Abstract
Polychlorinated biphenyls and their metabolites are environmental pollutants that are believed to have adverse health effects presumably by inducing oxidative stress. To determine if 1-(4-Chlorophenyl)-benzo-2,5-quinone (4-ClBQ: metabolite of 4-monochlorobiphenyl, PCB3) induced oxidative stress is associated with changes in the expression of specific antioxidant genes, mRNA levels of 92 oxidative stress-response genes were analyzed using TaqMan® Array Human Antioxidant Mechanisms (Life technologies), and results were verified by performing quantitative RT-PCR assays. The expression of selenoprotein P (sepp1) was found to be significantly downregulated (8–10-fold) in 4-ClBQ treated HaCaT human skin keratinocytes, which correlated with a significant increase in MitoSOX oxidation. Overexpression of Mn-superoxide dismutase, catalase, or treatment with N-acetyl-L-cysteine suppressed 4-ClBQ-induced toxicity. Sodium selenite supplementation also suppressed 4-ClBQ-induced decrease in sepp1 expression, which was associated with a significant inhibition in cell death. Furthermore, HaCaT cells overexpressing sepp1 were resistant to 4-ClBQ induced oxidative stress and toxicity. These results demonstrate that SEPP1 represents a previously unrecognized regulator of PCB induced biological effects. These results support the speculation that selenoproteins can be an attractive countermeasure for PCB induced adverse biological effects.
Keywords: PCB3, 4-ClBQ, HaCaT, Polychlorinated biphenyls, Selenoprotein P, Oxidative stress
Introduction
Polychlorinated biphenyls (PCBs)1 are a large group of persistent environmental pollutants. PCBs have been widely used in transformers and capacitors, plasticizers, hydraulic lubricants, flame retardants, and adhesives because of their extremely stable physical and chemical properties [1]. Due to their widespread applications, PCBs have been found in soil, air, water, and biological organisms [1]. A study conducted in 2003 reports that approximately 10% of the two million tons of PCBs produced commercially since 1929 is still present in the environment [2]. A concentration of 0.003–6.5 μM PCBs has been reported in the blood of individuals living in Anniston, Alabama, where a manufacturing plant existed from 1950s to 1970s [3]. Recent observations of a higher concentration of PCBs in indoor air and residents’ blood, building materials, and paints further elicited public concerns about the adverse biological effects of PCBs [4–6]. PCBs have been reported to cause skin toxicity, reproductive toxicity, and carcinogenicity in animals and human [1, 7].
4-monochlorobiphenyl (PCB3) is found in paints [4], soil [8], human blood [9], and recently in Chicago air [10]. PCB3 is metabolized to its hydroxylated form by cytochrome P450 enzymes, which further undergoes a second hydroxylation reaction resulting in PCB3 hydroquinone and quinone intermediates [11]. 1-(4-Chlorophenyl)-benzo-2,5-quinone (4-ClBQ), a quinone metabolite of PCB3, has been shown to generate reactive oxygen species (ROS: superoxide and hydrogen peroxide) via auto-oxidation and redox cycling [12]. Using electron paramagnetic resonance spectrometry, we have previously shown that 4-ClBQ treatment induces the production of a semiquinone radical which was associated with an increase in hydrogen peroxide levels in MCF-10A human mammary epithelial cells [13]. Prior treatments with antioxidants suppressed 4-ClBQ induced toxicity, suggesting that oxidative stress mediates 4-ClBQ induced toxicity in human mammary and prostate epithelial cells [13, 14].
Results from this study show that 4-ClBQ treatment increases cellular ROS levels in HaCaT human skin keratinocytes, which was associated with toxicity. Additional results identified selenoprotein P (SEPP1) as a previously unrecognized regulator for PCB induced toxicity.
Material and Methods
Chemicals and reagents
PCB3 and 4-ClBQ were provided by the Synthesis Core of the Iowa Superfund Research Project. The compounds were synthesized and purified as described previously [15]. Sodium selenite, N-acetyl-L-cysteine (NAC), polyethylene glycol-superoxide dismutase (PEG-SOD) and catalase (PEG-CAT) were purchased from Sigma Chemical Co (St. Louis, MO). MitoSOX Red and MitoTracker Green reagents were purchased from Molecular Probes (Eugene, OR).
Cell culture and treatments
Spontaneously immortalized human skin keratinocytes (HaCaT) were purchased from ATCC and cultured in DMEM with 10% fetal bovine serum. Dimethyl sulfoxide was used to prepare stock solutions of PCBs [13]. PCB treatments were carried out in serum-free DMEM for 24 h. Microscopic examination of cellular morphology was performed in 2% paraformaldehyde fixed cells. Cellular morphology of control and 4-ClBQ treated cells that were incubated with Phalloidin 488 (1:1000) and Hoechst (1:250) were recorded using Olympus CKX41 microscope (Olympus, Tokyo, Japan). Cell growth was assessed by counting cells using a Z1 Coulter Counter (Beckman Coulter, Fullerton, CA). A clonogenic assay was used to measure cell survival [14].
Immunoblotting
Total cellular proteins were separated on 12% SDS-PAGE and immunoblotting was performed using antibodies to human MnSOD (1:1000; Millipore, Billerica, MA) and catalase (1:1000; Athens Research and Technology, Athens, GA). ECL Plus reagent and Typhoon FLA 7000 (GE Healthcare, Waukesha, WI) were used for visualization and quantitation [16]. Actin protein levels were used for comparison.
For detection of SEPP1 protein levels, cell culture media collected from control and 4-ClBQ-treated cells were treated with acetone (1:4) and equal amounts of proteins were resolved on a 12% SDS-PAGE. Blots were incubated with primary antibody to human SEPP1 (1:200; Santa Cruz, CA) and analyzed as described above. A Coomassie-stained nonspecific polypeptide band was used for comparison.
Adenoviral infection
Replication deficient adenovirus containing CMV promoter driven human MnSOD cDNA (AdMnSOD), human catalase cDNA (AdCAT) or control plasmid DNA without any insert cDNA (AdEmpty) were obtained from the University of Iowa DNA-vector Core Facility. Cells were infected with 50 multiplicity of infection (MOI) of adenovirus following our previously published method [17]. Immunoblotting was used to evaluate MnSOD and catalase expression.
Flow cytometry assays
Cells were treated with 4-ClBQ for 24 h and mitochondrial ROS levels were determined by flow cytometry measurements of MitoSOX Red oxidation; MitoTracker Green uptake was used to measure mitochondrial mass [14]. The mean fluorescence intensity (MFI) of 10,000 cells was analyzed for each sample and corrected for autofluorescence. PEG-SOD and PEG-CAT (100 U/mL) were used to determine the specificity of MitoSOX Red oxidation for measurements of ROS (superoxide and hydrogen peroxide) levels.
Flow cytometry measurements of DNA content in ethanol-fixed and propidium iodide (35 μg/mL) stained control and 4-ClBQ treated cells were determined following our previously published protocols [17].
Human Antioxidant Mechanisms PCR array
A TaqMan® Array Human Antioxidant Mechanisms (Life technologies, Carlsbad, CA) was used to determine if the mRNA levels of any of the 92 most commonly studied oxidative stress response genes are altered in 4-ClBQ treated cells. Reverse transcription and PCR methods were performed following the manufacturer supplied protocols. Results were compiled using DataAssist™ software v3.0 (Applied Biosystems, Carlsbad, CA).
cDNA synthesis and quantitative polymerase chain reaction assay
cDNA synthesis and real-time PCR amplification were performed following our previously published methods [16]. Primer sequence for individual genes used in the PCR assay is shown in Supplemental Table 1.
Overexpression of sepp1 in HaCaT cells
Human sepp1 cDNA containing the ORF and two selenocysteine insertion sequence (SECIS) in its 3′-untranslated region was PCR amplified from the 7C1 pBluescript DNA (generous gift from Drs. Raymond F. Burk and Kristina E. Hill, Vanderbilt University). Restriction enzyme sites (Not I and Nco I) were included in the primer design for directional cloning of human sepp1 cDNA into the pShooter™ mammalian expression vector (Invitrogen, Grand Island, NY). The sequence analysis of sepp1 in the CMV-pShooter-sepp1 expression vector shows the presence of ORF (1–1146 nt; NM_005410.2), first (1386–1485 nt) and second (1821–1909 nt) SECIS sequences (Suppl. Fig. 4).
HaCaT cells were cultured to 70–80% confluence and then transfected with control (Empty; without any insert sequence) and CMV-pShooter-sepp1 plasmid DNAs using Metafectene®pro (Biontex, San Diego, CA). Flow cytometry measurements of GFP-fluorescence measured in cyto/pShooter™/GFP plasmid DNA transfected HaCaT cells showed approximately 25% transfection efficiency. Transgene expression was evaluated by measuring sepp1 mRNA and protein levels using quantitative RT-PCR and immunoblotting assays.
Statistical analysis
One-way analysis of variance followed by Tukey post-test (SPSS 19.0 software) were performed to evaluate statistical significance of results. Results are presented as mean ± SD. Results from at least n = 3 with P < 0.05 were considered significant.
Results
4-ClBQ treatment perturbs cellular morphology and increases mitochondrial ROS levels
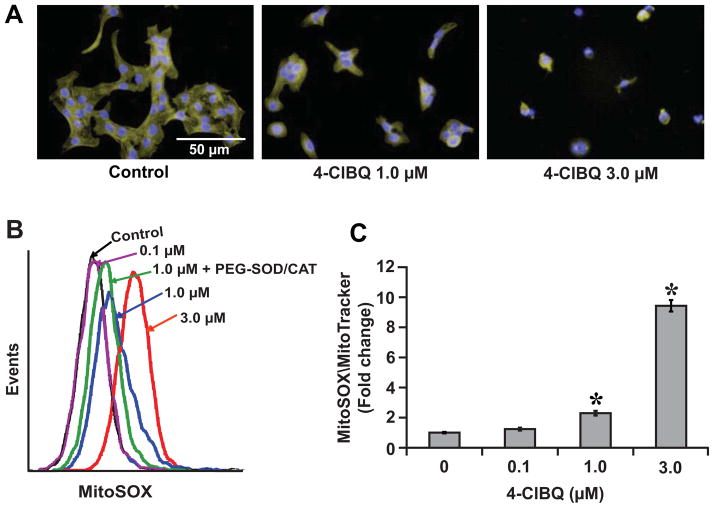
We have shown previously that 4-ClBQ treatment inhibits cellular proliferation and delays cell cycle progression as well as induces DNA damage and toxicity in human mammary and prostate epithelial cells [13, 16, 18]. Because PCB3, the parent compound of 4-ClBQ is a semi-volatile airborne PCB and skin is one of the target tissues, in this study we used HaCaT human skin keratinocytes to determine the biological effects of PCB3 and 4-ClBQ. A significant change in cellular morphology characterized by collapsed cytoplasm was observed in 4-ClBQ treated HaCaT cells (Fig. 1A). Using electron paramagnetic resonance spectrometry, we have previously shown the presence of a semiquinone radical in 4-ClBQ treated MCF-10A human mammary epithelial non-malignant cells which was associated with a significant increase in extracellular hydrogen peroxide levels [13]. To determine if 4-ClBQ treatment alters cellular ROS levels, control and 4-ClBQ treated HaCaT cells were incubated with MitoSOX Red or MitoTracker Green. The MFI of MitoSOX Red oxidation was comparable between control and 0.1 μM 4-ClBQ treated cells (Fig. 1B and 1C). However, the MFI increased 2-fold in 1.0 μM and 10-fold in 3.0 μM 4-ClBQ treated cells (Fig. 1C). 4-ClBQ induced increase in MitoSOX Red oxidation was suppressed in cells treated with PEG-SOD and PEG-CAT (Fig. 1B and Suppl. Fig. 1A), suggesting that the increase in MitoSOX Red oxidation is due to an increase in cellular superoxide and hydrogen peroxide levels. The increase in cellular ROS levels was evident as early as 4 h of 4-ClBQ treatment (Suppl. Fig. 1B). These results show that (a) 4-ClBQ treatment increases cellular ROS levels (superoxide and hydrogen peroxide) in HaCaT cells, and (b) mitochondria are the probable site of ROS generation.
Fig. 1.
4-ClBQ treatment perturbs cellular morphology and increases mitochondrial ROS levels. (A) Microscopic pictures of paraformaldehyde-fixed control and 4-ClBQ treated HaCaT cells that were stained with Phalloidin 488 and Hoechst; magnification: × 400; bars = 50 μm; n = 3. (B) Representative flow cytometry histograms of MitoSOX Red oxidation of control and 4-ClBQ treated HaCaT cells; PEG-SOD and PEG-catalase were used to determine the specificity of MitoSox Red oxidation for measurements of superoxide and hydrogen peroxide. (C) Flow cytometry measurements of MitoSOX Red oxidation and MitoTracker Green uptake in control and 4-ClBQ treated HaCaT cells at the end of 24 h treatment. MitoSOX Red oxidation was normalized to MitoTracker Green uptake in each sample, and the fold change in MFI was calculated relative to untreated cells. Asterisks represent statistical significance compared to untreated cells; P < 0.05, n = 3.
Oxidative stress mediates 4-ClBQ induced toxicity
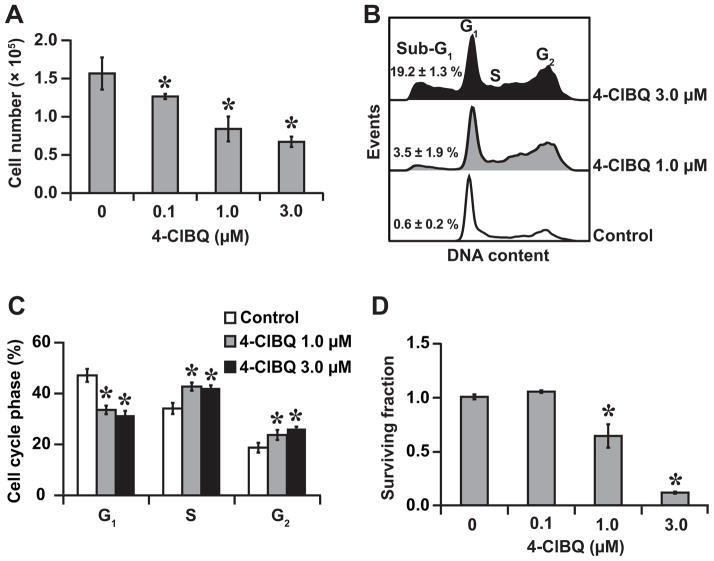
The significant change in cellular morphology and increase in mitochondrial ROS levels in 4-ClBQ treated HaCaT cells were associated with a dose dependent decrease in cell numbers (Fig. 2A). Because the decrease in cell numbers could represent a cytostatic and cytotoxic effect, flow cytometry measurements of DNA content were performed in control and 4-ClBQ treated cells. Cells treated with 4-ClBQ showed a dose dependent increase in the percentage of cells with sub-G1 DNA content (indicative of apoptosis), approximately 20% in 3.0 μM 4-ClBQ treated cells compared to less than 1% in control cells (Fig. 2B). 4-ClBQ treatment resulted in a significant accumulation of cells in S and G2 phases, which was associated with a corresponding decrease in the percentage of G1 cells (Fig. 2B and 2C), demonstrating that the 4-ClBQ treatment perturbs cell cycle progression in HaCaT cells.
Fig. 2.
4-ClBQ treatment perturbs cell cycle progression and induces cell death. (A) Cell numbers were counted at the end of 24 h treatments. (B) Representative histograms of DNA content that show an increase percentage of cells in the sub-G1, S, and G2 phases of 4-ClBQ treated cells. (C) Flow cytometry measurements of the percentage of cell cycle phases in control and 4-ClBQ treated cells. (D) Toxicity was measured using a clonogenic survival assay. Cell survival of 4-ClBQ treated cells was normalized to untreated cells. Asterisks represent statistical significance compared to untreated cells; P < 0.05, n = 3.
The cytotoxic effects of 4-ClBQ are also evident from results obtained from a clonogenic assay. Whereas 0.1 μM of 4-ClBQ treatment was found to be non-toxic, the surviving fraction decreased approximately 40% in 1.0 μM and 80% in 3.0 μM 4-ClBQ treated cells (Fig. 2D). It is interesting to note that 3- and 5-d treatments of PCB3, the parent compound of 4-ClBQ, did not result in any toxicity (Suppl. Fig. 2). These results show that while PCB3 is nontoxic, its metabolite 4-ClBQ exhibited both cytostatic and cytotoxic effects.
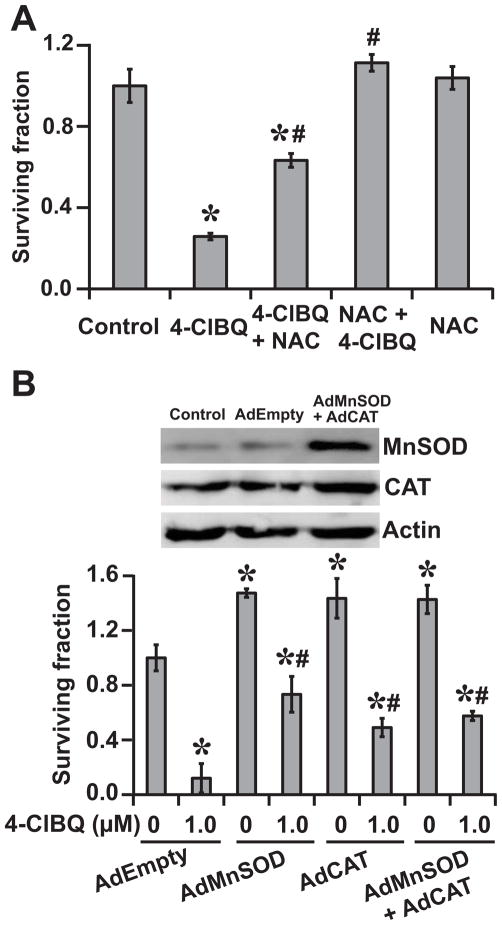
To determine if oxidative stress induces cytotoxicity in 4-ClBQ treated HaCaT cells, a clonogenic assay was used to measure cell survival in cells that were treated with 3.0 μM 4-ClBQ in presence and absence of 5.0 mM NAC. As shown before (Fig. 2D), 4-ClBQ treatment exhibited significant toxicity, which was suppressed in NAC treated cells (Fig. 3A). While pre-treatment of cells with NAC for 5 h before the addition of 4-ClBQ completely obliterated 4-ClBQ induced toxicity, NAC added 5 h after the 4-ClBQ treatment was also effective in suppressing toxicity.
Fig. 3.
Oxidative stress regulates 4-ClBQ induced toxicity. (A) Cell cultures were incubated with media containing 5.0 mM NAC 5 h prior or 5 h after the addition of 3.0 μM 4-ClBQ. Cell survival was determined by performing a clonogenic assay. Asterisks represent statistical significance compared to untreated cells; # represents statistical significance compared to 3.0 μM 4-ClBQ treated cells; P < 0.05; n = 3. (B) HaCaT cells were infected with adenoviruses AdEmpty (100 MOI), AdMnSOD (50 MOI), AdCAT (50 MOI) or AdMnSOD and AdCAT (50 MOI each). Transgene expression was measured using an immunoblotting assay. Cells were treated with 1.0 μM 4-ClBQ and survival was measured using a clonogenic assay. Surviving fraction was calculated relative to untreated AdEmpty infected cells. Asterisks represent statistical significance compared to AdEmpty infected untreated cells; # represents statistical significance compared to 1.0 μM 4-ClBQ treated AdEmpty infected cells; P < 0.05, n = 3.
The hypothesis of oxidative stress regulating 4-ClBQ induced toxicity was also evident from the results shown in Fig. 3B. Cell survival in AdEmpty infected and 4-ClBQ treated cells was found to be approximately 10%. However, overexpression of MnSOD and catalase alone or in combination was able to inhibit toxicity in 4-ClBQ treated cells. These results further demonstrate the causality of ROS (superoxide and hydrogen peroxide) regulating the toxicity of 4-ClBQ in HaCaT cells.
SEPP1 mitigates 4-ClBQ induced oxidative stress and toxicity
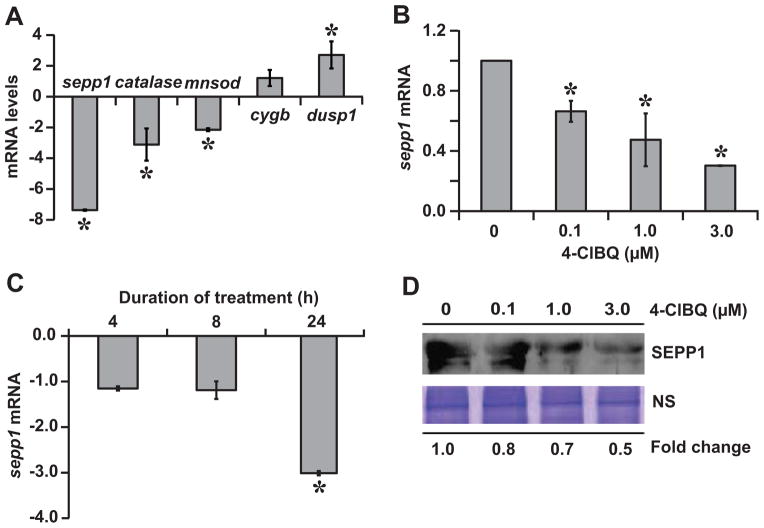
To determine if the 4-ClBQ treatment of HaCaT cells selectively affect expression of specific oxidative stress response genes, a TaqMan® Array Human Antioxidant Mechanisms was used to measure mRNA levels of the most commonly studied oxidative stress response genes in control and 4-ClBQ treated cells. These results showed approximately 2–3-fold decrease in mRNA levels of mnsod, catalase, glutathione peroxidase (gpx1, 4, and 5), thioredoxin-reductase-2, glutathione synthetase, glutathione reductase, and glutathione-S-transferase zeta-1, while mRNA levels of gpx2 and thioredoxin-reductase-1 did not change (data not shown). mRNA levels of sepp1 decreased more than 10-fold.
SEPP1 is a selenoprotein that has both antioxidant and selenium transport functions [19]. Therefore, we determined if SEPP1 regulates oxidative stress and toxicity of 4-ClBQ in HaCaT cells. Initially, a quantitative RT-PCR assay was performed to verify the PCR array results of sepp1 expression. Because PCBs have been shown earlier to negatively affect MnSOD and catalase activities [16, 20], we included mRNA levels of these two genes as positive controls. Cytoglobin (cygb) and dual-specificity phosphatase 1 (dusp1) mRNAs that showed significant increase (30-fold and 8-fold, respectively) in the PCR-array data were also included for comparison. 4-ClBQ treatment decreased mnsod (approx. 2-fold) and catalase (approx. 3-fold) mRNA levels (Fig. 4A). cygb mRNA levels did not change, while mRNA levels of dusp1 increased approximately 2-fold. sepp1 mRNA levels decreased approximately 30% in 0.1 μM and 70% in 3.0 μM 4-ClBQ treated cells (Fig. 4B). The decrease in sepp1 mRNA levels peaked at 24 h of 4-ClBQ treatment (Fig. 4C). A dose-dependent decrease in SEPP1 protein levels was also observed in 4-ClBQ treated HaCaT cells (Fig. 4D). These results show that 4-ClBQ treatments negatively affect sepp1 expression.
Fig. 4.
4-ClBQ treatment significantly inhibits sepp1 expression. (A) mRNA levels of sepp1, catalase, mnsod, cygb, and dusp1 that showed significant changes in the PCR-array were further verified by performing a quantitative RT-PCR assay; fold-change was calculated relative to individual mRNA levels in untreated cells. Quantitative RT-PCR measurements of sepp1 mRNA levels in (B) 24 h 4-ClBQ (0–3.0 μM) treated cells, and (C) 3.0 μM 4-ClBQ treated cells at the end of 4, 8, and 24 h of treatments. Fold change was calculated relative to time-matched untreated cells. Asterisks represent statistical significance compared to untreated cells; P < 0.05, n = 3. (D) Total proteins were precipitated from media collected from control and 4-ClBQ treated cells. SEPP1 protein levels were analyzed by immunoblotting. A Coomassie blue stained polypeptide band was used for loading correction.
In general, selenium is known to regulate the expression and function of selenoproteins. Therefore, we determined if supplementation of media with sodium selenite (10–100 nM) can suppress 4-ClBQ induced down-regulation of sepp1 expression. Whereas 10 and 50 nM of sodium-selenite were ineffective in suppressing 1.0 μM 4-ClBQ-induced decrease in sepp1 mRNA levels, 100 nM of sodium-selenite completely abrogated 4-ClBQ induced down-regulation of sepp1 expression (Fig. 5A and 5B; Suppl. Fig. 3). Furthermore, 100 nM of sodium-selenite protected cells from 1.0 μM 4-ClBQ induced toxicity: approximately 80% compared to 40% survival in cells that were treated with 4-ClBQ alone (Fig. 5C).
Fig. 5.
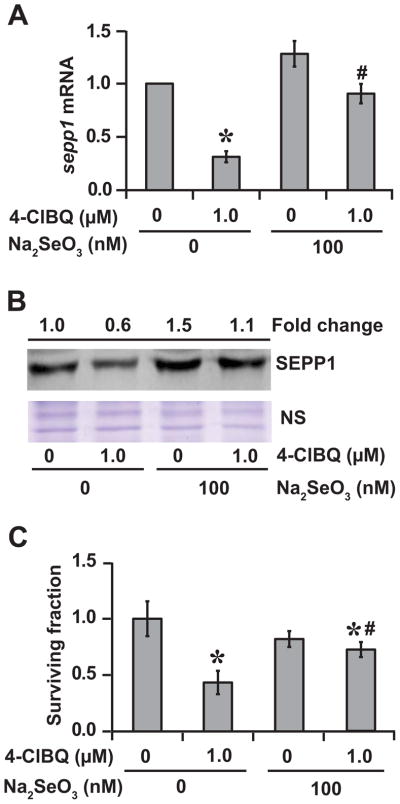
Sodium-selenite supplementation inhibits 4-ClBQ mediated down-regulation of sepp1 expression and protects HaCaT cells from 4-ClBQ induced toxicity. HaCaT cells were cultured in media supplemented with 100 nM of sodium selenite and then treated with 1.0 μM of 4-ClBQ for 24 h. (A) sepp1 mRNA levels were analyzed by performing a quantitative RT-PCR assay. Asterisks represent statistical significance compared to untreated cells; # represents statistical significance compared to 1.0 μM 4-ClBQ treated cells; P < 0.05; n = 3. (B) An immunoblotting assay was used to measure SEPP1 protein levels. A Coomassie blue stained polypeptide band was used for loading correction. (C) A clonogenic assay was used to measure cell survival. Asterisks represent statistical significance compared to untreated cells; # represents statistical significance compared to 1.0 μM 4-ClBQ treated cells; P < 0.05; n = 3.
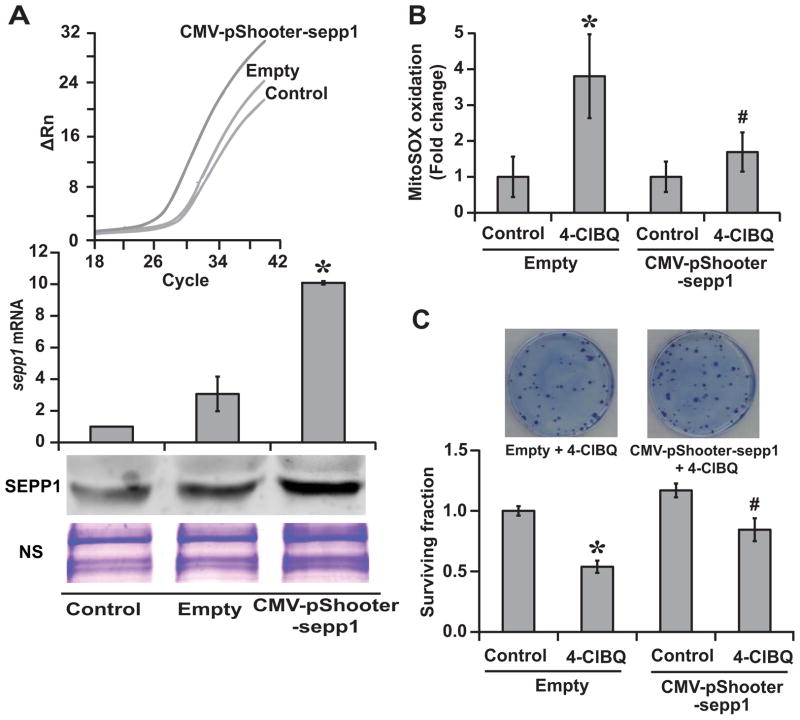
The causality of SEPP1 regulating 4-ClBQ induced oxidative stress and toxicity was further investigated in sepp1 overexpressing cells. HaCaT cells were transfected with plasmid DNA without an insert (Empty) or CMV-pShooter-sepp1 plasmid DNA that contains sepp1 ORF and two SECIS sequences (Suppl. Fig. 4). Cells were continued in culture in media supplemented with 30 nM sodium-selenite prior to and during the 4-ClBQ treatment. We selected a concentration of 30 nM sodium-selenite because this dose of sodium-selenite alone was ineffective in suppressing 4-ClBQ induced down-regulation of sepp1 expression. sepp1 mRNA levels increased approximately 8–10-fold in cells transfected with CMV-pShooter-sepp1 plasmid DNAs compared to untreated control cells (Fig. 6A). SEPP1 protein levels increased approximately 3-fold. 4-ClBQ- induced increase in MitoSOX Red oxidation was significantly suppressed in sepp1 overexpressing cells (Fig. 6B). Results from a clonogenic assay showed approximately 50% survival in 4-ClBQ treated cells that were transfected with the Empty-vector compared to more than 80% survival in 4-ClBQ treated sepp1 overexpressing cells (Fig. 6C). These results demonstrate the causality of SEPP1 regulating 4-ClBQ induced oxidative stress and toxicity in HaCaT cells.
Fig. 6.
Overexpression of sepp1 abrogates 4-ClBQ induced oxidative stress and cytotoxicity in HaCaT cells. Cells were transfected with CMV-pShooter-sepp1 plasmid DNA that contains the ORF and two SECIS sequences of human sepp1 cDNA. Cells transfected with plasmid DNAs without an insert sequence (Empty) were included as controls. Transfected cells were cultured in 30 nM sodium-selenite supplemented media prior to and during the 4-ClBQ treatment. (A) Transgene expression was evaluated by measuring sepp1 mRNA and protein levels using quantitative RT-PCR and immunoblotting assays. Upper panel shows CT plot of sepp1 amplification in Empty and CMV-pShooter-sepp1 plasmid DNA transfected cells. Asterisks represent statistical significance compared to untreated cells. P < 0.05; n = 3. (B) Flow cytometry measurements of MitoSOX Red oxidation in control and 1.0 μM 4-ClBQ treated cells transfected with Empty or CMV-pShooter-sepp1 plasmid DNA. Fold change was calculated relative to corresponding untreated cells. Asterisks represent statistical significance compared to untreated cells; # represents statistical significance compared to 1.0 μM 4-ClBQ treated empty-vector transfected cells. P < 0.05; n = 3. (C) A clonogenic assay was used to measure cell survival in 1.0 μM 4-ClBQ treated cells transfected with Empty or CMV-pShooter-sepp1 plasmid DNA. Surviving fraction was calculated relative to untreated cells transfected with Empty vector. Representative dishes showing a higher number of colonies in sepp1 overexpressing 4-ClBQ treated cells are included for comparison. Asterisks represent statistical significance compared to untreated empty-vector transfected cells; # represents statistical significance compared to 1.0 μM 4-ClBQ treated empty-vector transfected cells; P < 0.05; n = 3.
Discussion
The first report of PCB induced skin toxicity was related to the “Yusho disease” in Japan where individuals who ingested PCB-contaminated rice oil exhibited severe skin toxicity (e.g. pigmentation and acne) [21]. PCB3 is an airborne non-dioxin like PCB that has recently been detected in Chicago air [10]. In this study, we determined the cellular effects of PCB3 and its metabolite 4-ClBQ in HaCaT human skin keratinocytes. Our results show that while PCB3 is non-toxic, 4-ClBQ treatment induces oxidative stress and toxicity in HaCaT cells. 4-ClBQ treatment significantly inhibits sepp1 expression and HaCaT cells overexpressing sepp1 are resistant to 4-ClBQ induced oxidative stress and toxicity.
4-ClBQ treatment results in a significant change in cellular morphology of HaCaT cells (Fig. 1A). The change in cellular morphology was accompanied with a significant increase in mitochondrial ROS levels (based on MitoSOX Red oxidation). Suppression of MitoSOX Red oxidation in HaCaT cells pre-treated with PEG-SOD and PEG-CAT suggests that the 4-ClBQ induced increase in MitoSOX Red oxidation could be the result of an increase in cellular superoxide and hydrogen peroxide levels (Fig. 1B and 1C, Suppl. Fig. 1A). 4-ClBQ induced increase in cellular ROS levels was associated with a significant increase in the percentage of cells in the S and G2-phase (Fig. 2B and 2C), indicating that the decrease in cell number in 4-ClBQ treated cells could be due to a delay in cell cycle progression through S and G2 phases. 4-ClBQ treatment also results in cell death as evident from results obtained from the flow cytometry measurements of the percentage of cells with sub-G1 DNA content (indicative of apoptosis) and a clonogenic assay designed to assess the reproductive capacity of cells (Fig. 2B and 2D). Whereas results from both of these assays show the cytotoxicity of 4-ClBQ, the increase in the percentage of cells with sub-G1 DNA content suggest that apoptosis could be one of the cell death pathways regulating this toxicity. The apoptotic mode of cell death was also observed in macrophage J774A.1 cells that were treated with non-dioxin like PCBs (PCB101, PCB153, and PCB180) alone or in combination [22]. These authors have shown that the PCB induced apoptosis was associated with a significant decrease in Bcl-2 (anti-apoptotic) and increase in Bax (pro-apoptotic) protein levels, suggesting that the cytotoxic effects of non-dioxin PCBs could be mediated by the mitochondria-dependent apoptotic pathway. These earlier results are consistent with our observation of a significantly higher mitochondrial ROS levels in 4-ClBQ treated cells that correlated with toxicity (Fig. 1B and 1C, and Fig. 2). The observation of increased cellular ROS levels regulating 4-ClBQ induced cell death is further evident from the results shown in Fig. 3. NAC added 5 h prior to or 5 h after the 4-ClBQ treatment inhibits 4-ClBQ induced toxicity (Fig. 3A). Interestingly, these results also show that antioxidants (e.g. NAC) added after the 4-ClBQ treatment can rescue HaCaT cells from 4-ClBQ induced toxicity. While NAC is a non-specific antioxidant, results shown in Fig. 3B suggest that the 4-ClBQ induced toxicity could be mediated by an increase in cellular superoxide and hydrogen peroxide levels. Adenovirus mediated overexpression of MnSOD and catalase individually or in combination protects HaCaT cells from 4-ClBQ induced toxicity. These results suggest that an increase in cellular ROS levels, presumably of mitochondrial origin regulates 4-ClBQ induced toxicity in HaCaT cells.
Results from the PCR array and quantitative RT-PCR assays identified SEPP1 as a previously unrecognized regulator of 4-ClBQ induced toxicity (Figs. 4–6). SEPP1 is one of the twenty five human selenoproteins that has both antioxidant and selenium transport functions [19]. It is believed that the redox-motif (UxxC) in the N-terminal confers the antioxidant properties of SEPP1, while the other 9 selenocysteines in the C-terminal give its selenium transport properties. SEPP1 is an extracellular glycoprotein that carries approximately 50% of selenium content in human plasma [23], and as such plasma SEPP1 levels have been proposed as a reliable biomarker for assessing selenium nutritional requirement [24]. Results from a quantitative RT-PCR assay showed both dose and duration dependent decrease in sepp1 mRNA levels in 4-ClBQ treated HaCaT cells (Fig. 4B and 4C), which was associated with a corresponding decrease in SEPP1 protein levels (Fig. 4D). The mechanisms regulating sepp1 expression in 4-ClBQ treated cells are currently unknown. However, a recent study reports decreased hepatic levels of selenium and zinc in PCB126 treated rat livers correlating with a decrease in Se-GPx1 activity [25]. Previous studies also report decreased levels of hepatic selenium following PCB77 [26] and TCDD treatments [27]. PCB77 induced decrease in selenium was associated with a decrease in rat liver Se-GPx1 activity and gpx1 mRNA levels [26]. These previous literature reports and results presented here suggest that PCB induced decrease in selenoprotein expression could be a major mechanism of PCB-induced toxicity.
The relationship between selenoproteins and PCB induced biological effects is also evident from results shown in Fig. 5. Pre-treatment of HaCaT cells with 100 nM of sodium-selenite suppressed 4-ClBQ induced decrease in sepp1 expression, which was also associated with a significant protection from 4-ClBQ induced toxicity. The protective effect of selenium has also been reported earlier by Hassan et al [27]. Hepatic lipid peroxidation was significantly higher in TCDD fed rats that were deficient in selenium. Intravenous delivery of sodium-selenite increased plasma SEPP1 levels and suppressed diquat-induced liver injury [28]. Selenium-supplementation in drinking water inhibits cadmium induced downregulation of testicular sepp1 expression and lipid peroxidation in rats [29]. Furthermore, hepatocyte-derived SEPP1 protects primary human astrocyte against tert-butyl hydroperoxide-induced oxidative stress and cell death [30]. Thus, SEPP1 could be a major selenoprotein regulating PCB induced oxidative stress and toxicity.
Because selenium-supplementation is anticipated to affect any one of the 25 selenoproteins, the specificity of the effect of sodium-selenite on sepp1 expression and protection from 4-ClBQ induced toxicity is not unequivocal. In fact, GPx1 activity in 1.0 μM 4-ClBQ treated cells was approximately 3 mU/mg protein compared to 6 mU/mg protein in control (Suppl. Fig. 5). GPx1 activity increased to approximately 10 mU/mg protein in cells cultured in media supplemented with 100 nM sodium-selenite, and 4-ClBQ treatment had no additional effects on GPx1 activity (Suppl. Fig. 5).
To further determine the specificity of SEPP1 regulating 4-ClBQ induced oxidative stress and toxicity, experiments were repeated using HaCaT cells overexpressing human sepp1 (Fig. 6). CMV-pShooter-sepp1 plasmid DNA transfected cells exhibited approximately 10-fold increase in sepp1 mRNA levels, and 3-fold increase in SEPP1 protein levels (Fig. 6A). It is interesting to note that sepp1 overexpression suppressed 4-ClBQ-induced increases in cellular ROS levels and toxicity (Fig. 6B and 6C). Considering SEPP1 a secretory protein, it is currently unknown how overexpression of SEPP1 suppressed 4-ClBQ induced toxicity in HaCaT cells. Recent reports suggest that apolipoprotein E receptor 2 (ApoER2) in testis and megalin receptor in kidney tubule epithelial cells are required for SEPP1 uptake [31, 32]. Endocytosis of SEPP1 via ApoER2 and heparin sulfate proteoglycans has also been reported for rat L8 myoblasts cultured in vitro [33]. While specific mechanisms regulating SEPP1 uptake in HaCaT cells are currently under investigation, our results show sepp1 overexpression mitigates 4-ClBQ induced oxidative stress and toxicity in HaCaT human skin keratinocytes.
In summary, our results identify SEPP1 as a previously unrecognized regulator of PCB-induced oxidative stress and toxicity. Furthermore, these results also support the speculation that nutrient-based manipulations of selenoproteins can be an attractive countermeasure for PCB induced adverse biological effects.
Supplementary Material
4-monochlorobiphenyl (PCB3) is an environmental pollutant
1-(4-Chlorophenyl)-benzo-2,5-quinone (4-ClBQ), a metabolite of PCB3 is toxic
Selenoprotein P (SEPP1) regulates 4-ClBQ induced oxidative stress
Selenium supplementation and sepp1 overexpression ameliorate toxicity of 4-ClBQ
Acknowledgments
We thank Professors Raymond F. Burk and Kristina E. Hill at the Vanderbilt University for the 7C1 pBluescript DNA; Professors Larry W. Robertson and Hans J. Lehmler at the Occupational & Environmental Health University of Iowa for providing us with PCB3 and its metabolite 4-ClBQ; and the staff at the Flow Cytometry, and the Radiation and Free Radical Research Core facilities. This study was supported by NIEHS P42ES013661 and NIH 2R01CA111365. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
Footnotes
Abbreviations: 4-ClBQ, 1-(4-Chlorophenyl)-benzo-2,5-quinone; AdEmpty, adenovirus carrying a control vector without an insert; AdCAT, adenovirus carrying a human catalase cDNA; AdMnSOD, adenovirus carrying a human Mn-superoxide dismutase cDNA; MFI, mean fluorescence intensity; NAC, N-acetyl-L-cysteine; PCB3, 4-monochlorobiphenyl; PEG-CAT, polyethylene glycol-catalase; PEG-SOD, polyethylene glycol-superoxide dismutase; ROS, reactive oxygen species; SECIS, selenocysteine insertion sequence; SEPP1, selenoprotein P
Authors disclosure statement
The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Safe S. Toxicology, structure-function relationship, and human and environmental health impacts of Polychlorinated biphenyls: progress and problems. Environ Health Perspect. 1993;100:259–268. doi: 10.1289/ehp.93100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Polychlorinated biphenyls: human health aspects. Concise International Chemical Assessment Document. 2003;55:1–57. [Google Scholar]
- 3.Hansen LG, DeCaprio AP, Nisbet ICT. PCB congener comparisons reveal exposure histories for residents of Anniston, Alabama, USA. Fresenius Environ Bull. 2003;12:181–190. [Google Scholar]
- 4.Hu D, Hornbuckle KC. Inadvertent Polychlorinated biphenyls in commercial paint pigments. Environ Sci Technol. 2009;44:2822–2827. doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudel RA, Seryak LM, Brody JG. PCB-containing wood floor finish is a likely source of elevated PCBs in residents’ blood, household air and dust: a case study of exposure. Environ Health. 2008;7:2. doi: 10.1186/1476-069X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson M, Ottesen RT, Volden T. Building materials as a source of PCB pollution in Bergen, Norway. Sci Total Environ. 2004;325:139–144. doi: 10.1016/j.scitotenv.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Xiao W, Li K, Wu Q, Nishimura N, Chang X, Zhou Z. Influence of persistent thyroxine reduction on spermatogenesis in rats neonatally exposed to 2,2′,4,4′,5,5′-hexa-chlorobiphenyl. Birth Defects Res B Dev Reprod Toxicol. 2010;89:18–25. doi: 10.1002/bdrb.20213. [DOI] [PubMed] [Google Scholar]
- 8.Martinez A, Erdman NR, Rodenburg ZL, Eastling PM, Hornbuckle KC. Spatial distribution of chlordanes and PCB congeners in soil in Cedar Rapids, Iowa, USA. Environ Pollut. 2012;161:222–228. doi: 10.1016/j.envpol.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeCaprio AP, Johnson GW, Tarbell AM, Carpenter DO, Chiarenzelli JR, Morse GS, Santiago-Rivera AL, Schymura MJ. Polychlorinated biphenyl (PCB) exposure assessment by multivariate statistical analysis of serum congener profiles in an adult Native American population. Environ Res. 2005;98:284–302. doi: 10.1016/j.envres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Hu D, Lehmler HJ, Martinez A, Wang K, Hornbuckle KC. Atmospheric PCB congeners across Chicago. Atmos environ. 2010;44:1550–1557. doi: 10.1016/j.atmosenv.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLean MR, Bauer U, Amaro AR, Robertson LW. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem Res Toxicol. 1996;9: 158–164. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- 12.Song Y, Wagner BA, Witmer JR, Lehmler HJ, Buettner GR. Nonenzymatic displacement of chlorine and formation of free radicals upon the reaction of glutathione with PCB quinones. Proc Natl Acad Sci USA. 2009;106:9725–9730. doi: 10.1073/pnas.0810352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkatesha VA, Venkataraman S, Sarsour EH, Kalen AL, Buettner GR, Robertson LW, Lehmler HJ, Goswami PC. Catalase ameliorates Polychlorinated biphenyl-induced cytotoxicity in nonmalignant human breast epithelial cells. Free Radic Biol Med. 2008;45:1094–1102. doi: 10.1016/j.freeradbiomed.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Kalen AL, Li L, Lehmler HJ, Robertson LW, Goswami PC, Spitz DR, Aykin-Burns N. Polychlorinated-biphenyl-induced oxidative stress and cytotoxicity can be mitigated by antioxidants after exposure. Free Radic Biol Med. 2009;47:1762–1771. doi: 10.1016/j.freeradbiomed.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehmler HJ, Robertson LW. Synthesis of Polychlorinated biphenyls (PCBs) using the Suzuki-coupling. Chemosphere. 2001;45:137–143. doi: 10.1016/s0045-6535(00)00546-4. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhuri L, Sarsour EH, Kalen AL, Aykin-Burns N, Spitz DR, Goswami PC. Polychlorinated biphenyl induced ROS signaling delays the entry of quiescent human breast epithelial cells into the proliferative cycle. Free Radic Biol Med. 2010;49:40–49. doi: 10.1016/j.freeradbiomed.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarsour EH, Agarwal M, Pandita TK, Oberley LW, Goswami PC. Manganese superoxide dismutase protects the proliferative capacity of confluent normal human fibroblasts. J Biol Chem. 2005;280:18033–18041. doi: 10.1074/jbc.M501939200. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhuri L, Sarsour EH, Goswami PC. 2-(4-Chlorophenyl)benzo-1,4-quinone induced ROS-signaling inhibits proliferation in human non-malignant prostate epithelial cells. Environ Int. 2010;36:924–930. doi: 10.1016/j.envint.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burk RF, Hill KE. Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr. 2005;25:215–235. doi: 10.1146/annurev.nutr.24.012003.132120. [DOI] [PubMed] [Google Scholar]
- 20.Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J. Polychlorinated biphenyl (Aroclor 1254) inhibits testosterone biosynthesis and antioxidant enzymes in cultured rat Leydig cells. Reprod Toxicol. 2008;25:447–454. doi: 10.1016/j.reprotox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Kuratsune M, Yoshimura T, Matsuzaka J, Yamaguchi A. Epidemiologic study on Yusho, a poisoning caused by ingestion of rice oil contaminated with a commercial brand of Polychlorinated biphenyls. Environ Health Perspect. 1972;1:119–128. doi: 10.1289/ehp.7201119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrante MC, Mattace Raso G, Esposito E, Bianco G, Iacono A, Clausi MT, Amero P, Santoro A, Simeoli R, Autore G, Meli R. Effects of non-dioxin-like Polychlorinated biphenyl congeners (PCB 101, PCB 153 and PCB 180) alone or mixed on J774A.1 macrophage cell line: modification of apoptotic pathway. Toxicol Lett. 2011;202:61–68. doi: 10.1016/j.toxlet.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Takahashi K. Characterization of selenoprotein P as a selenium supply protein. Eur J Biochem. 2002;269:5746–5751. doi: 10.1046/j.1432-1033.2002.03298.x. [DOI] [PubMed] [Google Scholar]
- 24.Xia Y, Hill KE, Li P, Xu J, Zhou D, Motley AK, Wang L, Byrne DW, Burk RF. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: a placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am J Clin Nutr. 2010;92:525–531. doi: 10.3945/ajcn.2010.29642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai I, Chai Y, Simmons D, Luthe G, Coleman MC, Spitz D, Haschek WM, Ludewig G, Robertson LW. Acute toxicity of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: effects on hepatic oxidative stress, glutathione and metals status. Environ Int. 2010;36:918–923. doi: 10.1016/j.envint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twaroski TP, O’Brien ML, Robertson LW. Effects of selected Polychlorinated biphenyl (PCB) congeners on hepatic glutathione, glutathione-related enzymes, and selenium status: implications for oxidative stress. Biochem Pharmacol. 2001;62:273–281. doi: 10.1016/s0006-2952(01)00668-2. [DOI] [PubMed] [Google Scholar]
- 27.Hassan MQ, Stohs SJ, Murray WJ, Birt DF. Dietary selenium, glutathione peroxidase activity, and toxicity of 2,3,7,8-tetrachloro-dibenzo-p-dioxin. J Toxicol Environ Health. 1985;15:405–415. doi: 10.1080/15287398509530668. [DOI] [PubMed] [Google Scholar]
- 28.Burk RF, Hill KE, Awad JA, Morrow JD, Kato T, Cockell KA, Lyons PR. Pathogenesis of diquat-induced liver necrosis in selenium-deficient rats: assessment of the roles of lipid peroxidation and selenoprotein P. Hepatology. 1995;21:561–569. [PubMed] [Google Scholar]
- 29.Messaoudi I, Banni M, Said L, Said K, Kerkeni A. Involvement of selenoprotein P and GPx4 gene expression in cadmium-induced testicular pathophysiology in rat. Chem Biol Interact. 2010;188:94–101. doi: 10.1016/j.cbi.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Steinbrenner H, Alili L, Bilgic E, Sies H, Brenneisen P. Involvement of selenoprotein P in protection of human astrocytes from oxidative damage. Free Radic Biol Med. 2006;40:1513–1523. doi: 10.1016/j.freeradbiomed.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Masiulis I, Quill TA, Burk RF, Herz J. Differential functions of the ApoER2 intracellular domain in selenium uptake and cell signaling. Biol Chem. 2009;390:67–73. doi: 10.1515/BC.2009.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson GE, Winfrey VP, Hill KE, Burk RF. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J Biol Chem. 2008;283:6854–6860. doi: 10.1074/jbc.M709945200. [DOI] [PubMed] [Google Scholar]
- 33.Kurokawa S, Hill KE, McDonald WH, Burk RF. Long isoform mouse selenoprotein P (Sepp1) supplies rat myoblast L8 cells with selenium via endocytosis mediated by heparin binding properties and apolipoprotein E receptor-2 (ApoER2) J Biol Chem. 2012;287:28717–28726. doi: 10.1074/jbc.M112.383521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.