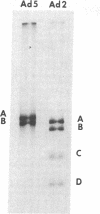
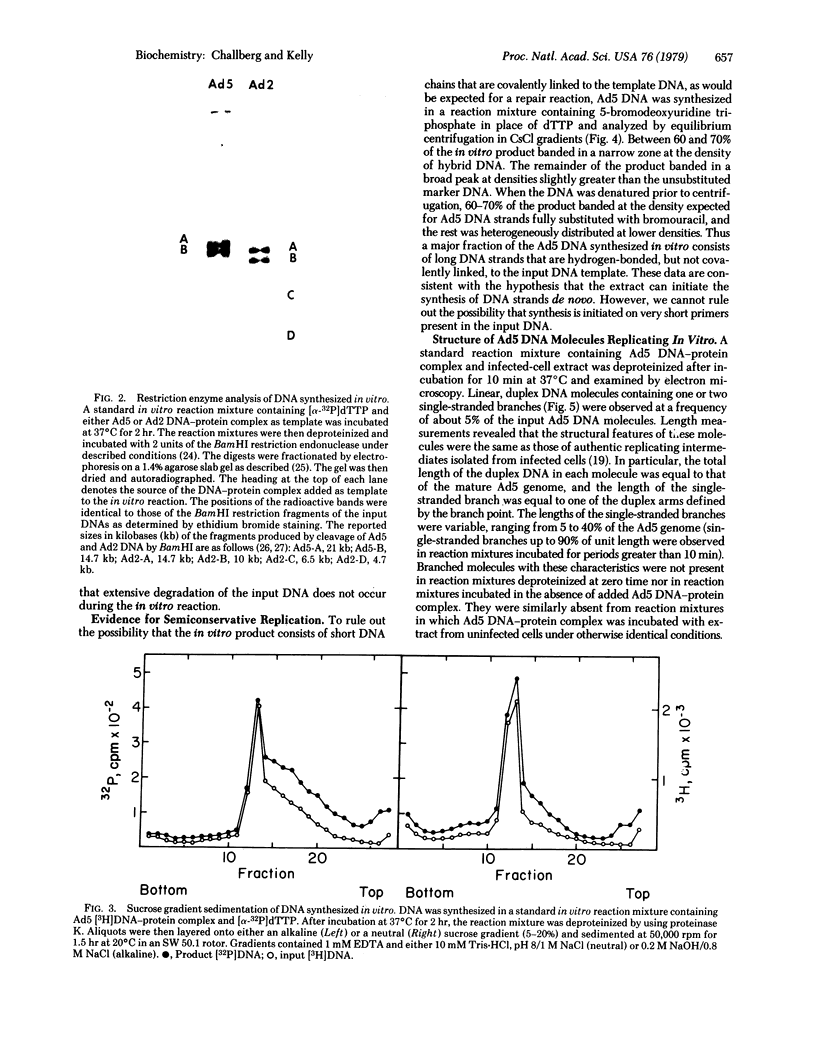
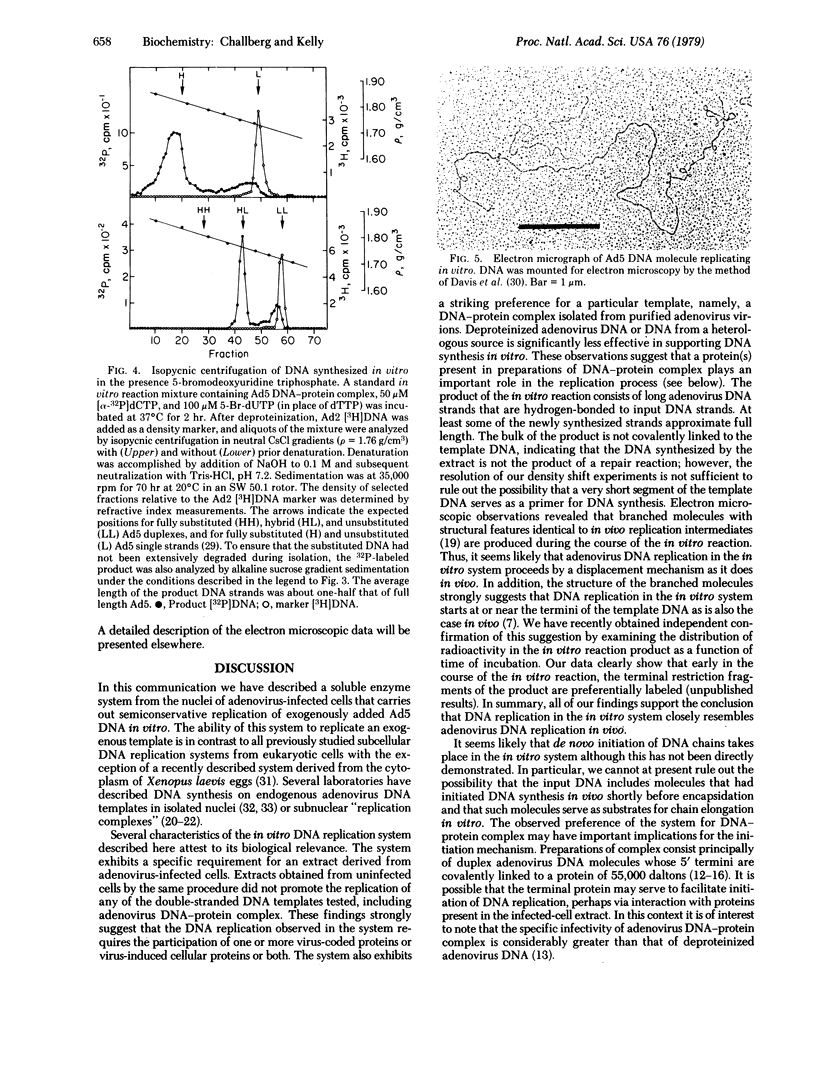
Abstract
A soluble extract from the nuclei of HeLa cells infected with adenovirus 5 (Ad5) carries out the semiconservative replication of exogenously added Ad5 DNA in vitro. Maximal DNA synthesis is observed when DNA-protein complex, isolated from Ad5 virions, is added as template. DNA-protein complex from virions of the closely related virus, adenovirus 2, is also active. In contrast, very little in vitro DNA synthesis is observed when deproteinized Ad5 DNA or DNA from a heterologous source (bacteriophage T7) is added as template. The product of the in vitro reaction consists of long Ad5 DNA strands that are hydrogen-bonded, but not covalently linked, to the input DNA template. During the course of the in vitro reaction, branched molecules with structural features identical to in vivo replication intermediates are formed. These findings support the conclusion that replication in the in vitro system closely resembles adenovirus DNA replication in vivo. The system provides an assay that should be useful for the purification and subsequent characterization of viral and cellular proteins involved in DNA replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALDWIN R. L., SHOOTER E. M. THE ALKALINE TRANSITION OF BU-CONTAINING DNA AND ITS BEARING ON THE REPLICATION OF DNA. J Mol Biol. 1963 Nov;7:511–526. doi: 10.1016/s0022-2836(63)80098-4. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Krauss M. R., Reeder R. H. DNA synthesis in a multi-enzyme system from Xenopus laevis eggs. Cell. 1978 Feb;13(2):307–318. doi: 10.1016/0092-8674(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Flint S. J., Williams J. F., Sharp P. A. Adenovirus transcription. IV. Synthesis of viral-specific RNA in human cells infected with temperature-sensitive mutants of adenovirus 5. J Virol. 1976 Sep;19(3):879–889. doi: 10.1128/jvi.19.3.879-889.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brison O., Kedinger C., Wilhelm J. Enzymatic properties of viral replication complexes isolated from adenovirus type 2-infected HeLa cell nuclei. J Virol. 1977 Nov;24(2):423–435. doi: 10.1128/jvi.24.2.423-435.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W., Nathans D. The isolation of simian virus 40 variants with specifically altered genomes. Proc Natl Acad Sci U S A. 1974 Mar;71(3):942–946. doi: 10.1073/pnas.71.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Beard P., Berg P. Synthesis of Superhelical Simian Virus 40 Deoxyribonucleic Acid in Cell Lysates*. J Biol Chem. 1975 Jun 10;250(11):4340–4347. [PubMed] [Google Scholar]
- Flint J. The topography and transcription of the adenovirus genome. Cell. 1977 Feb;10(2):153–166. doi: 10.1016/0092-8674(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Hierholzer J. C., Rose J. A. Mapping of base sequence heterologies between genomes from different adenovirus serotypes. Virology. 1973 Aug;54(2):414–426. doi: 10.1016/0042-6822(73)90153-0. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. A unique form of terminal redundancy in adenovirus DNA molecules. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2391–2395. doi: 10.1073/pnas.69.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L. DNA replication. Annu Rev Biochem. 1975;44:45–78. doi: 10.1146/annurev.bi.44.070175.000401. [DOI] [PubMed] [Google Scholar]
- Geider K. Molecular aspects of DNA replication in Escherichia coli systems. Curr Top Microbiol Immunol. 1976;74:55–112. doi: 10.1007/978-3-642-66336-9_3. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. M., Kelinman R. E., Horwitz M. S. Replication of adenovirus type 2 DNA in vitro. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4425–4429. doi: 10.1073/pnas.74.10.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lechner R. L., Kelly T. J., Jr The structure of replicating adenovirus 2 DNA molecules. Cell. 1977 Dec;12(4):1007–1020. doi: 10.1016/0092-8674(77)90165-9. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R., Padmanabhan R. V. Specific interaction of a protein(s) at or near the termini of adenovirus 2 DNA. Biochem Biophys Res Commun. 1977 Apr 25;75(4):955–964. doi: 10.1016/0006-291x(77)91475-9. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Williams J., Sharp P. A., Grodzicker T. Physical mapping of temperature-sensitive mutations of adenoviruses. J Mol Biol. 1975 Sep 25;97(3):369–390. doi: 10.1016/s0022-2836(75)80046-5. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Maat J., van Ormondt H., Sussenbach J. S. The nucleotide sequence at the termini of adenovirus type 5 DNA. Nucleic Acids Res. 1977 Dec;4(12):4371–4389. doi: 10.1093/nar/4.12.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C. Studies on the mechanism of replication of adenovirus DNA. I. The effect of hydroxyurea. Virology. 1973 Jul;54(1):299–303. doi: 10.1016/0042-6822(73)90142-6. [DOI] [PubMed] [Google Scholar]
- Wickner S. H. DNA replication proteins of Escherichia coli. Annu Rev Biochem. 1978;47:1163–1191. doi: 10.1146/annurev.bi.47.070178.005503. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L. Adenovirus DNA: structure and function of a novel replicon. Cell. 1978 Aug;14(4):761–773. doi: 10.1016/0092-8674(78)90332-x. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Replication of polyoma DNA in isolated nuclei. I. Characterization of the system from mouse fibroblast 3T6 cells. J Mol Biol. 1972 Dec 30;72(3):523–537. doi: 10.1016/0022-2836(72)90172-6. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Adenovirus-2 DNA contains an inverted terminal repetition. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3054–3057. doi: 10.1073/pnas.69.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Arens M., Green M. Adenovirus deoxyribonucleic acid replication. Isolation of a soluble replication system and analysis of the in vitro DNA product. J Biol Chem. 1977 Nov 25;252(22):7940–7946. [PubMed] [Google Scholar]
- van der Vliet P. C., Sussenbach J. S. The mechanism of adenovirus-DNA synthesis in isolated nuclei. Eur J Biochem. 1972 Nov 7;30(3):584–592. doi: 10.1111/j.1432-1033.1972.tb02130.x. [DOI] [PubMed] [Google Scholar]