Abstract
Introduction
Heart-rate variability reflects autonomic nervous system tone as well as the overall health of the baroreflex system. We hypothesized that loss of complexity in heart-rate variability upon ICU admission would be associated with unsuccessful early resuscitation of sepsis.
Methods
We prospectively enrolled patients admitted to ICUs with severe sepsis or septic shock from 2009 to 2011. We studied 30 minutes of EKG, sampled at 500 Hz, at ICU admission and calculated heart-rate complexity via detrended fluctuation analysis. Primary outcome was vasopressor independence at 24 hours after ICU admission. Secondary outcome was 28-day mortality.
Results
We studied 48 patients, of whom 60% were vasopressor independent at 24 hours. Five (10%) died within 28 days. The ratio of fractal alpha parameters was associated with both vasopressor independence and 28-day mortality (p=0.04) after controlling for mean heart rate. In the optimal model, SOFA score and the long-term fractal alpha parameter were associated with vasopressor independence.
Conclusions
Loss of complexity in heart rate variability is associated with worse outcome early in severe sepsis and septic shock. Further work should evaluate whether complexity of heart rate variability (HRV) could guide treatment in sepsis.
Keywords: Sepsis, Shock, Physiological Variability, Heart Rate Variability
Introduction
Severe sepsis and septic shock, the life-threatening manifestations of severe infection, afflict 750,000 patients annually in the USA with an associated mortality of 25–50%.1,2 While current consensus emphasizes early intervention to control sepsis,3,4 which intermediate endpoints and predictors are most useful for guiding interventions is not clear; nor are early markers of severity well established. Non-invasive assessments of cardiovascular function may be useful in this regard because cardiovascular dysfunction is common and central to overall organ dysfunction. The sinus node of the heart integrates many inputs to determine the interval between successive heartbeats. Heart rate variability (HRV), a measure of both sympathovagal balance and the overall integrity/health of the baroreflex system, has been shown to offer unique insights into severity of illness in primary cardiac disease,5–7 acute trauma8 and sepsis.9,10 Connections between the parasympathetic nervous system and inflammation suggest the possibility that HRV and inflammation may be interdependent.11 In addition to allowing identification of balance between the sympathetic and parasympathetic nervous systems, HRV exhibits nonlinear patterns of complexity, including fractal self-similarity across time scales.12,13 Fractal complexity is commonly measured via detrended fluctuation analysis13 (DFA, depicted in detail in eFigure 1), which measures the amount of fluctuation/variability as a function of the number of inter-beat intervals measured. In health, the relationship between fluctuation and number of inter-beat intervals for small numbers of inter-beat intervals (measured by a short-term fractal scaling coefficient usually called α1) is similar to the relationship between fluctuation and number of inter-beat intervals for large numbers of inter-beat intervals (measured by a long-term fractal scaling coefficient usually called α2). The ratio of the short-term scaling coefficient α1 and the long-term scaling coefficient α2 approaches 1 in health but is commonly less than 1 in disease. Whether the fractal scaling coefficients or their ratio measured early in severe sepsis or septic shock is related to outcome is not yet known.
Observations about complexity are not only theoretical: Moorman and colleagues have demonstrated in a randomized trial that monitoring the complexity of HRV (primarily via entropy, a measure of complexity derived from information theory) improved outcomes among premature infants at risk for sepsis in a neonatal ICU.14,15 We hypothesized that measures of HRV, with an emphasis on the fractal scaling coefficients from DFA, obtained within the first 6 hours of ICU admission among patients with severe sepsis and septic shock would predict liberation from vasopressor medications 24 hours after ICU admission, which we defined a priori as a clinically meaningful measure of successful early resuscitation.
The Intermountain Institutional Review Board approved this study; we obtained written, informed consent from all patients or legally authorized representatives. We have reported early results from this work in abstract form.16
Materials and Methods
Setting
Two intensive-care units, the 24-bed Shock Trauma ICU, and the 12-bed Respiratory ICU, at Intermountain Medical Center, a 452-bed tertiary-care, academic hospital in Murray, Utah, USA.
Patients
We prospectively identified adult (> 15 years of age) patients with severe sepsis or septic shock (as defined in consensus guidelines17) admitted to study ICUs from September 2009 to May 2011. We excluded pregnant patients, patients with admission DNR/DNI orders, and patients with non-sinus rhythm. We only included patients the first time they were admitted to a study ICU with sepsis during the study period. Patients were only included at the time of their initial admission to the ICU; we excluded patients who developed sepsis after their admission to the ICU.
Clinical Data
We calculated admission APACHE II18 and SOFA19 scores in all study patients. Infusion rates of vasopressors (norepinephrine, epinephrine, dopamine, phenylephrine, and vasopressin) are automatically uploaded in real-time to the hospital Electronic Medical Record (EMR) as part of routine clinical care. We analyzed all vasopressors administered during the first six hours after ICU admission, converting them to norepinephrine equivalent dosages according to standard equivalencies.20 We also noted symptomatic congestive heart failure at admission or left ventricular ejection fraction < 45% (when clinical echocardiogram was obtained).
Physiological Data Acquisition and Processing
We sampled EKG (lead II) from bedside Philips Intellivue monitors using the synchronization slave port, and digitized, at 500 Hz, that analog signal using a National Instruments (Austin, Texas) DAQ™ device and custom-written software within the LabView™ development environment. We identified the peak of the R wave using custom-written software within the LabView™ development environment. We identified (and excluded) ectopic and post-ectopic beats as well as uninterpretable segments of EKG signal (generally related to patient motion or displacement of electrodes) using the same custom-written software. We thereby generated tachograms—time series of RR intervals in milliseconds—for each patient for six hours.
Using standard algorithms implemented within the LabView™ development environment, we calculated metrics from the time-, frequency-, and complexity- domains of tachograms across the first six hours after ICU admission (the relevant equations are presented in the Appendix; the DFA technique is also depicted in eFigure 1). Because we were interested in early prediction, we calculated measures from the first 30 minutes of EKG recording for our calculations.
Clinical Outcomes
We chose as our primary outcome vasopressor independence at 24 hours after ICU admission, an a priori clinical outcome we felt represented successful early resuscitation of sepsis.21 To meet criteria for vasopressor independence at 24 hours, a patient had to be alive and liberated from vasopressor therapy from 24 through 48 hours after ICU admission. Secondary outcomes included all-cause 28-day mortality, vasopressor-free days and ICU-free days, all measured at 28 days after ICU admission. We determined all-cause 28-day mortality from the Intermountain Death Record, which incorporates data from Utah state vital statistics.
Statistical Methods
HRV metrics (mean, number and proportion of consecutive beats separated by more than 50msec, sample entropy, Poincare plot standard deviations, fractal exponents from detrended fluctuation analysis, power spectral density measurements including total power and power across the high-, low- and very low-frequency bands; predictors described in the Appendix) were incorporated as predictors alongside APACHE II and SOFA scores, age, sex, exposure to vasopressors, presence of shock into a multivariate naïve Bayesian classification model22 of primary (vasopressor independence at 24 hours) and secondary (28-day mortality) study outcomes. (We selected naïve Bayesian classification over logistic regression given the relatively small number of deaths in our study cohort and prior experience with the technique.) We employed a forward selection process to select the predictors which improved the out-of-bag area under the receiver operating characteristic curve (AUC) of the classification.23,24 The technique assesses two and three-way interaction terms and protects against collinearity. Final model discriminatory performance was assessed using the out-of-bag AUC over 2,000 bootstrap samples. This resulted in two separate models of outcome, one for the primary outcome of vasopressor independence at 24 hours, the other for the secondary outcome of 28-day mortality. For each model, predictors were selected on the basis of whether a given predictor improved the AUC of the given model. We display the association between significant predictors and the primary outcomes using effect plots, which describe the associations in greater detail than simple parameter estimates/odds ratios.25 Statistical analysis and hypothesis testing was performed within the R statistical package (version 2.12).26
Results
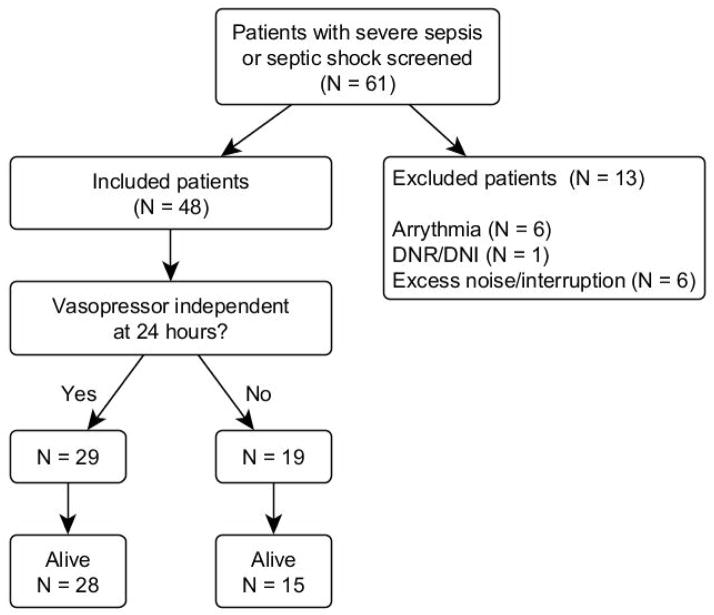
We enrolled 48 patients who met inclusion criteria. Figure 1 depicts the flow of patients through screening and analysis. Table 1 depicts patient demographics, measures of disease severity, and sources of sepsis. The majority of patients (60%) were vasopressor independent by 24 hours; five (10%) patients died. Forty-two (88%) patients were admitted to the ICU from an Emergency Department; the other six patients were admitted from the hospital ward. Thirty (63%) of patients had shock requiring vasopressors at time of enrollment. Table 2 depicts the baseline measures of HRV, divided into time domain, frequency domain, and complexity domain measures.
Figure 1.
Summary of Study Enrollment
Flow diagram depicting how the 48 patients included in the study were identified.
Table 1.
Demographics, Severity and Outcome
| Attribute | Central Tendency and Variation* |
|---|---|
| Age (years) | 57 (40–63) |
| Female sex (%) | 54 |
| Admission APACHE II (points) | 18.5 (14.8–29.2) |
| Day 1 SOFA (points) | 9 (6–11) |
| Day 2 SOFA (points) | 4 (3–10) |
| Day 3 SOFA (points) | 3 (2–7) |
| Vasopressors at ICU admission (%) | 63 |
| Vasopressor independent at 24 hours (%) | 60 |
| 28-day Mortality (%) | 10 |
| Vasopressor-free days at 28 days | 27 (25–28) |
| Baseline symptomatic CHF (%) | 6 |
| Ejection fraction < 45% (%) | 15 |
| Sepsis source (%) | |
| Pneumonia (N=14) | 29 |
| Urinary (N=11) | 23 |
| Skin/soft tissue (N=8) | 17 |
| Other/uncertain (N=7) | 15 |
| Abdominal (N=4) | 8 |
| Bacteremia (N=4) | 8 |
Mean (standard deviation) or median (inter-quartile range), depending on normality of the data
Table 2.
Measures of RR interval time series, first 30 minutes
| Attribute | Central Tendency | Spread* |
|---|---|---|
| Time Domain Measures | ||
| Mean RR (msec) | 633.7 | 121.5 |
| Variance | 155 | 69–523 |
| Coefficient of Variation | 0.26 | 0.1–0.7 |
| NN50 | 2.0 | 0–22 |
| PNN50 | 0.09 | 0–0.7 |
| RMSSD | 8.0 | 4.0–15.5 |
| Frequency Domain Measures** | ||
| Auto-regressive | ||
| High-frequency power | 0.37 | 0.2–0.5 |
| Low-frequency power | 0.63 | 0.5–0.8 |
| Ratio of Low- to High- frequency power | 1.74 | 1.1–5.6 |
| Complexity Domain Measures | ||
| Poincare standard deviation 1 | 5.7 | 2.8–10.9 |
| Poincare standard deviation 2 | 24.23 | 18.2–38.8 |
| Sample Entropy | 0.82 | 0.4 |
| DFA short-term coefficient | 0.98 | 0.4 |
| DFA long-term coefficient | 1.10 | 0.3 |
| Ratio of DFA coefficients | 0.87 | 0.6–1.2 |
Inter-quartile range for non-normal data; standard deviation for normal data.
All values are normalized.
NN50: number of RR intervals that differ from the preceding by at least 50msec; PNN50: proportion of RR intervals that differ from the preceding by at least 50msec; RMSSD: Root mean square difference of successive RR intervals; DFA: Detrended Fluctuation Analysis.
Primary Outcome
On bivariate logistic regression, the ratio of short-term to long-term fractal scaling coefficients (α1/α2) was associated (p = 0.05) with vasopressor independence at 24 hours, after controlling for mean heart rate.
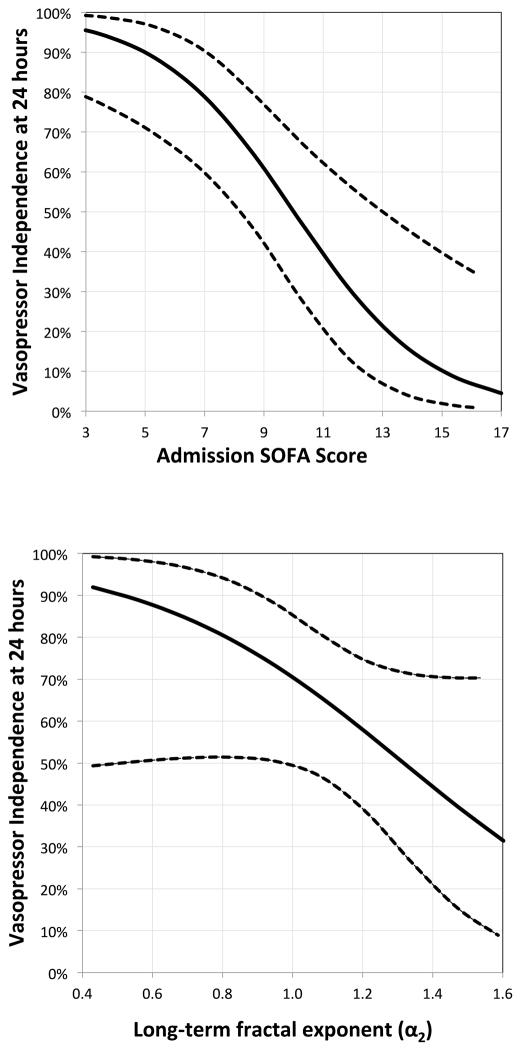
The multivariate naïve Bayesian model of vasopressor independence that optimized the AUC incorporated admission SOFA score and the long-term fractal exponent (α2). The resulting out-of-bag AUC (95% CI) for the model was 0.86 (0.68–0.99). Figure 2 displays effect plots of predictors that were significantly associated with vasopressor independence at 24 hours. Admission SOFA score and ratio of short-term and long-term fractal scaling (α1/α2) coefficients displayed moderate correlation (adjusted R2 = 0.17, p = 0.002; Spearman correlation coefficient −0.4). Other metrics of HRV complexity, including Sample Entropy and the standard deviations of the Poincare plot, were not associated with either the primary or secondary outcome.
Figure 2.
Effect Plots for Predictors of Vasopressor Independence at 24 hours
Effect plots specifying the association, with other predictors held constant, of a given predictor from the multivariate regression model of vasopressor independence at 24 hours. Dotted lines represent 95% confidence intervals for the association.
Secondary Outcomes
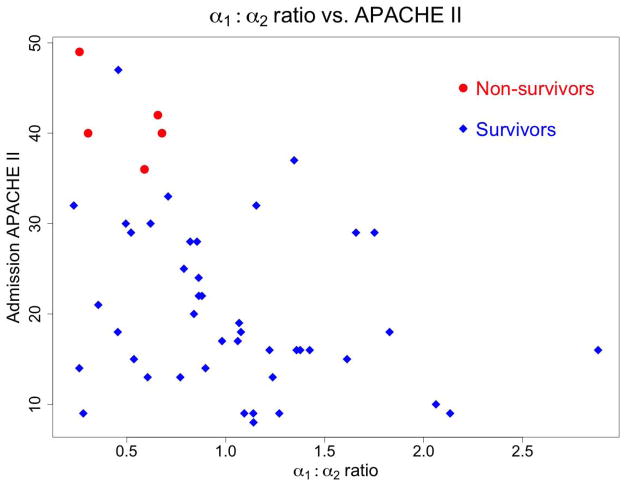
On bivariate logistic regression, the ratio of short-term to long-term fractal scaling coefficients (α1/α2) was associated (p = 0.04) with 28-day mortality, after controlling for mean heart rate. The multivariate naïve Bayesian model of 28-day mortality that optimized the AUC incorporated only admission APACHE II score. The resulting out-of-bag AUC (95% CI) for the model of 28-day mortality was 0.97 (0.88–1.00). Admission APACHE II score and ratio of short-term and long-term fractal scaling (α1/α2) coefficients displayed moderate correlation (adjusted R2 = 0.14, p = 0.006; Spearman correlation coefficient −0.4). Figure 3 depicts the relationships between 28-day mortality, APACHE II, and the ratio of fractal α coefficients from DFA. All deaths occurred among patients whose ratio of short-term and long-term fractal scaling coefficients (α1/α2) was < 0.75 and whose admission APACHE II was > 35. (Within our study cohort, no patients died between 28 days and 60 days; mortality outcomes are thus identical for 28-day and 60-day mortality.)
Figure 3.
α1: α2 Ratio vs. APACHE II Score
Ratio of fractal exponents as compared to the admission APACHE II score. Circles indicate non-survivors, while diamonds depict survivors. Note the clustering of non-survivors in the left upper quadrant, with high APACHE II and low ratio of fractal exponents.
Short-term and long-term fractal scaling coefficients correlated modestly with normalized power spectra for low (α1) and very low (α2) frequency (adjusted R2 = 0.21 for each comparison). eFigure 2 depicts the association between the ratio of LFν/VLFν and the ratio of α1/α2 (slope=0.5, adjusted R2= 0.42, p < 0.01). The LFν/VLFν ratio was not significantly associated with either the primary (p=0.2) or the secondary (p=0.07) outcome.
Discussion
Substantial research on the autonomic nervous system, including especially its baroreflex control system, has suggested that increased complexity in HRV denotes relative health, while a loss of complexity is associated with pathologic states or poor prognosis.27–31 Our prospective study of 48 ICU-admitted patients with early severe sepsis and septic shock confirms this finding, while observing that much of this effect is collinear with overall disease severity and multiple organ dysfunction, as assessed by the SOFA (associated with vasopressor independence) or APACHE II (associated with 28-day mortality) scores.
Other studies have suggested that loss of complexity or changes in sympathovagal balance in HRV heralds the onset of sepsis in at-risk populations32,33 predicts development of shock and/or organ dysfunction among patients with initial severe sepsis,10,30,34 or identifies differential rates of stress during sedation interruption.35 Our study suggests that loss of fractal complexity, as assessed by the scaling coefficients (“fractal exponents”) from detrended fluctuation analysis, is associated with the success of early resuscitation in early severe sepsis and septic shock. Our endpoint, while intermediate, was defined a priori and represents a reasonable clinical definition of early successful resuscitation of sepsis.
Loss of complexity in HRV likely derives from multiple sources, including reduction in variance in variables with high mean values, intrinsic pacemaker dysfunction especially related to the “funny current”36, disordered regulation of respiration, autonomic nervous system dysfunction and parasympathetic coupling to immune function.37 Which of those mechanisms might be amenable to therapeutic intervention is a matter for future research. Although it may be early on the basis of animal data alone, some authors have begun to suggest equipoise for study of the administration of, e.g., beta blockers38 in early sepsis or perhaps manipulation of the cholinergic anti-inflammatory pathway.11
This study does not allow us to decide whether the loss of complexity of HRV is causal or epiphenomenal: is the loss of complexity of HRV merely something that happens to people with severe general organ dysfunction, or is it a cause of worse organ dysfunction? Larger cohorts and, ultimately, therapeutic trials, will be required to answer that question definitively. While we studied patients early in their ICU stay, we were unable to demonstrate whether HRV patterns were related to the phase in the natural history of sepsis at which the patients were admitted to the ICU. Patients presenting very late in the course of their sepsis to the hospital may have different HRV patterns than those patients who present early in the course of their sepsis.
Current therapies for severe sepsis and septic shock (other than control of the inciting infection) are limited to volume expansion and administration of intravenous catecholamines. Noninvasive assessment of the balance of the cardiovascular system may be relevant to improving the clinical use of these therapies early in the treatment of sepsis. Patients with a particular profile of HRV may benefit from particular vasoactive agents or particular combinations of such agents. This question should be evaluated in further studies. Further studies should also investigate the stability over time and optimal window size for measurements of HRV commplexity in clinical cohorts.
In elegant analytical work corroborated by experiments in healthy volunteers, Francis and colleagues demonstrated that the short- and long-term scaling coefficients of detrended fluctuation analysis represent, respectively, the normalized low frequency power (LFν) and very low frequency power (VLFν) in the power spectrum of HRV.39 Our cohort partially corroborates these findings. Unfortunately, measures from the power spectrum were less predictive of clinical outcome than the ratio of fractal exponents despite the moderate correlation. Power spectral density measurements assume stationarity in the underlying time series (i.e., the mean heart rate does not change significantly), while detrended fluctuation analysis does not require stationarity. We suspect that the lack of stationarity, related to changes in the average heart rate in our critically ill patients, accounts for the difference between our results and theirs. Our inclusion of older patients may also affect the association between power spectral density measurements and clinical outcome. It is not clear why Sample Entropy and standard deviations on the Poincare plot were less associated with outcome than the scaling coefficients of detrended fluctuation analysis. These methods do measure slightly different elements of HRV complexity. Future research should clarify which elements of which metrics of HRV complexity are most relevant in specific clinical circumstances.
We acknowledge that we studied patients at different phases of their severe sepsis or septic shock, a persistent problem with clinical studies of sepsis. While we focused on the very early phase of ICU admission, patients present to medical care at various phases of infection. While this reality complicates most clinical research in acute sepsis, we chose this population because they are a clinically important population and our research questions relate to best management of patients early in their ICU stay.
In summary, in a prospective cohort of 48 patients with severe sepsis or septic shock, the long-term fractal exponent (α2), a standard measure of complexity in HRV was associated with successful early resuscitation, even after control for covariates.
Supplementary Material
Acknowledgments
This study was funded by National Institute of General Medical Sciences (1K23GM094465 to SMB), National Heart, Lung, and Blood Institute (1K23HL092161 to MTR), Intermountain Research and Medical Foundation, and the Easton Fund.
Abbreviations
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- AUC
Area Under the Curve
- CI
Confidence Interval
- EMR
Electronic Medical Record
- HF
High Frequency
- HRV
Heart Rate Variability
- ICU
Intensive Care Unit
- LF
Low Frequency
- SOFA
Sequential Organ Failure Assessment
- VLF
Very Low Frequency
Footnotes
Competing Interests:
The authors declare that they have no competing interests.
Authors‘ contributions:
SB organized and designed the study, participated in patient recruitment, data acquisition and data analysis, as well as drafting the manuscript. QT participated in software development, data analysis, assisted in drafting the manuscript and revised for important intellectual content. JJ helped design the study, analyze the data and revised the manuscript for important intellectual content. DK helped acquire and analyze data and revised the manuscript for important intellectual content. KK helped design the study, acquire and analyze data and revised the manuscript for important intellectual content. ML assisted in patient recruitment, study design and revision of the manuscript. MR participated in study development, data analysis and revision of the manuscript for important intellectual content. CG assisted in patient recruitment, study design and revision of the manuscript manuscript for important intellectual content. SB participated in software development, data acquisition and analysis and revision of the manuscript for important intellectual content. VM assisted in study inception and design, data analysis and manuscript revision for important intellectual content. AM participated in study design, data analysis and manuscript revision for important intellectual content. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Samuel M. Brown, Email: Samuel.Brown@imail.org.
Quinn Tate, Email: Quinn.Tate@utah.edu.
Jason P. Jones, Email: Jason.P.Jones@kp.org.
Daniel Knox, Email: Daniel.Knox@utah.edu.
Kathryn G. Kuttler, Email: Kathryn.Kuttler@imail.org.
Michael Lanspa, Email: Michael.Lanspa@imail.org.
Matthew T. Rondina, Email: Matthew.Rondina@hsc.utah.edu.
Colin K. Grissom, Email: Colin.Grissom@imail.org.
Subhasis Behera, Email: subhasis.behera@gmail.com.
V.J. Mathews, Email: Mathews@ece.utah.edu.
Alan Morris, Email: Alan.Morris@imail.org.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 3.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 5.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 6.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 7.La Rovere MT, Pinna GD, Maestri R, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–70. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 8.Russell JA, Walley KR, Gordon AC, et al. Interaction of vasopressin infusion, corticosteroid treatment, and mortality of septic shock. Critical care medicine. 2009;37:811–8. doi: 10.1097/CCM.0b013e3181961ace. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad S, Ramsay T, Huebsch L, et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS ONE. 2009;4:e6642. doi: 10.1371/journal.pone.0006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen WL, Chen JH, Huang CC, Kuo CD, Huang CI, Lee LS. Heart rate variability measures as predictors of in-hospital mortality in ED patients with sepsis. Am J Emerg Med. 2008;26:395–401. doi: 10.1016/j.ajem.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. Journal of internal medicine. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberger AL, Amaral LA, Hausdorff JM, Ivanov P, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci U S A. 2002;99 (Suppl 1):2466–72. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82–7. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 14.Moorman JR, Delos JB, Flower AA, et al. Cardiovascular oscillations at the bedside: early diagnosis of neonatal sepsis using heart rate characteristics monitoring. Physiological measurement. 2011;32:1821–32. doi: 10.1088/0967-3334/32/11/S08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moorman JR, Carlo WA, Kattwinkel J, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatr. 2011;159:900–6. e1. doi: 10.1016/j.jpeds.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown SM, Behera S, Jones J, et al. The Short-term Fractal Exponent of Heart-Rate Variability Is Associated with Mortality in Severe Sepsis and Septic Shock. International Conference on Complexity in Acute Illness; Bonn, Germany. 2011. [Google Scholar]
- 17.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 20.Brown SM, Lanspa MJ, Jones JP, et al. Survival after shock requiring high-dose vasopressor therapy. Chest. 2013;143:664–71. doi: 10.1378/chest.12-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piccinni P, Dan M, Barbacini S, et al. Early isovolaemic haemofiltration in oliguric patients with septic shock. Intensive care medicine. 2006;32:80–6. doi: 10.1007/s00134-005-2815-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H. The Optimality of Naive Bayes. In: Barr V, Markov Z, editors. Seventeenth International Florida Artificial Intelligence Research Society Conference; 2004 May 12–14; Miami Beach, FL: AAAI Press; pp. 562–7. [Google Scholar]
- 23.Efron B, Tibshirani R. Improvements on cross-validation: The. 632+ bootstrap method. Journal of the American Statistical Association. 1997;92:548–60. [Google Scholar]
- 24.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 25.Fox J. Effect displays in R for generalised linear models. Journal of Statistical Software. 2003;8:1–27. [Google Scholar]
- 26.Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 27.Riordan WP, Jr, Norris PR, Jenkins JM, Morris JA., Jr Early loss of heart rate complexity predicts mortality regardless of mechanism, anatomic location, or severity of injury in 2178 trauma patients. J Surg Res. 2009;156:283–9. doi: 10.1016/j.jss.2009.03.086. [DOI] [PubMed] [Google Scholar]
- 28.Norris PR, Canter JA, Jenkins JM, Moore JH, Williams AE, Morris JA., Jr Personalized Medicine: Genetic Variation and Loss of Physiologic Complexity Are Associated With Mortality in 644 Trauma Patients. Ann Surg. 2009 doi: 10.1097/SLA.0b013e3181b8fb1f. [DOI] [PubMed] [Google Scholar]
- 29.Gang Y, Malik M. Heart rate variability in critical care medicine. Curr Opin Crit Care. 2002;8:371–5. doi: 10.1097/00075198-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Pontet J, Contreras P, Curbelo A, et al. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. J Crit Care. 2003;18:156–63. doi: 10.1016/j.jcrc.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Buchan CA, Bravi A, Seely AJ. Variability analysis and the diagnosis, management, and treatment of sepsis. Curr Infect Dis Rep. 2012;14:512–21. doi: 10.1007/s11908-012-0282-4. [DOI] [PubMed] [Google Scholar]
- 32.Beuchee A, Carrault G, Bansard JY, Boutaric E, Betremieux P, Pladys P. Uncorrelated randomness of the heart rate is associated with sepsis in sick premature infants. Neonatology. 2009;96:109–14. doi: 10.1159/000208792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bravi A, Green G, Longtin A, Seely AJ. Monitoring and Identification of Sepsis Development through a Composite Measure of Heart Rate Variability. PLoS ONE. 2012;7:e45666. doi: 10.1371/journal.pone.0045666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnaby D, Ferrick K, Kaplan DT, Shah S, Bijur P, Gallagher EJ. Heart rate variability in emergency department patients with sepsis. Acad Emerg Med. 2002;9:661–70. doi: 10.1111/j.1553-2712.2002.tb02143.x. [DOI] [PubMed] [Google Scholar]
- 35.Bradley BD, Green G, Ramsay T, Seely AJ. Impact of sedation and organ failure on continuous heart and respiratory rate variability monitoring in critically ill patients: A pilot study*. Crit Care Med. 2013;41:433–44. doi: 10.1097/CCM.0b013e31826a47de. [DOI] [PubMed] [Google Scholar]
- 36.Papaioannou VE, Verkerk AO, Amin AS, de Bakker JM. Intracardiac origin of Heart Rate Variability, Pacemaker Funny Current and their Possible Association with Critical Illness. Curr Cardiol Rev. 2012 doi: 10.2174/157340313805076359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werdan K, Schmidt H, Ebelt H, et al. Impaired regulation of cardiac function in sepsis, SIRS, and MODS. Can J Physiol Pharmacol. 2009;87:266–74. doi: 10.1139/Y09-012. [DOI] [PubMed] [Google Scholar]
- 38.Rudiger A. Beta-block the septic heart. Crit Care Med. 2010;38:S608–12. doi: 10.1097/CCM.0b013e3181f204ca. [DOI] [PubMed] [Google Scholar]
- 39.Francis DP, Willson K, Georgiadou P, et al. Physiological basis of fractal complexity properties of heart rate variability in man. J Physiol. 2002;542:619–29. doi: 10.1113/jphysiol.2001.013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.