Abstract
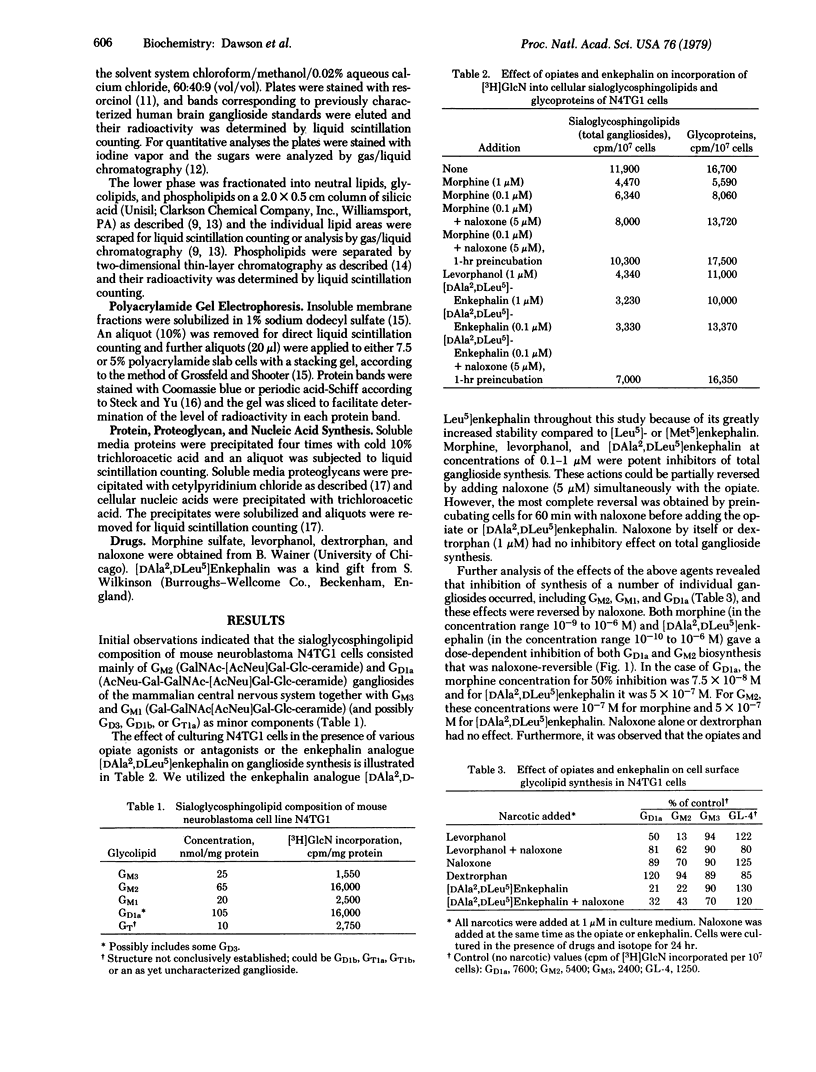
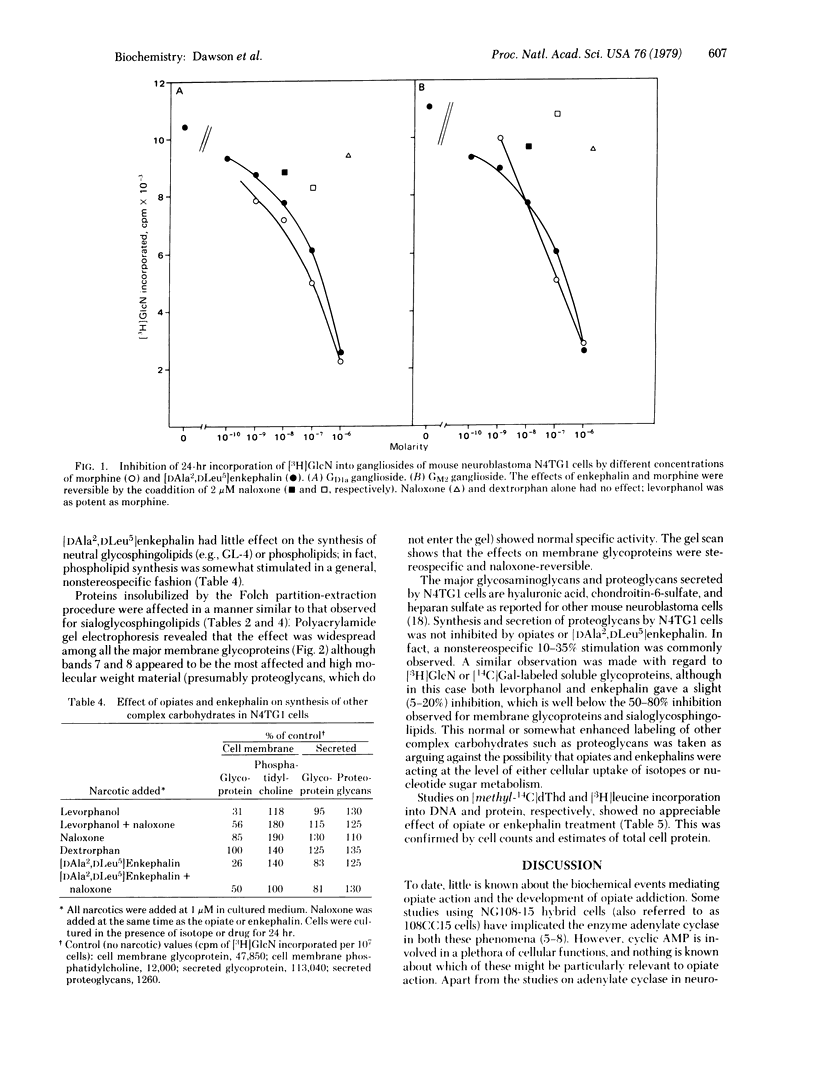
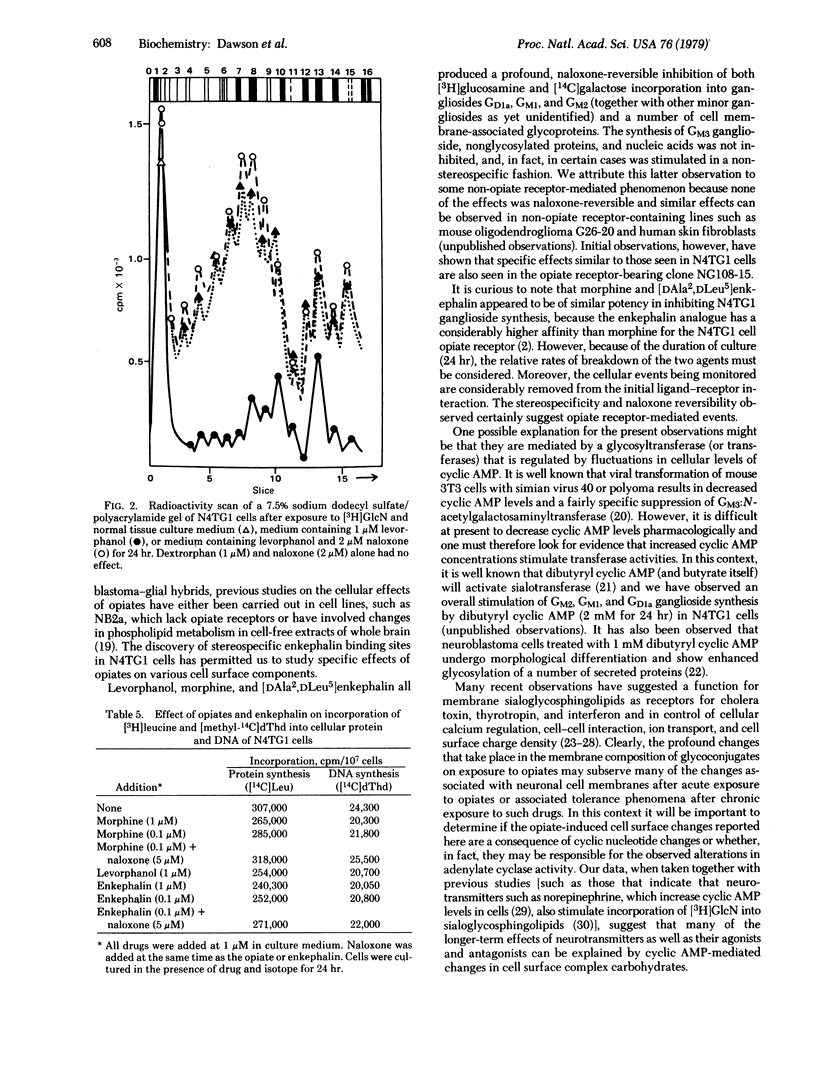
When mouse neuroblastoma clonal cell line N4TG1 cells were cultured in the presence of opiates or enkephalins, in the range 10(-6)-10(-10) M for 24 hr, a dose-dependent inhibition of the incorporation of [3H]glucosamine and [14C]-galactose into sialoglycosphingolipids and glycoproteins was observed. The gangliosides most affected comigrated in thinlayer chromatographic systems with GM2 (GalNAc[AcNeu]-Gal-Glc-ceramide), GM1 (Gal-GalNAc[AcNeu]Gal-Glc-ceramide), and GDla (AcNeu-Gal-GalNAc[AcNeu]Gal-Glc-ceramide). The effects were stereospecific and naloxone-reversible. Polyacrylamide gel electrophoresis revealed that the synthesis of a large number of membrane glycoproteins was also stereospecifically inhibited. Synthesis of other proteins and glycoproteins, proteoglycans, DNA, and membrane phospholipids and the rate of cell division were not altered in any specific or stereospecific manner. Moreover, clonal cell lines (neuroblastomas and oligodendroglioma) and human skin fibroblasts, which do not possess opiate receptors, did not respond to opiates or enkephalins in a stereospecific manner.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt M., Fischer K., Moroder L., Wunsch E., Hamprecht B. Enkephalin evokes biochemical correlates of opiate tolerance and dependence in neuroblastoma x glioma hybrid cells. FEBS Lett. 1976 Sep 15;68(1):38–40. doi: 10.1016/0014-5793(76)80399-7. [DOI] [PubMed] [Google Scholar]
- Cumar F. A., Brady R. O., Kolodny E. H., McFarland V. W., Mora P. T. Enzymatic block in the synthesis of gangliosides in DNA virus-transformed tumorigenic mouse cell lines. Proc Natl Acad Sci U S A. 1970 Oct;67(2):757–764. doi: 10.1073/pnas.67.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Kernes S. M., Miller R. J., Wainer B. Evidence for the noninvolvement of sulfogalactosylceramide (cerebroside sulfate) in the enkephalin (opiate) receptor. J Biol Chem. 1978 Nov 25;253(22):7999–8001. [PubMed] [Google Scholar]
- Dawson G., Matalon R., Dorfman A. Glycosphingolipids in cultured human skin fibroblasts. I. Characterization and metabolism in normal fibroblasts. J Biol Chem. 1972 Sep 25;247(18):5944–5950. [PubMed] [Google Scholar]
- Dawson G., Stoolmiller A. C. Comparison of the ganglioside composition of established mouse neuroblastoma cell strains grown in vivo and in tissue culture. J Neurochem. 1976 Jan;26(1):225–226. doi: 10.1111/j.1471-4159.1976.tb04466.x. [DOI] [PubMed] [Google Scholar]
- Dorfman A., Ho P. L. Synthesis of acid mucopolysaccharides by glial tumor cells in tissue culture. Proc Natl Acad Sci U S A. 1970 Jun;66(2):495–499. doi: 10.1073/pnas.66.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P. H., Bradley R. M., Henneberry R. C. Butyrate-induced glycolipid biosynthesis in HeLa cells: properties of the induced sialyltransferase. Arch Biochem Biophys. 1976 Feb;172(2):618–626. doi: 10.1016/0003-9861(76)90116-8. [DOI] [PubMed] [Google Scholar]
- Grossfeld R. M., Shooter E. M. A study of the changes in protein composition of mouse brain during ontogenetic development. J Neurochem. 1971 Dec;18(12):2265–2277. doi: 10.1111/j.1471-4159.1971.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Hamprecht B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma-glioma cell hybrids in cell culture. Int Rev Cytol. 1977;49:99–170. doi: 10.1016/s0074-7696(08)61948-8. [DOI] [PubMed] [Google Scholar]
- Hansson C. G., Karlsson K. A., Samuelsson B. E. The identification of sulphatides in human erythrocyte membrane and their relation to sodium-potassium dependent adenosine triphosphatase. J Biochem. 1978 Mar;83(3):813–819. doi: 10.1093/oxfordjournals.jbchem.a131977. [DOI] [PubMed] [Google Scholar]
- Klee W. A., Nirenberg M. A neuroblastoma times glioma hybrid cell line with morphine receptors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3474–3477. doi: 10.1073/pnas.71.9.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee W. A., Nirenberg M. Mode of action of endogenous opiate peptides. Nature. 1976 Oct 14;263(5578):609–612. doi: 10.1038/263609a0. [DOI] [PubMed] [Google Scholar]
- Miller R. J., Chang K. J., Cooper B., Cuatrecasas P. Radioimmunoassay and characterization of enkephalins in rat tissues. J Biol Chem. 1978 Jan 25;253(2):531–538. [PubMed] [Google Scholar]
- Minna J., Nelson P., Peacock J., Glazer D., Nirenberg M. Genes for neuronal properties expressed in neuroblastoma x L cell hybrids. Proc Natl Acad Sci U S A. 1971 Jan;68(1):234–239. doi: 10.1073/pnas.68.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Fishman P. H., Manganiello V. C., Vaughan M., Brady R. O. Functional incorporation of ganglioside into intact cells: induction of choleragen responsiveness. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1034–1037. doi: 10.1073/pnas.73.4.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin B. R., Pacuszka T., Lee G., Kohn L. D., Brady R. O., Fishman P. H. Thyroid gangliosides with high affinity for thyrotropin: potential role in thyroid regulation. Science. 1978 Jan 6;199(4324):77–79. doi: 10.1126/science.199.4324.77. [DOI] [PubMed] [Google Scholar]
- Natsuki R., Hitzemann R., Loh H. Effects of morphine on the incorporation of [14C]serine into phospholipid via the base-exchange reaction. Mol Pharmacol. 1978 May;14(3):448–453. [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Schubert D., Tarikas H., LaCorbiere M. Neurotransmitter regulation of adenosine 3',5'-monophosphate in clonal nerve, glia, and muscle cell lines. Science. 1976 Apr 30;192(4238):471–473. doi: 10.1126/science.176728. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Klee W. A., Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3092–3096. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- Truding R., Shelanski M. L., Morell P. Glycoproteins released into the culture medium of differentiating murine neuroblastoma cells. J Biol Chem. 1975 Dec 25;250(24):9348–9354. [PubMed] [Google Scholar]
- Vance D. E., Sweeley C. C. Quantitative determination of the neutral glycosyl ceramides in human blood. J Lipid Res. 1967 Nov;8(6):621–630. [PubMed] [Google Scholar]


