Abstract
Controversial reports on the role of autophagy as a survival or cell death mechanism in dopaminergic cell death induced by parkinsonian toxins exist. We investigated the alterations in autophagic flux and the role of autophagy protein 5 (Atg5)-dependent autophagy in dopaminergic cell death induced by parkinsonian toxins. Dopaminergic cell death induced by the mitochondrial complex I inhibitors 1-methyl-4-phenylpyridinium (MPP+) and rotenone, the pesticide paraquat, and the dopamine analog 6-hydroxydopamine (6-OHDA) was paralleled by increased autophagosome accumulation. However, when compared with basal autophagy levels using chloroquine, autophagosome accumulation was a result of impaired autophagic flux. Only 6-OHDA induced an increase in autophagosome formation. Overexpression of a dominant negative form of Atg5 increased paraquat- and MPP+-induced cell death. Stimulation of mammalian target of rapamycin (mTOR)-dependent signaling protected against cell death induced by paraquat, whereas MPP+-induced toxicity was enhanced by wortmannin, a phosphoinositide 3-kinase class III inhibitor, rapamycin, and trehalose, an mTOR-independent autophagy activator. Modulation of autophagy by either pharmacological or genetic approaches had no effect on rotenone or 6-OHDA toxicity. Cell death induced by parkinsonian neurotoxins was inhibited by the pan caspase inhibitor (Z-VAD), but only caspase-3 inhibition was able to decrease MPP+-induced cell death. Finally, inhibition of the lysosomal hydrolases, cathepsins, increased the toxicity by paraquat and MPP+, supporting a protective role of Atg5-dependent autophagy and lysosomes degradation pathways on dopaminegic cell death. These results demonstrate that in dopaminergic cells, Atg5-dependent autophagy acts as a protective mechanism during apoptotic cell death induced by paraquat and MPP+ but not during rotenone or 6-OHDA toxicity.
Key Words: autophagy, apoptosis, Atg5, cathepsins, paraquat, rotenone, MPP+, 6-hydroxydopamine, neurodegeneration, Parkinson’s disease.
Parkinson’s disease (PD) is the second most common neurodegenerative disorder worldwide, and its prevalence increases exponentially from 65 to 90 years of age. A fraction of PD occurrence is related to specific mutations, whereas over 90% of PD occurs most commonly in a sporadic (idiopathic) form, without a clearly defined genetic basis, and only a vaguely delineated pathogenesis that is thought to be linked to unknown environmental causes. However, it is clear that not a single environmental toxicant can be the cause of PD. Thus, it is now considered that sporadic PD arises from the convergence of genetic susceptibility, environmental exposures, and aging (Horowitz and Greenamyre, 2010; Vance et al., 2010). A dysfunction in the electron transport chain has been found in PD brains. Thus, inhibitors of complex I activity are well-accepted toxicological models to understand dopaminergic cell death pathways (Cannon and Greenamyre, 2010). Recent epidemiological data also suggest a link between the exposure to environmental toxicants, such as paraquat and rotenone, and an increased risk of developing PD (Franco et al., 2010; Tanner et al., 2011). PD is characterized by the irreversible loss of dopaminergic neurons in the substantia nigra pars compacta (Schapira, 2009), and although the underlying mechanisms and etiology of PD are not fully understood, mitochondrial dysfunction, oxidative stress, and protein aggregation are suggested as causative factors of neuronal death (Levy et al., 2009; Przedborski, 2005; Yao and Wood, 2009).
Macroautophagy (here referred as autophagy) is an essential pathway for macromolecule and organelle turnover. It is initiated by an isolation membrane/phagophore surrounding cytoplasmic constituents in an enclosed double-membraned structure called autophagosome. Subsequently, the autophagosome fuses with a lysosome forming an autolysosome, whose content undergoes degradation by lysosomal hydrolases (He and Klionsky, 2009). Autophagy in neurodegenerative diseases has gained attention in the past years. Pioneer studies evidenced that impairment of autophagy, specifically in the nervous system, leads to neurodegeneration accompanied by polyubiquitinated protein aggregation in cytoplasmic inclusion bodies (Hara et al., 2006; Komatsu et al., 2006). Moreover, morphological features of autophagy have been observed in postmortem human samples of PD patients (Anglade et al., 1997).
Autophagy and chaperone-mediated autophagy have been proposed to prevent α-synuclein accumulation (Cuervo et al., 2004; Webb et al., 2003), whereas Parkin and PTEN-induced putative kinase 1 have been shown to regulate mitochondrial targeted autophagy (mitophagy) (Dagda et al., 2009; Lee et al., 2010; Narendra et al., 2010; Vives-Bauza et al., 2010). On the other hand, less is known regarding the role of autophagy in dopaminergic cell death induced by environmental/mitochondrial toxins (parkinsonian mimetics). Autophagy acts as a protective mechanism promoting cell survival in response to a variety of stressors, whereas only in very few cases, autophagy participates as a programmed cell death mechanism (Shen et al., 2012). The pesticide paraquat, the mitochondrial complex I inhibitors 1-methyl-4-phenylpyridinium (MPP+) and rotenone, and the dopamine oxidation product 6-hydroxydopamine (6-OHDA), which are used in vitro and in vivo as experimental PD models, have been shown to induce the accumulation of autophagosomes. However, whether this is associated with an increase in or a disruption of autophagic flux has not been addressed in detail. Furthermore, conclusions regarding the role of autophagy in dopaminergic cell death induced by these parkinsonian mimetics have been reached, in many cases, only by the use of nonspecific pharmacological stimulators or inhibitors. Contradictory, both autophagy-dependent cell death and survival mechanisms have been reported to regulate dopaminergic cell death induced by MPP+ (Chu et al., 2007; Dehay et al., 2010), whereas both stimulation and inhibition of autophagy have been reported to be induced by paraquat (Gonzalez-Polo et al., 2007; Wills et al., 2012).
In this work, we determined the role of autophagy in dopaminergic cell death induced by parkinsonian mimetics. We demonstrated that paraquat and complex I inhibition by MPP+ and rotenone impair autophagic flux. Autophagy protein 5 (Atg5)-dependent autophagy and lysosomal hydrolase activity were observed to act as protective mechanisms during dopaminergic cell death in response to paraquat and MPP+. Only 6-OHDA was shown to stimulate autophagy flux. However, neither pharmacological inhibition of autophagy nor impairment of Atg5-dependent autophagy regulated rotenone or 6-OHDA toxicity.
MATERIALS AND METHODS
Cell culture and reagents.
The human dopaminergic neuroblastoma cell line SK-N-SH was purchased from the American Type Culture Collection. SK-N-SH cells were originally established from a biopsy of a neuroblastoma patient. SK-N-SH cells, from which SH-SY5Y cells were derived, have been reported to present significant levels of dopamine β-hydroxylase activity (Biedler et al., 1973) and low levels of tyrosine hydroxylase activity (Klongpanichapak et al., 2008; West et al., 1977). SK-N-SH cells express significant levels of the dopamine transporter (SLC6A3), which are not affected by retinoic acid-induced differentiation (unpublished data). Cells were cultured as explained in Rodriguez-Rocha et al., (2012). Paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride, 856177), MPP+ (D048), rotenone (R8875), 6-OHDA (Cat No. 162957), and chloroquine (CQ, C6628) were obtained from Sigma/Aldrich. Rapamycin was from LC Laboratories (R-5000). Wortmannin (AC32859) and D-trehalose (AC30987) were from Acros Organics. Staurosporine, Z-VAD-FMK (219007), Ac-DEVD-CMK (218750), Ac-DMQD-CHO (235421), and Z-FG-NHO-Bz (219415) were from Calbiochem EMD Millipore.
Western immunoblotting (WB).
Cells were lysed in either 1% SDS or radio immunoprecipitation assay (RIPA) buffer (20mM Tris-HCl, 150mM NaCl, 1mM Na2EDTA, 1mM ethylene glycol tetraacetic acid (EGTA), 1% Triton X-100) containing protease inhibitors (Halt Protease Inhibitor Cocktail, Thermo/Pierce). Samples were sonicated and centrifuged, and pellets were discarded. Twenty-five to fifty micrograms of protein per sample were loaded separated by SDS-polyacrylamide gel electrophoresis, and transferred to PVDF or nitrocellulose membranes. Blots were incubated with the appropriate primary antibody (1:1000): LC3B (Sigma/Aldrich, L7543), Atg5 (Cell Signaling, 8540), and cleaved caspase-3 (Cell Signaling, 9664) at 4°C overnight. Peroxidase-conjugated secondary anti-rabbit or anti-mouse antibodies (1:2000 or 1:5000, Thermo/Pierce or Cell Signaling) were used, and bands were detected using enhanced chemiluminescence (ECL) WB substrate (GE Life Sciences or Thermo/Pierce). Blots were subsequently reprobed for Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Cell Signaling, 2118) or β-actin (ThermoScientific, MA5-15739) to verify equal protein loading.
EGFP-LC3 stable transfection.
EGFP-LC3 plasmid generously donated by Dr Tamotsu Yoshimori (Osaka University, Japan) was linearized with Nco I/Stu I and transfected into SK-N-SH cells using FuGENE HD reagent (Promega). Stable cells overexpressing EGFP-LC3 were selected in complete medium containing 0.3mg/ml geneticin (G418, Acros Organics), and subsequently sorted out in a BD FACSaria cell sorter (BD Biosciences).
Recombinant adenoviral vectors.
The adenovirus Ad-EGFP-LC3 was a gift from Dr Aviva M. Tolkovsky (Cambridge Centre for Brain Repair, Cambridge, United Kingdom). The Ad-dnAtg5 was provided by Dr Gökhan S. Hotamisligil (Harvard School of Public Health, Boston, Massachusetts). Control adenovirus containing only the cytomegalovirus promoter (Ad-Empty) was used as control. Adenoviruses were amplified and titered in HEK293T cells according to previously established protocols (Barde et al., 2010; Rodriguez-Rocha et al., 2012). SK-N-SH cells were infected with adenoviral vectors at a multiplicity of infection (MOI) of 1.5 (Ad-Empty and Ad-dnAtg5) or 2.5 (Ad-EGFP-LC3) and treated with experimental conditions as indicated.
Fluorescence live-cell imaging.
Cells were grown on glass-bottom dishes (MatTek Corp) coated with poly-D-lysine (0.1mg/ml, Sigma/Aldrich). EGFP-LC3 was used to monitor autophagosome formation and LysoTracker Red DND-99 (500nM, Invitrogen, L-7528) to label lysosomes, and their fluorescence pattern was analyzed using an inverted (Olympus IX 81) confocal laser scanning microscope as follows: GFP, excitation laser wavelength: 488nm, emission filter wavelength: 505–525nm; and LysoTracker Red DND-99, ex laser wavelength: 543nm, em filter wavelength: 560–600nm. LysoTracker was removed after 15min of incubation, and fresh phenol red–free medium was added for image acquisition.
Transmission electron microscopy.
Cells were fixed with 2% glutaraldehyde in 0.2M 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid sodium salt (HEPES) (pH 7.4) for 2h at room temperature. Fixed cells were collected and postfixed with 1% OsO4 and stained in 2% uranyl acetate (Eskelinen, 2008). The pellets were dehydrated through a graduated ethanol series and embedded in Epon 812 (Electron Microscopic Sciences) for sectioning and observed under a transmission electron microscope (Hitachi H7500). A series of ultrastructural images were collected with a bottom-mount digital camera for the analysis of autophagosomes.
Evaluation of cell death and apoptosis.
Loss of cell viability was determined by propidium iodide (PI, 1 μg/ml, Sigma/Aldrich, P4170) uptake as a marker for loss of plasma membrane integrity using flow cytometry (FACS, fluorescence-activated cell sorting). PI was detected using FL-3 (488nm ex, 695/40nm em) or L2-4 (561nm ex, 615/25nm em). Cell death was represented as either percentage of cells with increased PI or mean PI fluorescence (AU). Externalization of phosphatidylserine was determined using Annexin V conjugated to Fluorescein isothiocyanate (FITC) (Trevigen). FITC fluorescence was analyzed on FL1 (488nm ex, 530-30nm em). Early apoptotic cells are defined as having Annexin V-positive (+), PI-negative (−) staining. Late-apoptotic and nonviable cells are both Annexin V and PI positive. The colorimetric measurement of lactate dehydrogenase (LDH) activity released from the cytosol of damaged cells into the supernatant was also used for the quantification of cell death according to the manufacturer’s protocol (Roche, 11644793001). After treatments, cell-free culture supernatant was assessed for LDH activity. Cells were lysed with a Triton X-100 solution at a final concentration of 1%, and LDH activity was also assessed to determine total LDH content. Absorbance (Abs) was measured at 490 and 630nm of reference in an ELx800 Absorbance Microplate Reader (Biotek). Total LDH activity was determined using the following equation: LDH release (%) = [Supernatant Abs × 2]/[Supernatant Abs + (2 × Lysed Abs)]. Results are expressed as percentage of LDH release and were normalized to control (untreated) cells.
Statistical analysis.
All experiment replicas were independent and performed on separate days. Collected data were analyzed according to statistical criteria by using paired or unpaired t test or 2-way ANOVA, and the appropriate parametric or nonparametric normality posttest using SIGMA-PLOT/STAT package. A probability value of p < .05 was considered statistically significant. Data were plotted as mean values of at least 3 independent experiments ± SEM using the same statistical package for data analysis. Flow cytometry plots and WB presented are representative of at least 3 independent experiments. Densitometry analysis of immunoblots was performed using ImageJ (NIH) v3.91 software (http://rsb.info.nih.gov/ij).
RESULTS
Autophagic Flux Is Impaired by Paraquat, MPP+, and Rotenone, and Increased by 6-OHDA
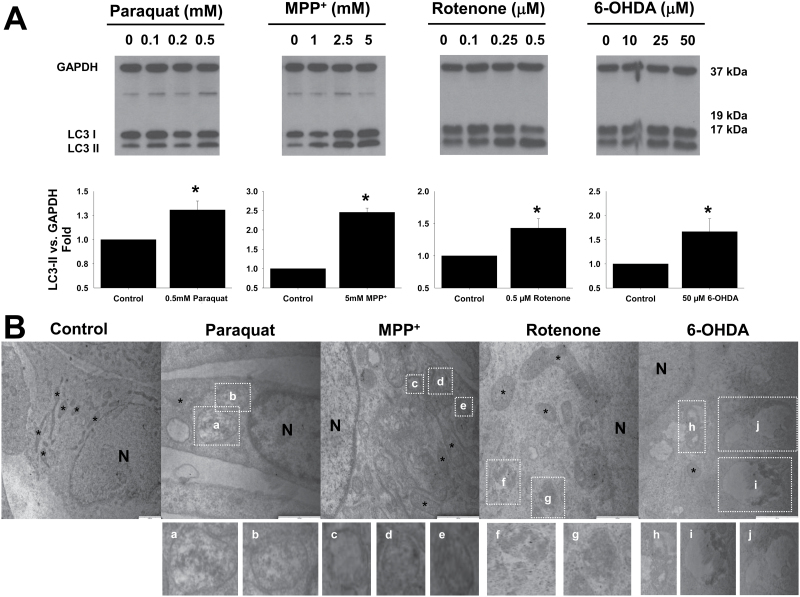
Accumulation of autophagosomes has been reported to parallel dopaminergic cell death induced by the parkinsonian mimetics paraquat, MPP+, rotenone, and 6-OHDA (Dagda et al., 2008; Gao et al., 2012; Gonzalez-Polo et al., 2007; Zhu et al., 2007). We observed that indeed, paraquat, MPP+, rotenone, and 6-OHDA induced a significant increase in autophagosome accumulation in dopaminergic cells in a dose-dependent manner, depicted by WB as the conversion of the microtubule-associated protein light chain 3 (LC3-I) to the autophagosomes marker LC3-II (Fig. 1A). Autophagosome accumulation was corroborated by electron microscopy (Fig. 1B). All neurotoxins induced, to a different extent, the accumulation of double-membrane vesicles (autophagosomes) and autolysosomes, in some of which their content could be evidenced under degradation.
Fig. 1.
Alterations in autophagic flux induced by PD toxins. A, Human dopaminergic SK-N-SH cells were treated with PD neurotoxins at the indicated concentrations for 24h. Accumulation of LC3-II was analyzed by WB in whole-cell lysates. B, Autophagy morphology was evaluated by TEM. Representative electron micrographs show typical features of double-membraned vesicles (autophagosomes [c–g]) and autolysosomes (a, b, h–j), which exhibited late autophagic compartment containing partially degraded, electron-dense materials, as evidenced in the magnified images of boxed areas. N, nucleus; *, mitochondria. Bars indicate 1-μm scale. Normal SK-N-SH (D) or cells transduced with 2.5 MOI Ad-EGFP-LC3 (C) were treated with PQ (0.5mM), MPP+ (2.5mM), rotenone (Rot, 4μM), or 6-OHDA (50μM) for 24h and 40μM CQ was added 2h before analysis. In (C), cells were stained with LysoTracker Red (500nM) for 15min prior to confocal microscopy imaging. Boxed areas (B and C) in the merged panels are enlarged and represented in the insets. Autophagolysosome formation is indicated by white arrowheads highlighting EGFP-LC3 and LysoTracker colocalization. D, WB is representative of 3 independent experiments. Data in bar graphs represent the ratio of LC3-II/GAPDH or /β-actin normalized against control (untreated) samples (A) or CQ-treated cells (D) and are means ± SEM of 3 independent experiments. *p < .05, significant difference between values of the corresponding drug-treated and untreated cells in the absence (A) or presence of CQ (D). Abbreviations: 6-OHDA, 6-hydroxydopamine; CQ, chloroquine; MOI, multiplicity of infection; MPP+ , 1-methyl-4-phenylpyridinium; PQ, paraquat; PD, Parkinson’s disease; TEM, transmission electron microscopy; WB, Western immunoblotting.
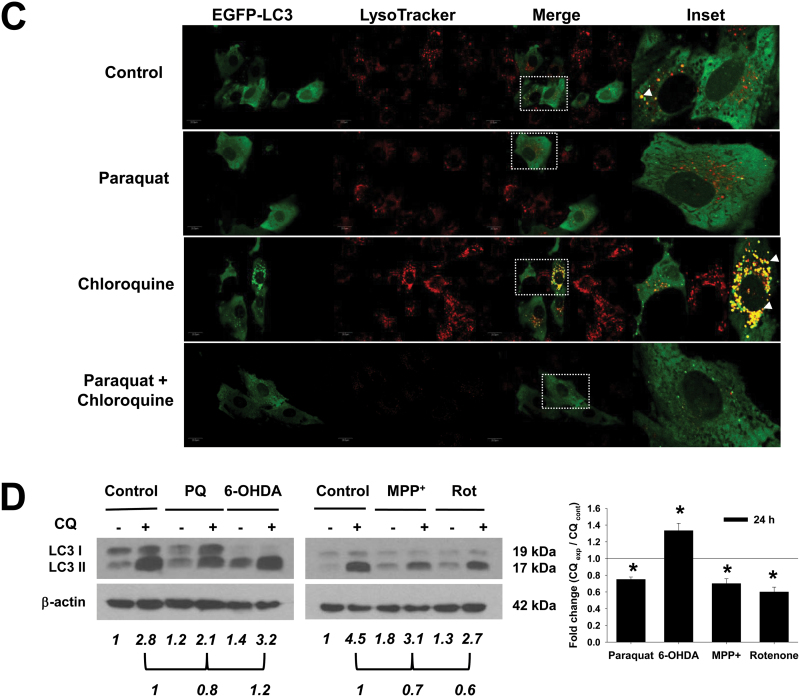
Accumulation of autophagosomes might be associated with either an increase in or an impairment of autophagic flux, defined as the complete process of autophagy, beginning with the formation of the phagophore and ending after the fusion of the autophagosome with the lysosome for the subsequent degradation of the lysosomal cargo. Autophagic flux is inferred by WB analysis of LC3-II turnover in the presence and absence of inhibitors of lysosomal or vacuolar degradation. CQ is usually described in the literature as an inhibitor of the fusion of autophagosomes with lysosomes. However, several reports demonstrate that, as a lysosomotropic agent, CQ actually inhibits the acid-dependent degradation of the autolysosome content without affecting autophagosome-lysosome fusion, resulting in an accumulation of autophagolysosomes that cannot be cleared (Amaravadi et al., 2007; Glaumann and Ahlberg, 1987; Maclean et al., 2008; Poole and Ohkuma, 1981) (Fig. 7). As shown in Figure 1C, CQ leads to the accumulation of lysosomes in cells overexpressing EGFP-LC3 and stained with lysotracker, but it does not impair autophagosome-lysosome fusion.
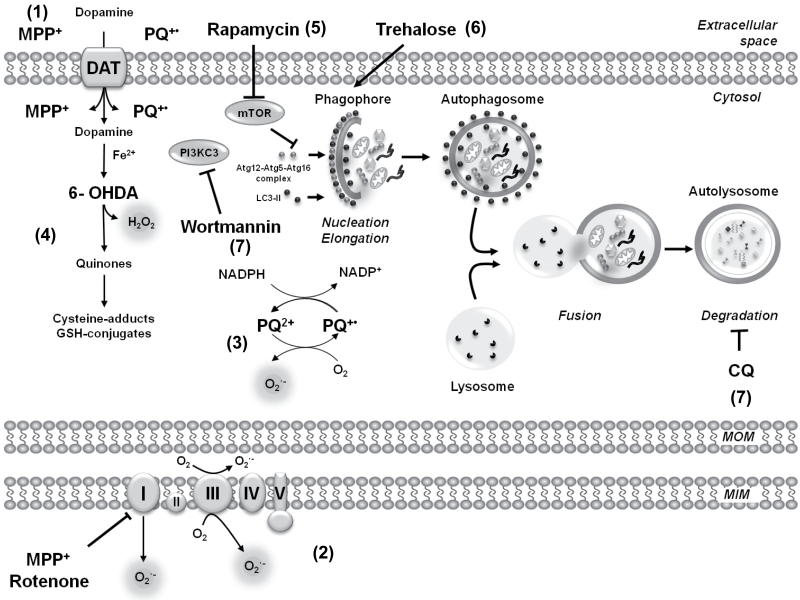
Fig. 7.
Putative targets for neurotoxicants and pharmacological modulators of autophagy. The parkinsonian mimetics PQ, the mitochondrial complex I inhibitors MPP+ and rotenone, and the dopamine analog 6-OHDA are used in vitro and in vivo as experimental PD models. (1) DAT have been shown to uptake MPP+ and 6-OHDA, whereas DAT-mediated PQ uptake is still controversial. (2) MPP+ and rotenone are widely used inhibitors of complex I. However, recent evidence suggests that their toxic effects might be mediated by distinct processes and/or that complex I-independent mechanisms might also be involved. (3) PQ has been proposed to generate superoxide anion (O2 •−) formation both in the cytosol and in the mitochondrial matrix. (4) 6-OHDA toxicity has been shown to be mediated by reactive oxygen species (ROS) formation via a nonenzymatic autooxidation process. 6-OHDA autooxidation also generates quinones, which have the capacity to react with thiol-containing molecules (cysteine and glutathione [GSH]). (5) Rapamycin is a well-established stimulator of autophagy via the inhibition of the serine/threonine kinase mTOR, whereas trehalose (6) acts as an mTOR-independent autophagy activator. (7) The formation of autophagosome precursors requires class III PI3Ks and is prevented by PI3K inhibitors such as wortmannin. However, as summarized in Supplementary Table 1, these pharmacological modulators have a number of secondary/nonautophagy-related effects; thus, conclusions based solely on pharmacological regulation of autophagy might be misleading. (8) The lysosomotropic agent, CQ, inhibits the acid-dependent degradation of the autolysosome content without affecting autophagosome-lysosome fusion, which results in the accumulation of autophagolysosomes that cannot be cleared. Abbreviations: 6-OHDA, 6-hydroxydopamine; CQ, chloroquine; DAT, dopamine transporters; MPP+ , 1-methyl-4-phenylpyridinium; MIM, mitochondrial inner membrane; MOM, mitochondrial outer membrane; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinase; PQ, paraquat.
Autophagic flux was blocked with CQ to evaluate basal autophagy levels and contrast them with the effects of neurotoxins. CQ induces the accumulation of autolysosomes as early as 2–4h of incubation (data not shown). Cells were treated with the parkinsonian mimetics for 24h, and CQ was added 2h before LC3 analysis by WB. In the presence of CQ, paraquat, MPP+, and rotenone induced a decrease in LC3-II accumulation compared with CQ alone (Figs. 1C and 1D). Only 6-OHDA increased LC3-II levels in the presence of CQ. These results demonstrate that paraquat and the complex I inhibitors, MPP+ and rotenone, induce an accumulation of autophagosomes by impairment of autophagy flux, whereas 6-OHDA does it by increasing autophagy rate.
MODULATION OF AUTOPHAGY BY PHARMACOLOGICAL AND GENETIC APPROACHES
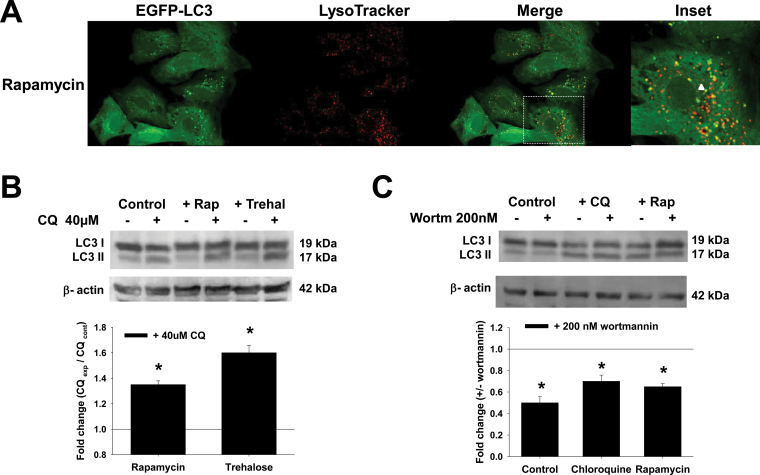
Rapamycin is a well-established stimulator of autophagy via the inhibition of the serine/threonine kinase mammalian target of rapamycin (mTOR). Recently, a novel function of trehalose as an mTOR-independent autophagy activator was described (Sarkar et al., 2007) (Fig. 7). Rapamycin and trehalose induced the accumulation of autophagosomes and an increase in autophagic flux in dopaminergic cells (Figs. 2A–C). The formation of autophagosome precursors requires class III phosphoinositide 3-kinases (PI3Ks) and is prevented by PI3K inhibitors such as 3-methyladenine (3-MA) and wortmannin (Petiot et al., 2000; Rubinsztein et al., 2007) (Fig. 7). 3-MA, but not wortmannin, has been shown to both stimulate and inhibit autophagy depending on the concentration used; therefore, we decided to use wortmannin in our studies (Wu et al., 2010). Wortmannin reduces the levels of basal autophagy compared with CQ, as well as the accumulation of autophagosomes upon rapamycin treatment (Fig. 2C). Most of the studies addressing the role of autophagy in dopaminergic cell death induced by parkinsonian mimetics have used similar pharmacological approaches. However, it is important to state that there are a number of secondary signaling pathways modulated by such pharmacological tools (see Supplementary Table 1).
Fig. 2.
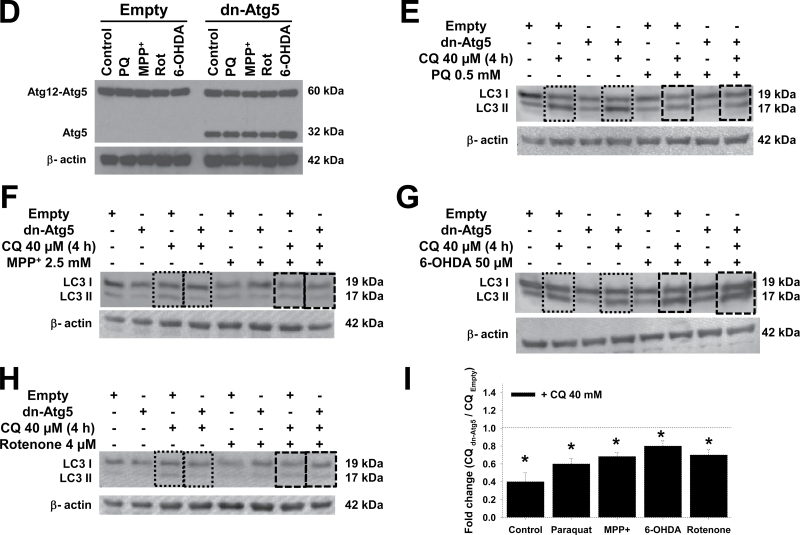
Pharmacological and genetic approaches used to modulate autophagy flux and Atg5-dependent autophagy. A, Cells were transduced with Ad-EGFP-LC3 (2.5 MOI) for 24h and then treated with 5μM rapamycin for 24h. Cells were stained with LysoTracker and analyzed by confocal microscopy. The boxed area in the merged panel is enlarged and represented in the inset. Autophagolysosome formation is indicated by white arrowheads highlighting EGFP-LC3 and LysoTracker colocalization. B and C, Cells were treated with rapamycin (5μM) and trehalose (100mM) for 24h in the presence or absence of CQ (40μM for 4h before analysis) or wortmannin (200nM, 24h). D–H, Cells were infected for 24h with Ad-Empty or Ad-dnAtg5 at 1.5 MOI followed by treatment with PQ (0.5mM), MPP+ (2.5mM), rotenone (Rot, 4μM), or 6-OHDA (50μM) for 24h. LC3-II accumulation and Atg12-Atg5 complex were evaluated by WB. Blots are representative experiments. I, Autophagic flux was evaluated in cells treated with CQ (40μM) 4h prior to collection. Changes in LC3-II/β-actin signal induced by dnAtg5 with respect to cells infected with Empty virus in the presence of CQ, was evaluated in controls (dotted lines) or cells treated with parkinsonian mimetics (broken lines). *p < .05, significant difference between cells infected with dnAtg5 and Empty viruses. Abbreviations: 6-OHDA, 6-hydroxydopamine; CQ, chloroquine; dnAtg5, dominant negative form of Atg5; MOI, multiplicity of infection; MPP+ , 1-methyl-4-phenylpyridinium; PQ, paraquat; WB, Western immunoblotting.
The Atg12-Atg5 complex conjugation is essential for autophagosome formation, and functions as an E3-like ligase for LC3 lipidation (Hanada et al., 2007; Tanida, 2011) (Fig. 7). Recently, Atg5-independent autophagy has been reported to occur in response to starvation and DNA damage (Nishida et al., 2009). Overexpression of a dominant negative (dn) form of Atg5 (dnAtg5) has been previously demonstrated to effectively inhibit autophagy (Yang et al., 2010). We overexpressed dnAtg5 in cells treated with the parkinsonian toxins (Fig. 2D). dnAtg5 significantly reduced autophagic flux (in the presence of CQ) in control cells and in cells treated with the parkinsonian toxins (Figs. 2E–I).
Atg5-Dependent Autophagy Has a Protective Role in Response to Paraquat- and MPP+-Induced Cell Death but Not in Response to Rotenone- or 6-Ohda-Induced Cell Death
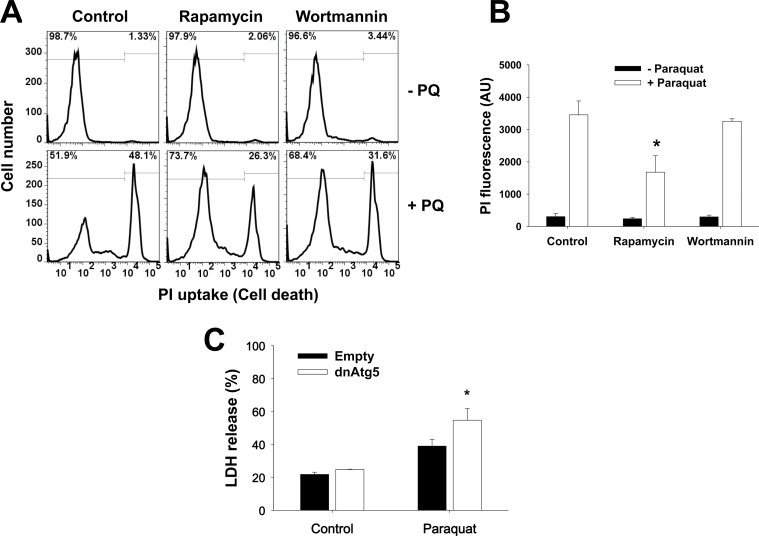
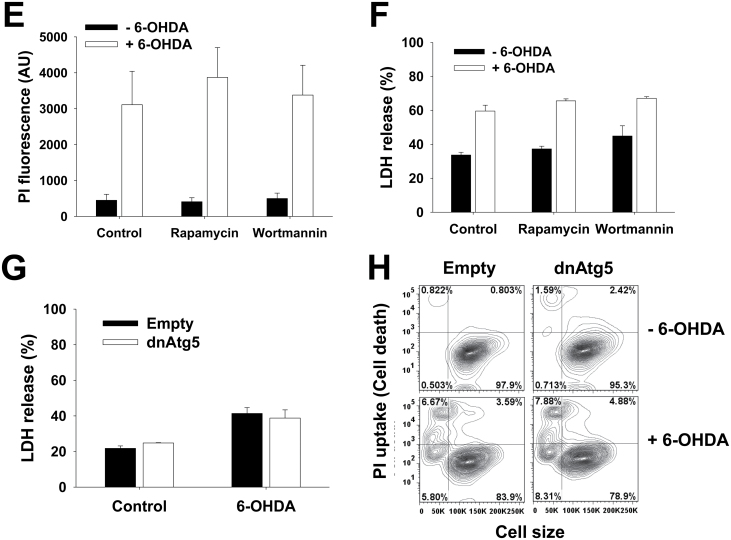
Using the pharmacological and genetic tools described above, we next decided to evaluate the role of autophagy in dopaminergic cell death induced by parkinsonian mimetics. Cell death was evaluated by FACS using PI uptake and by determining the activity of released LDH, both reliable markers of compromised membrane integrity and cell death. Rapamycin significantly decreased the toxicity induced by paraquat (Figs. 3A and 3B). Neither trehalose (data not shown) nor wortmannin showed an effect over paraquat toxicity (Figs. 3A and 3B). Blockage of Atg5-dependent autophagy by dnAtg5 increased cell death induced by paraquat (Fig. 3C).
Fig. 3.
Atg5-dependent autophagy protects against dopaminergic cell death induced by PQ. A and B, Cells were subjected to 1h pretreatment with rapamycin (5μM) or wortmannin (200nM), followed by exposure to PQ (0.5mM) for 48h. Loss of cell viability or plasma membrane integrity was analyzed by flow cytometry and is reflected as an increase in the number of cells with high PI fluorescence (histograms in A and bar graphs in B). C, Cells were infected with Ad-Empty or Ad-dnAtg5 at 1.5 MOI for 24h followed by treatment with PQ (0.5mM) for additional 48h. LDH activity was also assessed to evaluate cell death. Data in bar graphs represent changes in PI fluorescence intensity in AU (B), or percentage of released LDH (C), and are means ± SEM of 3–4 independent experiments. *p < .05, significant difference with respect to PQ-only treatment. Abbreviations: dnAtg5, dominant negative form of Atg5; LDH, lactate dehydrogenase; MOI, multiplicity of infection; PI, propidium iodide; PQ, paraquat.
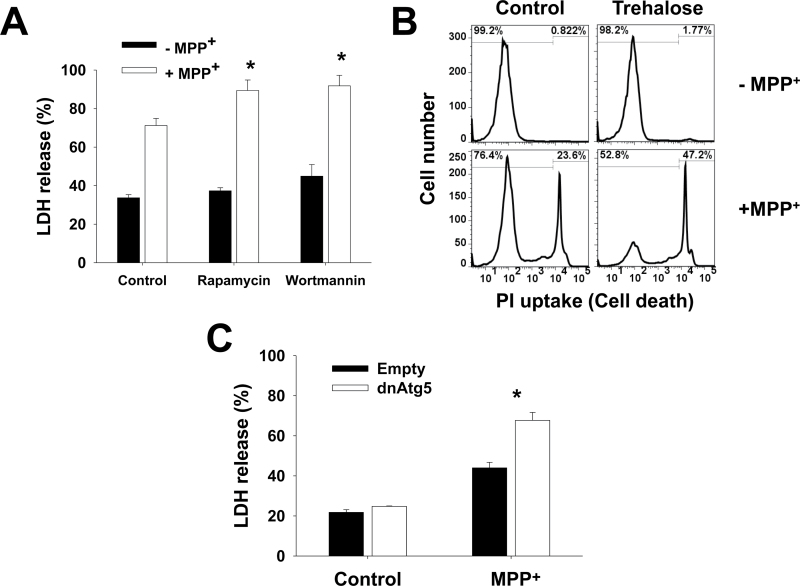
Paraquat and MPP+ are structurally similar. However, it has been clearly demonstrated that the toxicity exerted by these drugs is mediated by divergent mechanisms (Ramachandiran et al., 2007). Rapamycin and trehalose (Figs. 4A and 4B) augmented MPP+-induced cell death. Contradictory, wortmannin (Fig. 4A) or dnAtg5 (Fig. 4C) also led to increased cell death. These results demonstrate that inhibition of Atg5-dependent autophagy potentiates paraquat- and MPP+-induced cell death and thus autophagy seems to exert a protective effect against the toxicity of these neurotoxins. The discrepancy between these results and those obtained using both pharmacological stimulators (rapamycin and trehalose) inhibitors (wortmannin) can be ascribed to secondary or nonspecific effects of these agents (see Discussion section and Supplementary Table 1).
Fig. 4.
Atg5-dependent autophagy protects against MPP+-induced dopaminergic cell death. A and B, Cells were subjected to 1h pretreatment with rapamycin (5μM), wortmannin (200nM), or trehalose (100mM). In (C), cells were infected with Ad-Empty or Ad-dnAtg5 at 1.5 MOI for 24h. Then, cells were treated with MPP+ (2.5mM) for additional 48h. Loss of cell viability or plasma membrane integrity was determined by measurement of released LDH activity (A and C) or by PI uptake by flow cytometry (B) as explained in Figure 3. Histograms in (A) are representative of 3 independent experiments. Data in bar graphs represent percentage of released LDH activity, and are means ± SEM of 3 independent experiments. *p < .05, significant difference with respect to MPP+-only treatment. Abbreviations: dnAtg5, dominant negative form of Atg5; LDH, lactate dehydrogenase; MOI, multiplicity of infection; MPP+ , 1-methyl-4-phenylpyridinium; PI, propidium iodide.
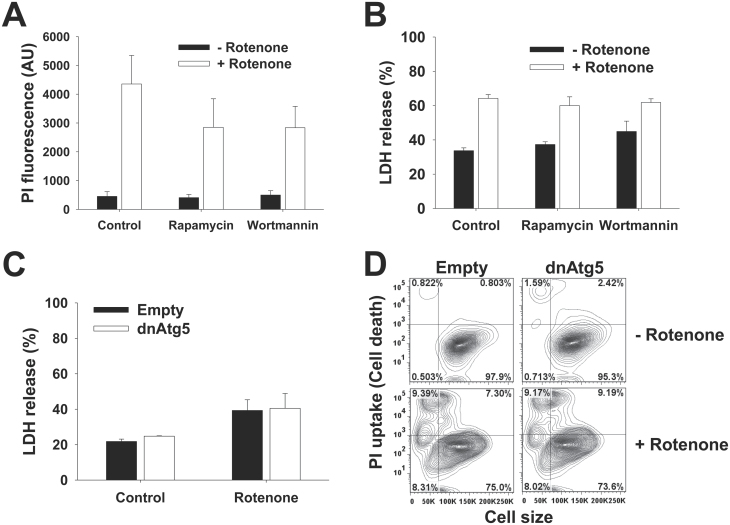
As complex I inhibitors, it is usually considered that both MPP+ and rotenone share similar mechanisms to induce dopaminergic toxicity (Martinez and Greenamyre, 2012) (Fig. 7). However, recent reports suggest that rotenone and MPP+ induce distinct effects on cellular bioenergetics and cell death (Giordano et al., 2012). In contrast to MPP+ and paraquat, neither the pharmacological modulators of autophagy, nor overexpression of dnAtg5 had an effect on rotenone toxicity (Figs. 5A–D). 6-OHDA is a hydroxylated analog of dopamine used to induce selective cell death of dopaminergic cells. Similar to rotenone, 6-OHDA was affected by neither treatment with rapamycin, trehalose (not shown), or wortmannin, nor by overexpression of dnAtg5 (Figs. 5E–H).
Fig. 5.
Rotenone and 6-OHDA toxicity is not affected by pharmacological modulators of autophagy, or impairment of Atg5-dependent pathway. In (A, B, E, and F), cells were subjected to 1h pretreatment with rapamycin (5μM) or wortmannin (200nM). In (C, D, G, and H), cells were infected with Ad-Empty or Ad-dnAtg5 at 1.5 MOI for 24h. Then, cells were exposed to rotenone (4μM) (A–D) or 6-OHDA (50μM) (E–H) for 48h. Loss of cell viability or plasma membrane integrity was determined by measurement of released LDH activity (B and C, and F and G) or by PI uptake analysis with flow cytometry (A, D, E, and H) as explained in Figure 3. D and H, Contour plots represent changes in PI uptake simultaneously compared with cell shrinkage as a marker for apoptotic volume decrease and are representative of 3 independent experiments. Data in bar graphs represent percentage of released LDH activity and are means ± SEM of 3 independent experiments. Abbreviations: 6-OHDA, 6-hydroxydopamine; dnAtg5, dominant negative form of Atg5; LDH, lactate dehydrogenase; MOI, multiplicity of infection; PI, propidium iodide.
CASPASE-DEPENDENT CELL DEATH INDUCED BY PARKINSONIAN MIMETICS IS REGULATED BY ATG5-DEPENDENT AUTOPHAGY AND CATHEPSIN ACTIVITY
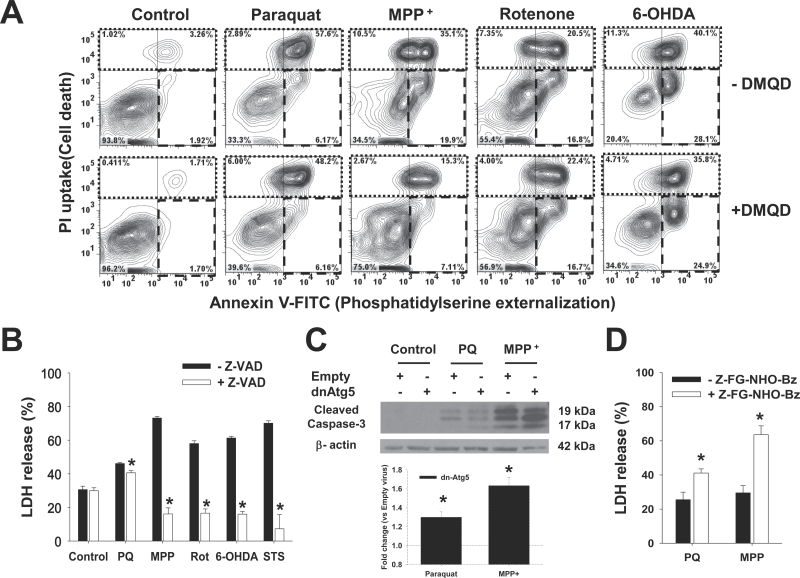
Dopaminergic cell death induced by parkinsonian neurotoxins has been previously demonstrated to be mediated by caspase-dependent apoptosis (Ahmadi et al., 2003; Fei et al., 2008; Hartley et al., 1994; Hartmann et al., 2000; Lotharius et al., 1999; Viswanath et al., 2001). However, some reports have questioned this idea suggesting that either caspase-independent apoptosis or autophagic cell death mediate the toxicity of parkinsonian mimetics (Chu et al., 2005; Han et al., 2003; Kang et al., 2012; Li et al., 2005; Lotharius et al., 1999; Ramachandiran et al., 2007; Rodriguez-Blanco et al., 2012; Zhu et al., 2007). Thus, we wanted to evaluate the role of apoptosis in dopaminergic cell death induced by neurotoxins and the role of Atg5-dependent autophagy. Apoptosis was determined by detection of phosphatidylserine externalization (Annexin V-FITC) in the absence of lost plasma membrane integrity (PI staining). As shown in Figure 6A (Annexin V-FITC/PI contour plots), all neurotoxins induced apoptotic cell death to a different extent, evidenced by the appearance of an Annexin V + / PI - population (lower right quadrant in broken line). Inhibition of the executioner caspase-3 with DMQD-CHO peptide only decreased the cell death triggered by MPP+ treatment. Similar results were obtained with the irreversible caspase-3/-7–like inhibitor DEVD-FMK (not shown). In contrast, the irreversible pan-caspase inhibitor Z-VAD-FMK significantly reduced cell death induced by all neurotoxins (Fig. 6B). Because dnAtg5 increased the cell death induced by paraquat and MPP+, we examined the influence of autophagy in the activation of caspases. Both paraquat- and MPP+-induced caspase-3 activation/cleavage was increased by blocking autophagy with dnAtg5 (Fig. 6C), and this effect was more prominent in MPP+ toxicity, which correlates with the selective effect of the caspase-3 inhibitor on MPP+-induced cell death.
Fig. 6.
Caspases mediate cell death induced by the PQ, MPP+, rotenone, and 6-OHDA, whereas lysosomal hydrolase activity only regulates PQ and MPP+ toxicity. Cells were pretreated with the caspase-3 inhibitor Ac-DMQD-CHO (A), the pan-caspase inhibitor Z-VAD-FMK (B), or the cathepsin inhibitor Z-FG-NHO-Bz (D) at 50μM for 1h. In (C), cells were infected with Ad-Empty or Ad-dnAtg5 at 1.5 MOI for 24h. Then, cells were treated with PQ (0.5mM), MPP+ (2.5mM), rotenone (Rot, 4μM), 6-OHDA (50μM), or STS (20nM) for 48h. A, Apoptosis was measured by Annexin V-FITC/PI staining. In contour plots, early apoptosis is identified as an increase in the number of Annexin V (+)/PI (−) cells (lower right quadrant, broken line region). Late-apoptotic and necrotic cells are identified as Annexin V (+)/PI (+) (upper left quadrant, dotted line region). Plots are representative of 3 independent experiments. In (B and D), cell death was determined by measuring released LDH activity, and data represent percentage of released LDH and are means ± SEM of 3 independent experiments. *p < .05, significant difference with respect to the corresponding neurotoxin treatments in the absence of caspase or cathepsin inhibitors. C, Representative WB analysis of caspase-3 activation. Inset bar graph represents the densitometric analysis of cleaved caspase-3/β-actin normalized with respect to the corresponding Empty virus + drug treatment values. Abbreviations: 6-OHDA, 6-hydroxydopamine; dnAtg5, dominant negative form of Atg5; LDH, lactate dehydrogenase; MOI, multiplicity of infection; MPP+ , 1-methyl-4-phenylpyridinium; PI, propidium iodide; PQ, paraquat; STS, staurosporine; WB, Western immunoblotting.
The degradation of cellular constituents by autophagy is executed inside the lysosomal (autolysosomal) compartment, and the efficiency of lysosomal degradation determines autophagic flux (Fig. 7). Lysosomes contain more than 50 acid hydrolases, including peptidases, phosphatases, nucleases, glycosidases, proteases, and lipases, which digest most macromolecules in the cell (Kaminskyy and Zhivotovsky, 2012). The major function of lysosomal proteases is not to kill the cell but to maintain cellular homeostasis by recycling cellular content (Turk and Turk, 2009). Cathepsins B, L, and D are the most abundant lysosomal proteases (Kaminskyy and Zhivotovsky, 2012). Inhibition of lysosomal enzymes with Z-FG-NHO-Bz augmented paraquat- and MPP+-mediated cell death (Fig. 6D) suggesting that lysosomal degradation/turnover of subcellular components is involved in the protective effects of Atg5-dependent autophagy against MPP+ and paraquat toxicity.
DISCUSSION
The observation that accumulation of autophagosome structures is found in postmortem PD brains has prompted researchers to study the role of autophagy in PD progression (Anglade et al., 1997). A number of findings have demonstrated the importance of autophagy pathways in regulating the accumulation of aggregated proteins, mitochondrial function, and cell survival/death in experimental PD models, highlighting the complexity of the interrelationship between autophagy pathways and dopaminergic neurodegeneration (Harris and Rubinsztein, 2012; Wong and Cuervo, 2010). However, a number of important questions still remain unsolved. Parkinsonian mimetics such as dopamine analogs (6-OHDA), environmental toxicants (pesticides and metals), and mitochondrial toxins (complex I inhibitors) have been widely used to understand the sensitivity of dopaminergic cells to the prooxidant properties of dopamine, environmental exposures, and mitochondrial dysfunction, respectively. To date, contradictory results still exist regarding the alterations in autophagy flux induced by parkinsonian mimetics. Furthermore, the role of autophagy in cell death induced by parkinsonian neurotoxins and the exact signaling mechanisms involved in the regulation of autophagy in dopaminergic cell death are still unclear. In this work, we demonstrated that autophagy is regulated distinctively by neurotoxins. Although complex I inhibitors and paraquat decrease autophagic flux, the toxicity of 6-OHDA is associated with a clear increase in the autophagic rate. Atg5-dependent autophagy and lysosomal hydrolase activity were shown to exert a protective effect against MPP+ and paraquat toxicity. In contrast, cell death induced by rotenone and 6-OHDA did not seem either to be regulated by Atg5-dependent signaling or to respond to pharmacological regulation of autophagy.
Autophagic flux refers to the entire process of autophagy including the delivery of autophagosomes’ cargo to lysosomes, its subsequent degradation, and the release of the resulting macromolecules back into the cytosol (Fig. 7). By responding to perturbations in the extracellular environment, cells tune autophagic flux to meet intracellular metabolic demands. Accumulation of autophagosomes can be associated with increased autophagic flux, impaired autophagosolysosome clearance, or both. Paraquat has been reported to stimulate or inhibit autophagy (Gonzalez-Polo et al., 2007; Wills et al., 2012). 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone, and 6-OHDA have also been reported to increase autophagy (Dadakhujaev et al., 2010; Dehay et al., 2010; Gao et al., 2012; Li et al., 2011; Meredith et al., 2009; Pan et al., 2009; Rodriguez-Blanco et al., 2012; Solesio et al., 2012). However, conclusions in these studies have been reached without proper analysis of the autophagic rate that requires the presence of autophagic flux inhibitors. Here, we have clearly demonstrated that paraquat and the complex I inhibitors MPP+ and rotenone impair autophagy flux. Similar to what was reported by Dagda et al. (2008), we also found that 6-OHDA induced a clear increase in autophagic flux. In contrast, a previous study reported an increase in autophagic flux in response to MPP+ in the presence of bafilomycin (Zhu et al., 2007).
A number of complex I inhibitors have been described to date (Friedrich et al., 1994; Hollingworth et al., 1994; Miyoshi, 1998; Schapira, 2010) (see http://www.oxphos.org/index.php?option=com_content&task=view&id=77&Itemid=75 for a comprehensive list of inhibitors). Unfortunately, very little research has been done on the effect on autophagy flux of other complex I inhibitors besides MPP+ or rotenone. For example, ranolazine, capsaicin, deguelin, and the oxidative phosphorylation uncoupler carbonyl cyanide m-chlorophenyl hydrazone induce an increase in autophagosome formation, but these studies did not evaluate if this effect was associated with an increase in autophagy rate or an impairment in the autophagy flux (Huang et al., 2010; Kwon et al., 2011; Oh and Lim, 2009; Yang et al., 2013). Similar to the observations made here that MPP+ and rotenone inhibit autophagy flux, haloperidol, another complex I-inhibitor, was shown to reduce autophagolysosome formation in primary neuronal cultures (Park et al., 2012).
We have demonstrated that the toxicity of paraquat, MPP+, and rotenone is associated with a decrease in autophagy flux. However, the mechanisms involved remain unclear. Contradictory results exist on the effect of parkinsonian mimetics on mTOR signaling, a major signaling regulator of basal autophagy. For example, paraquat has been shown to increase (Niso-Santano et al., 2011) autophagy by dephosphorylation of mTOR, whereas a different study reports on the inhibitory effect of paraquat on autophagy by an increase in mTOR signaling (Wills et al., 2012). MPTP/MPP+ has been consistently reported to inhibit mTOR signaling (Deguil et al., 2007; Rieker et al., 2011; Rodriguez-Blanco et al., 2012). An interesting study showed that chronic rotenone exposure impairs the ability of rapamycin to induce autophagy, suggesting that alterations in cellular metabolism by rotenone impair autophagic activity (Yu et al., 2009). Multiple signaling pathways regulate/trigger autophagy (He and Klionsky, 2009; Yang and Klionsky, 2010); thus, the identification of the mechanisms by which paraquat and complex I inhibitors impair autophagy requires a more thorough study. Autophagy has been demonstrated to require ATP as energy source (Sakai and Ogawa, 1982; Schellens and Meijer, 1991). Thus, it is plausible that inhibition of autophagy by these neurotoxins is linked directly to ATP depletion (Kang et al., 2012; Mizuno et al., 1987), which does not seem to occur during 6-OHDA-induced toxicity (Kang et al., 2012). The importance of basal autophagy and neuronal degeneration was demonstrated by elegant studies where impairment in basal autophagy in the nervous system leads to neurodegeneration accompanied by polyubiquitinated protein aggregation in cytoplasmic inclusion bodies (Hara et al., 2006; Komatsu et al., 2006).
Autophagy acts as a protective mechanism promoting cell survival in response to a variety of stressors, and only in very few cases, it participates as a programmed cell death mechanism (Shen et al., 2012). Contradictory findings have been reported on the role of autophagy-dependent cell death and survival mechanisms in dopaminergic cell death induced by MPP+ (Chu et al., 2007; Dehay et al., 2010). A protective role for autophagy in paraquat, rotenone, and 6-OHDA has been postulated in most studies so far (Dadakhujaev et al., 2010; Dehay et al., 2010; Gonzalez-Polo et al., 2007, 2009; Niso-Santano et al., 2011; Pan et al., 2009; Xiong et al., 2011). However, contradictory results have suggested that autophagic cell death participates in MPP+/MPTP toxicity based on the inhibitory and stimulatory effects of bafylomycin (Rodriguez-Blanco et al., 2012) and rapamycin (Chu et al., 2007), respectively. 6-OHDA-induced dopaminergic cell death was also associated with autophagic cell death by the inhibitory effect of 3-MA. We found that rapamycin stimulated MPP+ toxicity, which suggests a role of autophagic cell death. However, wortmannin, considered an inhibitor of autophagosome formation, had a similar stimulatory effect. In contrast, paraquat toxicity was only shown to be reduced by rapamycin, but wortmannin had no effect on paraquat-induced cell death, whereas neither 6-OHDA nor rotenone were shown to be modulated by these agents. As summarized in Supplementary Table 1 and Figure 7, both rapamycin and PI3K inhibitors wortmannin, LY294002, and 3-MA, widely used to modulate autophagy, have a variety of secondary/nonautophagy-related effects. Interestingly, rapamycin has been shown to protect against the accumulation of aggregated proteins independently of autophagy by reducing protein synthesis (King et al., 2008). Thus, we consider that conclusions based solely on pharmacological regulation of autophagy might be misleading.
To date, a wide variety of signaling molecules have been shown to regulate autophagy (He and Klionsky, 2009; Yang and Klionsky, 2010). Yeast genetic studies identified Atg proteins as core components of the molecular machinery involved in the formation of autophagosomes (Mizushima et al., 2011). The Atg12-Atg5 and Atg8 (LC3)-phosphatidylethanolamine (PE) ubiquitin-like conjugation systems function at the late stages of autophagosome formation (Fig. 7). LC3 and Atg5 are not required for the initiation of autophagy but mediate phagophore expansion and autophagosome formation. An Atg5/Atg7-independent macroautophagy process induced by etoposide and starvation has been recently reported, which seem to depend on Atg1 (Ulk1) and Atg6 (beclin 1) signaling. Conversely, Ulk1/2- and beclin-independent autophagy pathways seem to require Atg5 signaling (Cheong et al., 2011; Grishchuk et al., 2011; Seo et al., 2011; Smith et al., 2010). Atg5 knockdown was reported to prevent the protective effects of rapamycin against rotenone-induced cell death (Pan et al., 2009). In contrast, Atg7, Atg5, and LC3, but not beclin 1, knockdown were shown to protect SH-SY5Y cells against MPP+ (Zhu et al., 2007). In contrast, in our results, we demonstrated that dnAtg5 significantly enhanced the toxicity of paraquat and MPP+ but not that of rotenone. In addition, dnAtg5 had no effect on 6-OHDA toxicity. The difference between this and the studies described above might relate to the experimental approach used. siRNA-mediated knockdown of Atg5 might only partially reduce its expression levels (Ahmadi et al., 2003; Zhu et al., 2007), and it has been shown that low levels of Atg5 expression are sufficient to maintain autophagy (Hosokawa et al., 2007). Thus, a better approach may be the use of the dn (K130R) version used in our study (Yang et al., 2010).
The degradation of the autophagosome cargo is executed inside the lysosomal compartment by the activity of a number of hydrolases (Kaminskyy and Zhivotovsky, 2012), which maintain cellular homeostasis by recycling cellular content (Turk and Turk, 2009). Cathepsins B, L, and D are the most abundant lysosomal proteases (Kaminskyy and Zhivotovsky, 2012), and despite their ubiquitous expression, combined deficiency of cathepsins B and L induces neurodegeneration (Felbor et al., 2002). Using an inhibitor of cathepsins B/L/S, we demonstrated the correlation between inhibition of autophagy and lysosomal hydrolase activity and increased cell death induced by paraquat and MPP+. Inhibition of cathepsins L and D has been previously reported to decrease 6-OHDA toxicity (Lee et al., 2007; Xiang et al., 2011), whereas we observed no effect of cathepsin inhibition in rotenone- or 6-OHDA-induced cell death.
A number of studies have demonstrated the role of caspase-dependent apoptosis in dopaminergic cell death induced by parkinsonian toxins (Kermer et al., 2004; Mattson, 2006; Okouchi et al., 2007). However, with the discovery of autophagy and the establishment of the concept of autophagic cell death, many reports have rushed to suggest that cell death induced by parkinsonian mimetics might involve caspase-independent pathways, particularly autophagic cell death (Chu et al., 2005, 2007; Han et al., 2003; Harbison et al., 2011; Li et al., 2011; Ramachandiran et al., 2007; Rodriguez-Blanco et al., 2012). We observed that inhibition of caspases protects against the toxicity induced by paraquat, MPP+, rotenone, and 6-OHDA. Interestingly, only caspase-3 was shown to play a role in MPP+ toxicity. We also report that impairment of Atg5-mediated autophagy enhances the cleavage/activity of caspase-3 induced by MPP+. These findings agree with a number of studies that have indeed shown that inhibition of autophagy stimulates caspase-dependent cell death (Boya et al., 2005; Eisenberg-Lerner et al., 2009; Hou et al., 2010; Loos et al., 2011).
An interesting observation is that although Atg5-dependent autophagy and lysosomal hydrolase activity protect against MPP+-induced cell death, the toxicity induced by rotenone was not affected by these mechanisms. Rotenone and MPP+ are complex I inhibitors whose toxic effect were initially largely referred to be mediated by similar mechanisms. Interestingly, recent reports have demonstrated that rotenone and MPP+ actually exert distinct effects on alterations in cellular metabolism and activation of signaling cascades, supporting the idea that their toxicity is mediated by distinct mechanisms (Giordano et al., 2012; Song et al., 2012; Yacoubian et al., 2010).
All together, our results demonstrated that paraquat, MPP+, and rotenone impair autophagic flux and that 6-OHDA is the only parkinsonian toxin capable of increasing autophagic rate. Furthermore, we demonstrated, for the first time, a protective role of Atg5-dependent autophagy in paraquat and MPP+ toxicity, which correlates with the activity of cathepsins. In contrast, neither pharmacological modulation of autophagy nor impairment of Atg5-dependent signaling seem to affect rotenone or 6-OHDA toxicity. We also conclude that findings based only on the pharmacological regulation of autophagy might be misleading based on the reported secondary/nonautophagy-related effects of agents such as rapamycin, and PI3K inhibitors (Supplementary Table 1). Finally, we observed that cell death induced by parkinsonian mimetics is largely mediated by caspase-dependent apoptosis, discarding the role of autophagic cell death in this process.
SUPPLEMENTARY DATA
Supplementary data (references Chen and Wang [2001], El-Kholy et al. [2003], Ethier and Madison [2002], Ferby et al. [1996], Foster and Fingar [2010], Gong et al. [2012], Halvorsen et al. [2002], Hazeki et al. [2006], Hsuan et al. [2006], Hou et al. [2012], King et al. [2001], Klionsky et al. [2012], Kofman et al. [2012], Lan et al. [2012], Malagelada et al. [2010], Mnich et al. [2010], Niso-Santano et al. [2006], Nakanishi et al. [1992], Renna et al. [2010], Sagi et al. [2007], Salinas et al. [2003], Sun et al. [2004], Tsai et al. [2012], Wang et al. [2010], and Wu et al. [2009] are cited in Supplementary Table 1) are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health Grant (P20RR17675); Centers of Biomedical Research Excellence; Scientist Development Grant of the American Heart Association (12SDG12090015); Research Council Interdisciplinary Grant; Life Sciences Grant Program of the University of Nebraska-Lincoln.
Supplementary Material
ACKNOWLEDGMENTS
EGFP-LC3 plasmid was generously donated by Dr Tamotsu Yoshimori (Osaka University, Japan). The adenovirus Ad-EGFP-LC3 was a gift from Dr Aviva M. Tolkovsky (Cambridge Centre for Brain Repair, Cambridge, United Kingdom). The Ad-dnAtg5 was kindly provided by Dr Gökhan S. Hotamisligil (Harvard School of Public Health, Boston, MA). We would like to thank Dr Charles A. Kuszynski, Daniel Shea, and Zhi Hong Gill at the Nebraska Center for Virology for their help in the flow cytometry analyses and cell sorting, as well as Terri Fangman for her help in the fluorescence live-cell imaging.
REFERENCES
- Ahmadi F. A., Linseman D. A., Grammatopoulos T. N., Jones S. M., Bouchard R. J., Freed C. R., Heidenreich K. A., Zawada W. M. (2003). The pesticide rotenone induces caspase-3-mediated apoptosis in ventral mesencephalic dopaminergic neurons. J. Neurochem. 87, 914–921 [DOI] [PubMed] [Google Scholar]
- Amaravadi R. K., Yu D., Lum J. J., Bui T., Christophorou M. A., Evan G. I., Thomas-Tikhonenko A., Thompson C. B. (2007). Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Invest. 117, 326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglade P., Vyas S., Javoy-Agid F., Herrero M. T., Michel P. P., Marquez J., Mouatt-Prigent A., Ruberg M., Hirsch E. C., Agid Y. (1997). Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol. Histopathol. 12, 25–31 [PubMed] [Google Scholar]
- Barde I., Salmon P., Trono D. (2010). Production and titration of lentiviral vectors. Curr. Protoc. Neurosci. Chapter 4, Unit 4.21. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. (1973). Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 33, 2643–2652 [PubMed] [Google Scholar]
- Boya P., Gonzalez-Polo R. A., Casares N., Perfettini J. L., Dessen P., Larochette N., Metivier D., Meley D., Souquere S., Yoshimori T., et al. (2005). Inhibition of macroautophagy triggers apoptosis. Mol. Cell Biol. 25, 1025–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. R., Greenamyre J. T. (2010). Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog. Brain Res. 184, 17–33 [DOI] [PubMed] [Google Scholar]
- Chen X., Wang Z. (2001). Regulation of epidermal growth factor receptor endocytosis by wortmannin through activation of Rab5 rather than inhibition of phosphatidylinositol 3-kinase. EMBO Rep. 2, 842–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong H., Lindsten T., Wu J., Lu C., Thompson C. B. (2011). Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc. Natl. Acad. Sci. U.S.A. 108, 11121–11126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. T., Zhu J. H., Cao G., Signore A., Wang S., Chen J. (2005). Apoptosis inducing factor mediates caspase-independent 1-methyl-4- phenylpyridinium toxicity in dopaminergic cells. J. Neurochem. 94, 1685–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. T., Zhu J., Dagda R. (2007). Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: Implications for neurodegeneration and cell death. Autophagy 3, 663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo A. M., Stefanis L., Fredenburg R., Lansbury P. T., Sulzer D. (2004). Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295 [DOI] [PubMed] [Google Scholar]
- Dadakhujaev S., Noh H. S., Jung E. J., Cha J. Y., Baek S. M., Ha J. H., Kim D. R. (2010). Autophagy protects the rotenone-induced cell death in alpha-synuclein overexpressing SH-SY5Y cells. Neurosci. Lett. 472, 47–52 [DOI] [PubMed] [Google Scholar]
- Dagda R. K., Cherra S. J., III, Kulich S. M., Tandon A., Park D., Chu C. T. (2009). Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J. Biol. Chem. 284, 13843–13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda R. K., Zhu J., Kulich S. M., Chu C. T. (2008). Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: Implications for Parkinson’s disease. Autophagy 4, 770–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguil J., Jailloux D., Page G., Fauconneau B., Houeto J. L., Philippe M., Muller J. M., Pain S. (2007). Neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) in MPP+-induced alteration of translational control in Neuro-2a neuroblastoma cells. J. Neurosci. Res. 85, 2017–2025 [DOI] [PubMed] [Google Scholar]
- Dehay B., Bove J., Rodriguez-Muela N., Perier C., Recasens A., Boya P., Vila M. (2010). Pathogenic lysosomal depletion in Parkinson’s disease. J. Neurosci. 30, 12535–12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg-Lerner A., Bialik S., Simon H. U., Kimchi A. (2009). Life and death partners: Apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 16, 966–975 [DOI] [PubMed] [Google Scholar]
- El-Kholy W., Macdonald P. E., Lin J. H., Wang J., Fox J. M., Light P. E., Wang Q., Tsushima R. G., Wheeler M. B. (2003). The phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks K(V) currents via a direct mechanism. FASEB J. 17, 720–722 [DOI] [PubMed] [Google Scholar]
- Eskelinen E. L. (2008). Fine structure of the autophagosome. Methods Mol. Biol. 445, 11–28 [DOI] [PubMed] [Google Scholar]
- Ethier M. F., Madison J. M. (2002). LY294002, but not wortmannin, increases intracellular calcium and inhibits calcium transients in bovine and human airway smooth muscle cells. Cell Calcium 32, 31–38 [DOI] [PubMed] [Google Scholar]
- Fei Q., McCormack A. L., Di Monte D. A., Ethell D. W. (2008). Paraquat neurotoxicity is mediated by a Bak-dependent mechanism. J. Biol. Chem. 283, 3357–3364 [DOI] [PubMed] [Google Scholar]
- Felbor U., Kessler B., Mothes W., Goebel H. H., Ploegh H. L., Bronson R. T., Olsen B. R. (2002). Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc. Natl. Acad. Sci. U.S.A. 99, 7883–7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferby I. M., Waga I., Hoshino M., Kume K., Shimizu T. (1996). Wortmannin inhibits mitogen-activated protein kinase activation by platelet-activating factor through a mechanism independent of p85/p110-type phosphatidylinositol 3-kinase. J. Biol. Chem. 271, 11684–11688 [DOI] [PubMed] [Google Scholar]
- Foster K. G., Fingar D. C. (2010). Mammalian target of rapamycin (mTOR): Conducting the cellular signaling symphony. J. Biol. Chem. 285, 14071–14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R., Li S., Rodriguez-Rocha H., Burns M., Panayiotidis M. I. (2010). Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chem. Biol. Interact. 188, 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich T., Ohnishi T., Forche E., Kunze B., Jansen R., Trowitzsch W., Hofle G., Reichenbach H., Weiss H. (1994). Two binding sites for naturally occurring inhibitors in mitochondrial and bacterial NADH:ubiquinone oxidoreductase (complex I). Biochem. Soc. Trans. 22, 226–230 [DOI] [PubMed] [Google Scholar]
- Gao H., Yang W., Qi Z., Lu L., Duan C., Zhao C., Yang H. (2012). DJ-1 protects dopaminergic neurons against rotenone-induced apoptosis by enhancing ERK-dependent mitophagy. J. Mol. Biol. 423, 232–248 [DOI] [PubMed] [Google Scholar]
- Giordano S., Lee J., Darley-Usmar V. M., Zhang J. (2012). Distinct effects of rotenone, 1-methyl-4-phenylpyridinium and 6-hydroxydopamine on cellular bioenergetics and cell death. PLoS One 7, e44610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaumann H., Ahlberg J. (1987). Comparison of different autophagic vacuoles with regard to ultrastructure, enzymatic composition, and degradation capacity--formation of crinosomes. Exp. Mol. Pathol. 47, 346–362 [DOI] [PubMed] [Google Scholar]
- Gong L., Zhang Q. L., Zhang N., Hua W. Y., Huang Y. X., Di P. W., Huang T., Xu X. S., Liu C. F., Hu L. F., et al. (2012). Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson’s disease: Linking to Akt/GSK3beta signaling pathway. J. Neurochem. 123, 876–885 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Polo R., Niso-Santano M., Moran J. M., Ortiz-Ortiz M. A., Bravo-San Pedro J. M., Soler G., Fuentes J. M. (2009). Silencing DJ-1 reveals its contribution in paraquat-induced autophagy. J. Neurochem. 109, 889–898 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Polo R. A., Niso-Santano M., Ortiz-Ortiz M. A., Gomez-Martin A., Moran J. M., Garcia-Rubio L., Francisco-Morcillo J., Zaragoza C., Soler G., Fuentes J. M. (2007). Inhibition of paraquat-induced autophagy accelerates the apoptotic cell death in neuroblastoma SH-SY5Y cells. Toxicol. Sci. 97, 448–458 [DOI] [PubMed] [Google Scholar]
- Grishchuk Y., Ginet V., Truttmann A. C., Clarke P. G., Puyal J. (2011). Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy 7, 1115–1131 [DOI] [PubMed] [Google Scholar]
- Halvorsen E. M., Dennis J., Keeney P., Sturgill T. W., Tuttle J. B., Bennett J. B., Jr (2002). Methylpyridinium (MPP(+))- and nerve growth factor-induced changes in pro- and anti-apoptotic signaling pathways in SH-SY5Y neuroblastoma cells. Brain Res. 952, 98–110 [DOI] [PubMed] [Google Scholar]
- Han B. S., Hong H. S., Choi W. S., Markelonis G. J., Oh T. H., Oh Y. J. (2003). Caspase-dependent and -independent cell death pathways in primary cultures of mesencephalic dopaminergic neurons after neurotoxin treatment. J. Neurosci. 23, 5069–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada T., Noda N. N., Satomi Y., Ichimura Y., Fujioka Y., Takao T., Inagaki F., Ohsumi Y. (2007). The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 282, 37298–37302 [DOI] [PubMed] [Google Scholar]
- Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., et al. (2006). Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 [DOI] [PubMed] [Google Scholar]
- Harbison R. A., Ryan K. R., Wilkins H. M., Schroeder E. K., Loucks F. A., Bouchard R. J., Linseman D. A. (2011). Calpain plays a central role in 1-methyl-4-phenylpyridinium (MPP+)-induced neurotoxicity in cerebellar granule neurons. Neurotox. Res. 19, 374–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H., Rubinsztein D. C. (2012). Control of autophagy as a therapy for neurodegenerative disease. Nat. Rev. Neurol. 8, 108–117 [DOI] [PubMed] [Google Scholar]
- Hartley A., Stone J. M., Heron C., Cooper J. M., Schapira A. H. (1994). Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: Relevance to Parkinson’s disease. J. Neurochem. 63, 1987–1990 [DOI] [PubMed] [Google Scholar]
- Hartmann A., Hunot S., Michel P. P., Muriel M. P., Vyas S., Faucheux B. A., Mouatt-Prigent A., Turmel H., Srinivasan A., Ruberg M., et al. (2000). Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 97, 2875–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeki K., Kinoshita S., Matsumura T., Nigorikawa K., Kubo H., Hazeki O. (2006). Opposite effects of wortmannin and 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride on toll-like receptor-mediated nitric oxide production: Negative regulation of nuclear factor-{kappa}B by phosphoinositide 3-kinase. Mol. Pharmacol. 69, 1717–1724 [DOI] [PubMed] [Google Scholar]
- He C., Klionsky D. J. (2009). Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth R. M., Ahammadsahib K. I., Gadelhak G., McLaughlin J. L. (1994). New inhibitors of complex I of the mitochondrial electron transport chain with activity as pesticides. Biochem. Soc. Trans. 22, 230–233 [DOI] [PubMed] [Google Scholar]
- Horowitz M. P., Greenamyre J. T. (2010). Gene-environment interactions in Parkinson’s disease: The importance of animal modeling. Clin. Pharmacol. Ther. 88, 467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa N., Hara Y., Mizushima N. (2007). Generation of cell lines with tetracycline-regulated autophagy and a role for autophagy in controlling cell size. FEBS Lett. 581, 2623–2629 [DOI] [PubMed] [Google Scholar]
- Hou H., Zhang Y., Huang Y., Yi Q., Lv L., Zhang T., Chen D., Hao Q., Shi Q. (2012). Inhibitors of phosphatidylinositol 3’-kinases promote mitotic cell death in HeLa cells. PLoS One 7, e35665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W., Han J., Lu C., Goldstein L. A., Rabinowich H. (2010). Autophagic degradation of active caspase-8: A crosstalk mechanism between autophagy and apoptosis. Autophagy 6, 891–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuan S. L., Klintworth H. M., Xia Z. (2006). Basic fibroblast growth factor protects against rotenone-induced dopaminergic cell death through activation of extracellular signal-regulated kinases 1/2 and phosphatidylinositol-3 kinase pathways. J. Neurosci. 26, 4481–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Yitzhaki S., Perry C. N., Liu W., Giricz Z., Mentzer R. M., Jr, Gottlieb R. A. (2010). Autophagy induced by ischemic preconditioning is essential for cardioprotection. J. Cardiovasc. Transl. Res. 3, 365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminskyy V., Zhivotovsky B. (2012). Proteases in autophagy. Biochim. Biophys. Acta 1824, 44–50 [DOI] [PubMed] [Google Scholar]
- Kang H., Han B. S., Kim S. J., Oh Y. J. (2012). Mechanisms to prevent caspase activation in rotenone-induced dopaminergic neurodegeneration: Role of ATP depletion and procaspase-9 degradation. Apoptosis. 17, 449–462 [DOI] [PubMed] [Google Scholar]
- Kermer P., Liman J., Weishaupt J. H., Bahr M. (2004). Neuronal apoptosis in neurodegenerative diseases: From basic research to clinical application. Neurodegener. Dis. 1, 9–19 [DOI] [PubMed] [Google Scholar]
- King M. A., Hands S., Hafiz F., Mizushima N., Tolkovsky A. M., Wyttenbach A. (2008). Rapamycin inhibits polyglutamine aggregation independently of autophagy by reducing protein synthesis. Mol. Pharmacol. 73, 1052–1063 [DOI] [PubMed] [Google Scholar]
- King T. D., Bijur G. N., Jope R. S. (2001). Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithium. Brain Res. 919, 106–114 [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Abdalla F. C., Abeliovich H., Abraham R. T., Acevedo-Arozena A., Adeli K., Agholme L., Agnello M., Agostinis P., Aguirre-Ghiso J. A., et al. (2012). Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8, 445–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klongpanichapak S., Phansuwan-Pujito P., Ebadi M., Govitrapong P. (2008). Melatonin inhibits amphetamine-induced increase in alpha-synuclein and decrease in phosphorylated tyrosine hydroxylase in SK-N-SH cells. Neurosci. Lett. 436, 309–313 [DOI] [PubMed] [Google Scholar]
- Kofman A. E., McGraw M. R., Payne C. J. (2012). Rapamycin increases oxidative stress response gene expression in adult stem cells. Aging (Albany NY) 4, 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., Tanida I., Ueno T., Koike M., Uchiyama Y., Kominami E., et al. (2006). Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884 [DOI] [PubMed] [Google Scholar]
- Kwon K. Y., Viollet B., Yoo O. J. (2011). CCCP induces autophagy in an AMPK-independent manner. Biochem. Biophys. Res. Commun. 416, 343–348 [DOI] [PubMed] [Google Scholar]
- Lan D. M., Liu F. T., Zhao J., Chen Y., Wu J. J., Ding Z. T., Yue Z. Y., Ren H. M., Jiang Y. P., Wang J. (2012). Effect of trehalose on PC12 cells overexpressing wild-type or A53T mutant alpha-synuclein. Neurochem. Res. 37, 2025–2032 [DOI] [PubMed] [Google Scholar]
- Lee D. C., Womble T. A., Mason C. W., Jackson I. M., Lamango N. S., Severs W. B., Palm D. E. (2007). 6-Hydroxydopamine induces cystatin C-mediated cysteine protease suppression and cathepsin D activation. Neurochem. Int. 50, 607–618 [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Nagano Y., Taylor J. P., Lim K. L., Yao T. P. (2010). Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J. Cell Biol. 189, 671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O. A., Malagelada C., Greene L. A. (2009). Cell death pathways in Parkinson’s disease: Proximal triggers, distal effectors, and final steps. Apoptosis 14, 478–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Spletter M. L., Johnson D. A., Wright L. S., Svendsen C. N., Johnson J. A. (2005). Rotenone-induced caspase 9/3-independent and -dependent cell death in undifferentiated and differentiated human neural stem cells. J. Neurochem. 92, 462–476 [DOI] [PubMed] [Google Scholar]
- Li L., Wang X., Fei X., Xia L., Qin Z., Liang Z. (2011). Parkinson’s disease involves autophagy and abnormal distribution of cathepsin L. Neurosci. Lett. 489, 62–67 [DOI] [PubMed] [Google Scholar]
- Loos B., Genade S., Ellis B., Lochner A., Engelbrecht A. M. (2011). At the core of survival: Autophagy delays the onset of both apoptotic and necrotic cell death in a model of ischemic cell injury. Exp. Cell Res. 317, 1437–1453 [DOI] [PubMed] [Google Scholar]
- Lotharius J., Dugan L. L., O’Malley K. L. (1999). Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J. Neurosci. 19, 1284–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean K. H., Dorsey F. C., Cleveland J. L., Kastan M. B. (2008). Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J. Clin. Invest. 118, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagelada C., Jin Z. H., Jackson-Lewis V., Przedborski S., Greene L. A. (2010). Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J. Neurosci. 30, 1166–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez T. N., Greenamyre J. T. (2012). Toxin models of mitochondrial dysfunction in Parkinson’s disease. Antioxid. Redox Signal. 16, 920–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P. (2006). Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid. Redox Signal. 8, 1997–2006 [DOI] [PubMed] [Google Scholar]
- Meredith G. E., Totterdell S., Beales M., Meshul C. K. (2009). Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson’s disease. Exp. Neurol. 219, 334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H. (1998). Structure-activity relationships of some complex I inhibitors. Biochim. Biophys. Acta 1364, 236–244 [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Suzuki K., Sone N., Saitoh T. (1987). Inhibition of ATP synthesis by 1-methyl-4-phenylpyridinium ion (MPP+) in isolated mitochondria from mouse brains. Neurosci. Lett. 81, 204–208 [DOI] [PubMed] [Google Scholar]
- Mizushima N., Yoshimori T., Ohsumi Y. (2011). The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132 [DOI] [PubMed] [Google Scholar]
- Mnich K., Finn D. P., Dowd E., Gorman A. M. (2010). Inhibition by anandamide of 6-hydroxydopamine-induced cell death in PC12 cells. Int. J. Cell Biol. 2010, 818497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S., Kakita S., Takahashi I., Kawahara K., Tsukuda E., Sano T., Yamada K., Yoshida M., Kase H., Matsuda Y., et al. (1992). Wortmannin, a microbial product inhibitor of myosin light chain kinase. J. Biol. Chem. 267, 2157–2163 [PubMed] [Google Scholar]
- Narendra D. P., Jin S. M., Tanaka A., Suen D. F., Gautier C. A., Shen J., Cookson M. R., Youle R. J. (2010). PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y., Arakawa S., Fujitani K., Yamaguchi H., Mizuta T., Kanaseki T., Komatsu M., Otsu K., Tsujimoto Y., Shimizu S. (2009). Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461, 654–658 [DOI] [PubMed] [Google Scholar]
- Niso-Santano M., Bravo-San Pedro J. M., Gomez-Sanchez R., Climent V., Soler G., Fuentes J. M., Gonzalez-Polo R. A. (2011). ASK1 overexpression accelerates paraquat-induced autophagy via endoplasmic reticulum stress. Toxicol. Sci. 119, 156–168 [DOI] [PubMed] [Google Scholar]
- Niso-Santano M., Moran J. M., Garcia-Rubio L., Gomez-Martin A., Gonzalez-Polo R. A., Soler G., Fuentes J. M. (2006). Low concentrations of paraquat induces early activation of extracellular signal-regulated kinase 1/2, protein kinase B, and c-Jun N-terminal kinase 1/2 pathways: Role of c-Jun N-terminal kinase in paraquat-induced cell death. Toxicol. Sci. 92, 507–515 [DOI] [PubMed] [Google Scholar]
- Oh S. H., Lim S. C. (2009). Endoplasmic reticulum stress-mediated autophagy/apoptosis induced by capsaicin (8-methyl-N-vanillyl-6-nonenamide) and dihydrocapsaicin is regulated by the extent of c-Jun NH2-terminal kinase/extracellular signal-regulated kinase activation in WI38 lung epithelial fibroblast cells. J. Pharmacol. Exp. Ther. 329, 112–122 [DOI] [PubMed] [Google Scholar]
- Okouchi M., Ekshyyan O., Maracine M., Aw T. Y. (2007). Neuronal apoptosis in neurodegeneration. Antioxid. Redox Signal. 9, 1059–1096 [DOI] [PubMed] [Google Scholar]
- Pan T., Rawal P., Wu Y., Xie W., Jankovic J., Le W. (2009). Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience 164, 541–551 [DOI] [PubMed] [Google Scholar]
- Park J., Chung S., An H., Kim J., Seo J., Kim D. H., Yoon S. Y. (2012). Haloperidol and clozapine block formation of autophagolysosomes in rat primary neurons. Neuroscience 209, 64–73 [DOI] [PubMed] [Google Scholar]
- Petiot A., Ogier-Denis E., Blommaart E. F., Meijer A. J., Codogno P. (2000). Distinct classes of phosphatidylinositol 3’-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 275, 992–998 [DOI] [PubMed] [Google Scholar]
- Poole B., Ohkuma S. (1981). Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J. Cell Biol. 90, 665–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S. (2005). Pathogenesis of nigral cell death in Parkinson’s disease. Parkinsonism Relat. Disord. 11(Suppl. 1), S3–S7 [DOI] [PubMed] [Google Scholar]
- Ramachandiran S., Hansen J. M., Jones D. P., Richardson J. R., Miller G. W. (2007). Divergent mechanisms of paraquat, MPP+, and rotenone toxicity: Oxidation of thioredoxin and caspase-3 activation. Toxicol. Sci. 95, 163–171 [DOI] [PubMed] [Google Scholar]
- Renna M., Jimenez-Sanchez M., Sarkar S., Rubinsztein D. C. (2010). Chemical inducers of autophagy that enhance the clearance of mutant proteins in neurodegenerative diseases. J. Biol. Chem. 285, 11061–11067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieker C., Engblom D., Kreiner G., Domanskyi A., Schober A., Stotz S., Neumann M., Yuan X., Grummt I., Schutz G., et al. (2011). Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J. Neurosci. 31, 453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Blanco J., Martin V., Garcia-Santos G., Herrera F., Casado-Zapico S., Antolin I., Rodriguez C. (2012). Cooperative action of JNK and AKT/mTOR in 1-methyl-4-phenylpyridinium-induced autophagy of neuronal PC12 cells. J. Neurosci. Res. 90, 1850–1860 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rocha H., Garcia Garcia A., Zavala-Flores L., Li S., Madayiputhiya N., Franco R. (2012). Glutaredoxin 1 protects dopaminergic cells by increased protein glutathionylation in experimental Parkinson’s disease. Antioxid. Redox Signal. 17, 1676–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein D. C., Gestwicki J. E., Murphy L. O., Klionsky D. J. (2007). Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov. 6, 304–312 [DOI] [PubMed] [Google Scholar]
- Sagi Y., Mandel S., Amit T., Youdim M. B. (2007). Activation of tyrosine kinase receptor signaling pathway by rasagiline facilitates neurorescue and restoration of nigrostriatal dopamine neurons in post-MPTP-induced parkinsonism. Neurobiol. Dis. 25, 35–44 [DOI] [PubMed] [Google Scholar]
- Sakai M., Ogawa K. (1982). Energy-dependent lysosomal wrapping mechanism (LWM) during autophagolysosome formation. Histochemistry 76, 479–488 [DOI] [PubMed] [Google Scholar]
- Salinas M., Diaz R., Abraham N. G., Ruiz de Galarreta C. M., Cuadrado A. (2003). Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J. Biol. Chem. 278, 13898–13904 [DOI] [PubMed] [Google Scholar]
- Sarkar S., Davies J. E., Huang Z., Tunnacliffe A., Rubinsztein D. C. (2007). Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J. Biol. Chem. 282, 5641–5652 [DOI] [PubMed] [Google Scholar]
- Schapira A. H. (2009). Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol. Sci. 30, 41–47 [DOI] [PubMed] [Google Scholar]
- Schapira A. H. (2010). Complex I: Inhibitors, inhibition and neurodegeneration. Exp. Neurol. 224, 331–335 [DOI] [PubMed] [Google Scholar]
- Schellens J. P., Meijer A. J. (1991). Energy depletion and autophagy. Cytochemical and biochemical studies in isolated rat hepatocytes. Histochem. J. 23, 460–466 [DOI] [PubMed] [Google Scholar]
- Seo G., Kim S. K., Byun Y. J., Oh E., Jeong S. W., Chae G. T., Lee S. B. (2011). Hydrogen peroxide induces Beclin 1-independent autophagic cell death by suppressing the mTOR pathway via promoting the ubiquitination and degradation of Rheb in GSH-depleted RAW 264.7 cells. Free Radic. Res. 45, 389–399 [DOI] [PubMed] [Google Scholar]
- Shen S., Kepp O., Kroemer G. (2012). The end of autophagic cell death? Autophagy 8, 1–3 [DOI] [PubMed] [Google Scholar]
- Smith D. M., Patel S., Raffoul F., Haller E., Mills G. B., Nanjundan M. (2010). Arsenic trioxide induces a beclin-1-independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell Death Differ. 17, 1867–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solesio M. E., Saez-Atienzar S., Jordan J., Galindo M. F. (2012). Characterization of mitophagy in the 6-hydoxydopamine Parkinson’s disease model. Toxicol. Sci. 129, 411–420 [DOI] [PubMed] [Google Scholar]
- Song J. X., Shaw P. C., Wong N. S., Sze C. W., Yao X. S., Tang C. W., Tong Y., Zhang Y. B. (2012). Chrysotoxine, a novel bibenzyl compound selectively antagonizes MPP(+), but not rotenone, neurotoxicity in dopaminergic SH-SY5Y cells. Neurosci. Lett. 521, 76–81 [DOI] [PubMed] [Google Scholar]
- Sun H., Oudit G. Y., Ramirez R. J., Costantini D., Backx P. H. (2004). The phosphoinositide 3-kinase inhibitor LY294002 enhances cardiac myocyte contractility via a direct inhibition of Ik,slow currents. Cardiovasc. Res. 62, 509–520 [DOI] [PubMed] [Google Scholar]
- Tanida I. (2011). Autophagosome formation and molecular mechanism of autophagy. Antioxid. Redox Signal. 14, 2201–2214 [DOI] [PubMed] [Google Scholar]
- Tanner C. M., Kamel F., Ross G. W., Hoppin J. A., Goldman S. M., Korell M., Marras C., Bhudhikanok G. S., Kasten M., Chade A. R., et al. (2011). Rotenone, Paraquat and Parkinson’s Disease. Environ. Health Perspect. 119, 866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K. D., Chang W. W., Lin C. C., Hsu S. C., Lee Y. J., Chen W., Shieh J. C., Lin T. H. (2012). Differential effects of LY294002 and wortmannin on inducible nitric oxide synthase expression in glomerular mesangial cells. Int. Immunopharmacol. 12, 471–480 [DOI] [PubMed] [Google Scholar]
- Turk B., Turk V. (2009). Lysosomes as “suicide bags” in cell death: Myth or reality? J. Biol. Chem. 284, 21783–21787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance J. M., Ali S., Bradley W. G., Singer C., Di Monte D. A. (2010). Gene-environment interactions in Parkinson’s disease and other forms of parkinsonism. Neurotoxicology 31, 598–602 [DOI] [PubMed] [Google Scholar]
- Viswanath V., Wu Y., Boonplueang R., Chen S., Stevenson F. F., Yantiri F., Yang L., Beal M. F., Andersen J. K. (2001). Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease. J. Neurosci. 21, 9519–9528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C., Zhou C., Huang Y., Cui M., de Vries R. L., Kim J., May J., Tocilescu M. A., Liu W., Ko H. S., et al. (2010). PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. U.S.A. 107, 378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Yang H. J., Xia Y. Y., Feng Z. W. (2010). Insulin-like growth factor 1 protects human neuroblastoma cells SH-EP1 against MPP+-induced apoptosis by AKT/GSK-3beta/JNK signaling. Apoptosis 15, 1470–1479 [DOI] [PubMed] [Google Scholar]
- Webb J. L., Ravikumar B., Atkins J., Skepper J. N., Rubinsztein D. C. (2003). Alpha-Synuclein is degraded by both autophagy and the proteasome. J. Biol. Chem. 278, 25009–25013 [DOI] [PubMed] [Google Scholar]
- West G. J., Uki J., Herschman H. R., Seeger R. C. (1977). Adrenergic, cholinergic, and inactive human neuroblastoma cell lines with the action-potential Na+ ionophore. Cancer Res. 37, 1372–1376 [PubMed] [Google Scholar]
- Wills J., Credle J., Oaks A. W., Duka V., Lee J. H., Jones J., Sidhu A. (2012). Paraquat, but not maneb, induces synucleinopathy and tauopathy in striata of mice through inhibition of proteasomal and autophagic pathways. PLoS One 7, e30745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E., Cuervo A. M. (2010). Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 13, 805–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Jiang C., Shen Q., Hu Y. (2009). Systematic gene expression profile of hypothalamus in calorie-restricted mice implicates the involvement of mTOR signaling in neuroprotective activity. Mech. Ageing Dev. 130, 602–610 [DOI] [PubMed] [Google Scholar]
- Wu Y. T., Tan H. L., Shui G., Bauvy C., Huang Q., Wenk M. R., Ong C. N., Codogno P., Shen H. M. (2010). Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J. Biol. Chem. 285, 10850–10861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang B., Fei X., Zhuang W., Fang Y., Qin Z., Liang Z. (2011). Cathepsin L is involved in 6-hydroxydopamine induced apoptosis of SH-SY5Y neuroblastoma cells. Brain Res. 1387, 29–38 [DOI] [PubMed] [Google Scholar]
- Xiong N., Jia M., Chen C., Xiong J., Zhang Z., Huang J., Hou L., Yang H., Cao X., Liang Z., et al. (2011). Potential autophagy enhancers attenuate rotenone-induced toxicity in SH-SY5Y. Neuroscience 199, 292–302 [DOI] [PubMed] [Google Scholar]
- Yacoubian T. A., Slone S. R., Harrington A. J., Hamamichi S., Schieltz J. M., Caldwell K. A., Caldwell G. A., Standaert D. G. (2010). Differential neuroprotective effects of 14-3-3 proteins in models of Parkinson’s disease. Cell Death Dis. 1, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Li P., Fu S., Calay E. S., Hotamisligil G. S. (2010). Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 11, 467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. L., Ji C., Bi Z. G., Lu C. C., Wang R., Gu B., Cheng L. (2013). Deguelin induces both apoptosis and autophagy in cultured head and neck squamous cell carcinoma cells. PLoS One 8, e54736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Klionsky D. J. (2010). Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 22, 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z., Wood N. W. (2009). Cell death pathways in Parkinson’s disease: Role of mitochondria. Antioxid. Redox Signal. 11, 2135–2149 [DOI] [PubMed] [Google Scholar]
- Yu W. H., Dorado B., Figueroa H. Y., Wang L., Planel E., Cookson M. R., Clark L. N., Duff K. E. (2009). Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric alpha-synuclein. Am. J. Pathol. 175, 736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. H., Horbinski C., Guo F., Watkins S., Uchiyama Y., Chu C. T. (2007). Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am. J. Pathol. 170, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.