Abstract
The weaver mutation in mice results in a severe ataxia that is attributable to the degeneration of cerebellar granule cells and dopaminergic neurons in the substantia nigra. Recent genetic studies indicate that the GIRK2 gene is altered in weaver. This gene codes for a G-protein-activated, inwardly rectifying K+ channel protein (8). The mutation results in a single amino acid substitution (glycine-->serine) in the pore-forming H5 region of the channel. The functional consequences of this mutation appear to depend upon the co-expression of other GIRK subunits--leading to either a gain or loss of function. Here, we show that G-protein-activated inwardly rectifying K+ currents are significantly reduced in cerebellar granule cells from animals carrying the mutant allele. The reduction is most pronounced in homozygous neurons. These findings suggest that the death of neurons in weaver is attributable to the loss of GIRK2-mediated currents, not to the expression of a nonspecific cation current.
Full text
PDF
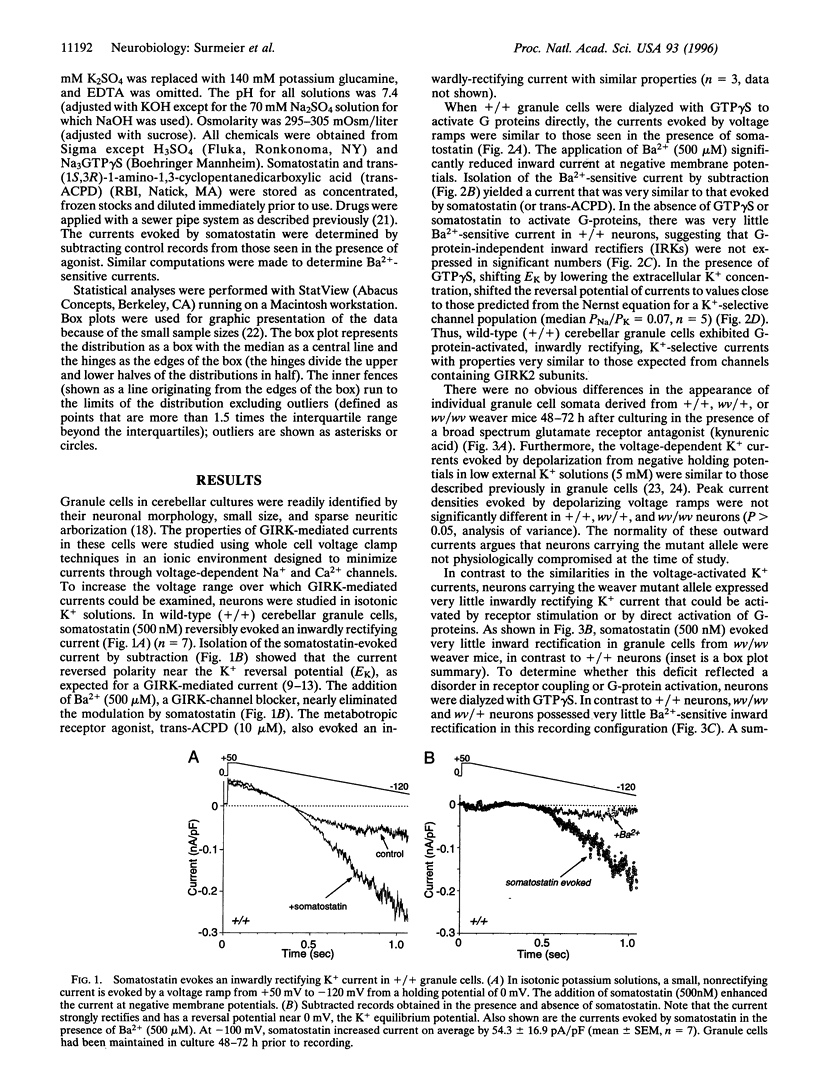
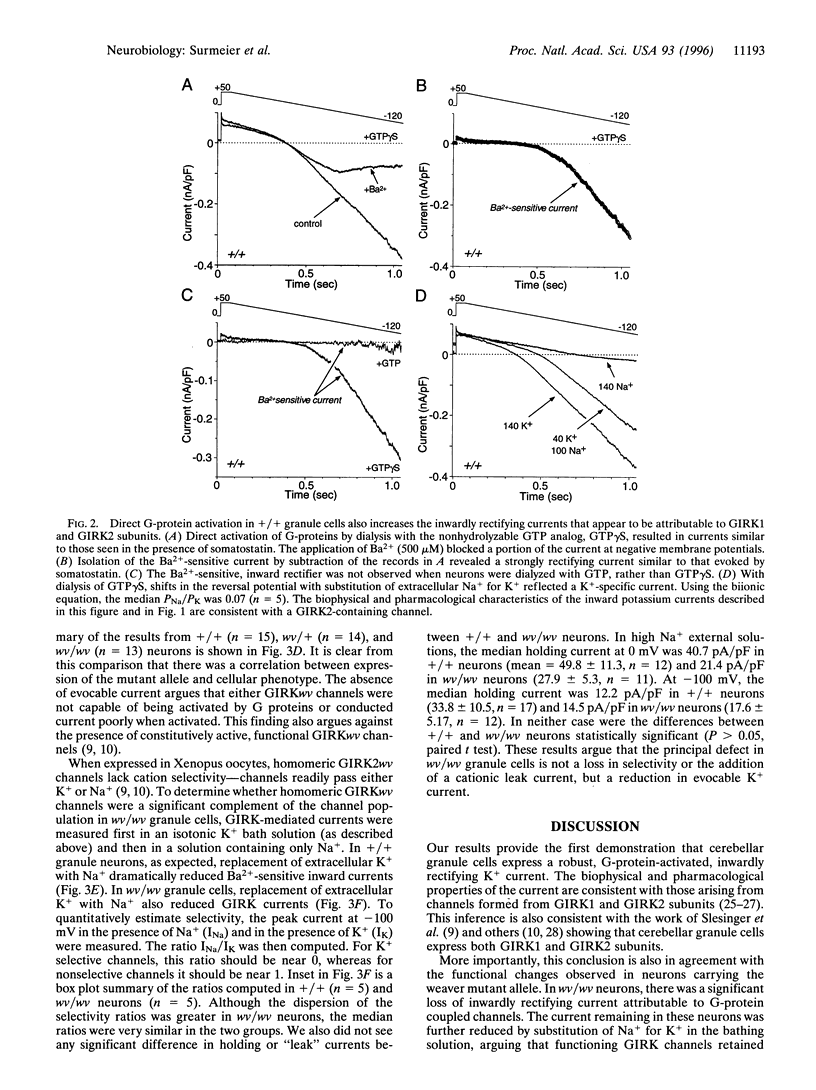
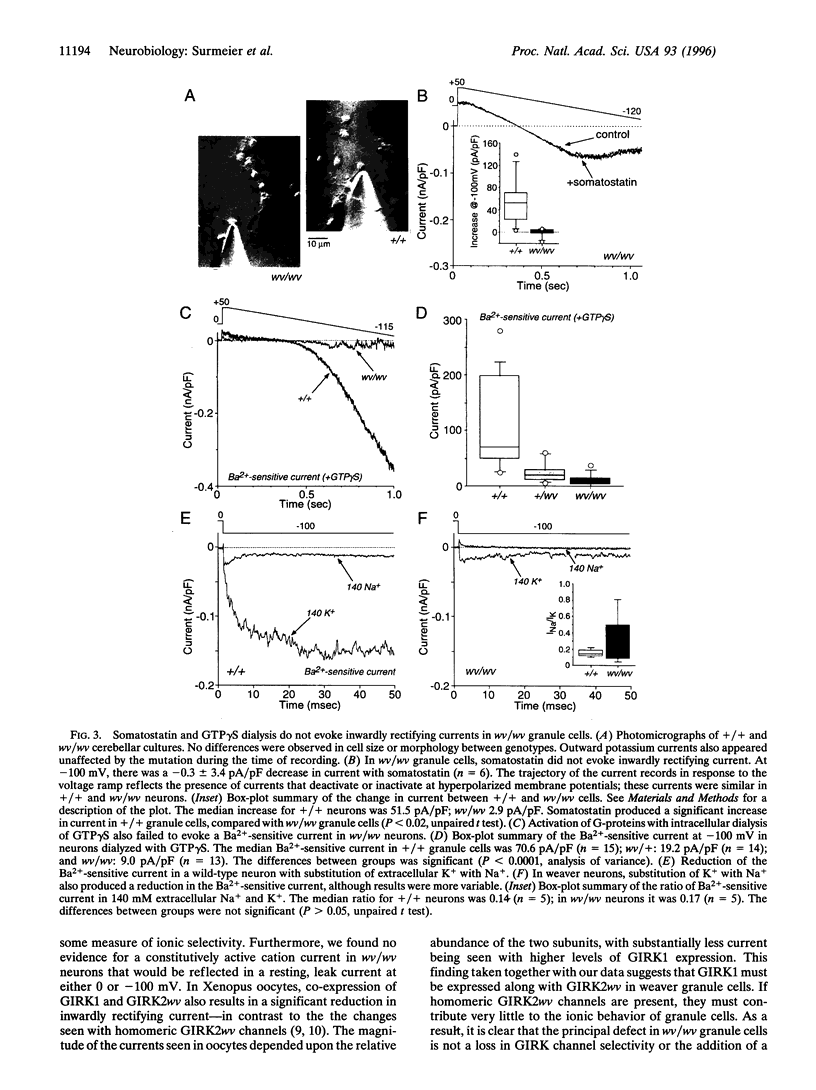

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkley D. S., Rakic L. L., Chaffee J. K., Wong D. L. Cell separation by velocity sedimentation of postnatal mouse cerebellum. J Cell Physiol. 1973 Apr;81(2):271–279. doi: 10.1002/jcp.1040810215. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995 Feb;18(2):58–60. [PubMed] [Google Scholar]
- Dascal N., Schreibmayer W., Lim N. F., Wang W., Chavkin C., DiMagno L., Labarca C., Kieffer B. L., Gaveriaux-Ruff C., Trollinger D. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10235–10239. doi: 10.1073/pnas.90.21.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F., Lesage F., Guillemare E., Fink M., Hugnot J. P., Bigay J., Lazdunski M., Romey G., Barhanin J. Heterologous multimeric assembly is essential for K+ channel activity of neuronal and cardiac G-protein-activated inward rectifiers. Biochem Biophys Res Commun. 1995 Jul 17;212(2):657–663. doi: 10.1006/bbrc.1995.2019. [DOI] [PubMed] [Google Scholar]
- Farrant M., Feldmeyer D., Takahashi T., Cull-Candy S. G. NMDA-receptor channel diversity in the developing cerebellum. Nature. 1994 Mar 24;368(6469):335–339. doi: 10.1038/368335a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Lu Z., Abramson T., MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994 Apr;66(4):1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K., Trenkner E. Regional differences in cytoarchitecture of the weaver cerebellum suggest a new model for weaver gene action. Neuroscience. 1987 Dec;23(3):871–885. doi: 10.1016/0306-4522(87)90164-3. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine in the basal ganglia. Its role and therapeutic implications (including the clinical use of L-DOPA). Br Med Bull. 1973 May;29(2):172–178. doi: 10.1093/oxfordjournals.bmb.a070990. [DOI] [PubMed] [Google Scholar]
- Inoue M., Nakajima S., Nakajima Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J Physiol. 1988 Dec;407:177–198. doi: 10.1113/jphysiol.1988.sp017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalonen T., Johansson S., Holopainen I., Oja S. S., Arhem P. Single-channel and whole-cell currents in rat cerebellar granule cells. Brain Res. 1990 Dec 3;535(1):33–38. doi: 10.1016/0006-8993(90)91820-7. [DOI] [PubMed] [Google Scholar]
- Kawamoto J. C., Barrett J. N. Cryopreservation of primary neurons for tissue culture. Brain Res. 1986 Oct 1;384(1):84–93. doi: 10.1016/0006-8993(86)91222-9. [DOI] [PubMed] [Google Scholar]
- Kinney G. A., Slater N. T. Potentiation of NMDA receptor-mediated transmission in turtle cerebellar granule cells by activation of metabotropic glutamate receptors. J Neurophysiol. 1993 Feb;69(2):585–594. doi: 10.1152/jn.1993.69.2.585. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Ikeda K., Ichikawa T., Abe S., Togashi S., Kumanishi T. Molecular cloning of a mouse G-protein-activated K+ channel (mGIRK1) and distinct distributions of three GIRK (GIRK1, 2 and 3) mRNAs in mouse brain. Biochem Biophys Res Commun. 1995 Mar 28;208(3):1166–1173. doi: 10.1006/bbrc.1995.1456. [DOI] [PubMed] [Google Scholar]
- Kofuji P., Davidson N., Lester H. A. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P., Hofer M., Millen K. J., Millonig J. H., Davidson N., Lester H. A., Hatten M. E. Functional analysis of the weaver mutant GIRK2 K+ channel and rescue of weaver granule cells. Neuron. 1996 May;16(5):941–952. doi: 10.1016/s0896-6273(00)80117-8. [DOI] [PubMed] [Google Scholar]
- Komuro H., Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993 Apr 2;260(5104):95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G., Gordon E. A., Wickman K., Velimirović B., Krapivinsky L., Clapham D. E. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995 Mar 9;374(6518):135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Reuveny E., Slesinger P. A., Jan Y. N., Jan L. Y. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993 Aug 26;364(6440):802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- Lesage F., Duprat F., Fink M., Guillemare E., Coppola T., Lazdunski M., Hugnot J. P. Cloning provides evidence for a family of inward rectifier and G-protein coupled K+ channels in the brain. FEBS Lett. 1994 Oct 10;353(1):37–42. doi: 10.1016/0014-5793(94)01007-2. [DOI] [PubMed] [Google Scholar]
- Navarro B., Kennedy M. E., Velimirovíc B., Bhat D., Peterson A. S., Clapham D. E. Nonselective and G betagamma-insensitive weaver K+ channels. Science. 1996 Jun 28;272(5270):1950–1953. doi: 10.1126/science.272.5270.1950. [DOI] [PubMed] [Google Scholar]
- Patil N., Cox D. R., Bhat D., Faham M., Myers R. M., Peterson A. S. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat Genet. 1995 Oct;11(2):126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol. 1973 Nov 15;152(2):103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- Roffler-Tarlov S., Martin B., Graybiel A. M., Kauer J. S. Cell death in the midbrain of the murine mutation weaver. J Neurosci. 1996 Mar 1;16(5):1819–1826. doi: 10.1523/JNEUROSCI.16-05-01819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D. J., Slater N. T. The developmental onset of NMDA receptor-channel activity during neuronal migration. Neuropharmacology. 1993 Nov;32(11):1239–1248. doi: 10.1016/0028-3908(93)90018-x. [DOI] [PubMed] [Google Scholar]
- Schmidt M. J., Sawyer B. D., Perry K. W., Fuller R. W., Foreman M. M., Ghetti B. Dopamine deficiency in the weaver mutant mouse. J Neurosci. 1982 Mar;2(3):376–380. doi: 10.1523/JNEUROSCI.02-03-00376.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slesinger P. A., Patil N., Liao Y. J., Jan Y. N., Jan L. Y., Cox D. R. Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron. 1996 Feb;16(2):321–331. doi: 10.1016/s0896-6273(00)80050-1. [DOI] [PubMed] [Google Scholar]
- Smeyne R. J., Goldowitz D. Development and death of external granular layer cells in the weaver mouse cerebellum: a quantitative study. J Neurosci. 1989 May;9(5):1608–1620. doi: 10.1523/JNEUROSCI.09-05-01608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne R. J., Goldowitz D. Postnatal development of the wild-type and weaver cerebellum after embryonic administration of propylthiouracil (PTU). Brain Res Dev Brain Res. 1990 Jul 1;54(2):282–286. doi: 10.1016/0165-3806(90)90151-n. [DOI] [PubMed] [Google Scholar]
- Surmeier D. J., Bargas J., Hemmings H. C., Jr, Nairn A. C., Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995 Feb;14(2):385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Trenkner E., Sidman R. L. Histogenesis of mouse cerebellum in microwell cultures. Cell reaggregation and migration, fiber and synapse formation. J Cell Biol. 1977 Dec;75(3):915–940. doi: 10.1083/jcb.75.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenkner E. The role of taurine and glutamate during early postnatal cerebellar development of normal and weaver mutant mice. Adv Exp Med Biol. 1990;268:239–244. doi: 10.1007/978-1-4684-5769-8_27. [DOI] [PubMed] [Google Scholar]
- Triarhou L. C., Norton J., Ghetti B. Mesencephalic dopamine cell deficit involves areas A8, A9 and A10 in weaver mutant mice. Exp Brain Res. 1988;70(2):256–265. doi: 10.1007/BF00248351. [DOI] [PubMed] [Google Scholar]
- Yang J., Jan Y. N., Jan L. Y. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron. 1995 May;14(5):1047–1054. doi: 10.1016/0896-6273(95)90343-7. [DOI] [PubMed] [Google Scholar]
- Zegarra-Moran O., Moran O. Properties of the transient potassium currents in cerebellar granule cells. Exp Brain Res. 1994;98(2):298–304. doi: 10.1007/BF00228417. [DOI] [PubMed] [Google Scholar]



