Abstract
The flagellar motor of Escherichia coli has been shown to adapt to changes in the steady-state level of the chemotaxis response regulator, CheY-P, by adjusting the number of molecules to which CheY-P binds, FliM. Previous measurements of motor ultrasensitivity have been made on cells containing different amounts of CheY-P, and thus different amounts of FliM. Here, we designed an experiment to measure the sensitivity of motors containing fixed amounts of FliM, finding Hill coefficients about twice as large as those observed before. This ultrasensitivity provides further insights into the motor switching mechanism and plays important roles in chemotaxis signal amplification and coordination of multiple motors. The Hill coefficients observed here appear to be the highest known for allosteric protein complexes, either biological or synthetic. Extreme motor ultrasensitivity broadens our understanding of mechanisms of allostery, and serves as an inspiration for future design of synthetic protein switches.
Keywords: cooperativity, allostery, molecular motor, switch, chemotaxis
Introduction
Cells of Escherichia coli are propelled by several helical filaments, each driven at its base by a reversible rotary motor. When all the motors of a cell rotate counterclockwise (CCW), the filaments form a bundle and the cell swims smoothly. When one or more motors rotate clockwise (CW), their filaments come out of the bundle and the cell changes course 1. The cell performs chemotaxis in a biased random walk 2, by modulating the direction of flagellar rotation 3; 4. Chemoreceptors in the cell membrane sense changes in the concentrations of environmental chemical attractants or repellents, regulating the activity of a kinase that phosphorylates a response regulator, CheY 5; 6. CheY-P binds to a component of the switch complex at the base of the flagellar motor, FliM, increasing the fraction of time that the motor spins CW (raising the CW bias) 7; 8.
The motor is very sensitive to the concentration of CheY-P, [CheY-P]. This sensitivity is commonly characterized by a Hill coefficient, n, obtained by fitting the motor CW bias vs. [CheY-P] relationship with the Hill function 1/(1+(K1/2/[CheY-P])n)), where K1/2 is the concentration at which CW bias is 0.5. Measurements in bacterial populations reported values of the Hill coefficient ranging from 3.5 to 5.5 9; 10. Measurements with single cells removed complications due to averaging over cell populations and reported a value of the Hill coefficient of 10.3 11. Recently, the flagellar motor was found to partially adapt to variations in the concentration of CheY-P by changing the number of FliM subunits in its switch complex 12. Therefore, the measurements made with single cells were actually measurements of cells that had adapted to different levels of CheY-P, representing an average over motors with different complements of FliM. To learn the actual response of the motor, the input-output relationship should be measured for motors with a fixed number of FliM subunits.
Results
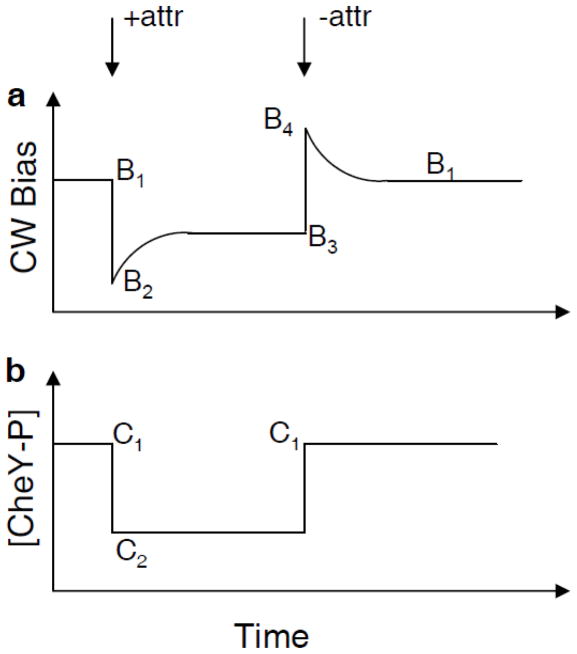
Here, we designed an experiment that used a bead assay to accomplish this task, Fig. 1. Panel a shows the CW bias and panel b the intracellular CheY-P concentration, [CheY-P], both as a function of time. The bias was measured by the bead assay, while the CheY-P concentration was inferred from the bias measurement. We used a cheR cheB strain, so that when the attractant was added, the intracellular CheY-P concentration changed from C1 to C2 and remained constant 12; 13. In contrast, the CW bias changed from B1 to B2 and slowly adapted to B3 12. When the attractant was removed, the intracellular CheY-P concentration changed from C2 back to the pre-stimulus value C1 13, while the CW bias changed from B3 to B4 and slowly adapted back to the pre-stimulus value B1. Since the motor was adapted before attractant addition and removal, C1 and C2 can be extracted from B1 and B3 using the relationship measured by Cluzel et al. 11, which is a relationship for adapted motors. At the instant of attractant addition, the motor has yet to adapt, so (C1, B1) and (C2, B2) correspond to the input-output of a motor with a fixed number of FliM subunits, a motor with an adapted CW bias B1. Similarly, at the instant of attractant removal, (C2, B3) and (C1, B4) correspond to the input-output of a motor with a fixed number of FliM subunits, a motor with an adapted CW bias B3. We performed single-motor measurements on a population of cells with various pre-stimulus biases, B1, and various responses to attractant addition and removal, B2, B3, and B4. Since there is a one-to-one relationship between the number of FliM subunits in a motor and the adapted CW bias 12, we can group the data points according to the adapted CW bias (B1 and B3) and obtain a full input-output relationship.
Fig. 1.
Schematic of the response of cheR cheB cells to step addition and removal of a non-metabolizable attractant. (a) Motor CW bias as a function of time. (b) Intracellular CheY-P concentration as a function of time.
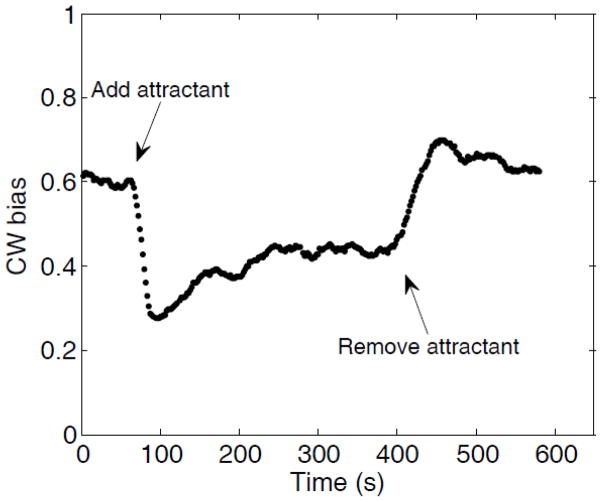
An example of the CW bias as a function of time using the bead assay is shown in Fig. 2, which shows the averaged responses of nine motors on different cells to stepwise addition and removal of 0.5 mM MeAsp, added near 70 s and removed near 400 s.
Fig. 2.
Motor response of 9 cheR cheB cells to stepwise addition and removal of chemical attractant (0.5 mM MeAsp), monitored by the bead assay. The attractant was added and removed at the times indicated by the arrows.
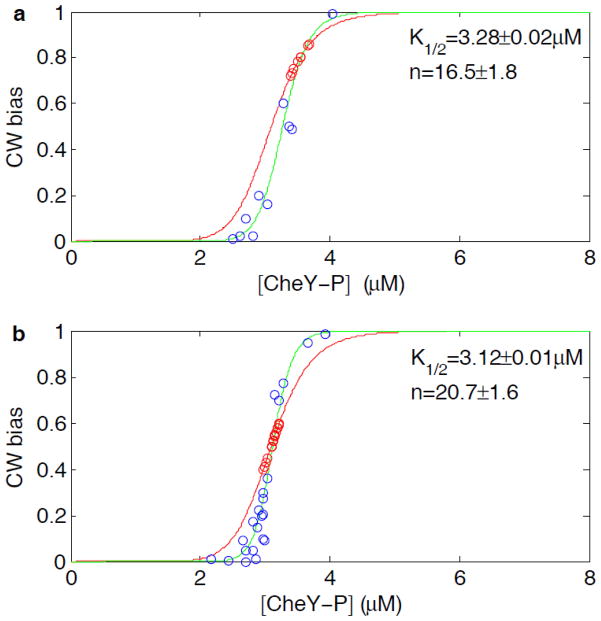
Two measured input-output relationships are shown in Fig. 3, corresponding to motors with an adapted CW bias 0.8 ± 0.1 (panel a) and 0.5 ± 0.1 (panel b). In each figure, the red curve shows the Hill function obtained by Cluzel et al. 11 with Hill coefficient 10.3. Data points below the red curve were obtained from attractant addition, while data points above the red curve were obtained from attractant removal. Data points on the red curve, shown with red symbols, were the initial values for the adapted motors. The fits to a Hill function are shown by the blue curves, yielding a Hill coefficient 16.5 ± 1.8 and dissociation constant 3.28 ± 0.02 μM for motors with an adapted bias of 0.8 (panel a), and a Hill coefficient 20.7 ± 1.6 and dissociation constant of 3.12 ± 0.01 μM for motors with an adapted bias of 0.5 (panel b). The sensitivities (Hill coefficients) are about twice that of the previous measurement made with single cells. In the Monod-Wyman-Changeux (MWC) model of the motor 10; 14, the sensitivity (Hill coefficients) increases with the number of FliM units N, while K1/2 decreases with N 15. Our results thus suggest that the number of FliM units in motors with an adapted bias of 0.5 is larger than that in motor with an adapted bias of 0.8, consistent with the previous study of motor adaptation 12.
Fig. 3.
True input-output relationships of the motor. Blue and red circles are measurements in this study, and green curves are the corresponding fits with the Hill function. Red curves are the the Hill function fit to the data of Cluzel et al. 11. (a) Input-output relationship for motors with an adapted CW bias of 0.8 ± 0.1. (b) Input-output relationship for motors with an adapted CW bias of 0.5 ± 0.1.
In the previous study 12, we used the curve measured by Cluzel et al. as the relationship for pre-adapted motors with a specific number of FliM units, and based on this, we constructed a new relationship for the motor with a larger number of FliM units. According to the present study, the curve for the pre-adapted motor with a specific number of FliM units is actually about a factor of two steeper than what Cluzel et al. measured.
In the conformational spread model of the motor switch 16, this ultrasensitivity (a sensitivity larger than 16) can be explained by the large number of protomers (N ~ 34), a high bi-stability of the protomer structure (with the energy difference between CW and CCW states of each protomer, Ea, larger than 1 kT), and a strong coupling between the protomers (with the coupling energy between neighboring protomers, EJ, larger than 4 kT); see Supplementary Materials. The ultrasensitivity observed here is even more impressive considering the fact that the motor keeps remodeling itself by varying the number of FliM units. How does the motor switch maintain such variability while exhibiting a high stability with strong coupling between protomers? The FliG proteins, another component of the motor switch, might confer this high stability while turn-over of the FliM proteins provides the variability. This is in line with a recent discovery showing non-exchange of FliG proteins in the motor for hours 17, while turn-over of FliM proteins happens in tens of seconds 18.
The conformational spread model predicts that a motor exhibiting a sensitivity of 16 would show a CheY-P binding cooperativity of about 9 (see Supplemental Materials). The highest binding cooperativity reported thus far is less than 2 8. A more careful measurement of the CheY-P binding cooperativity is needed. If higher binding cooperativities are not found, one might have to consider non-equilibrium models for switching, such as the one proposed by Tu 19.
Discussion
Sensitivity of the motor contributes to signal amplification in chemotaxis. The ultrasensitivity measured in this study shows that it plays a more important role than previously thought. Moreover, ultrasensitivity of the motor is critical for coordination of multiple motors in a cell by fluctuations or bursts in the intracellular concentration of CheY-P. The rotational bias between adjacent motors was observed to be correlated over a time scale of about 10 s 20. This was attributed to slow fluctuations in the steady state [CheY-P] due to fluctuations in the receptor adaptation kinetics, and was found to enhance the sensitivity of bacterium to very shallow gradients of attractants 21. This steady-state [CheY-P] fluctuation remains to be shown directly. Ultrasensitivity of the motor makes the bias correlation possible even with [CheY-P] fluctuations of small amplitude. Recently, coordinated switching between adjacent motors was documented on a subsecond timescale, and was postulated to arise from a burst of CheY-P propagating from the cell pole that drives the motor bias from 0% to 100% CW or vice versa 22. Such CheY-P bursts also remain to be shown directly. High motor ultrasensitivity alleviates the requirement of large bursts. Therefore, previous studies of the motor bias correlation 20; 21; 22 and previous proposals for the motor coordination mechanism 22 should be revisited using our new values for motor sensitivity.
The ultrasensitivity (high switching cooperativity) observed here (Hill coefficient n ~21) is easily the highest found among allosteric protein complexes, e.g., hemoglobin (n ~ 3) 23, aspartate carbamoyltransferase (n < 3) 24, cytochrome P450 (n < 4) 25, the oligomeric chaperon GroEL (n ~ 3) 26, ion channels (n ≤ 3) 27, and synthetic protein switches (n ≤ 4) 28. We expect this ultrasensitivity will provide further insights into mechanisms of allostery and inspire future design of synthetic protein switches.
Materials and Methods
Strain JY35 [cheR cheB fliC] is a derivative of E. coli K12 strain RP437 29. The plasmid pKAF131 carrying the sticky fliC allele under control of the native fliC promoter 30 was transformed into JY35, yielding the strain used for this study. The bead assay was described previously 12; 31. Briefly, cells were grown at 33 °C in T-broth to an OD600 between 0.45 and 0.50, washed twice with motility buffer (10 mM potassium phosphate, 0.1 mM EDTA, 1 μM methionine, 10 mM lactic acid, pH 7.0), sheared to truncate flagella, and concentrated by a factor of 2. The sheared cells were immobilized on a glass coverslip coated with poly-L-lysine (0.01%, P4707, Sigma, St. Louis, MO) and 1.0-μm-diameter polystyrene latex beads (2.69%, 07310, Polysciences, Warrington, PA) were attached to the truncated flagella. The coverslip was installed as the top window of a flow chamber 32, and a constant flow of buffer (400 μl/min) was maintained by a syringe pump (Pump-22, Harvard Apparatus, Holliston, MA). The attractant used in this study was 0.5 mM α-methyl-D,L-aspartate (MeAsp) in motility buffer. Rotation of the bead was monitored with a laser dark-field setup described previously 33. For each experiment, the bead was monitored for ~70 s in motility buffer, for ~330 s in the attractant solution, and again for about ~200 s in motility buffer. Data were analyzed using custom scripts in Matlab, and curves were fit with the nonlinear least square method in Matlab.
Supplementary Material
Acknowledgments
We thank Richard Branch for helpful comments. This work was supported by National Institutes of Health Grant AI016478.
References
- 1.Turner L, Ryu W, Berg HC. Real-time imaging of fluorescent flagellar filaments. J Bacteriol. 2000;182:2793–2801. doi: 10.1128/jb.182.10.2793-2801.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg HC, Brown DA. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972;239:500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- 3.Berg HC, Anderson RA. Bacteria swim by rotating their flagellar filaments. Nature. 1973;245:380–384. doi: 10.1038/245380a0. [DOI] [PubMed] [Google Scholar]
- 4.Larsen SH, Reader RW, Kort EN, Tso W, Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974;249:74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- 5.Hazelbauer GL, Falke JJ, Parkinson JS. Baterial chemoreceptors: high -performance signaling in networked arrays. Trends Biochem Sci. 2008;33:9–19. doi: 10.1016/j.tibs.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sourjik V. Receptor clustering and signal processing in E. coli chemotaxis. Trends Microbiol. 2004;12:569–576. doi: 10.1016/j.tim.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Welch M, Oosawa K, Aizawa S, Eisenbach M. Phosphorylation-dependent binding of a signal molecule to the flagellar switch of bacteria. Proc Natl Acad Sci USA. 1993;90:8787–8791. doi: 10.1073/pnas.90.19.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sourjik V, Berg HC. Binding of the Escherichia coli response regulator CheY to its target measured in vivo by fluorescence resonance energy transfer. Proc Natl Acad Sci USA. 2002;99:12669–12674. doi: 10.1073/pnas.192463199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scharf BE, Fahrner KA, Turner L, Berg HC. Control of direction of flagellar rotation in bacterial chemotaxis. Proc Natl Acad Sci USA. 1998;95:201–206. doi: 10.1073/pnas.95.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alon U, Camarena L, Surette MG, Aguera y Arcas B, Liu Y, Leibler S, Stock JB. Response regulator output in bacterial chemotaxis. EMBO J. 1998;17:4238–4248. doi: 10.1093/emboj/17.15.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cluzel P, Surette M, Leibler S. An ultrasensitive bacterial motor revealed by monitoring signaling proteins in single cells. Science. 2000;287:1652–1655. doi: 10.1126/science.287.5458.1652. [DOI] [PubMed] [Google Scholar]
- 12.Yuan J, Branch RW, Hosu BG, Berg HC. Adaptation at the output of the chemotaxis signalling pathway. Nature. 2012;484:233–236. doi: 10.1038/nature10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sourjik V, Berg HC. Receptor sensitivity in bacterial chemotaxis. Proc Natl Acad Sci USA. 2002;99:123–127. doi: 10.1073/pnas.011589998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 15.Sourjik V, Berg HC. Functional interactions between receptors in bacterial chemotaxis. Nature. 2004;428:437–441. doi: 10.1038/nature02406. [DOI] [PubMed] [Google Scholar]
- 16.Duke TAJ, Le Novere N, Bray D. Conformational spread in a ring of proteins: a stochastic approach to allostery. J Mol Biol. 2001;308:541–553. doi: 10.1006/jmbi.2001.4610. [DOI] [PubMed] [Google Scholar]
- 17.Fukuoka H, Inoue Y, Terasawa S, Takahashi H, Ishijima A. Exchange of rotor components in functioning bacterial flagellar motor. Biochem Biophys Res Commun. 2010;394:130–135. doi: 10.1016/j.bbrc.2010.02.129. [DOI] [PubMed] [Google Scholar]
- 18.Delalez NJ, Wadhams GH, Rosser G, Xue Q, Brown MT, Dobbie IM, Berry RM, Leake MC, Armitage JP. Signal-dependent turnover of the bacterial flagellar switch protein FliM. Proc Natl Acad Sci USA. 2010;107:11347–11351. doi: 10.1073/pnas.1000284107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu Y. The nonequilibrium mechanism for ultrasensitivity in a biological switch: Sensing by Maxwell’s demons. Proc Natl Acad Sci USA. 2008;105:11737–11741. doi: 10.1073/pnas.0804641105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishihara A, Segall JE, Block SM, Berg HC. Coordination of flagella on filamentous cells of Escherichia coli. J Bacteriol. 1983;155:228–237. doi: 10.1128/jb.155.1.228-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sneddon MW, Pontius W, Emonet T. Stochastic coordination of multiple actuators reduces latency and improves chemotactic response in bacteria. Proc Natl Acad Sci USA. 2012;109:805–810. doi: 10.1073/pnas.1113706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terasawa S, Fukuoka H, Inoue Y, Sagawa T, Takahashi H, Ishijima A. Coordinated reversal of flagellar motors on a single Escherichia coli cell. Biophys J. 2011;100:2193–2200. doi: 10.1016/j.bpj.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyman J. Allosteric effects in Hemoglobin. Cold Spring Harb Sysmp Quant Biol. 1963;28:483–489. [Google Scholar]
- 24.Eisensteini E, Markby DW, Schachman HK. Changes in stability and allosteric properties of aspartate transcarbamoylase resulting from amino acid substitutions in the zinc-binding domain of the regulatory chains. Proc Natl Acad Sci USA. 1989;86:3094–3098. doi: 10.1073/pnas.86.9.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueng Y, Kuwabara T, Chun Y, Guengerich FP. Cooperativity in oxidations catalyzed by cytochrome P450 3A4. Biochemistry. 1997;36:370–381. doi: 10.1021/bi962359z. [DOI] [PubMed] [Google Scholar]
- 26.Gray TE, Fersht AR. Cooperativity in ATP hydrolysis by GroEL is increased by GroES. FEBS Lett. 1991;292:254–258. doi: 10.1016/0014-5793(91)80878-7. [DOI] [PubMed] [Google Scholar]
- 27.Liman ER, Buck LB. A second subunit of the olfactory cyclic nucleotide-gated channel confers high sensitivity to cAMP. Neuron. 1994;13:611–621. doi: 10.1016/0896-6273(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 28.Dueber JE, Mirsky EA, Lim WA. Engineering synthetic signaling proteins with ultrasensitive input/output control. Nature biotechnol. 2007;25:660–662. doi: 10.1038/nbt1308. [DOI] [PubMed] [Google Scholar]
- 29.Parkinson JS. Complementation analysis and deletion mapping of Escherichia coli mutants defective in chemotaxis. J Bacteriol. 1978;135:45–53. doi: 10.1128/jb.135.1.45-53.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan J, Fahrner KA, Turner L, Berg HC. Asymmetry in the clockwise and counter-clockwise rotation of the bacterial flagellar motor. Proc Natl Acad Sci USA. 2010;107:12846–12949. doi: 10.1073/pnas.1007333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan J, Berg HC. Resurrection of the flagellar motor near zero load. Proc Natl Acad Sci USA. 2008;105:1182–1185. doi: 10.1073/pnas.0711539105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg HC, Block SM. A miniature flow cell designed for rapid exchange of media under high-power microscope objectives. J Gen Microbiol. 1984;130:2915–2920. doi: 10.1099/00221287-130-11-2915. [DOI] [PubMed] [Google Scholar]
- 33.Yuan J, Fahrner KA, Berg HC. Switching of the bacterial flagellar motor near zero load. J Mol Biol. 2009;390:394–400. doi: 10.1016/j.jmb.2009.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.