that MT formation was virtually identical in cells expressing Pre-29-mut compared to control (Suppl. Figure 4D). To further confirm specificity, we carried out reciprocal loss-of-function where C2C12 MB were administered antisense RNA oligos (Anti-29), which were seen to successfully decrease endogenous miR-29 (Suppl. Figure 4E). Compared to the control (Anti-NC), addition of Anti-29 caused a significant reduction in myogenic activity (Figure 4D) that correlated tightly with decreases in MyHC, troponin, and MT formation (Figure 4E, 4F and Suppl. Figure 4F). Taken together, results suggest that miR-29 functions in muscle cells as an enhancer of differentiation.
Figure 4. miR-29 accelerates muscle differentiation.
(A) C2C12 cells were transfected with 0.2µg of MyHC-Luc or Troponin-Luc reporters along with pCMV-LacZ and 50µM of precursor control oligos (Pre-NC) or miR-29c (Pre-29c) oligos. Cells were differentiated for 48h and luciferase was determined and normalized to β-Galactosidase. Relative activity is shown with respect to control cells where normalized luciferase values were set to 1. The data represents the average of three independent experiments ± S.D. (B) C2C12 MB were transfected with Pre-NC or Pre-29c oligos. Cells were then maintained as MB or differentiated into MT. Lysates were prepared and probed for MyHC and troponin T. (C) MB were transfected with Pre-NC or Pre-29a, b, or c members and differentiated (DM) for 1 or 2 days (d), at which time cells were immunostained for MyHC. Scale bar = 100µm. Cell morphology was visualized by phase-contrast microscopy; scale bar = 200µm. (D) C2C12 cells transfected with a MyHC-Luc along with anti-miR control (Anti-NC) or anti-miR-29c (Anti-29c). Cells were then maintained as MB or differentiated into MT for 48hrs at which time luciferase activity was determined. (E) MB were administered Anti-NC or Anti-29c and then MyHC and troponin were probed in cells maintained as MB or differentiated into MT. (F) MB were transfected with Anti-NC or Anti-29c oligos and cells were subsequently differentiated for 3 days at which time cultures were stained for MyHC. Positively stained cells were counted from a minimum of 10 randomly chosen fields from 3 individual plates.
miR-29 regulates myogenesis through feedback inhibition of YY1
We subsequently investigated the mechanism through which miR-29 regulates differentiation by searching for targets that might mediate its effect. Remarkably, one of the protein targets consistently predicted by three algorithms, TargetScan (Lewis et al., 2003), miRanda (Enright et al., 2003) and PicTar (Krek et al., 2005), was YY1. Based on this information, and our results showing an inverse relationship in expression between miR-29 and YY1 during myogenesis (Figure 3), we speculated that miR-29 acts in a feedback loop to suppress YY1 and its anti-myogenic activity.
A search for miR-29 binding sites within the YY1 3’UTR revealed that all miR-29 family members were predicted to hybridize to an evolutionarily conserved site among vertebrate species (Figure 5A, Suppl. Figure 5A). Secondary structure analysis also showed a favorable minimum free energy in the formation of the miR-29:YY1 3’UTR duplex stem-loop (Figure 5B). Furthermore, a perfect match exists between the seed region of miR-29 and the 3’ UTR of YY1, suggesting that miR-29 is involved in translational repression of YY1. This was tested by cloning a 500bp fragment of the YY1 3’UTR downstream of the firefly luciferase (Luc) gene. Co-transfections of this reporter (WT) with each of the miR-29 family members caused similar repressions of luciferase (Figure 5C). This was specific to miR-29 binding since luciferase was less affected when transfections were repeated with an irrelevant miR or with a mutant lacking the miR-29 binding site in the YY1 3’UTR. We also observed similar miR-29 mediated downregulation of luciferase when the full length YY1 3’UTR was inserted downstream of the reporter (Suppl. Figure 5B). At the functional level, we predicted that miR-29 binding to the YY1 3’UTR would lead to YY1 translational repression. Indeed, introduction of Pre-29 caused YY1 protein, but not RNA to be reduced, while Anti-29 resulted in the opposite effect (Figure 5D, Suppl. Figure 5C). To address whether YY1 targeting by miR-29 was relevant in vivo, we administered Anti-29 oligos in hindlimb muscles of neonatal mice and subsequently probed for YY1. Although some variability was observed with control oligos, addition of Anti-29 led to reproducible increases in YY1 (Figure 5E and Suppl. Figure 5D). To ascertain the significance of these changes in YY1, we used siRNA to knockdown YY1 in differentiating C2C12 cells. Consistent with the role of YY1 as a negative regulator of myogenesis, depletion of YY1 caused a 2.3 fold increase in myotube formation (Figure 5F). Given that a similar phenotype was seen upon miR-29 overexpression in differentiating MB suggests that miR-29 action in skeletal muscle is largely mediated through negative feedback on YY1.
Figure 5. miR-29 suppresses YY1 through binding to its 3’UTR.
(A) Predicted target site of miR-29c (green) in the 3’UTR of mouse YY1 (red) with the seed region underlined. (B) Predicted folding structure from mFOLD between miR-29c (green) and YY1 3’UTR (red). The minimal free energy (mfe) is indicated as well as the seed region shown by a line and arrow. (C) A wild type (WT) luciferase reporter was generated by fusing a ~ 500bp fragment of the YY1 3’UTR encompassing the miR-29 binding site downstream of the luciferase (Luc) reporter gene. The mutant plasmid was generated by deleting the miR-29 binding site. WT or Mutant reporter constructs were then transfected into MB with indicated precursor miRNA oligos. Luciferase was determined at 48h post-transfection and normalized to β-Galactosidase. Data represent the average of three independent experiments ± S.D. (D) MB were transfected with either precursor (Pre-NC) or Anti-miR control (Anti-NC) or precursor miR-29c (Pre-29) or anti-miR-29c (Anti-29) oligos. YY1 protein was then probed in extracts from cells differentiated for 48h. Blots were stripped and reprobed for α-tubulin. (E) P5 neonatal mice were injected with anti-miR control (Anti-NC) or anti-miR-29c (Anti-29) oligos into lower limb muscles. 48h post-injection, lysates were probed for YY1. (F) MB were transfected with siRNA control or siRNA to YY1. Cells where then differentiated for 24h, at which time they were photographed under phase contrast (scale bar = 200µm) or immunostained for MyHC (scale bar = 100µm). Numbers indicate averages of MyHC positive cells counted from a minimum of 10 randomly chosen fields.
miR-29 functions as a tumor suppressor in Rhabdomyosarcoma
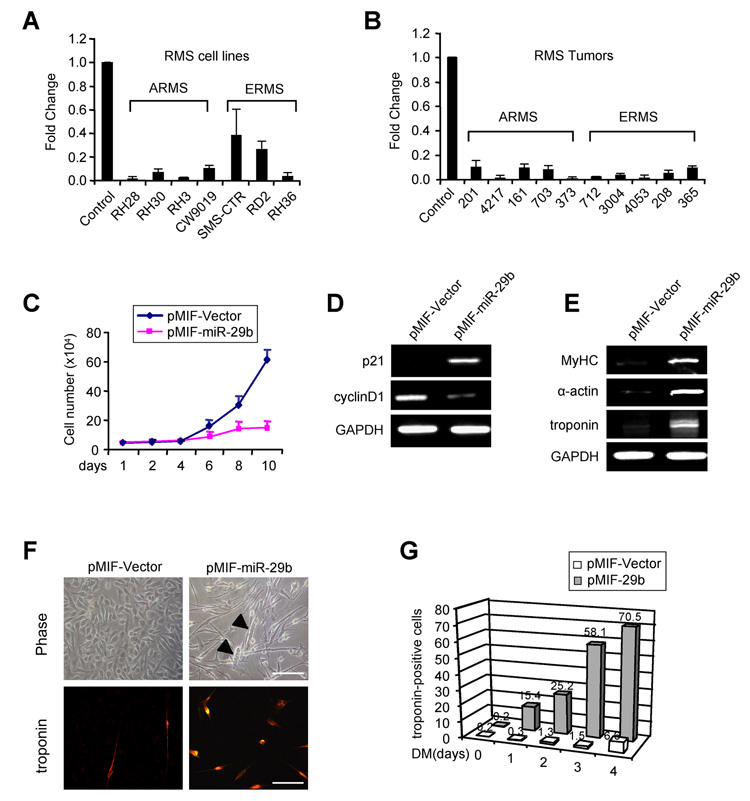
RMS cells are prohibited from terminal differentiation despite their commitment to a myogenic pathway. Our result showing that miR-29 promotes myogenesis led us to posit whether miR-29 might be a contributing factor in RMS. Functionally, miR-29 acts as a tumor suppressor through its pro-apoptotic activity and targeting of pro-survival products TCL (Pekarsky et al., 2006) and Mcl-1 (Mott et al., 2007). To address the role of miR-29 in RMS, we examined its expression in cell lines derived from alveolar (ARMS) and embryonal (ERMS) RMS tumors. Significantly, miR-29b expression was strongly reduced in each of the RMS cell lines (Figure 6A), indicating that regulation of miR-29 does not discriminate among RMS subtypes. Consistent with these results, miR-29b was also highly suppressed in primary RMS tumors, and similar to cell lines this reduction did not discriminate between ARMS and ERMS (Figure 6B). To assess the specificity of this regulation with respect to miR-29, expression of other muscle associated microRNAs, miR-1, and miR-206 were examined in RMS. Whereas miR-1 was also reduced in most tumors, no uniform pattern of regulation occurred with miR-206 (Suppl. Figure 6A and 6B), suggesting that RMS is not associated with a general own regulation of muscle-associated miRNAs.
Figure 6. miR-29 functions as a tumor suppressor in RMS.
(A) Normal human skeletal muscle cells were cultured along with ARMS and ERMS cell lines and qRT-PCR was performed to measure miR-29b. (B) Total RNAs were obtained from ten RMS patient tumors (numbers represent patient identification) and expression of miR-29b was measured by qRT-PCR. Total RNAs from human skeletal muscle were used as the control. (C) RH30 cells were transduced with vector or miR-29b expressing lentiviruses, and stable cell lines were generated. The cell number was determined by Trypan blue staining over the course of ten days. (D) Total RNAs were extracted from vector or miR-29b expressing RH30 cells and semi-quantitative RT-PCR was performed probing for p21CIP/WAF1 and cyclinD1. GAPDH was used as a control. (E) Same analysis as in (D) was done for differentiation markers, MyHC, α-actin and troponin T. (F) Phase contrast images of control and miR-29 expressing RH30 cells. Arrows indicate myotubelike structures. Cells were treated with differentiation medium for 2 days and immunostained for troponin. Scale bars = 200µm. (G) Vector control or miR-29 expressing RH30 cells were differentiated (DM) for 0, 1, 2, 3, or 4 days, and subsequently immunostained for troponin. The graph represents average number of troponin positive cells that were counted from a minimum of 10 randomly chosen fields from 3 culture plates.
To further investigate the tumor suppressor role of miR-29 in RMS, we tested the effects of ectopic expression of miR-29 in the RH30 ARMS cell line. Addition of Pre-29b oligos in these cells caused a two-fold reduction in cell growth compared to Pre-NC control (Suppl. Figure 6C). This difference in cell number was further enhanced when miR-29b was stably expressed using lentiviral (pMIF) infection (Figure 6C). Decreases in proliferation correlated with regulation of cell cycle proteins, cyclin-dependent kinase inhibitor p21CIP/WAF1 and cyclin D1 (Figure 6D). This regulation was also not specific to ARMS since a similar defect in growth was observed when Pre-29b was added to RD2 ERMS cells (data not shown). In addition, miR-29 caused only a mild effect on apoptosis and was not seen to affect expression of the pro-survival protein, Mcl-1 (Suppl. Figure 6D). Significantly, although RH30 tumor cells fail to undergo terminal differentiation, stable expression of miR-29b resulted in increased levels of differentiation markers, MyHC, α-actin and troponin (Figure 6E). Morphologically, the cell shape changed from a round to an elongated appearance, suggestive of an increased differentiating tendency (Figure 6F). Indeed, upon addition of differentiation medium, multinucleated myotube-like cells were readily formed in miR-29b expressing RH30 cells at levels approximately 10 times that of vector (Figure 6G and Suppl. Figure 6E). This effect was not specific to ARMS since similar differentiation-accelerating effects by miR-29b were observed in ERMS RD2 cells (Suppl. Figure 6F and 6G). These results indicate that miR-29 limits RMS cell growth through its promotion of myogenic differentiation.
Next, we evaluated the anti-tumor effect of miR-29 in vivo by establishing RH30 tumors in immunocompromised mice. Tumors were allowed to reach a size of at least 0.1 cm3, at which time 1 × 109 PFU pMIF-vector or pMIF-miR-29b-expressing lentiviruses were administered intra-tumorally and tumor size was monitored daily for a period of 3 weeks. Whereas tumors injected with vector control virus grew rapidly with a doubling time of 2 days, tumors injected with miR-29b showed visibly slower growth, with cell doubling occurring at around 6 days (Figure 7A). Differences in tumor volumes became more apparent after 8 days post-injection and persisted until the experimental endpoint, where control tumors were on average 1.9 times larger than those injected with miR-29b (n=6; p=0.0029). In some animals, tumors were resected to evaluate the mechanism of miR-29 action. H&E staining from both groups revealed densely packed, small, round cells with scant cytoplasm, consistent with the ARMS origin of the RH30 cell line (Figure 7B). Significantly, intra-tumoral addition of miR-29 stimulated myogenic differentiation as evidenced by the increased expression of MyHC, α-actin and troponin (Figure 7C). This was associated with reduced cellular proliferation since Ki67 and phospho-Histone H3 (P-H3) staining were 2.3 and 1.9 fold lower respectively in miR-29 injected tumors versus control (Figure 7B and Figure 7D, p<0.05). Similar to in vitro analysis, we again found no clear evidence of apoptosis by TUNEL staining in miR-29 expressing tumors (Figure 7D), suggesting that in comparison to other malignancies the pro-apoptotic activity of miR-29 may not be a primary tumor suppressor mechanism in RMS. To verify that results were not a consequence of lentivirus delivery, RMS tumors were re-established in nude mice and subsequently injected with Pre-29b compared to control Pre-NC oligos. Analogous to lentiviral delivery, tumors administered with Pre-29b were on average 2.2 times smaller then controls (Figure 7E; n=4, p=0.0047). Collectively, these data support that miR-29 is a tumor suppressor in RMS functioning through its pro-differentiation activity.
Figure 7. miR-29 inhibits RMS tumor growth in vivo.
(A) RH30 tumors were established in mice and then subjected injected with vector or miR-29b expressing lentiviruses. Tumor volume was recorded daily for 21 days. (B) Tumors were sectioned for stained for histology with H&E or with proliferation markers Ki67 and phospho-Histone H3 (P-H3). Apoptotic cells were stained by a standard TUNEL assay. (C) During the course of miRNA treatment, 3 tumors from vector control and miR-29b injected groups were resected. Total RNAs were prepared and semi-quantitative RT-PCR was performed probing for differentiation markers. (D) Ki-67, P-H3 and apoptotic cells were quantitated by counting positively stained cells from 10 randomly chosen fields from six sections per tumor. *p<0.05. (E) RH30 xenograft tumors were established as in (A) and then injected with precursor control miR (Pre-NC) or miR-29b (Pre-29b) oligos. Tumor volumes were recorded for 21 days.
Evidence of dysregulation of NF-κB–YY1–miR-29 circuitry in RMS
To further address the mechanism of miR-29 tumor suppressor activity in RMS, we considered YY1 as a likely downstream effector since this was a miR-29 target identified in differentiating muscle cells. Dysregulated expression of YY1 has also been associated with multiple cancers (Baritaki et al., 2007; de Nigris et al., 2006; Seligson et al., 2005). In line with our hypothesis, higher levels of YY1 were detected in RMS cell lines (Figure 8A) as well as in patient tumors (Suppl. Figure 7A) compared to normal adjacent tissues (Figure 8B). Significantly, these results coincided strongly with lower levels of miR-29 (Figure 6), implying that tumor suppressor activity of miR-29 occurred by targeting YY1. In support of this notion, expression of miR-29b in RH30 cells caused a concomitant decrease in YY1 (Suppl. Figure 7B), and similar to what was observed in C2C12 MB, downregulation of YY1 in RH30 cells occurred through the conserved binding site in its 3’UTR, as ectopic expression of miR-29 repressed luciferase reporter activity containing the wild type, but not mutant miR-29 binding site (Suppl. Figure 7C). Furthermore, depletion of YY1 with siRNA in RH30 cells and xenograft tumors induced differentiation markers (Figure 8C and Figure 8D, respectively) comparable to what was observed with reconstituted miR-29. These data indicate that loss of miR-29 in RMS leads to dysregulated expression of YY1 thus favoring an undifferentiated phenotype.
Figure 8. NF-κBY–Y1–miR-29 circuitry is dysregulated in RMS.
(A) Extracts were prepared from normal human muscle and RMS cell lines and immunoblots were performed probing for YY1, Ezh2, and p65. (B) Lysates were prepared five RMS patient tumors and adjacent normal muscle tissue and probed for YY1, Ezh2, and p65 proteins. (C) RH30 cells were transfected with siRNA control oligos or siRNA-YY1. Cells were differentiated and semi-quantitative RT-PCR was performed probing for differentiation markers, or GAPDH used as a control. (D) RH30 tumors were established in nude mice and then injected with siRNA oligos every 3 days for 1 week. RNA and protein lysates were prepared from tumors and subsequently probed for MyHC, α-actin and troponin by RT-PCR and YY1 by Western. (E) ChIPs with either an YY1 antibody or control IgG were performed on chromatins isolated from human skeletal muscle cells (control) or RH30 cells. Precipitated DNA fragments were amplified with oligonucleotides spanning regions A-D of the human miR-29b/c regulatory region. Total inputs are indicated. (F) RH30 cells were infected with adenoviruses expressing vector control or IκBα-SR. YY1 and miR-29b levels were measured at 48h post-infection by qRT-PCR. (G) RH30 cells were infected with control or IκBá-SR adenovirus and differentiation markers were probed by qRT-PCR. (H) RH30 cells were transduced with vector or IκBα-SR expressing retroviruses to generate stable cell lines. Cells were then treated with differentiation medium for 2 days and immunostained for troponin or quantified for myofibrillar expression. (I) The model depicts the role of the NF-κB-YY1-miR-29 regulatory circuit in both normal myogenic differentiation and RMS. In myogenesis this circuit involves constitutive activity of NF-κB in myoblasts regulating YY1, which subsequently epigentically suppresses miR-29 and maintains cells in an undifferentiated state. As differentiation ensues, downregulation of the NF-κB–YY1 pathway leads to upregulation of miR-29 that in turns further decreases YY1 levels to ensure proper differentiation into myotubes. In RMS, this circuit becomes dysregulated due to an increase in the NF-κB–YY1 pathway that constitutively represses miR-29. In the absence of miR-29 tumor suppressor activity, YY1 is left uncontrolled thereby impairing differentiation leading to Rhabdomyosarcomagenesis.
In probing into the mechanism underlying the down-regulation of miR-29 in RMS, we found that similar to YY1, Ezh2 was also pronouncedly elevated in RMS cell lines and patient tumors (Figures 8A and 8B). This suggested that epigenetic silencing by the PcG complex might account for loss of miR-29. Consistent with this thinking, YY1 and Ezh2 binding was detected on the miR-29b/c locus in RH30 cells, but interestingly this binding occurred on site “B” (Figure 8E), rather than the YY1 “D” binding site that was identified in C2C12 MB (Figure 1). The reason for this difference in binding site occupancy is not known. It is possible that such selectivity is dependent on the dosage of the PcG complex or the presence of a tumor factor that preferentially directs YY1 binding. YY1 knockdown by siRNA oligos in RH30 cells led to an increase in miR-29b and to a lesser extent, miR29c (Suppl. Figure 7D, 7E), supporting data above that YY1 is required for miR-29 suppression in RMS. Furthermore, this suppression was also regulated by NF-κB since addition of IB-SR lowered YY1 while concurrently increasing levels of miR-29b (Figure 8F). This was consistent with the increase in p65 detected in RMS cell lines and patient tumors (Figures 8A, 8B). Finally, we also observed that inactivation of NF-κB by stable expression of IκBα-SR in RH30 cells stimulated their myogenic potential as assessed by measurement of differentiation markers and quantitation of troponin positive cells (Figure 8G and 8H; Suppl. Figure 8A, 8B, and 8C), which mirrored the phenotype caused by miR-29 overexpression. Together, these data support that dysregulation of NF-κB–YY1–miR-29 circuitry is a contributing factor for RMS tumorigenesis by interfering with the muscle differentiation program.
DISCUSSION
This study was undertaken to investigate the potential interplay between the classical transcription factors NF-κB/YY1 and novel gene regulators miRNAs in both normal and abnormal myogenesis. Results led to identifying miR-29 as a target of the NF-κB–YY1 pathway. Although miRNA biology has greatly advanced in the last few years, much of the effort has focused on describing the targets of miRNAs rather than understanding the regulation of miRNA genes themselves. The nature of miRNA promoter/regulator elements remains one of the most interesting and open questions in the study of miRNA regulation, and their identification are likely to help unravel the regulatory networks involved by these miRNAs, such as in recently described embryonic stem cells (Marson et al., 2008). Our current study identified a regulatory element located ~20Kb upstream from the miR-29b/c cluster on chromosome 1. This element contains an YY1 binding site that also associates with transcriptional repressor factors Ezh2 and HDAC-1. We showed that this repressive complex binds specifically in myoblasts when miR-29b/c was at its lowest, and that loss of YY1 and Ezh2 binding during myogenesis leads to induction of miR-29. This suggests that YY1 and the PcG complex function through this regulatory element to epigenetically repress miR-29b/c transcription in progenitor muscle cells. Recent work showed that a similar PcG complex under NF-κB control is responsible for the silencing of multiple myofibrillar genes in myoblasts (Wang et al., 2007). Likewise, we found that miR-29 expression was also negatively regulated by NF-κB, suggesting that miR-29 regulation is controlled by an analogous mechanism to that described for protein-coding genes.
The difficulty of accurately identifying the transcriptional start site (TSS) of miRNAs has hindered studies of intergenic miRNA regulation. However, recently Chang and colleagues used RACE to characterize the TSS of miR-29b/c (Chang et al., 2008). Impressively, their results placed the TSS within the conserved regulatory region described in this report. The four YY1 sites that we identified are located in close proximity to the TSS. Site D, which is occupied by YY1 in myoblasts, lies 2Kb downstream of the pri-miR-29b/c starting site in the first intron, whereas the functional YY1 binding site B in RMS lies only 200bp upstream of the TSS. This is consistent with previous findings that map YY1 regulatory sites within the proximal promoter regions of myofibrillar genes (Wang et al., 2007), again supporting the argument that miR-29 can be regulated in a similar fashion to that of protein-coding genes.
The decrease in NF-κB and YY1 activities, in conjunction with an upregulation of miR- 29s during in vitro and in vivo differentiation of skeletal muscle cells argued that this family of miRNAs plays a role in normal myogenesis. Our results from combined gain-of-function and loss-of-function experiments support this notion. We further showed that the mechanism by which miR-29 family functions in this capacity is by targeting its own repressor, YY1. Reports have shown that YY1 mRNA and protein are downregulated during muscle differentiation (Caretti et al., 2004; Wang et al., 2007), in part through the concomitant reduction of p65 and the classical signaling pathway of NF-κB (Bakkar et al., 2008). Our current findings suggest that miR-29 is an additional factor ensuring YY1 downregulation during skeletal myogenesis. Therefore, similar to multiple myogenic transcription factors, cell cycle regulators, and signaling pathways, miR-29 represents a group of non-coding RNAs including muscle-specific miRs-1, 133, and 206 that participates in fine-tuning the fidelity of skeletal muscle cell differentiation.
The ability of miR-29 to stimulate normal myogenesis prompted us to ask whether it could also contribute to the aberrant myogenic differentiation and tumor development in RMS. Indeed, our results support that miR-29 acts as a tumor suppressor in skeletal muscle cells. This conclusion was derived from findings showing that miR-29 expression was dramatically reduced in RMS cancer cell lines and patient tumor samples. These data are consistent with a recently performed miRNA profiling study that identified the downregulation of miR-29 in four patients with ARMS (Subramanian et al., 2007). Further support for tumor suppressor actions of miR-29 in skeletal muscle came from our results showing that forced expression of miR-29 restored the potential to re-engage a muscle differentiation program and led to a reduction in RH30 proliferation. Significantly, the tumor suppressor activities of miR-29 were also confirmed in an RMS xenograft model. Similar to cultured cells, miR-29 expressing tumors displayed increased differentiation and reduced cellular proliferation. Thus, loss of miR-29 in RMS likely facilitates tumor development through an inhibition of myogenic differentiation. Previous studies have suggested that miR-29 is involved in B-cell chronic lymphocytic leukemia and cholangiocarcinoma by regulating an apoptotic pathway. However, our results in RMS did not reveal a significant effect on apoptosis, suggesting miR-29 exerts its tumor suppressor function in RMS through a distinguished mode than previously described.
In an attempt to elucidate the downstream oncogenes that mediate the tumor suppressor function of miR-29, we discovered that YY1 was highly induced in RMS cells and patient samples. YY1 was downregulated by ectopic expression of miR-29 in RH30 cells and the data support that this downregulation is mediated by a similar translational repression mechanism as we observed in C2C12 MB. These data further suggest that YY1 is a downstream effector of miR-29. YY1 is thought to function as an oncogene through the modulation of key genes involved in cell cycle regulation and apoptosis (Gordon et al., 2006). However, our results demonstrated that in RMS, the oncogenic activity of YY1 arise from its anti-differentiation property in myogenesis. Furthermore, YY1 has been shown to regulate p53 (Sui et al, 2004). Findings demonstrate that p53 is required for normal skeletal myogenesis and its inactivation correlates with RMS (Cam et al., 2006). Therefore, it is possible that deregulated expression of YY1 in RMS leads to p53 inactivation in muscle cells that contributes to their transformed phenotype predominantly by a block in myogenic differentiation. In addition to YY1, we have identified several sarcoma-associated oncogenes that qualify as miR-29 targets (Suppl. Table 2), suggesting that the tumor suppressor activity of miR-29 in muscle cells may occur through different mechanisms or that miR-29 may function as a tumor suppressor in multiple sarcomas other than RMS.
In contrast to the majority of tumor suppressor miRNAs that reside in fragile sites and exhibit loss of heterozygosity (Calin et al., 2004), miR-29 genes reside in chromosomal regions not typically described to undergo rearrangements (Barr, 2001; Douglass et al., 1985). This suggests that mechanisms other than chromosomal alterations exist to suppress miR-29. Our current findings support that such suppression results from a dysregulation of YY1 in RMS. YY1 is bound to the regulatory region of miR-29b/c in RMS cells, suggesting that suppression of miR-29b/c occurs by epigenetic silencing. Our discovery that Ezh2 levels are also elevated in RMS is consistent with findings that PcG protein contribute to oncogenesis (Sparmann and van Lohuizen, 2006). Interestingly, recent work has elucidated that miR-29 can target methyltransferases in lung tumor cells and that forced expression of miR-29 leads to reduced global DNA methylation (Fabbri et al., 2007). Thus, miR-29 might represent a unique class of miRNAs involved in cancer epigenomics with respect to their regulation and function.
Taken together, these results identify the involvement of miR-29 in a regulatory circuit relevant in both skeletal myogenesis and RMS. As modeled in Figure 8I, we propose that miR-29 and YY1 are interlinked by a mutual negative feedback loop, whereby miR-29 blocks YY1 translation and YY1 inhibits miR-29 transcription. This loop is further regulated by NF-κB that indirectly controls miR-29 through YY1. In normal proliferating myoblasts, constitutive NF-κB activity functions in part by maintaining levels of YY1 that repress miR-29 by an epigenetic mechanism. Once differentiation ensues, NF-κB activity is decreased leading to a transcriptional reduction in YY1 and subsequent derepression of miR-29. Increasing levels of miR-29, in turn, inhibit YY1 translation, thereby promoting myofibrillar gene expression. However, in RMS this regulatory circuit is compromised by constitutive activation of NF-κB–YY1 that, in combination with an elevated PcG activity, promotes sustained silencing of miR-29. In the absence of miR- 29, YY1 levels are further elevated to inhibit differentiation thereby facilitating tumor development. These findings not only provide insights in the interplay between transcription factors and miRNAs during muscle differentiation, but also have implications for the diagnosis and treatment of RMS.
EXPERIMENTAL PROCEDURES
Cell lines
C2C12 MB were cultured and differentiated as previously described (Guttridge et al., 1999). Human MB were obtained from Clonetics and maintained in F12 medium supplemented with 20% FBS and 4% Chicken Embryo Extract. Human RMS cell lines were maintained in RPMI medium supplemented with 10% FBS.
Transfection and infections
Transfections with miRNA precursor oligos and siRNA oligos were performed with Lipofectamine as suggested by the manufacturer (Invitrogen). For luciferase assays, cells were transfected in 12-well plates and luciferase activity was monitored as previously described (Guttridge et al., 1999). For lentivirus production, pMIF-cGFP-Zeo-vector or pMIF-cGFP-Zeo-miR-29b plasmids along with the packaging plasmid mix (pPACK) (System Biosciences) were transfected into 293TN cells maintained in 10% FBS. 48h after transfection, supernatant was harvested from these cells and viral titers were estimated by FACS analysis. Approximately 1 × 109 virus particles were used to transduce RH30 or RD2 cells, which were subsequently placed in 200µg/ml Zeocin for stable selection. Adenovirus particles expressing IκBα-SR or GFP-vector control (University of North Carolina Vector Core Facility) were used to infect RH30 cells. RNAs were prepared at 48hr post-infection and used for subsequent analysis. pBabe-vector and pBabe-IκBα-SR retroviruses were produced as previously described (Hertlein et al., 2005).
Oligonucleotides and plasmids
Precursor miRNA oligos were obtained from Ambion. 2-O’- methyl and 3’-Ammino-C6 modified Anti-miR oligos were obtained from Fidelity Systems. siRNA oligos against human or mouse YY1 were obtained from Santa Cruz technologies, and siRNA oligos against p65 was obtained from Dharmacon. In each case 50µM oligos were used for transient transfections. Details on the construction and use of plasmids can be found in Supplemental Methods.
RT-PCR and Real-time RT-PCR
Total RNAs from cells were extracted using TRIzol reagent (Invitrogen). Expression of mature miRNAs was determined using the miRNA-specific Taqman microRNA assay kit (Applied Biosystem) in an iCycler (Bio-Rad Laboratories). U6 was used for normalization.
Immunoblotting and Immunostaining
Cell extracts were prepared and used for immunoblotting as described (Guttridge et al., 1999). Source and dilutions for each antibody are as follows: YY1 (Santa Cruz Biotechnology; 1:1000), troponin T (Sigma; 1:1000), MyHC (Sigma; 1:1000), Ezh2 (Zymed; 1,000); p65 (Rockland; 1:10000), α-tubulin (Sigma; 1:1000), IκBα (Santa Cruz Biotechnology; 1:1000), and β-actin (Santa Cruz Biotechnology; 1:1000). For immunofluorescence of C2C12 cells, antibodies were MyHC or troponin T (Sigma; 1:500); For tumor sections, antibodies used were anti-phospho histone H3 (Cell Signaling; 1:200) and Ki-67 (Dako, 1:50).
EMSAs and ChIP assays
EMSAs and ChIPs were prepared as previously described (Wang et al., 2007). For ChIP assays 2 µg of antibodies against YY1 (Santa Cruz Biotechnology), Ezh2 (Zymed), HDAC-1 (Santa Cruz Biotechnology), trimethyl-histone H3-K27 (Upstate), SRF (Santa Cruz), Mef2 (Santa Cruz) or isotype IgG (Sigma) were used. Genomic DNA pellets were resuspended in 20 µl of water. PCR was performed with 2 µl of immunoprecipitated material and products were analyzed on an agarose gel visualized by a gel documentation system.
Tumor Samples
Specimens were obtained from the Cooperative Health Tumor Network in accordance with the Institutional Review Boards of the Ohio State University and Nationwide Children’s Hospital. Details on the characterization of each tumor specimen can be found in Supplemental Methods.
Animal studies
Mice were housed in the animal facilities of the Comprehensive Cancer Center under conventional conditions with constant temperature and humidity and fed a standard diet. Treatment of mice was in accordance with the institutional guidelines of The Ohio State University Animal Care. For Anti-miR oligo injection, P5 C57/BL6 mice were injected with 5 nM of Anti-NC or Anti-miR-29 oligos into the lower limb. Muscles were collected 48h postinjection and used for Westerns. For cardiotoxin studies, 4-week old mice were injected with 50 µl of cardiotoxin (CTX) at 10 µg/ml into the TA. Muscles were harvested and RNAs extracted for real-time RT-PCR analysis. For xenograft studies, 3-4 week old athymic nu/nu female mice (Charles Rivers, NCI) were injected subcutaneously with 3 × 106 RH30 cells. Tumor diameter was measured daily two-dimensionally with electronic calipers. Tumor volume was calculated using the following formula: (π/6) × Dl × Ds2, where Dl is the largest diameter and Ds is the smallest diameter. 5 µM of pre-miR oligos or siRNA oligos were pre-incubated with Lipofectamine (Invitrogen) for 15 minutes prior to injection into tumors in a final volume of 60µl of OPTI-EM (Invitrogen).
Bioinformatic and statistical analysis
Promoter sequences were retrieved from UCSC genome bioinformatics (http://genome.ucsc.edu/). Prediction of the transcription faction binding sites was performed using rVISTA sequence analysis tool (http://rvista.dcode.org/). Prediction of miRNA target was performed using three publicly available algorithms: TargetScan (http://www.targetscan.org/), miRanda (http://www.microrna.org/) and PicTar (http://pictar.bio.nyu.edu/). Statistical significance of tumor growth was assessed using a paired Student’s t-test.
Supplementary Material
ACKNOWLEDGEMENTS
This study is dedicated in memory to our friend and colleague, Stephen J. Qualman. We thank members of the Guttridge lab for their support and insight throughout the course of this project, especially E. Hall, W. He, and J. Wang. This study was supported by a Ruth L. Kirschstein F32 award to H.W. and NIH funding, AR052787 to D.C.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sahenk Z, et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C, Catoe H, Werner R. MIR-206 regulates connexin43 expression during skeletal muscle development. Nucleic Acids Res. 2006;34:5863–5871. doi: 10.1093/nar/gkl743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M, Acharyya S, Rudnicki MA, Hollenbach AD, Guttridge DC. IKK/NF-kB Regulates Skeletal Myogenesis Via a Signaling Switch to Inhibit Differentiation and Promote Mitochondrial Biogenesis. J. Cell. Biol. 2008;180:787–802. doi: 10.1083/jcb.200707179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baritaki S, Sifakis S, Huerta-Yepez S, Neonakis IK, Soufla G, Bonavida B, Spandidos DA. Overexpression of VEGF and TGF-beta1 mRNA in Pap smears correlates with progression of cervical intraepithelial neoplasia to cancer: implication of YY1 in cervical tumorigenesis and HPV infection. Int J Oncol. 2007;31:69–79. [PubMed] [Google Scholar]
- Barr FG. Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene. 2001;20:5736–5746. doi: 10.1038/sj.onc.1204599. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Breitfeld PP, Meyer WH. Rhabdomyosarcoma: new windows of opportunity. Oncologist. 2005;10:518–527. doi: 10.1634/theoncologist.10-7-518. [DOI] [PubMed] [Google Scholar]
- Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam H, Griesmann H, Beitzinger M, Hofmann L, Beinoraviciute-Kellner R, Sauer M, Huttinger-Kirchhof N, Oswald C, Friedl P, Gattenlohner S, et al. p53 family members in myogenic differentiation and rhabdomyosarcoma development. Cancer Cell. 2006;10:281–293. doi: 10.1016/j.ccr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Caretti G, Di Padova M, Micales B, Lyons GE, Sartorelli V. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 2004;18:2627–2638. doi: 10.1101/gad.1241904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nigris F, Botti C, de Chiara A, Rossiello R, Apice G, Fazioli F, Fiorito C, Sica V, Napoli C. Expression of transcription factor Yin Yang 1 in human osteosarcomas. Eur J Cancer. 2006;42:2420–2424. doi: 10.1016/j.ejca.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Douglass EC, Green AA, Hayes FA, Etcubanas E, Horowitz M, Wilimas JA. Chromosome 1 abnormalities: a common feature of pediatric solid tumors. J Natl Cancer Inst. 1985;75:51–54. [PubMed] [Google Scholar]
- Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5:R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25:1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- He L, Hamnnon GJ. MicroRNAS: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Hutvagner G. Small RNA asymmetry in RNAi: function in RISC assembly and gene regulation. FEBS Lett. 2005;579:5850–5857. doi: 10.1016/j.febslet.2005.08.071. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol. 2006;174:677–687. doi: 10.1083/jcb.200603008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Lee TC, Zhang Y, Schwartz RJ. Bifunctional transcriptional properties of YY1 in regulating muscle actin and c-myc gene expression during myogenesis. Oncogene. 1994;9:1047–1052. [PubMed] [Google Scholar]
- Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. Embo J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Liu N, Williams AH, Kim Y, McAnally J, Bezprozvannaya S, Sutherland LB, Richardson JA, Bassel-Duby R, Olson EN. An intragenic MEF2-dependent enhancer directs muscle-specific expression of microRNAs 1 and 133. Proc Natl Acad Sci U S A. 2007;104:20844–20849. doi: 10.1073/pnas.0710558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots GG, Ovcharenko I. rVISTA 2.0: evolutionary analysis of transcription factor binding sites. Nucleic Acids Res. 2004;32:W217–W221. doi: 10.1093/nar/gkh383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall CL, Helman LJ. High-dose chemotherapy for rhabdomyosarcoma: where do we go from here. J Pediatr Hematol Oncol. 2001;23:266–267. doi: 10.1097/00043426-200106000-00005. [DOI] [PubMed] [Google Scholar]
- Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK, Wernig M, Newman J, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563–568. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourelatos Z, Dostie J, Paushkin S, Sharma A, Charroux B, Abel L, Rappsilber J, Mann M, Dreyfuss G. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 2002;16:720–728. doi: 10.1101/gad.974702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak D, Agarwal S, Sumathi S, Chakrabarti SK, Naik PS, Khurana SM. Small but mighty RNA-mediated interference in plants. Indian J Exp Biol. 2005;43:7–24. [PubMed] [Google Scholar]
- Pekarsky Y, Santanam U, Cimmino A, Palamarchuk A, Efanov A, Maximov V, Volinia S, Alder H, Liu CG, Rassenti L, et al. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006;66:11590–11593. doi: 10.1158/0008-5472.CAN-06-3613. [DOI] [PubMed] [Google Scholar]
- Qualman SJ, Coffin CM, Newton WA, Hojo H, Triche TJ, Parham DM, Crist WM. Intergroup Rhabdomyosarcoma Study: update for pathologists. Pediatr Dev Pathol. 1998;1:550–561. doi: 10.1007/s100249900076. [DOI] [PubMed] [Google Scholar]
- Rao PK, Kumar RM, Farkhondeh M, Baskerville S, Lodish HF. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci U S A. 2006;103:8721–8726. doi: 10.1073/pnas.0602831103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenber MI, Georges SA, Asawachaicharn A, Analau E, Tapscott SJ. MyoD inhibits Fstl1 and Utrn expression by inducing transcription of miR-206. J Cell Biol. 2006;175:77–85. doi: 10.1083/jcb.200603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- Seligson D, Horvath S, Huerta-Yepez S, Hanna S, Garban H, Roberts A, Shi T, Liu X, Chia D, Goodglick L, Bonavida B. Expression of transcription factor Yin Yang 1 in prostate cancer. Int J Oncol. 2005;27:131–141. [PubMed] [Google Scholar]
- Shivdasani RA. MicroRNAs: regulators of gene expression and cell differentiation. Blood. 2006;108:3646–3653. doi: 10.1182/blood-2006-01-030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Lui WO, Lee CH, Espinosa I, Nielsen TO, Heinrich MC, Corless CL, Fire AZ, van de Rijn M. MicroRNA expression signature of human sarcomas. Oncogene. 2007;27:2015–2026. doi: 10.1038/sj.onc.1210836. [DOI] [PubMed] [Google Scholar]
- Sui G, Affar el B, Shi Y, Brignone C, Wall NR, Yin P, Donohoe M, Luke MP, Calvo D, Grossman SR, Shi Y. Ying Yang 1 is a negative regulator of p53. Cell. 2004;117:859–872. doi: 10.1016/j.cell.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Wang H, Hertlein E, Bakkar N, Sun H, Acharyya S, Wang J, Carathers M, Davuluri R, Guttridge DC. NF-kappaB regulation of YY1 inhibits skeletal myogenesis through transcriptional silencing of myofibrillar genes. Mol Cell Biol. 2007;27:4374–4387. doi: 10.1128/MCB.02020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender E, Kel AE, Kel OV, Karas H, Heinemeyer T, Dietze P, Knuppel R, Romaschenko AG, Kolchanov NA. TRANSFAC, TRRD and COMPEL: towards a federated database system on transcriptional regulation. Nucleic Acids Res. 1997;25:265–268. doi: 10.1093/nar/25.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Cai X, Cullen BR. Use of RNA polymerase II to transcribe artificial microRNAs. Methods Enzymol. 2005;392:371–380. doi: 10.1016/S0076-6879(04)92022-8. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.