Abstract
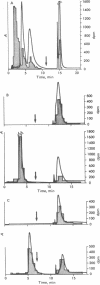
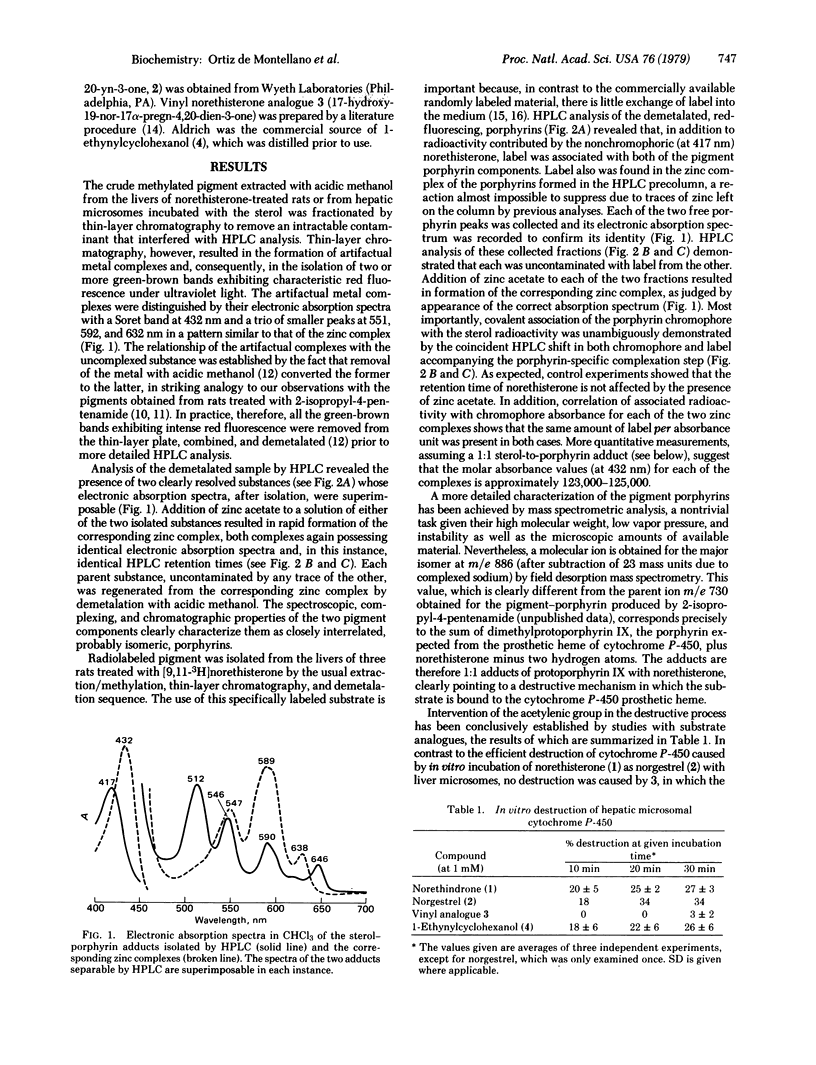
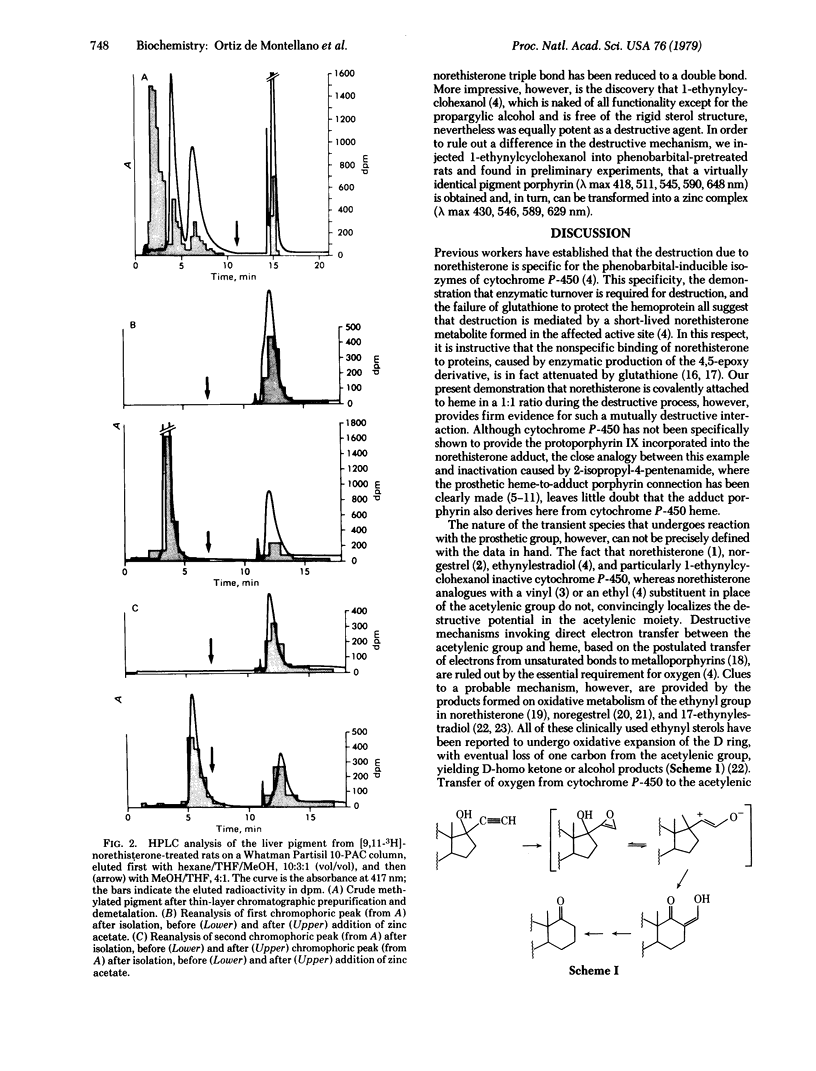
The hepatic pigment accumulated as a consequence of the self-catalyzed destruction of cytochrome P-450 by norethisterone (17-hydroxy-19-nor-17 alpha-pregn-4-en-20-yn-3-one), after acidic methylation and purification, consists of two virtually identical, probably isomeric, porphyrins. Radiolabeled norethisterone is incorporated into both porphyrin products. The major of the two porphyrins exhibits a mass spectrometric molecular ion exactly equivalent to the sum of norethisterone and dimethylprotoporphyrin IX, less two hydrogen atoms: unequivocably demonstrating covalent association of the sterol with this porphyrin in a 1:1 ratio. Cytochrome P-450 is therefore destroyed by self-catalyzed addition of norethisterone to its heme prosthetic group. Cytochrome P-450 is also destroyed by norgestrel (13-ethyl-17-hydroxyl-18, 19-dinor-17 alpha-pregn-4-en-20-yn-3-one) and by 1-ethynylcyclohexanol but not by 17-hydroxy-19-nor-17alpha-pregn-4,20-dien-3-one. The destructive potential is thus clearly a property of the propargylic alcohol function. A mechanism involving enzymatic oxidation of the triple bond is postulated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Aziz M. T., Williams K. I. Metabolism of 17-alpha-ethynylestradiol and its 3-methyl ether by the rabbit; an in vivo D-homoannulation. Steroids. 1969 Jun;13(6):809–820. doi: 10.1016/0039-128x(69)90076-2. [DOI] [PubMed] [Google Scholar]
- De Matteis F. Drug-induced destruction of cytochrome P-450. Drug Metab Dispos. 1973 Jan-Feb;1(1):267–274. [PubMed] [Google Scholar]
- De Matteis F., Unseld A. Increased liver haem degradation caused by foreign chemicals: a comparison of the effects of 2-allyl-2-isopropylacetamide and cobaltous chloride. Biochem Soc Trans. 1976;4(2):205–209. doi: 10.1042/bst0040205. [DOI] [PubMed] [Google Scholar]
- Freudenthal R. I., Amerson E., Martin J., Wall M. E. The effectof norethynodrel, norethindrone and ethynodiol diacetate on hepatic microsomal drug metabolism. Pharmacol Res Commun. 1974 Oct;6(5):457–468. doi: 10.1016/s0031-6989(74)80055-x. [DOI] [PubMed] [Google Scholar]
- Hanasono G. K., Fischer L. J. The excretion of tritium-labeled chlormadinone acetate, mestranol, norethindrone, and norethynodrel in rats and the enterohepatic circulation of metabolites. Drug Metab Dispos. 1974 Mar-Apr;2(2):159–168. [PubMed] [Google Scholar]
- Kappus H., Bolt H. M. Irreversible protein binding of norethisterone (norethindrone) epoxide. Steroids. 1976 Jan;27(1):29–45. doi: 10.1016/0039-128x(76)90067-2. [DOI] [PubMed] [Google Scholar]
- Kappus H., Remmer H. Metabolic activation of norethisterone (norethindrone) to an irreversibly protein-bound derivative by rat liver microsomes. Drug Metab Dispos. 1975 Sep-Oct;3(5):338–344. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin W., Jacobson M., Sernatinger E., Kuntzman R. Breakdown of cytochrome P-450 heme by secobarbital and other allyl-containing barbiturates. Drug Metab Dispos. 1973 Jan-Feb;1(1):275–285. [PubMed] [Google Scholar]
- McDonagh A. F., Pospisil R., Meyer U. A. Degradation of hepatic haem to porphyrins and oxophlorins in rats treated with 2-allyl-2-isopropylacetamide. Biochem Soc Trans. 1976;4(2):297–298. doi: 10.1042/bst0040297. [DOI] [PubMed] [Google Scholar]
- Onken D., Heublein D. Athinierte Steroide. Pharmazie. 1970 Jan 1;25(1):3–9. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Mico B. A., Yost G. S. Suicidal inactivation of cytochrome P-450. Formation of a heme-substrate covalent adduct. Biochem Biophys Res Commun. 1978 Jul 14;83(1):132–137. doi: 10.1016/0006-291x(78)90407-2. [DOI] [PubMed] [Google Scholar]
- Palmer K. H., Feierabend J. F., Baggett B., Wall M. E. Metabolic removal of a 17-alpha-ethynyl group from the antifertility steroid, norethindrone. J Pharmacol Exp Ther. 1969 Jun;167(2):217–222. [PubMed] [Google Scholar]
- Sisenwine S. F., Kimmel H. B., Liu A. L., Ruelius H. W. Stereoselective biotransformations of dl-norgestrel and its enantiomers in the African green monkey. Drug Metab Dispos. 1974 Jan-Feb;2(1):65–70. [PubMed] [Google Scholar]
- Sisenwine S. P., Kimmel H. B., Liu A. L., Ruelius H. W. Urinary metabolites of DL-norgestrel in women. Acta Endocrinol (Copenh) 1973 May;73(1):91–104. doi: 10.1530/acta.0.0730091. [DOI] [PubMed] [Google Scholar]
- White I. N., Muller-Eberhard U. Decreased liver cytochrome P-450 in rats caused by norethindrone or ethynyloestradiol. Biochem J. 1977 Jul 15;166(1):57–64. doi: 10.1042/bj1660057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Williams K. I. Metabolism of 2-3H- and 4-14C-17alpha-ethynylestradiol 3-methyl ether (mestranol) by women. Steroids. 1975 Dec;26(6):707–720. doi: 10.1016/0039-128x(75)90104-x. [DOI] [PubMed] [Google Scholar]