Abstract
The best validated susceptibility variants for Parkinson’s disease (PD) are located in the alpha-synuclein (SNCA) and microtubule-associated protein tau (MAPT) genes. Recently, a protective p.N551K-R1398H-K1423K haplotype in the leucine-rich repeat kinase 2 (LRRK2) gene was identified, with p.R1398H appearing to be the most likely functional variant. To date, the consistency of the protective effect of LRRK2 p.R1398H across MAPT and SNCA variant genotypes has not been assessed. To address this, we examined four SNCA variants (rs181489, rs356219, rs11931074, rs2583988), the MAPT H1-haplotype defining variant rs1052553, and LRRK2 p.R1398H (rs7133914) in Caucasian (N=10,322) and Asian (N=2,289) series. There was no evidence of an interaction of LRRK2 p.R1398H with MAPT or SNCA variants (all P≥0.10); the protective effect of p.R1398H was observed at similar magnitude across MAPT and SNCA genotypes, and the risk effects of MAPT and SNCA variants were observed consistently for LRRK2 p.R1398H genotypes. Our results indicate that the association of LRRK2 p.R1398H with PD is independent of SNCA and MAPT variants, and vice versa, in Caucasian and Asian populations.
Keywords: Parkinson disease, LRRK2, SNCA, MAPT, interaction, genetics
1. Introduction
With an estimated prevalence of between 1% and 2% in individuals older than 65, Parkinson’s disease (PD) is one of the most common age-related neurodegenerative disorders (de Lau and Breteler, 2006; Postuma and Montplaisir, 2009). Long thought of as a sporadic disease, PD now has a well-established genetic component which includes both disease-causing mutations as well as risk-modifying susceptibility variants (Gasser et al., 2011). Of the PD susceptibility variants that have been identified thus far, the best validated have involved those located in the α-synuclein (SNCA) gene, which also contains several pathogenic mutations that are linked to familial PD, and in the microtubule-associated protein tau (MAPT) gene (Gasser et al., 2011). More specifically, associations with PD have been identified in both Caucasian and Asian populations at the 3′ and 5′ ends of the SNCA gene (Mizuta et al., 2006; Mueller et al., 2005; Pankratz et al., 2009; Ross et al., 2007; Satake et al., 2009; Simón-Sánchez et al., 2009; Winkler et al., 2007), while the H1 haplotype in MAPT is associated with PD in Caucasians, but not in Asians owing to the almost complete absence of the H2 haplotype in that group (Evans et al., 2004; Healy et al., 2004; Skipper et al., 2004; Tobin et al., 2008; Wider et al., 2010).
Variation in the leucine-rich repeat kinase 2 (LRRK2) gene, which like SNCA harbors disease-causing mutations of its own, has also been associated with susceptibility to PD in both Caucasian and Asian populations. The majority of proposed LRRK2 PD risk variants have been relatively rare (minor allele frequencies [MAFs] between 1% and 5%) and have included p.G2385R and p.R1628P in Asian populations as well as the more recently identified p.A419V (Asians), and p.M1646T (Caucasians) (Di Fonzo et al., 2006; Farrer et al., 2007; Ross et al., 2008; Ross et al., 2011; Tan et al., 2010). The most common LRRK2 PD risk factor to date, identified by several groups including our own, has involved a 3-variant (p.N551K-R1398H-K1423K) protective haplotype in both populations (Ross et al., 2011; Tan et al., 2010). It has been shown that the p.1398H variant has reduced kinase activity in comparison to the wild type p.R1398 (Tan et al. 2010). Given these data, the p.R1398H (rs7133914) substitution, which occurs with a MAF of approximately 7% in Caucasians and 10% in Asians (Heckman et al., in press; Tan et al., 2010), is the most likely functional variant on the haplotype. The protective effect of p.R1398H appears strongest in Asians, where consistent odds ratios of 0.75 and 0.73 have been observed in studies by Tan et al. (2010) and Ross et al. (2011), with a similar odds ratio of 0.79 observed in a smaller study by Chen et al. (2011). In Caucasians, the odds ratio for p.R1398H observed in the aforementioned study by Ross et al. in a series of 6995 patients and 5595 controls was 0.89. This is very similar to the findings of a large meta-analysis of genome-wide association studies, where although not nominally significant, LRRK2 p.R1398H (MAF~6.7%) had a protective odds ratio of 0.92 and 95% confidence limits ranging from 0.83 to 1.02 in regard to susceptibility to PD (Nalls et al. 2011; Personal Communication).
In order to best determine risk of PD for a given individual and understand potential future therapeutic implications, it is important not only to identify individual genetic risk factors but also to understand how these risk factors interact with one another. However, sample sizes needed to reasonably evaluate evidence of such gene-gene interactions are usually fairly large and can be difficult to achieve. This is due to the fact that the risk factor of interest in an interaction study (presence of the genotype of interest for both variants) occurs much less frequently than the genotype for the individual variants, which can result in a lack of precision in estimated interaction effects. Collaboration between members of the Genetic Epidemiology of Parkinson’s Disease (GEO-PD) Consortium and the resulting large number of patients with PD and controls offers the opportunity to effectively examine how recognized susceptibility variants for PD may or may not interact with one another. Such a study was previously undertaken by the GEO-PD Consortium, where SNCA and MAPT variants were examined in relation to risk of PD and found to have independent effects (Elbaz et al., 2011). The identification of PD susceptibility variants in LRRK2 raises the question of whether the effects of these variants may be modified by those in SNCA or MAPT, or vice versa. The aim of this study was to evaluate the interaction of the common LRRK2 susceptibility variant p.R1398H with SNCA and MAPT variants in relation to risk of PD using Caucasian and Asian patient-control series obtained through the GEO-PD Consortium.
2. Methods
2.1. Subjects
As of 2013, the GEO-PD Consortium includes 57 sites from 29 countries and six continents who have agreed to share DNA and data for 38,686 patients with PD and 34,871 control subjects (http://www.geopd.org/). A total of 20 sites participating in the GEO-PD Consortium provided data to be used in the current study as part of a project initiated in 2009. The majority of the Caucasian subjects utilized in this study were also included in the previously mentioned GEO-PD SNCA-MAPT interaction study (Elbaz et al., 2011), and the subjects included in this study are a subset of those included in the previously referred to investigation of LRRK2 exonic variants in relation to PD (Ross et al., 2011). To be consistent with the association analysis in the latter study involving LRRK2 exonic variants, carriers of LRRK2 pathogenic variants (N=64) were excluded. Subjects were not genotyped for known pathogenic SNCA mutations and therefore this was not part of our exclusion criteria. In total, 7,342 patients with PD and 5,269 controls from 13 different countries on 4 continents were studied, and these subjects were divided into a Caucasian series (5,991 patients with PD, 4,331 controls, 16 sites, 10 countries) and an Asian series (1,351 patients with PD, 938 controls, 4 sites, 3 countries). Table 1 provides demographic information for the Caucasian and Asians series, while site-specific information is displayed in Supplementary Table 1.
Table 1.
Patient characteristics for the Caucasian and Asian series
| Variable | Patients with PD | Controls |
|---|---|---|
| Caucasian series | N=5,991 | N=4,331 |
| Age | 69 ± 11 (18 – 106) | 65 ± 15 (21 – 107) |
| Gender | ||
| Male | 3453 (58%) | 2045 (47%) |
| Female | 2538 (42%) | 2286 (53%) |
| Age at onset | 59 ± 12 (18 – 96) | NA |
| Asian series | N=1,351 | N=938 |
| Age | 61 ± 12 (20 – 91) | 60 ± 11 (23 – 89) |
| Gender | ||
| Male | 672 (50%) | 322 (34%) |
| Female | 679 (50%) | 616 (66%) |
| Age at onset | 54 ± 12 (20 – 89) | NA |
The sample mean ± SD (minimum – maximum) is given for age and age at onset. Information was unavailable regarding age in the Caucasian series (147 patients with PD, 21 controls) and Asian series (371 patients with PD, 298 controls). Information was unavailable regarding age at onset in the Caucasian series (723 patients) and Asian series (8 patients). NA=not applicable. SD=standard deviation.
Patients were diagnosed with PD using standard criteria (Bower et al., 1999; Gelb et al., 1999; Hughes et al., 1992). Controls were individuals free of PD or a related movement disorder at the time of examination. All subjects were unrelated within and between diagnosis groups. The Mayo Clinic Institutional Review Board approved the study, each individual site received local IRB approval, and all subjects provided informed consent.
2.2. Genetic analysis
Four SNCA variants (3′ end of gene: rs181489, rs356219, rs11931074; 5′ end of gene: rs2583988) as well as the MAPT H1-haplotype defining variant rs1052553 were genotyped due to consistently replicated associations with PD (Healy et al., 2004; Mizuta et al., 2006; Mueller et al., 2005; Pankratz et al., 2009; Ross et al., 2007; Satake et al., 2009; Skipper et al., 2004; Simón-Sánchez et al., 2009; Tobin et al., 2008; Wider et al., 2010; Winkler et al., 2007). These five variants were chosen for the aforementioned GEO-PD SNCA-MAPT interaction study (Elbaz et al., 2011). The REP1 polymorphism located in the SNCA promoter has also been associated with PD (Krüger et al., 1999; Maraganore et al., 2006), however due to the fact that the 263bp allele (which has shown the strongest association with PD) is relatively rare, we did not evaluate REP1 in the current study. The LRRK2 variant rs7133914 (p.R1398H) was also selected for inclusion due to the aforementioned findings demonstrating that is the most likely functional variant on a 3-variant haplotype (all 3 variants in strong linkage disequilibrium with r2>0.84 in controls) that affects risk of PD in a protective manner (Ross et al., 2011, Tan et al., 2010).
DNA was sourced from blood and was stored in a −80°C freezer. All samples were de-identified with an anonymous code from each site and only a minimal clinical dataset. All LRRK2 and SNCA genotyping was done using MassArray iPLEX chemistry and analyzed using Typer 4.0 (Sequenom, San Diego, CA). MAPT rs1052553 was genotyped using an ABI Taqman genotyping assay on an ABI 7900HT Fast Real-Time PCR system and analyzed using SDS 2.2.2 software (Applied Biosystems, Foster City, CA, USA). All genotyping was performed at the Mayo Clinic Florida neurogenetics laboratory (Jacksonville, FL, USA). Primer sequences are provided in Supplementary Table 2 for all variants except for MAPT rs1052553. Positive control DNA was run for each variant. Call rates in each series were >95%. There was no evidence of departure from Hardy Weinberg Equilibrium in controls for any of the sites (all P>0.05 after Bonferroni correction).
2.3 Statistical analysis
All analysis was performed separately for the Caucasian and Asian series. Associations of individual SNCA variants, MAPT rs1052553, and LRRK2 p.R1398H with PD, and pair-wise interactions of LRRK2 p.R1398H with SNCA and MAPT variants in relation to PD, were evaluated using odds ratios (ORs) and 95% confidence intervals (CIs) from fixed-effects logistic regression models adjusted for site. Interactions were evaluated on a multiplicative scale only because it has been shown that when at least one of the interacting factors is protective, biological interactions are expected to result in departure from multiplicative effects (Weinberg, 1986).
We considered LRRK2 p.R1398H under a dominant model (presence vs. absence of the minor allele) in all analyses owing to the very small number of homozygotes of the minor allele, while SNCA variants were evaluated under an additive model (effect of each additional minor allele), dominant model, recessive model (presence of two copies vs. zero or one copy of the minor allele) and genotype model (general comparison across genotypes). MAPT rs1052553 was also evaluated under additive, dominant, recessive, and genotype models, but with effects corresponding to the major allele to be consistent with previous reports where ORs correspond to the H1 risk allele. In Caucasians, three-gene interactions were also examined. Sensitivity of results to model adjustment for age and gender and to the use of random-effects models (DerSimonian and Laird, 1986) were also assessed when evaluating interactions. Between-site heterogeneity in interaction ORs was examined using chi-square tests based on the Q statistic, and also by estimating the I2 statistic, which measures the proportion of variation in interaction ORs between sites due to heterogeneity beyond chance (Higgins and Thompson, 2002).
A relatively large number of statistical tests of gene-gene interaction were performed in our analyses (24 in the Caucasian series and 8 in the Asian series). In order to adjust for multiple testing and control the family-wise error rate at 5%, we employed a Bonferroni correction separately for each series, after which p-values≤0.0021 (Caucasian series) and ≤0.00625 (Asian series) were considered as statistically significant. All statistical analyses were performed using R Statistical Software (version 2.14.0; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
A summary of allele and genotype frequencies for SNCA variants, MAPT rs1052553, and LRRK2 p.R1398H in our Caucasian and Asian patient-control series is provided in Supplementary Table 3, along with country-specific frequencies. The SNCA variants rs181489 and rs2583988 as well as MAPT rs1052553 were observed extremely rarely in Asian patients and controls and as such were not assessed in association analysis. SNCA variants were in relatively weak linkage disequilibrium in controls (r2 ≤ 0.32) with the exception of rs181489 and rs356219 in the Caucasian series (r2=0.58), rs181489 and rs2583988 in the Caucasian series (r2=0.53), and rs356219 and rs11931074 in the Asian series (r2=0.97).
In order to best interpret the results of gene-gene interaction analysis, it is helpful to first understand the effects of individual variants on risk of PD, and therefore single-variant associations with PD for the SNCA, MAPT, and LRRK2 variants, which have largely been reported before in the aforementioned GEO-PD studies (Elbaz et al., 2011, Ross et al., 2011), are displayed in Supplementary Table 4. As has been previously shown, all variants were significantly associated with PD.
Evaluations of pair-wise interactions of LRRK2 p.R1398H with SNCA variants and MAPT rs1052553 in relation to PD for the Caucasian series are shown in Table 2. To simplify our presentation of interaction results, we have focused on additive and genotype models for SNCA and MAPT variants in Table 2 since all of these variants had the strongest association with PD under an additive model except SNCA rs11931074 (which was also strongly associated with PD under an additive model), and because genotype models allow for the most general test of interaction. Gene-gene interactions under dominant and recessive models for SNCA and MAPT variants are shown in Supplementary Tables 5 and 6. In site-adjusted analyses, no interactions of LRRK2 p.R1398H with SNCA and MAPT variants approached significance after multiple testing adjustment under any statistical model (all interaction P≥0.10); the protective effect of p.R1398H on risk of PD observed in similar magnitude for different genotypes of SNCA and MAPT variants, while the risk effects of SNCA and MAPT variants were seen similarly for subjects with and without a copy of the minor allele for p.R1398H. All interaction ORs were close to 1.0 in magnitude indicating lack of any interaction with LRRK2 p.R1398H, the only exceptions involving rare genotypes for MAPT rs1052553 under a dominant model (Supplementary Table 5) and SNCA rs11931074 under a recessive model (Supplementary Table 6) which are best interpreted with caution owing to the non-significant interactions and very low genotype frequencies. The lack of interaction of LRRK2 p.R1398H with MAPT and SNCA variants was also observed when adjusting for age and gender (Supplementary Table 7) in those subjects with that information available (98%) and also when utilizing a random effects model (Supplementary Table 8). Results of country-specific interaction analysis are shown in Supplementary Table 9; between-site heterogeneity regarding interactions with LRRK2 p.R1398H was low for SNCA rs356219, rs11931074, and rs2583988 (I2=0%, P≥0.45) and moderate for SNCA rs181489 and MAPT rs1052553 (I2=25% to 36%, P≥0.075) (Supplementary Table 8).
Table 2.
Interactions of LRRK2 p.R1398H with SNCA and MAPT variants in regard to susceptibility to PD in the Caucasian series under additive and genotype models
| Test of association | |||||
|---|---|---|---|---|---|
|
| |||||
| Variant/Genotype | LRRK2 p.R1398H | Sample genotype count and frequency | OR (95% CI) | P-value | Test of interaction |
| SNCA rs181489 | |||||
| CC | GG | 3908 (39.9%) | 1.00 (reference) | NA | Additive model |
| CC | GA or AA | 599 (6.1%) | 0.82 (0.69 – 0.98) | 0.030 | OR: 1.06 |
| CT | GG | 3636 (37.1%) | 1.14 (1.04 – 1.25) | 0.0070 | 95% CI: 0.88 – 1.28 |
| CT | GA or AA | 542 (5.5%) | 1.08 (0.90 – 1.30) | 0.42 | P=0.52 |
| TT | GG | 967 (9.9%) | 1.65 (1.42 – 1.92) | 1.4E-10 | Genotype model 1 |
| TT | GA or AA | 136 (1.4%) | 1.40 (0.98 – 2.00) | 0.066 | P=0.14 |
| SNCA rs356219 | |||||
| AA | GG | 3087 (30.9%) | 1.00 (reference) | NA | Additive model |
| AA | GA or AA | 440 (4.4%) | 0.82 (0.67 – 1.01) | 0.060 | OR: 0.98 |
| AG | GG | 4142 (41.5%) | 1.15 (1.04 – 1.26) | 0.0060 | 95% CI: 0.82 – 1.17 |
| AG | GA or AA | 628 (6.3%) | 1.11 (0.93 – 1.32) | 0.27 | P=0.81 |
| GG | GG | 1476 (14.8%) | 1.51 (1.33 – 1.73) | 7.2E-10 | Genotype model 1 |
| GG | GA or AA | 219 (2.2%) | 1.10 (0.83 – 1.46) | 0.51 | P=0.32 |
| SNCA rs11931074 | |||||
| GG | GG | 7443 (74.6%) | 1.00 (reference) | NA | Additive model |
| GG | GA or AA | 1061 (10.5%) | 0.85 (0.74 – 0.97) | 0.017 | OR: 1.06 |
| GT | GG | 1300 (12.9%) | 1.34 (1.18 – 1.51) | 6.8E-6 | 95% CI: 0.79 – 1.43 |
| GT | GA or AA | 232 (2.3%) | 1.32 (1.00 – 1.74) | 0.052 | P=0.69 |
| TT | GG | 59 (0.6%) | 1.46 (0.84 – 2.62) | 0.19 | Genotype model 1 |
| TT | GA or AA | 12 (0.1%) | 0.67 (0.20 – 2.24) | 0.51 | P=0.61 |
| SNCA rs2583988 | |||||
| CC | GG | 4495 (44.6%) | 1.00 (reference) | NA | Additive model |
| CC | GA or AA | 677 (6.7%) | 0.82 (0.69 – 0.97) | 0.019 | OR: 1.07 |
| CT | GG | 3480 (34.6%) | 1.20 (1.09 – 1.31) | 0.0001 | 95% CI: 0.89 – 1.29 |
| CT | GA or AA | 500 (5.0%) | 1.13 (0.93 – 1.37) | 0.23 | P=0.47 |
| TT | GG | 800 (7.9%) | 1.42 (1.21 – 1.67) | 1.9E-5 | Genotype model 1 |
| TT | GA or AA | 117 (1.2%) | 1.22 (0.84 – 1.80) | 0.30 | P=0.56 |
| MAPT rs10525532 | |||||
| GG | GG | 364 (3.6%) | 1.00 (reference) | NA | Additive model |
| GG | GA or AA | 58 (0.6%) | 0.54 (0.30 – 0.97) | 0.041 | OR: 1.05 |
| GA | GG | 2617 (25.7%) | 1.10 (0.88 – 1.38) | 0.41 | 95% CI: 0.85 – 1.30 |
| GA | GA or AA | 398 (3.9%) | 1.05 (0.78 – 1.41) | 0.75 | P=0.65 |
| AA | GG | 5881 (58.0%) | 1.36 (1.10 – 1.70) | 0.0055 | Genotype model 1 |
| AA | GA or AA | 858 (8.4%) | 1.19 (0.92 – 1.53) | 0.19 | P=0.29 |
ORs and p-values result from fixed-effects logistic regression models. For tests of association, the two given variants were combined into one variable, and the model was adjusted for site. For tests of interaction, models included each of the two variants, their interaction, and site. Additive models and genotype models refer to the characterization of SNCA and MAPT variants; only dominant models were considered for LRRK2 p.R1398H due to the small number of rare homozygotes for this variant. Interaction ORs under an additive model are interpreted as the multiplicative increase in the effect of the minor allele for LRRK2 p.R1398H on PD corresponding to each additional risk allele for SNCA and MAPT variants, or alternatively as the multiplicative increase in the effect of each additional risk allele for SNCA and MAPT variants on PD corresponding to presence of the minor allele for LRRK2 p.R1398H.
Tests of interaction under a genotype model do not produce a single interaction OR, and therefore only a p-value is given.
The A allele for MAPT rs1052553 corresponds to the H1 haplotype. OR=odds ratio. CI=confidence interval.
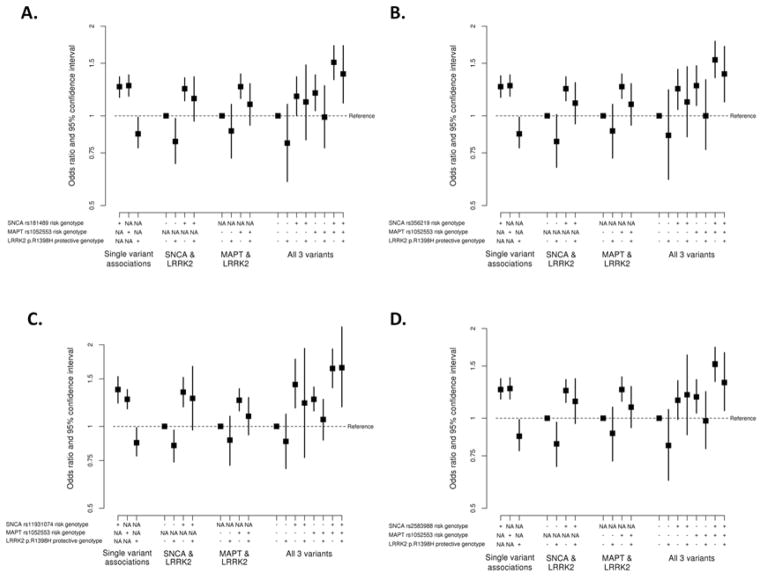
More detailed analysis combining genotypes across all three genes for SNCA variants, MAPT rs1052553, and LRRK2 p.R1398H in the Caucasian series is displayed in Supplementary Table 10 and Figure 1, where rare homozygotes were collapsed with heterozygotes for each variant in order to avoid extremely rare three-variant genotype combinations. There was no evidence of any interaction in these three-gene analyses (all P≥0.63).
Figure 1.
A) Individual and combined effects of SNCA rs181489, MAPT rs1052553, and LRRK2 p.R1398H on risk of PD in the Caucasian series. For SNCA rs181489, the risk genotype was CT or TT (i.e. presence of the minor allele); B) Individual and combined effects of SNCA rs356129, MAPT rs1052553, and LRRK2 p.R1398H on risk of PD in the Caucasian series. For SNCA rs356129, the risk genotype was AG or GG (i.e. presence of the minor allele); C) Individual and combined effects of SNCA rs11931074, MAPT rs1052553, and LRRK2 p.R1398H on risk of PD in the Caucasian series. For SNCA rs11931074, the risk genotype was GT or TT (i.e. presence of the minor allele); D) Individual and combined effects of SNCA rs2583988, MAPT rs1052553, and LRRK2 p.R1398H on risk of PD in the Caucasian series. For SNCA rs2583988, the risk genotype was CT or TT (i.e. presence of the minor allele). Figures 1A–1D) For MAPT rs1052553, the risk genotype was AA (i.e. presence of two copies of the major allele); for LRRK2 p.R1398H, the protective genotype was GA or AA (i.e. presence of the minor allele); NA indicates that a given SNP was not involved in the particular portion of the analysis.
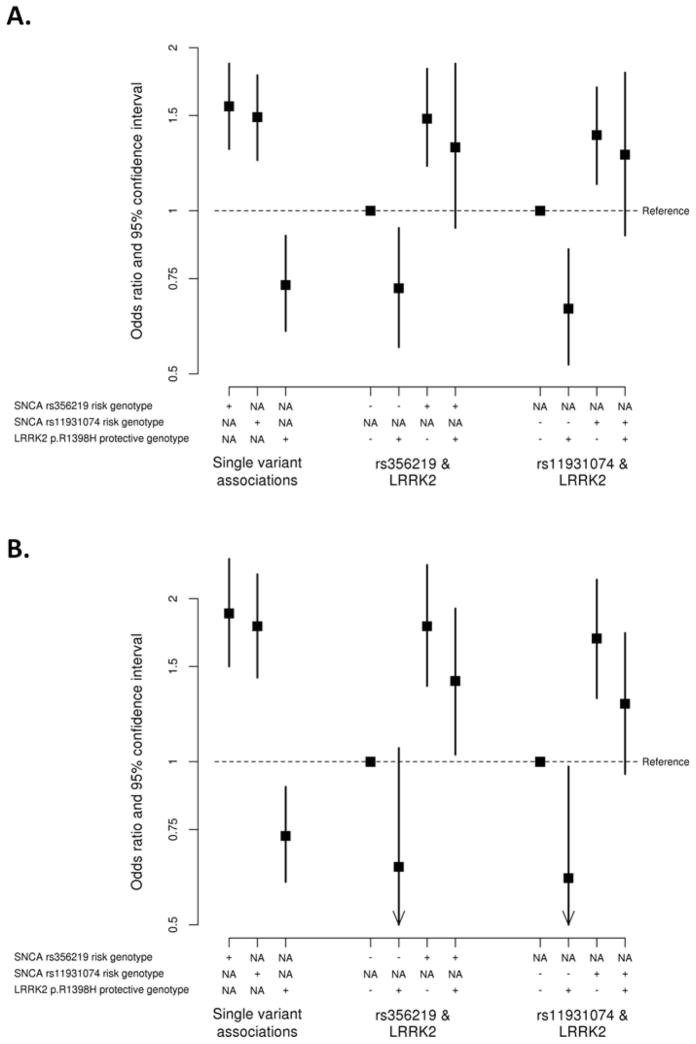
Interactions of LRRK2 p.R1398H with SNCA variants rs356219 and rs11931074 in the Asian series are examined in Table 3 in analysis adjusted for site. Individual effects of LRRK2 p.R1398H and SNCA variants on risk of PD were observed consistently across variants in the other gene, with no statistically significant evidence of gene-gene interaction (all interaction P≥0.14). All interaction ORs were between 1.17 and 1.39, indicating a slight but non-significant reduction of the protective effect of LRRK2 p.R1398H on risk of PD when the risk allele for SNCA variants was present, and a similar small and non-significant enhancement of the SNCA risk effects given the protective genotype for p.R1398H (Figure 2). Results were similar when adjusting for age and gender (Supplementary Table 7) in the subgroup of Asian individuals for whom that information was available (71%) and also under a random effects model (Supplementary Table 8). Interactions between LRRK2 p.R1398H and SNCA variants under additive and recessive models are shown in Supplementary Table 11 separately for each Asian country; between-site heterogeneity in interactions with LRRK2 p.R1398H was moderate for both SNCA rs356219 and rs11931074 in the Asian series (I2=46% to 55%, P≥0.084, Supplementary Table 8).
Table 3.
Interactions of LRRK2 p.R1398H with SNCA variants in regard to susceptibility to PD in the Asian series
| Test of association | |||||
|---|---|---|---|---|---|
|
| |||||
| Variant/Genotype | LRRK2 p.R1398H | Sample genotype count and frequency | OR (95% CI) | P-value | Test of interaction |
| Additive/Genotype models1 | |||||
| SNCA rs356219 | |||||
| AA | GG | 282 (12.9%) | 1.00 (reference) | N/A | Additive model |
| AA | GA or AA | 83 (3.8%) | 0.64 (0.39 – 1.06) | 0.087 | OR: 1.17 |
| AG | GG | 808 (37.0%) | 1.59 (1.21 – 2.09) | 0.0009 | 95% CI: 0.87 – 1.59 |
| AG | GA or AA | 232 (10.6%) | 1.19 (0.84 – 1.69) | 0.33 | P=0.30 |
| GG | GG | 623 (28.5%) | 2.09 (1.56 – 2.79) | 6E-7 | Genotype model 4 |
| GG | GA or AA | 156 (7.1%) | 1.84 (1.23 – 2.77) | 0.0031 | P=0.59 |
| SNCA rs11931074 | |||||
| GG | GG | 302 (13.3%) | 1.00 (reference) | N/A | Additive model |
| GG | GA or AA | 89 (3.9%) | 0.61 (0.37 – 0.98) | 0.044 | OR: 1.25 |
| GT | GG | 843 (37.2%) | 1.55 (1.19 – 2.02) | 0.0012 | 95% CI: 0.93 – 1.69 |
| GT | GA or AA | 243 (10.7%) | 1.06 (0.75 – 1.49) | 0.75 | P=0.14 |
| TT | GG | 630 (27.8%) | 1.90 (1.43 – 2.51) | 7.8E-6 | Genotype model 4 |
| TT | GA or AA | 158 (7.0%) | 1.75 (1.18 – 2.61) | 0.0059 | P=0.31 |
| Dominant model2 | |||||
| SNCA rs356219 | |||||
| AA | GG | 282 (12.9%) | 1.00 (reference) | N/A | OR: 1.23 95% CI: 0.71 – 2.14 P=0.47 |
| AA | GA or AA | 83 (3.8%) | 0.64 (0.39 – 1.06) | 0.087 | |
| AG or GG | GG | 1431 (65.5%) | 1.78 (1.38 – 2.31) | 1.10E-5 | |
| AG or GG | GA or AA | 388 (17.8%) | 1.41 (1.03 – 1.92) | 0.030 | |
| SNCA rs11931074 | |||||
| GG | GG | 302 (13.3%) | 1.00 (reference) | N/A | OR: 1.25 95% CI: 0.74 – 2.15 P=0.41 |
| GG | GA or AA | 89 (3.9%) | 0.61 (0.37 – 0.98) | 0.043 | |
| GT or TT | GG | 1473 (65.0%) | 1.69 (1.31 – 2.17) | 4.3E-5 | |
| GT or TT | GA or AA | 401 (17.7%) | 1.28 (0.95 – 1.73) | 0.11 | |
| Recessive model3 | |||||
| SNCA rs356219 | |||||
| AA or AG | GG | 1090 (49.9%) | 1.00 (reference) | N/A | OR: 1.22 95% CI: 0.78 – 1.92 P=0.38 |
| AA or AG | GA or AA | 315 (14.4%) | 0.72 (0.56 – 0.93) | 0.011 | |
| GG | GG | 623 (28.5%) | 1.48 (1.21 – 1.83) | 0.0002 | |
| GG | GA or AA | 156 (7.1%) | 1.31 (0.93 – 1.87) | 0.13 | |
| SNCA rs11931074 | |||||
| GG or GT | GG | 1145 (50.6%) | 1.00 (reference) | N/A | OR: 1.39 95% CI: 0.90 – 2.17 P=0.14 |
| GG or GT | GA or AA | 332 (14.7%) | 0.66 (0.52 – 0.85) | 0.0011 | |
| TT | GG | 630 (27.8%) | 1.38 (1.12 – 1.69) | 0.0020 | |
| TT | GA or AA | 158 (7.0%) | 1.27 (0.90 – 1.80) | 0.18 | |
ORs and p-values result from fixed-effects logistic regression models. For tests of association, the two given variants were combined into one variable, and the model was adjusted for site. For tests of interaction, models included each of the two variants, their interaction, and site. Additive models, genotype models, dominant models, and recessive models refer to the characterization of SNCA variants; only dominant models were considered for LRRK2 p.R1398H due to the small number of rare homozygotes for this variant.
Interaction ORs under an additive model are interpreted as the multiplicative increase in the effect of the minor allele for LRRK2 p.R1398H on PD corresponding to each additional risk allele for SNCA variants, or alternatively as the as the multiplicative increase in the effect of each additional risk allele for SNCA variants on PD corresponding to presence of the minor allele for LRRK2 p.R1398H.
Interaction ORs under a dominant model are interpreted as the multiplicative increase in the effect of the minor allele for LRRK2 p.R1398H on PD corresponding to presence of the risk allele for SNCA variants, or alternatively as the as the multiplicative increase in the effect of presence of the risk allele for SNCA variants on PD corresponding to presence of the minor allele for LRRK2 p.R1398H..
Interaction ORs under a recessive model are interpreted as the multiplicative increase in the effect of the minor allele for LRRK2 p.R1398H on PD corresponding to presence of two risk alleles for SNCA variants, or alternatively as the as the multiplicative increase in the effect of presence of two risk alleles for SNCA variants on PD corresponding to presence of the minor allele for LRRK2 p.R1398H.
Tests of interaction under a genotype model do not produce a single interaction OR, and therefore only a p-value is given. OR=odds ratio. CI=confidence interval.
Figure 2.
A) Individual and combined effects of SNCA rs356219, SNCA rs11931074, and LRRK2 p.R1398H on risk of PD in the Asian series. SNCA rs356219 and rs11931074 were considered under a recessive model (i.e. presence vs. absence of two copies of the minor allele). For SNCA rs356219, the risk genotype was GG. For SNCA rs11931074, the risk genotype was TT; B) Individual and combined effects of SNCA rs356219, SNCA rs11931074, and LRRK2 p.R1398H on risk of PD in the Asian series. SNCA rs356219 and rs11931074 were considered under a dominant model (i.e. presence vs. absence of the minor allele). For SNCA rs356219, the risk genotype was AG or GG. For SNCA rs11931074, the risk genotype was GT or TT. Figures 2A–2B) For LRRK2 p.R1398H, the protective genotype was GA or AA (i.e. presence of the minor allele); NA indicates that a given SNP was not involved in the particular portion of the analysis.
4. Discussion
Recently, a 3-variant (p.N551K-R1398H-K1423K) haplotype in the LRRK2 gene was shown to affect susceptibility to PD in a protective manner in both Caucasian and Asian populations (Ross et al., 2011; Tan et al., 2010). The p.R1398H substitution appears to be the most likely functional variant as it is located in the conserved Roc domain and there is supporting evidence of reduced kinase activity (Tan et al., 2010). While a number of previous investigations have examined interactions between the well-validated PD susceptibility variants located in the SNCA and MAPT genes (Biernacka et al., 2011; Elbaz et al., 2011; Goris et al., 2007; Mamah et al., 2005; McCulloch et al., 2008; Simón-Sánchez et al., 2009; Trotta et al., 2012; Wider et al., 2011), no report to date has examined interactions of LRRK2 p.R1398H with SNCA and MAPT variants. The results of our large case-control study involving both Caucasian and Asian individuals indicate that the protective effect of LRRK2 p.R1398H is observed consistently for different SNCA and MAPT genotypes, while similarly, the SNCA and MAPT risk effects are observed for individuals with and without the protective p.R1398H allele.
Despite the relatively large number of interactions and statistical models considered, the independent effects on PD risk for LRRK2 p.R1398H, MAPT rs1052553, and SNCA variants were observed with a very high level of consistency in our study. This was most apparent in the large Caucasian series, where all interaction odds ratios were between 0.80 and 1.13, with the exception of the two aforementioned instances involving rare genotypes for MAPT rs1052553 and SNCA rs11931074. Additionally, between-site heterogeneity in interaction effects was low to moderate in Caucasians. Although the protective effect of LRRK2 p.R1398H on risk of PD was observed consistently across SNCA variant genotypes in Asians, perhaps the least convincing evidence of lack of gene-gene interaction was observed in this series. Though not approaching significance even before adjustment for multiple testing, the magnitude of this observed protective effect was slightly smaller when the risk genotype for SNCA variants was present, while conversely the observed risk effects of SNCA variants were marginally stronger in individuals with the protective p.R1398H genotypes. Additionally, heterogeneity in interaction effects between sites was highest in the Asian series. However, it is important to highlight that it would be very unusual to observe a complete lack of gene-gene interaction (i.e. interaction odds ratio equal to 1) in all scenarios simply due to natural sampling variability, particularly given the number of possible interactions that were examined. Nonetheless, given the smaller size of our Asian series in comparison to the Caucasian series, it will be important to validate our findings in larger series of Asian individuals.
Recent studies have supported our earlier work indicating that the effects of SNCA and MAPT variants on PD risk are independent of one another (Biernacka et al., 2011; Trotta et al., 2012; Wider et al., 2011). Though our current study is the first to date to examine the potential interaction of the protective LRRK2 p.R1398H substitution with MAPT and SNCA variants in regard to risk of PD, previous studies have evaluated interactions with, or combined effects of, LRRK2 variants and those in SNCA and MAPT. In their analysis of 1098 patients with PD and 1098 matched controls from the United States (a subset of which were also used in the current study), Biernacka et al. (2011) found no statistically significant evidence of gene-gene interaction when considering 8 intronic LRRK2 variants, 10 SNCA variants (eight intronic, one 3′ downstream, and 5′ Rep1), and 8 MAPT variants (six intronic, one 3′ UTR, and H1/H2). Wang et al. (2012) concluded that other genes, including MAPT and SNCA, modified LRRK2-related risk for PD in a Chinese cohort of 2,013 sporadic PD patients and 1,971 controls. This was based on findings that in comparison to individuals harboring only the LRRK2 p.G2385R or p.R1628P risk variants, the risk of PD is increased in individuals with these and other PD risk variants. However, it is unclear whether this represents independent or interactive effects, and the sample sizes of the combined risk-variant groups examined were quite small. The results of these studies are consistent with those of our own, with the effect of LRRK2 variants on PD susceptibility appearing to be independent of SNCA and MAPT risk factors for PD.
While the strengths of our study, including the large sample size and inclusion of subjects from a variety of different populations, are important to highlight, several limitations should also be acknowledged. A key question is whether the lack of interaction of LRRK2 p.R1398H with SNCA and MAPT variants is a consequence of sample size or the frequencies of the examined variants. In order to assess the possibility of a false-negative association, it is most helpful to examine 95% confidence limits for observed interaction odds ratio estimates (Goodman and Berlin, 1994). These confidence limits were generally relatively tight in the larger Caucasian series indicating a lack of a biologically significant interaction in this population, but were wider in the Asian series, further highlighting the need for validation of our findings in that series. Additionally, as is generally the case for large-scale collaborative studies attempting to address a focused research question that involves a small number of genetic variants, without available genome-wide population control markers, population stratification could potentially have had an impact on our results. However, this potential limitation is lessened by the fact that our logistic regression models were adjusted by site, which makes any possible population stratification a site-specific issue. Other limitations of our study include the different diagnostic criteria across the different sites and the lack of a standardized inclusion/exclusion criteria for patients with PD and controls.
In conclusion, our study provides evidence that the effect of LRRK2 p.R1398H on risk of PD is independent of the MAPT H1-haplotype defining variant rs1052553 and SNCA variants, and vice versa. This lack of gene-gene interaction was apparent in both our large Caucasian patient-control series and our smaller Asian series. Evaluation of interactions involving individuals of other ethnic backgrounds, other rarer LRRK2 susceptibility variants, and PD susceptibility variants at other loci (Lill et al., 2012) is needed in order to move toward a fuller understanding of the genetic architecture of PD susceptibility.
Supplementary Material
Acknowledgments
This work was supported by a grant from The Michael J. Fox Foundation for Parkinson’s Research (OAR, MJF). Original funding for the GEO-PD was supported by a grant from The Michael J. Fox Foundation for Parkinson’s Research Edmond J. Safra Global Genetics Consortia program (DMM). The Mayo Clinic Jacksonville is a Morris K. Udall Center of Excellence in Parkinson’s Disease Research [grant number P50 NS072187] and was supported by a the gift from the family of Carl Edward Bolch, Jr., and Susan Bass Bolch (RJU, ZKW, OAR). Owen A. Ross, PhD, acknowledges funding support from the National Institutes of Health [grant number R01 NS078086]. This research was undertaken, in part, thanks to funding from the Canada Excellence Research Chairs program (MJF; CVG). Leading Edge Endowment Funds, provided by the Province of British Columbia, LifeLabs, and Genome BC, support the Dr. Donald Rix BC Leadership Chair (MJF). Demetrius M. Maraganore, MD acknowledges the National Institutes of Health for funding support [grant number R01ES10751]. Studies at individual sites were supported by a number of different funding agencies world-wide including; Italian Ministry of Health (Ricerca Corrente, Ricerca Finalizzata); the Swedish Parkinson Academy; the Swedish Parkinson Foundation; the Federal Ministry for Education and Research [BMBF, NGFNplus; 01GS08134] (RK); the NGFNplus (Neuron-Parkinson-subproject 7) (SG); CHRU de Lille, Univ Lille 2, Inserm; French Ministry PHRCs (1994/, 2002/1918/2005/1914); Association France Parkinson (2005); Fondation de France 2004-013306; Fondation de la Recherche Médicale (2006); PPF (synucléothèque 2005-2009); the 2 Centres de Ressources Biologiques (IPL-Lille, CHRU-Lille) and its scientific committee (AD, MCCH, Philippe Amouyel, Florence Pasquier, Régis Bordet); funding from France-Parkinson Association and the program “Investissement d’avenir” ANR-10-IAIHU-06; the Swedish Research Council; the Swedish Society for Medical Research; the Swedish Society of Medicine; funds from the Karolinska Institutet and the Parkinson Foundation in Sweden (KW); the National Institutes of Health and National Institute of Neurological Disorders and Stroke [grant numbers 1RC2NS070276, NS057567, and P50NS072187]; Mayo Clinic Florida Research Committee CR programs (MJF) (ZKW); the Geriatric Medical Foundation of Queensland (GDM); a career development award from the Volkswagen Foundation and from the Hermann and Lilly Schilling Foundation (CK); the Research Committee of University of Thessaly (Code: 2845); and Laboratory of Neurogenetics, Biomedicine Department, CERETETH, Larissa, Greece (Code: 01-04-207) (GH, ED).
A number of people must be acknowledged for their contributions to make this work possible; Ferdinanda Annesi, PhD; Patrizia Tarantino, PhD (Institute of Neurological Sciences, National Research Council); Monica Gagliardi, PhD, (Institute of Neurological Sciences, National Research Council, Cosenza Italy), Chiara Riva, PhD (Department of Neuroscience and Biomedical Technologies, University of Milano-Bicocca, Monza, Italy); Roberto Piolti, MD (Department of Neurology, Ospedale San Gerardo, Monza, Italy); Alessandro Ferraris MD, PhD (IRCCS Casa Sollievo della Sofferenza Hospital, Mendel Laboratory, San Giovanni Rotondo, Italy); Aurélie Duflot, (UMR837 Inserm-Univ Lille 2, CHRU de Lille), Jean-Philippe Legendre, Nawal Waucquier (Neurologie et Pathologie du Mouvement, Clinique de Neurologie du CHU de Lille). Anna Rita Bentivoglio, MD, PhD, Tamara Ialongo, MD, PhD, Arianna Guidubaldi, MD, Carla Piano, MD (Institute of Neurology, Catholic University, Rome, Italy); Phil Hyu Lee MD, PhD (Department of Neurology, Yonsei University College of Medicine, Seoul, Korea); Jan Reimer (Department of Neurology, Skåne University Hospital, Sweden); Hiroyo Yoshino, PhD, Manabu Funayama, PhD, Yuanzhe Li, MD, PhD (Juntendo University School of Medicine, Tokyo, Japan). From the Queensland Parkinson’s Project: R.S. Boyle and A. Sellbach (Princess Alexandra Hospital, Brisbane), J. D. O’Sullivan (Royal Brisbane and Women’s Hospital, Brisbane), G.T. Sutherland, G.A. Siebert and N.N.W. Dissanayaka (Eskitis Institute for Cell and Molecular Therapies, Griffith University, Nathan, QLD). Finally, we would like to acknowledge all the patients and control subjects who kindly donated DNA to make collaborative studies like these possible.
Footnotes
A full list of GEO-PD consortium member sites is provided in the Supplementary Text.
Disclosure Statement
JOA, MJF, and ZKW report holding a patent on LRRK2 genetic variability and MJF has received royalties for licensing of genetically modified LRRK2 mouse models. DMM declares a patent pending entitled Methods to treat PD. CK and RK declare receiving payment in their role as consultants for Centogene and Takeda Pharmaceutical, respectively. All other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Biernacka JM, Armasu SM, Cunningham JM, Ahlskog JE, Chung SJ, Maraganore DM. Do interactions between SNCA, MAPT, and LRRK2 genes contribute to Parkinson’s disease susceptibility? Parkinsonism Relat Disord. 2011;17:730–736. doi: 10.1016/j.parkreldis.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmstead County, Minnesota, 1976–1990. Neurology. 1999;52:1214–1220. doi: 10.1212/wnl.52.6.1214. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang S, Liu Y, Hong H, Wang H, Zheng Y, Zhou H, Chen J, Xian W, He Y, Li J, Liu Z, Pei Z, Zeng J. LRRK2 R1398H polymorphism is associated with decreased risk of Parkinson’s disease in a Han Chinese population. Parkinsonism Relat Disord. 2011;17:291–292. doi: 10.1016/j.parkreldis.2010.11.012. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Di Fonzo A, Wu-Chou YH, Lu CS, van Doeselaar M, Simons EJ, Rohé CF, Chang HC, Chen RS, Weng YH, Vanacore N, Breedveld GJ, Oostra BA, Bonifati V. A common missense variant in the LRRK2 gene, Gly2385Arg, associated with Parkinson’s disease risk in Taiwan. Neurogenetics. 2006;7:133–138. doi: 10.1007/s10048-006-0041-5. [DOI] [PubMed] [Google Scholar]
- Elbaz A, Ross OA, Ioannidis JP, Soto-Ortolaza AI, Moisan F, Aasly J, Annesi G, Bozi M, Brighina L, Chartier-Harlin MC, Destée A, Ferrarese C, Ferraris A, Gibson JM, Gispert S, Hadjigeorgiou GM, Jasinska-Myga B, Klein C, Krüger R, Lambert JC, Lohmnan K, van de Loo S, Loriot MA, Lynch T, Mellick GD, Mutez E, Nilsson C, Opala G, Puschmann A, Quattrone A, Sharma M, Silburn PA, Stefanis L, Uitti RJ, Valente EM, Vilariño-Güell C, Wirdefeldt K, Wszolek ZK, Xiromerisiou G, Maraganore DM, Farrer MJ Genetic Epidemiology of Parkinson’s Disease (GEO-PD) Consortium. Independent and joint effects of the MAPT and SNCA genes in Parkinson disease. Ann Neurol. 2011;69:778–792. doi: 10.1002/ana.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W, Fung HC, Steele J, Eerola J, Tienari P, Pittman A, Silva Rd, Myers A, Vrieze FW, Singleton A, Hardy J. The tau H2 haplotype is almost exclusively Caucasian in origin. Neurosci Lett. 2004;369:183–185. doi: 10.1016/j.neulet.2004.05.119. [DOI] [PubMed] [Google Scholar]
- Farrer MJ, Stone JT, Lin CH, Dächsel JC, Hulihan MM, Haugarvoll K, Ross OA, Wu RM. Lrrk2 G2385R is an ancestral risk factor for Parkinson’s disease in Asia. Parkinsonism Relat Disord. 2007;13:89–92. doi: 10.1016/j.parkreldis.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Gasser T, Hardy J, Mizuno Y. Milestones in PD genetics. Mov Disord. 2011;26:1042–1048. doi: 10.1002/mds.23637. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med. 1994;121:200–206. doi: 10.7326/0003-4819-121-3-199408010-00008. [DOI] [PubMed] [Google Scholar]
- Goris A, Williams-Gray CH, Clark GR, Foltynie T, Lewis SJ, Brown J, Ban M, Spillantini MG, Compston A, Burn DJ, Chinnery PF, Barker RA, Sawcer SJ. Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson’s disease. Ann Neurol. 2007;62:145–153. doi: 10.1002/ana.21192. [DOI] [PubMed] [Google Scholar]
- Healy DG, Abou-Sleiman PM, Lees AJ, Casas JP, Quinn N, Bhatia K, Hingorani AD, Wood NW. Tau gene and Parkinson’s disease: a case-control study and meta-analysis. J Neurol Neurosurg Psychiatry. 2004;75:962–965. doi: 10.1136/jnnp.2003.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman MG, Soto-Ortolaza AI, Aasly JO, Abahuni N, Annesi G, Bacon JA, Bardien S, Bozi M, Brice A, Brighina L, Carr J, Chartier-Harlin MC, Dardiotis E, Dickson DW, Diehl NN, Elbaz A, Ferrarese C, Fiske B, Gibson JM, Gibson R, Hadjigeorgiou GM, Hattori N, Ioannidis JP, Boczarska-Jedynak M, Jasinska-Myga B, Jeon BS, Kim YJ, Klein C, Kruger R, Kyratzi E, Lesage S, Lin CH, Lynch T, Maraganore DM, Mellick GD, Mutez E, Nilsson C, Opala G, Park SS, Petrucci S, Puschmann A, Quattrone A, Sharma M, Silburn PA, Sohn YH, Stefanis L, Tadic V, Theuns J, Tomiyama H, Uitti RJ, Valente EM, Van Broeckhoven C, van de Loo S, Vassilatis DK, Vilariño-Güell C, White LR, Wirdefeldt K, Wszolek ZK, Wu RM, Hentati F, Farrer MJ, Ross OA on behalf of the Genetic Epidemiology of Parkinson’s Disease (GEO-PD) Consortium. Population-specific frequencies for LRRK2 susceptibility variants in the genetic epidemiology of Parkinson’s disease (GEO-PD) consortium. Mov Disord. doi: 10.1002/mds.25600. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinic-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Parkinson Disease Genomics Consortium. Nalls MA, Plagnov V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simón-Sánchez J, Schulte C, Lesage S, Sveinbjörnsdóttir S, Stefánsson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB, Wood NW. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger R, Vieira-Saecker AM, Kuhn W, Berg D, Müller T, Kühnl N, Fichs GA, Storch A, Hungs M, Woitalla D, Przuntek H, Epplen JT, Schöls L, Riess O. Increased susceptibility to sporadic Parkinson’s disease by a certain combined alpha-synuclein/apolipoprotein E genotype. Ann Neurol. 1999;45:611–617. doi: 10.1002/1531-8249(199905)45:5<611::aid-ana9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lill CM, Roehr JT, McQueen MB, Kavvoura FK, Bagade S, Schjeide BM, Schjeide LM, Meissner E, Zauft U, Allen NC, Liu T, Schilling M, Anderson KJ, Beecham G, Berg D, Biernacka JM, Brice A, DeStefano AL, Do CB, Eriksson N, Factor SA, Farrer MJ, Foroud T, Gasser T, Hamza T, Hardy JA, Heutink P, Hill-Burns EM, Klein C, Latourelle JC, Maraganore DM, Martin ER, Martinez M, Myers RH, Nalls MA, Pankratz N, Payami H, Satake W, Scott WK, Sharma M, Singleton AB, Stefansson K, Toda T, Tung JY, Vance J, Wood NW, Zabetian CP, Young P, Tanzi RE, Khoury MJ, Zipp F, Lehrach H, Ioannidis JP, Bertram L 23andMe Genetic Epidemiology of Parkinson’s Disease Consortium; International Parkinson’s Disease Genomics Consortium; Parkinson’s Disease GWAS Consortium; Welcome Trust Case Control Consortium 2 (WTCCC2) Comprehensive research synopsis and systemic meta-analyses in Parkinson’s disease genetics: The PDGene database. PLoS Genet. 2012;8:e10002548. doi: 10.1371/journal.pgen.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah CE, Lesnick TG, Lincoln SJ, Strain KJ, de Andrade M, Bower JH, Ahlskog JE, Rocca WA, Farrer MJ, Maraganore DM. Interaction of alpha-synuclein and tau genotypes in Parkinson’s disease. Ann Neurol. 2005;57:439–443. doi: 10.1002/ana.20387. [DOI] [PubMed] [Google Scholar]
- Maraganore DM, de Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Krüger R, Rocca WA, Schneider NK, Lesnick TG, Lincoln SJ, Hulihan MM, Aasly JO, Ashizawa T, Chartier-Harlin MC, Checkoway H, Ferrarese C, Hadjigeorgiou G, Hattori N, Kawakami H, Lambert JC, Lynch T, Mellick GD, Papapetropoulus S, Parsian A, Quattrone A, Riess O, Tan EK, Van Broeckhoven C Genetic Epidemiology of Parkinson’s Disease (GEO-PD) Consortium. Collaborative analysis of alpha-synuclein gene promoter variability in Parkinson disease. JAMA. 2006;296:661–670. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- McCulloch CC, Kay DM, Factor SA, Samii A, Nutt JG, Higgins DS, Griffith A, Roberts JW, Leis BC, Montimurro JS, Zabetian CP, Payami H. Exploring gene-environment interactions in Parkinson’s disease. Hum Genet. 2008;123:257–265. doi: 10.1007/s00439-008-0466-z. [DOI] [PubMed] [Google Scholar]
- Mizuta I, Satake W, Nakabayashi Y, Ito C, Suzuki S, Momose Y, Nagai Y, Oka A, Inoko H, Fukae J, Saito Y, Sawabe M, Murayama S, Yamamoto M, Hattori N, Murata M, Toda T. Multiple candidate gene analysis identifies alpha-synuclein as a susceptibility gene for sporadic Parkinson’s disease. Hum Mol Genet. 2006;15:1151–1158. doi: 10.1093/hmg/ddl030. [DOI] [PubMed] [Google Scholar]
- Mueller JC, Fuchs J, Hofer A, Zimprich A, Lichtner P, Illig T, Berg D, Wüllner U, Meitinger T, Gasser T. Multiple regions of alpha-synuclein are associated with Parkinson’s disease. Ann Neurol. 2005;57:535–541. doi: 10.1002/ana.20438. [DOI] [PubMed] [Google Scholar]
- Pankratz N, Wilk JB, Latourelle JC, DeStefano AL, Halter C, Pugh EW, Doheny KF, Gusella JF, Nichols WC, Foroud T, Myers RH PSG-PROGENI, GenePD Investigators Coordinators, Molecular Genetic Laboratories. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet. 2009;124:593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Montplaisir J. Predicting Parkinson’s disease - why, when, and how? Parkinsonism Relat Disord. 2009;15(Suppl 3):S105–109. doi: 10.1016/S1353-8020(09)70793-X. [DOI] [PubMed] [Google Scholar]
- Ross OA, Gosal D, Stone JT, Lincoln SJ, Heckman MG, Irvine GB, Johnston JA, Gibson JM, Farrer MJ, Lynch T. Familial genes in sporadic disease: common variants of alpha-synuclein gene associate with Parkinson’s disease. Mech Aging Dev. 2007;128:378–382. doi: 10.1016/j.mad.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross OA, Wu YR, Lee MC, Funayama M, Chen ML, Soto AI, Mata IF, Lee-Chen GJ, Chen CM, Tang M, Zhao Y, Hattori N, Farrer MJ, Tan EK, Wu RM. Analysis of Lrrk2 R1628P as a risk factor for Parkinson’s disease. Ann Neurol. 2008;64:88–92. doi: 10.1002/ana.21405. [DOI] [PubMed] [Google Scholar]
- Ross OA, Soto-Ortolaza AI, Heckman MG, Aasly JO, Abahuni N, Annesi G, Bacon JA, Bardien S, Bozi M, Brice A, Brighina L, Van Broeckhoven C, Carr J, Chartier-Harlin MC, Dardiotis E, Dickson DW, Diehl NN, Elbaz A, Ferrarese C, Ferraris A, Fiske B, Gibson JM, Gibson R, Hadjigeorgiou GM, Hattori N, Ioannidis JP, Jasinska-Myga B, Jeon BS, Kim YJ, Klein C, Kruger R, Kyratzi E, Lesage S, Lin CH, Lynch T, Maraganore DM, Mellick GD, Mutez E, Nilsson C, Opala G, Park SS, Puschmann A, Quattrone A, Sharma M, Silburn PA, Sohn YH, Stefanis L, Tadic V, Theuns J, Tomiyama H, Uitti RJ, Valente EM, van de Loo S, Vassilatis DK, Vilariño-Güell C, White LR, Wirdefeldt K, Wszolek ZK, Wu RM, Farrer MJ Genetic Epidemiology of Parkinson’s Disease (GEO-PD) Consortium. Association of LRRK2 exonic variants with susceptibility to Parkinson’s disease: a case control study. Lancet Neurol. 2011;10:898–908. doi: 10.1016/S1474-4422(11)70175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- Simón-Sánchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Krüger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chancock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper L, Wilkes K, Toft M, Baker M, Lincoln S, Hulihan M, Ross OA, Hutton M, Aasly J, Farrer MJ. Linkage disequilibrium and association of MAPT H1 in Parkinson disease. Am J Hum Genet. 2004;75:669–677. doi: 10.1086/424492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EK, Peng R, Teo YY, Tan LC, Angeles D, Ho P, Chen ML, Lin CH, Mao XY, Chang XL, Prakash KM, Liu JJ, Au WL, Le WD, Jankovic J, Burgunder JM, Zhao Y, Wu RM. Multiple LRRK2 variants modulate risk of Parkinson disease: a Chinese multicenter study. Hum Mutat. 2010;31:561–568. doi: 10.1002/humu.21225. [DOI] [PubMed] [Google Scholar]
- Tobin JE, Latourelle JC, Lew MF, Klein C, Suchowersky O, Shill HA, Golbe LI, Mark MH, Growdon JH, Wooten GF, Racette BA, Perlmutter JS, Watts R, Guttman M, Baker KB, Goldwurm S, Pezzoli G, Singer C, Saint-Hilaire MH, Hendricks AE, Williamson S, Nagle MW, Wilk JB, Massood T, Laramie JM, DeStefano AL, Litvan I, Nicholson G, Corbett A, Isaacson S, Burn DJ, Chinnery PF, Pramstaller PP, Sherman S, Al-hinti J, Drasby E, Nance M, Moller AT, Ostergaard K, Roxburgh R, Snow B, Slevin JT, Cambi F, Gusella JF, Myers RH. Haplotypes and gene expression implicate the MAPT region for Parkinson disease: the GenePD Study. Neurology. 2008;71:28–34. doi: 10.1212/01.wnl.0000304051.01650.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta L, Guella I, Soldà G, Sironi F, Tesei S, Canesi M, Pezzoli G, Goldwurn S, Duga S, Asselta R. SNCA and MAPT genes: Independent and joint effects in Parkinson disease in the Italian population. Parkinsonism Relat Disord. 2012;18:257–262. doi: 10.1016/j.parkreldis.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Cai Y, Zheng Z, Tang BS, Xu Y, Wang T, Ma J, Chen SD, Langston JW, Tanner CM, Chan P Chinese Parkinson Study Group (CPSG) Penetrance of LRRK2 G2385R and R1628P is modified by common PD-associated genetic variants. Parkinsonism Relat Disord. 2012;18:958–963. doi: 10.1016/j.parkreldis.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Weinberg CR. Applicability of the simple independent action model to epidemiologic studies involving two factors and a dichotomous outcome. Am J Epidemiol. 1986;123:162–173. doi: 10.1093/oxfordjournals.aje.a114211. [DOI] [PubMed] [Google Scholar]
- Wider C, Vilariño-Güell C, Jasinska-Myga B, Heckman MG, Soto-Ortolaza AI, Cobb SA, Aasly JO, Gibson JM, Lynch T, Uitti RJ, Wszolek ZK, Farrer MJ, Ross OA. Association of the MAPT locus with Parkinson’s disease. Eur J Neurol. 2010;17:483–486. doi: 10.1111/j.1468-1331.2009.02847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wider C, Vilariño-Güell C, Heckman MG, Jasinska-Myga B, Ortolaza-Soto AI, Diehl NN, Crook JE, Cobb SA, Bacon JA, Aasly JO, Gibson JM, Lynch T, Uitti RJ, Wszolek ZK, Farrer MJ, Ross OA. SNCA, MAPT, and GSK3B in Parkinson disease: a gene-gene interaction study. Eur J Neurol. 2011;18:876–881. doi: 10.1111/j.1468-1331.2010.03297.x. [DOI] [PubMed] [Google Scholar]
- Winkler S, Hagenah J, Lincoln S, Heckman M, Haugarvoll K, Lohmann-Hedrich K, Kostic V, Farrer M, Klein C. alpha-Synuclein and Parkinson disease susceptibility. Neurology. 2007;69:1745–1750. doi: 10.1212/01.wnl.0000275524.15125.f4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.