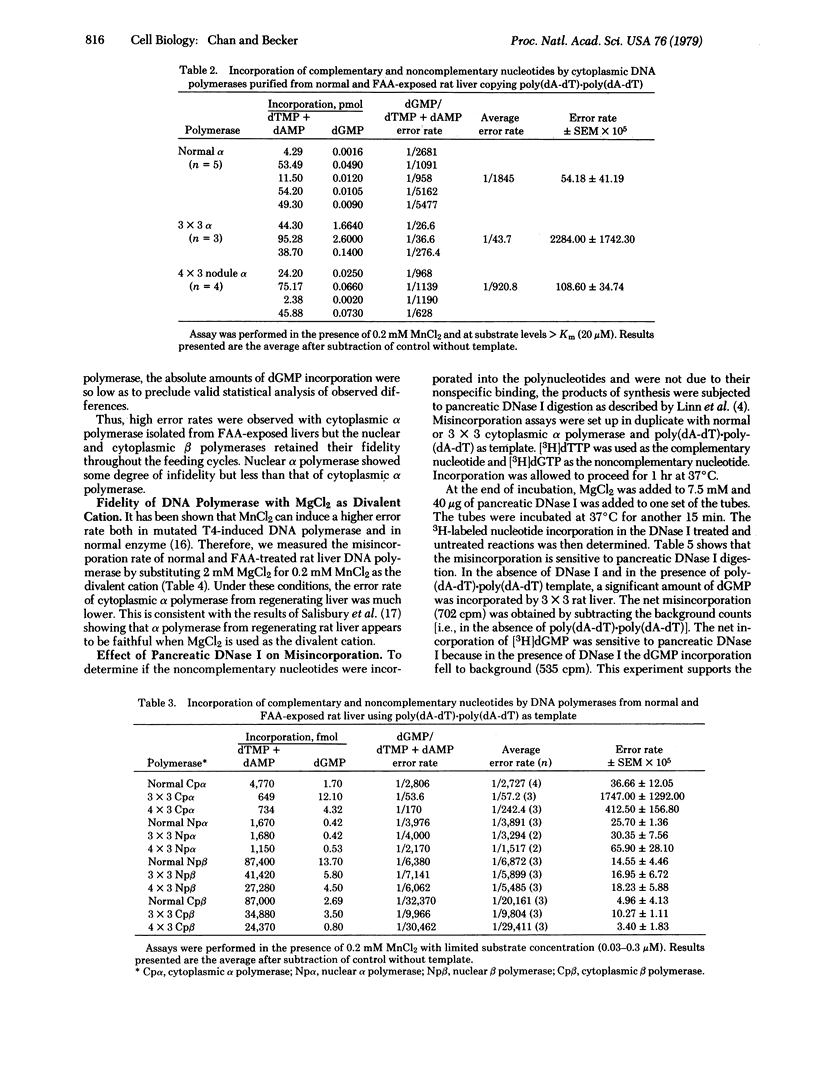
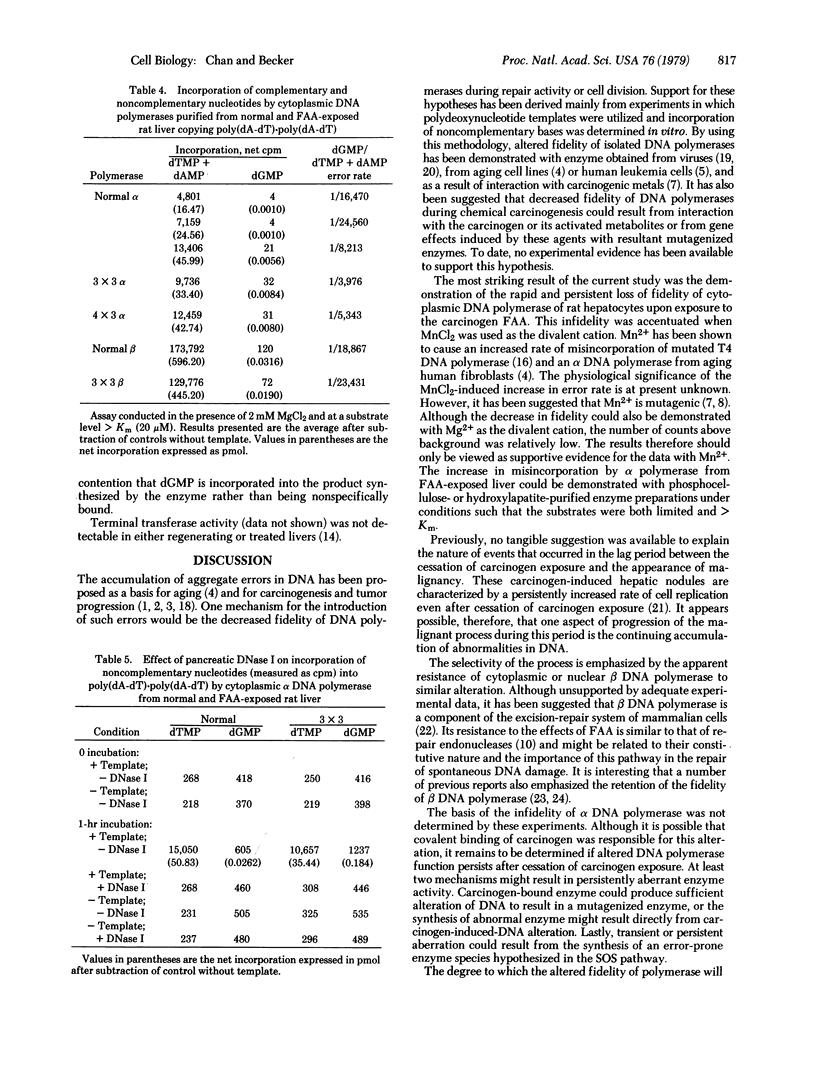
Abstract
alpha and beta DNA polymerases (DNA nucleotidyltransferase; deoxynucleosidetriphosphate:DNA deoxynucleotidyltransferase, EC 2.7.7.7) were isolated from nuclear and cytoplasmic fractions of rat livers exposed to a carcinogenic regimen with the hepatocarcinogen N-2-fluorenylacetamide and from 24-hr regenerating liver. The fidelity of polymerization of these enzymes was compared by determining the incorporation of noncomplementary deoxyribonucleoside triphosphates (misincorporation) on a poly(dA-dT).poly(dA-dT) template, with MnCl2 and MgCl2 as divalent cations. Our initial studies indicate that the cytoplasmic alpha polymerases from carcinogen-exposed rat livers were strikingly error-prone whereas the nuclear and cytoplasmic beta polymerases retained their fidelity throughout the feeding cycles. The misincorporation was significantly accentuated by MnCl2 compared with that obtained with MgCl2 as divalent cation. The products were sensitive to pancreatic DNase I digestion, indicating that the noncomplementary bases had been incorporated by the polymerization process. Nuclear alpha polymerase showed some degree of infidelity but less than that of cytoplasmic alpha polymerase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Silverstone A. E., Kung P. C., Harrison T. A., McCaffrey R. P. Specialized DNA polymerases in lymphoid cells. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):63–72. doi: 10.1101/sqb.1977.041.01.010. [DOI] [PubMed] [Google Scholar]
- Battula N., Loeb L. A. The infidelity of avian myeloblastosis virus deoxyribonucleic acid polymerase in polynucleotide replication. J Biol Chem. 1974 Jul 10;249(13):4086–4093. [PubMed] [Google Scholar]
- Becker F. F., Klein K. M. The effect of L-asparaginase on mitotic activity during N-2-fluorenylacetamide hepatocarcinogenesis: subpopulations of nodular cells. Cancer Res. 1971 Feb;31(2):169–173. [PubMed] [Google Scholar]
- Bertazzoni U., Stefanini M., Noy G. P., Giulotto E., Nuzzo F., Falaschi A., Spadari S. Variations of DNA polymerase-alpha and -beta during prolonged stimulation of human lymphocytes. Proc Natl Acad Sci U S A. 1976 Mar;73(3):785–789. doi: 10.1073/pnas.73.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Rodriguez L. W., Becker F. F. Endogenous DNA polymerase activity in fractionated rat lever chromatin. Nucleic Acids Res. 1977 Aug;4(8):2683–2695. doi: 10.1093/nar/4.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Srivastava B. I. Antigenic relationships in calf thymus and human leukemic cell terminal deoxynucleotidyl transferase. Biochim Biophys Acta. 1976 Oct 18;447(3):353–359. doi: 10.1016/0005-2787(76)90058-7. [DOI] [PubMed] [Google Scholar]
- Farber E. Carcinogenesis--cellular evolution as a unifying thread: Presidential address. Cancer Res. 1973 Nov;33(11):2537–2550. [PubMed] [Google Scholar]
- Fry M., Weisman-Shomer P. Altered nuclear deoxyribonucleic acid alpha-polymerases in senescent cultured chick embryo fibroblasts. Biochemistry. 1976 Sep 21;15(19):4319–4329. doi: 10.1021/bi00664a028. [DOI] [PubMed] [Google Scholar]
- Hachmann H. J., Lezius A. G. High-molecular-weight DNA polymerases from mouse myeloma. Purification and properties of three enzymes. Eur J Biochem. 1975 Jan 2;50(2):357–366. doi: 10.1111/j.1432-1033.1975.tb09811.x. [DOI] [PubMed] [Google Scholar]
- Hall Z. W., Lehman I. R. An in vitro transversion by a mutationally altered T4-induced DNA polymerase. J Mol Biol. 1968 Sep 28;36(3):321–333. doi: 10.1016/0022-2836(68)90158-7. [DOI] [PubMed] [Google Scholar]
- Holmer A. M., Hesslewood I. P., Johnston I. R. The occurrence of multiple activities in the high-molecular-weight DNA polymerase fraction of mammalian tissues. A preliminary study of some of their properties. Eur J Biochem. 1974 Apr 16;43(3):487–499. doi: 10.1111/j.1432-1033.1974.tb03436.x. [DOI] [PubMed] [Google Scholar]
- Lieberman M. W. Approaches to the analysis of fidelity of DNA repair in mammalian cells. Int Rev Cytol. 1976;45:1–23. doi: 10.1016/s0074-7696(08)60076-5. [DOI] [PubMed] [Google Scholar]
- Linn S., Kairis M., Holliday R. Decreased fidelity of DNA polymerase activity isolated from aging human fibroblasts. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2818–2822. doi: 10.1073/pnas.73.8.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Springgate C. F., Battula N. Errors in DNA replication as a basis of malignant changes. Cancer Res. 1974 Sep;34(9):2311–2321. [PubMed] [Google Scholar]
- Lynch W. E., Surrey S., Lieberman I. Nuclear deoxyribonucleic acid polymerases of liver. J Biol Chem. 1975 Oct 25;250(20):8179–8183. [PubMed] [Google Scholar]
- Matsukage A., Sivarajan M., Wilson S. H. Studies on DNA alpha-polymerase of mouse myeloma: partial purification and comparison of three molecular forms of the enzyme. Biochemistry. 1976 Nov 30;15(24):5305–5314. doi: 10.1021/bi00669a017. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Incorporation of noncomplementary nucleotides at high frequencies by ribodeoxyvirus DNA polymerases and Escherichia coli DNA polymerase I. Biochemistry. 1976 Apr 6;15(7):1510–1516. doi: 10.1021/bi00652a023. [DOI] [PubMed] [Google Scholar]
- Mordoh J., Fridlender B. R. A modified nuclear DNA polymerase in a case of acute lymphoblastic leukemia. Biochem Biophys Res Commun. 1975 Dec 1;67(3):888–896. doi: 10.1016/0006-291x(75)90760-3. [DOI] [PubMed] [Google Scholar]
- Nelson R. L., Mason H. S. An explicit hypothesis for chemical carcinogenesis. J Theor Biol. 1972 Oct;37(1):197–200. doi: 10.1016/0022-5193(72)90127-0. [DOI] [PubMed] [Google Scholar]
- Noy G. P., Weissbach A. HeLa cell DNA polymerases: the effect of cycloheximide in vivo and detection of a new form of DNA polymerase alpha. Biochim Biophys Acta. 1977 Jul 5;477(1):70–83. doi: 10.1016/0005-2787(77)90161-7. [DOI] [PubMed] [Google Scholar]
- Salisbury J. G., O'Connor P. J., Saffhill R. Molecular size and fidelity of DNA polymerase alpha from the regenerating liver of the rat. Biochim Biophys Acta. 1978 Jan 26;517(1):181–185. doi: 10.1016/0005-2787(78)90045-x. [DOI] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Infidelity of DNA synthesis in vitro: screening for potential metal mutagens or carcinogens. Science. 1976 Dec 24;194(4272):1434–1436. doi: 10.1126/science.1006310. [DOI] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. On the fidelity of DNA replication. Effect of metal activators during synthesis with avian myeloblastosis virus DNA polymerase. J Biol Chem. 1977 Jun 10;252(11):3605–3610. [PubMed] [Google Scholar]
- Springgate C. F., Loeb L. A. Mutagenic DNA polymerase in human leukemic cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):245–249. doi: 10.1073/pnas.70.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava B. I. Deoxynucleotide-polymerizing enzymes in normal and malignant human cells. Cancer Res. 1974 May;34(5):1015–1026. [PubMed] [Google Scholar]
- Srivastava B. I. Fidelity of deoxyribonucleic acid polymerases from normal and leukaemic human cells in polydeoxynucleotide replication. Biochem J. 1974 Aug;141(2):585–587. doi: 10.1042/bj1410585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teebor G. W., Becker F. F. Regression and persistence of hyperplastic hepatic nodules induced by N-2-Fluorenylacetamide and their relationship to hepatocarcinogenesis. Cancer Res. 1971 Jan;31(1):1–3. [PubMed] [Google Scholar]
- Teebor G. W., Duker N. J., Becker F. F. Normal endonuclease activities for damaged DNA during hepatocarcinogenesis. Biochim Biophys Acta. 1977 Jul 15;477(2):125–131. doi: 10.1016/0005-2787(77)90228-3. [DOI] [PubMed] [Google Scholar]
- Wang T. S., Eichler D. C., Korn D. Effect of Mn2+ on the in vitro activity of human deoxyribonucleic acid polymerase beta. Biochemistry. 1977 Nov 1;16(22):4927–4934. doi: 10.1021/bi00641a029. [DOI] [PubMed] [Google Scholar]
- Wheldon T. E., Kirk J. An error cascade mechanism for tumour progression. J Theor Biol. 1973 Nov 5;42(1):107–111. doi: 10.1016/0022-5193(73)90150-1. [DOI] [PubMed] [Google Scholar]


