Abstract
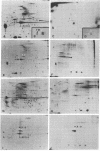
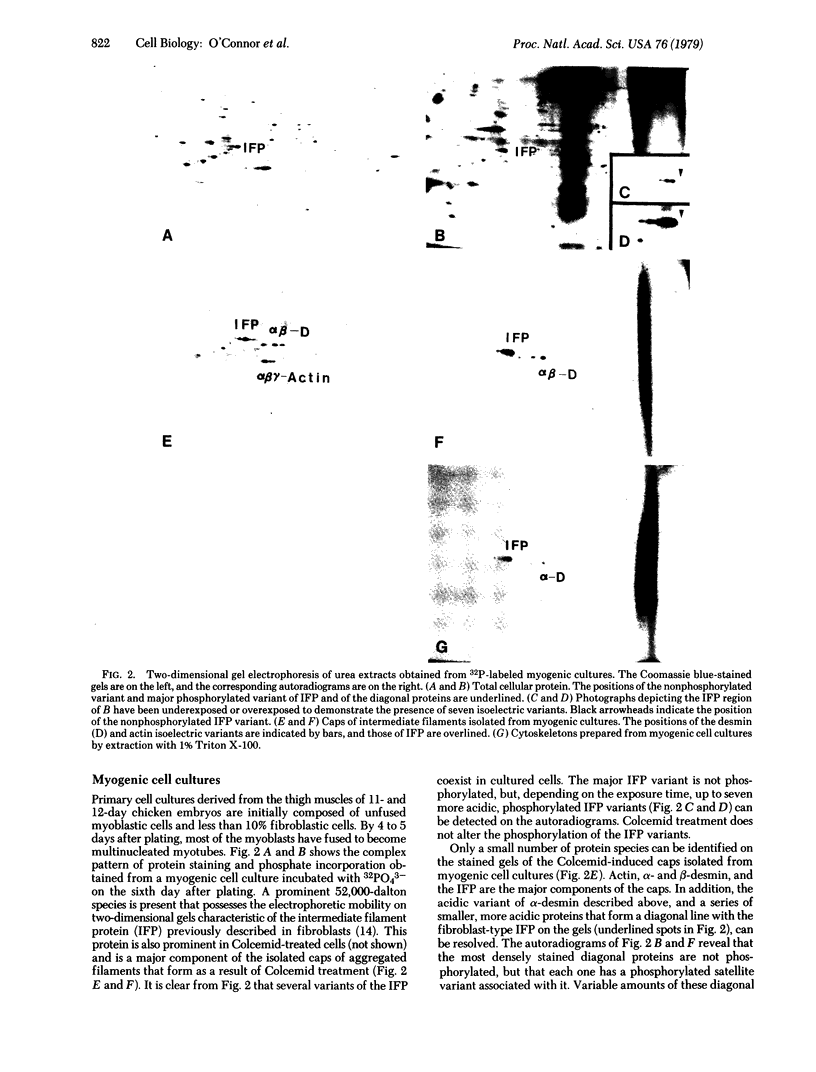
The phosphorylation of the subunit proteins of intermediate (10-nm) filaments has been investigated in chicken muscle and nonmuscle cells by using a two-dimensional gel electrophoresis system. Desmin, the 50,000-dalton subunit protein of the intermediate filaments of muscle, had previously been shown to exist as two major isoelectric variants—α and β—in smooth, skeletal, and cardiac chicken muscle. Incubation of skeletal and smooth muscle tissue with 32PO43- reveals that the acidic variant, α-desmin, and three other desmin variants are phosphorylated in vivo and in vitro. Under the same conditions, minor components of α- and β-tropomyosin from skeletal muscle, but not smooth muscle, are also phosphorylated. Both the phosphorylated desmin variants and the nonphosphorylated β-desmin variant remain insoluble under conditions that solubilize actin and myosin filaments, but leave Z-discs and intermediate filaments insoluble. Primary cultures of embryonic chicken muscle labeled with 32PO43- possess, in addition to the desmin variants described above, a major nonphosphorylated and multiple phosphorylated variants of the 52,000-dalton, fibroblast-type intermediate filament protein (IFP). Filamentous cytoskeletons, prepared from primary myogenic cultures by Triton X-100 extraction, contain actin and all of the phosphorylated and nonphosphorylated variants of both desmin and the IFP. Similarly, these proteins are the major components of the caps of aggregated 10-nm filaments isolated from the same cell cultures previously exposed to Colcemid. These results demonstrate that a nonphosphorylated and several phosphorylated variants of desmin and IFP are present in assembled structures in muscle and nonmuscle cells.
Keywords: desmin, tropomyosin, two-dimensional gel electrophoresis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S., Levinson W., Spudich J. A. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5(2):119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- Chacko S., Conti M. A., Adelstein R. S. Effect of phosphorylation of smooth muscle myosin on actin activation and Ca2+ regulation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):129–133. doi: 10.1073/pnas.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke P. H., Chase R. H. Potassium chloride-insoluble myofilaments in vertebrate smooth muscle cells. Exp Cell Res. 1971 Jun;66(2):417–425. doi: 10.1016/0014-4827(71)90696-3. [DOI] [PubMed] [Google Scholar]
- Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. J Cell Biol. 1976 Mar;68(3):539–556. doi: 10.1083/jcb.68.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Isolation from peripheral nerve of a protein similar to the glial fibrillary acidic protein. FEBS Lett. 1976 Jul 15;66(2):281–284. doi: 10.1016/0014-5793(76)80522-4. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Croop J., Dienstman S., Ishikawa H., Somlyo A. P. Effects of cytochaslasin B and colcemide on myogenic cultures. Proc Natl Acad Sci U S A. 1975 Feb;72(2):513–517. doi: 10.1073/pnas.72.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Lazarides E. Invariance and heterogeneity in the major structural and regulatory proteins of chick muscle cells revealed by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1450–1454. doi: 10.1073/pnas.74.4.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Balzer D. R., Jr Specificity of desmin to avian and mammalian muscle cells. Cell. 1978 Jun;14(2):429–438. doi: 10.1016/0092-8674(78)90128-9. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Granger B. L. Fluorescent localization of membrane sites in glycerinated chicken skeletal muscle fibers and the relationship of these sites to the protein composition of the Z disc. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3683–3687. doi: 10.1073/pnas.75.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Hubbard B. D. Immunological characterization of the subunit of the 100 A filaments from muscle cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4344–4348. doi: 10.1073/pnas.73.12.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. The distribution of desmin (100 A) filaments in primary cultures of embryonic chick cardiac cells. Exp Cell Res. 1978 Mar 15;112(2):265–273. doi: 10.1016/0014-4827(78)90209-4. [DOI] [PubMed] [Google Scholar]
- Mak A., Smillie L. B., Bárány M. Specific phosphorylation at serine-283 of alpha tropomyosin from frog skeletal and rabbit skeletal and cardiac muscle. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3588–3592. doi: 10.1073/pnas.75.8.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M. Resolution of simian virus 40 proteins in whole cell extracts by two-dimensional electrophoresis: heterogeneity of the major capsid protein. Cell. 1976 Oct;9(2):289–298. doi: 10.1016/0092-8674(76)90119-7. [DOI] [PubMed] [Google Scholar]
- O'Neill M. C., Stockdale F. E. A kinetic analysis of myogenesis in vitro. J Cell Biol. 1972 Jan;52(1):52–65. doi: 10.1083/jcb.52.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant H. C., Shecket G., Gainer H., Lasek R. J. Neurofilament protein is phosphorylated in the squid giant axon. J Cell Biol. 1978 Aug;78(2):R23–R27. doi: 10.1083/jcb.78.2.r23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder J. C., Bray D., Gratzer W. B. Control of interaction of spectrin and actin by phosphorylation. Nature. 1977 Dec 22;270(5639):752–754. doi: 10.1038/270752a0. [DOI] [PubMed] [Google Scholar]
- Rash J. E., Biesele J. J., Gey G. O. Three classes of filaments in cardiac differentiation. J Ultrastruct Res. 1970 Dec;33(5):408–435. doi: 10.1016/s0022-5320(70)90171-1. [DOI] [PubMed] [Google Scholar]
- Schachner M., Hedley-Whyte E. T., Hsu D. W., Schoonmaker G., Bignami A. Ultrastructural localization of glial fibrillary acidic protein in mouse cerebellum by immunoperoxidase labeling. J Cell Biol. 1977 Oct;75(1):67–73. doi: 10.1083/jcb.75.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer W. W., Lynch R. G. Immunofluorescence studies of neurofilaments in the rat and human peripheral and central nervous system. J Cell Biol. 1977 Jul;74(1):241–250. doi: 10.1083/jcb.74.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V., Sobieszek A. Studies on the function and composition of the 10-NM(100-A) filaments of vertebrate smooth muscle. J Cell Sci. 1977 Feb;23:243–268. doi: 10.1242/jcs.23.1.243. [DOI] [PubMed] [Google Scholar]
- Starger J. M., Brown W. E., Goldman A. E., Goldman R. D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978 Jul;78(1):93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]