Many animals, including insects, are known to use visual landmarks to orient in their environment. In Drosophila melanogaster, behavioral genetics studies have identified a higher brain structure called the central complex as being required for the fly’s innate responses to vertical visual features1 and its short- and long-term memory for visual patterns2–4. But whether and how neurons of the fly central complex represent visual features is unknown. We used two-photon calcium imaging in head-fixed walking and flying flies to probe visuomotor responses of ring neurons—a class of central complex neurons that have been implicated in landmark-driven spatial memory in walking flies2,3 and memory for visual patterns in tethered flying flies5. We found that dendrites of ring neurons are visually responsive and arranged retinotopically. Ring neuron receptive fields comprise both excitatory and inhibitory subfields, resembling those of simple cells in mammalian primary visual cortex. Ring neurons show strong and, in some cases, direction-selective orientation tuning, with a notable preference for vertically oriented features similar to those that evoke innate responses in flies1,2. Visual responses were diminished during flight, but, in contrast with the hypothesized role of the central complex in the control of locomotion6, not modulated during walking. Taken together, these results suggest that ring neurons represent behaviorally relevant visual features in the fly’s environment, enabling downstream central complex circuits to produce appropriate motor commands6. More broadly, this study opens the door to mechanistic investigations of circuit computations underlying visually guided action selection in the Drosophila central complex.
Flies display a variety of visual pattern- and position-dependent behaviors, including stripe fixation2, short-term orientation memory2, pattern learning4, and place learning3. Common to these behaviors is a need to detect and respond to specific features in the insect’s visual surroundings. In addition, all these behaviors require the central complex1–5, a deep brain region that has also been implicated in motor control6. We used two-photon calcium imaging in genetically targeted populations of central complex input neurons in behaving flies to investigate their potential visuomotor role. We focused on the dendritic responses of ring neurons—neurons that connect the lateral triangle (LTr) to the ellipsoid body (EB)7–9 (Fig. 1a), and that have been specifically implicated in visuomotor memory2,3.
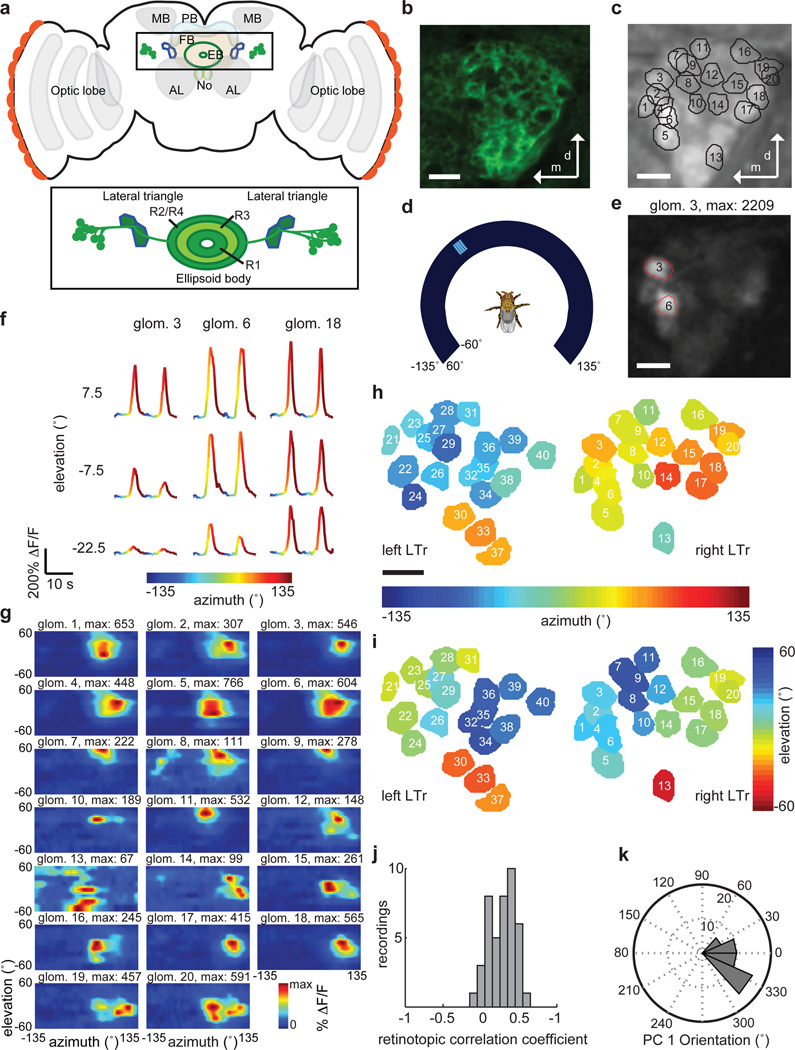
Figure 1. Drosophila ellipsoid body ring neurons arborize in visually responsive LTr microglomeruli that show a retinotopic organization.
a, Schematic of fly central brain showing antennal lobe (AL), mushroom bodies calyces (MB) and optic lobes along with sub-structures of the central complex: ellipsoid body (EB), fan-shaped body (FB), protocerebral bridge (PB), and noduli (NO). Inset: EB ring neurons (R1–R4) and LTr. b, Frame of a confocal stack showing LTr microglomeruli labeled by pan-neuronal GFP expression. c, Projection of two-photon calcium imaging video of LTr with overlay of microglomeruli selected based on responses to moving visual stimuli. 20±5 microglomeruli delineated in individual planes of focus; 30±4 microglomeruli over multiple planes of focus (n = 11 flies) (see Methods). d, Schematic of RF mapping setup with fly positioned in center of curved visual display. e, Sample frame from trial showing responses in selected glomeruli (red outlines). f, Calcium transients of three LTr microglomeruli in response to visual stimulus moving left to right in front of the fly at different elevations. g, Two-dimensional response maps (two-trial averages) for all microglomeruli shown in c. h, LTr microglomeruli from left and right hemisphere of same fly, colored according to center of RF in azimuth, and i, elevation. j, Histogram of correlation coefficients between RF center and anatomical position. For n = 42 focal planes with 20±5 glomeruli, correlation is significantly different than for randomly arranged microglomeruli (r = 0.27±0.17, p = 2.3·10−17, n = 11 flies), indicating retinotopy in the organization of microglomeruli across flies. k, Histogram of primary retinotopic axis of LTr map as found by principal component analysis (see Methods, n = 42 focal planes, 11 flies). All scale bars: 5µm.
Electron microscopy in the locust has shown that dendrites of ring neuron analogs arborize in specialized structures in the LTr called microglomeruli, where they are contacted by axonal projections from visual areas10. Confocal images of the Drosophila LTr labeled with GFP under the control of a pan-neuronal driver line, R57C1011, revealed a similar dense microglomerular substructure in the region (Fig. 1b, Supplementary Videos 1–4).
Do LTr microglomeruli respond to visual input? We used two-photon imaging with the calcium indicator GCaMP expressed pan-neuronally to record neural activity in the LTr of head-fixed Drosophila placed at the center of a visual arena (Fig. 1c,d). Flies were presented with small bright vertical bars moving horizontally at different elevations, and we recorded LTr calcium transients from multiple planes of focus on one or both sides of the brain in a single experiment (see Methods). Calcium transients showed strong temporal correlations at the spatial scale expected of LTr microglomeruli (Fig. 1e, Supplementary Video 5; Extended Data Fig. 1a). Visual stimuli evoked robust calcium transients in a subset of microglomeruli, but only when the localized stimuli were in specific spatial locations around the fly (Fig. 1f). We computed receptive fields (RFs) for responsive microglomeruli, and found that a majority of RFs are centered in the ipsilateral visual hemifield (Fig. 1g–i, Extended Data Fig. 1b and Methods). Finally, LTr microglomeruli are clustered retinotopically, and principal component analysis based on RF centers suggests that they form a spatial map with axes that are almost parallel to the fly’s visual field (Fig. 1j,k, Extended Data Fig. 1c–e).
We next examined the anatomical relationship between the LTr and individual classes of ring neurons that send arbors to the region8,12. We studied dendritic arborization patterns of ring neurons targeted by EB1-GAL4, which labels R2 ring neurons required for pattern memory5, and c232-GAL4, which labels R3/R4d neurons required for spatial memory2 (Supplementary Video 1,2 for R3/R4d; Supplementary Video 3,4 for R2). In agreement with past anatomical work9, different ring neuron classes arborize in specific contiguous parts of the LTr (Extended Data Fig. 2a,b). Each ring neuron in these classes extends dendrites into a single microglomerulus in the LTr, and sends axonal arbors throughout a class-specific ring of the EB (Fig. 2a, Extended Data Fig. 2c–e, see also7,12).
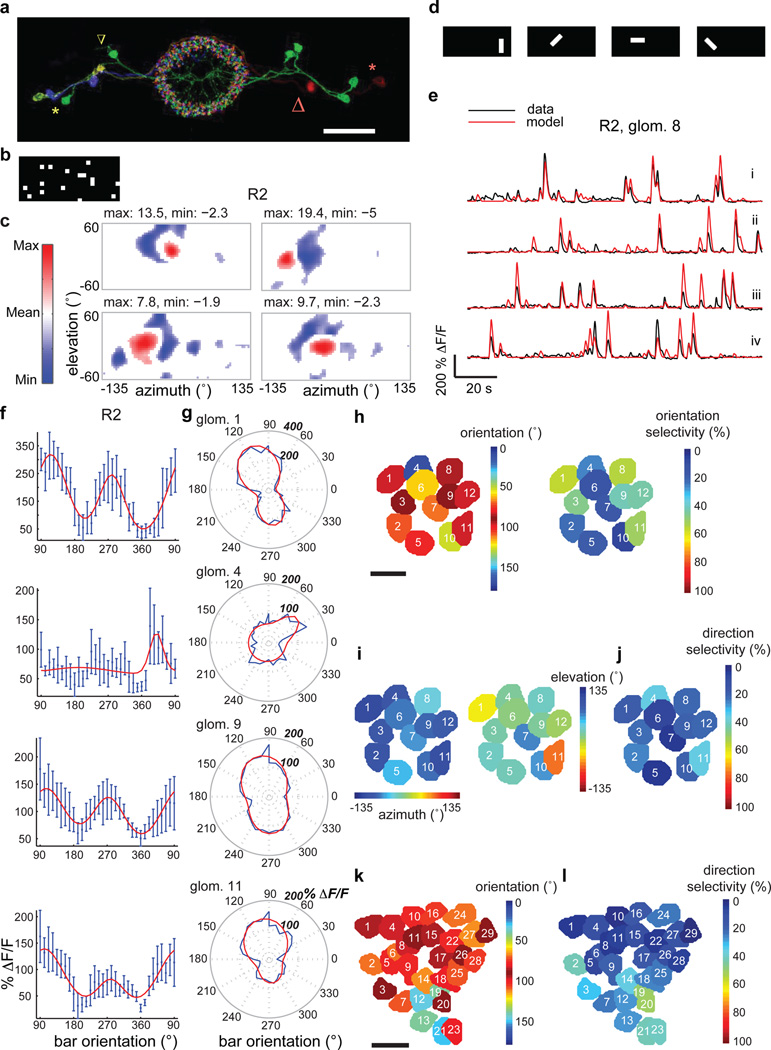
Figure 2. Ring neurons are tuned to specific visual features and orientations.
a, Multicolor FLP-out of R2 neurons showing three cell bodies on each side along with their color-matched microglomeruli (green, light green and purple at left; two green and one red at right). Red and yellow stars mark two cell bodies (one on each side, lateral) and arrows of like color their respective LTr microglomeruli (medial). All neurons send processes throughout EB rings. Scale bar: 30µm. b, Example frame of white noise stimulus used for RF mapping using reverse correlation. c, Sample RFs of R2 microglomeruli. Red subfields: excitatory responses > 30% of maximum; blue subfields: inhibitory responses < 30% of minimum of mean-subtracted weighted average. See Extended Data Fig. 8 for all RFs. d, Bright bars with four different orientations used as test features. e, Modeled and actual (black) ΔF/F changes of an R2 microglomerulus in response to differently oriented bars (fly 2 in Extended Data Fig. 7). In red: (i) trial used for fitting parameters, and, (ii)–(iv) tests. f, Orientation tuning curves for R2 neurons (two-trial average, fit in red). 90° corresponds to back-to-front movement of vertical bar, 270° to front-to-back movement of vertical bar. Error bars: standard deviation. g, Polar plots of orientation tuning data and fits (red) for data shown in f. h, Microglomeruli of R2 neurons colored by orientation preference (collapsed to 0°–180°) and orientation selectivity (two-trial average). i, Same microglomeruli as in h colored by azimuth and elevation of center of their excitatory RFs, measured using horizontally moving bars as described in Extended Data Fig. 1 (two-trial average). j, Direction selectivity of same microglomeruli (two-trial average). k, Preferred orientation (collapsed to 0°–180°) and l, direction selectivity of microglomeruli in pan-neuronal line (both four-trial average). See Methods for analysis details. Scale bar for h–k: 5µm.
To understand whether different types of ring neurons have distinctive visual response properties, we mapped RFs for dendritic microglomeruli of R3/R4d and R2 ring neurons (Extended Data Fig. 3a–c, and Extended Data Fig. 3d–f respectively). We found visual responses in ~7/40 c232-GAL4-labeled microglomeruli—corresponding to ~7/20 R4d microglomeruli (Extended Data Fig. 3a)—and ~14/20 of R2 microglomeruli labeled by EB1-GAL4. RFs for R2 and R4d neurons cover large parts of the visual field, with highest density near the midline of the ipsilateral visual field (Extended Data Fig. 3g,h). In summary, R4d and R2 microglomeruli appear to have similar visual response properties and overlapping RFs, but with peak sensitivity in different parts of the visual field (Extended Data Fig. 3i–k).
We next probed the fine structure of microglomerular RFs using sparse white noise stimuli (Fig. 2b, see Methods). Reverse correlation of microglomerular responses revealed prominent inhibitory subfields in the RFs (Fig. 2c for R2, Fig. 3a for R4d). The spatial scales of RF structure we observe is within the range for visual features that evoke strong innate responses in flies, and that are used for visual pattern learning in tethered flies2,3. To test the validity of these white-noise-based RFs, we used them to predict responses to novel bar stimuli (Fig. 2d, see Methods). The predicted responses captured much of the temporal and spatial variation in the data (Fig. 2e), with high correlations between estimated and actual responses (Extended Data Fig. 4a,b).
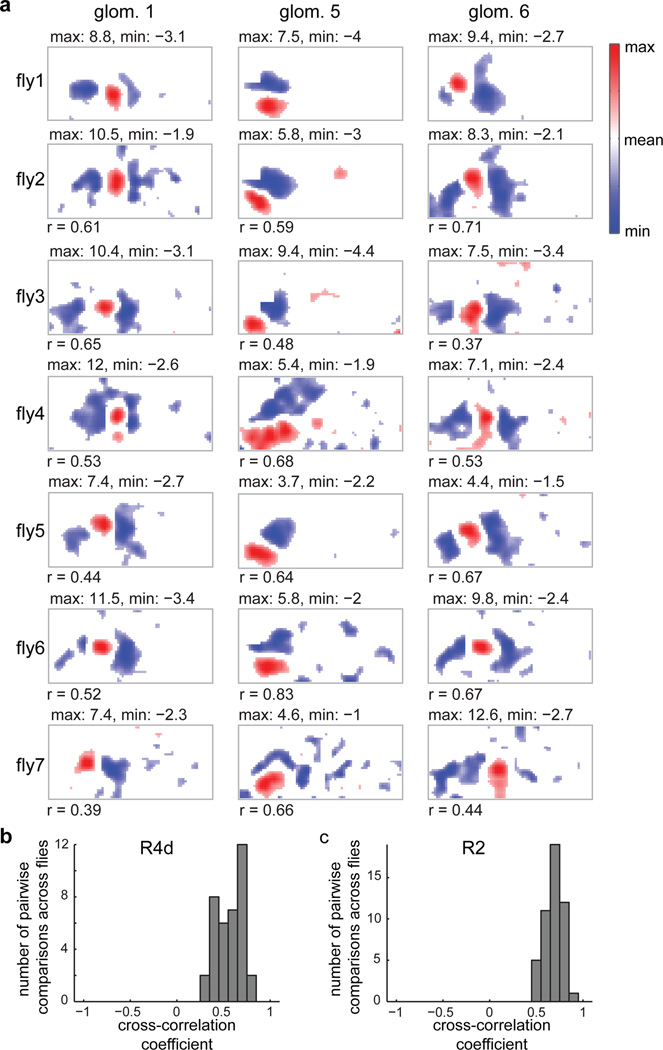
Figure 3. Ring neuron LTr microglomeruli show stereotyped RF properties across flies.
a, Subset of RFs measured in R4d neurons across seven flies aligned by similarity (see Extended Data Fig. 8b for full set). Numbers below RFs are cross-correlations with top RF in column as template. b, Histogram of cross-correlation values calculated for R4d neuron RFs with best-matched template. c, Histogram of cross-correlation values for R2 neurons (n = 6 flies, see Extended Data Fig. 8a for RFs).
Noting that the RF structure of ring neuron inputs resembles those of simple cells in mammalian primary visual cortex13, we next asked if these neurons share other response properties. Indeed, when we presented flies with a series of moving oriented bars, we found strong orientation tuning in microglomerular response patterns (Fig. 2f,g). As expected for RF structures with both excitatory and inhibitory lobes, microglomeruli also showed orientation tuning when presented with bars of opposite contrast, i.e., dark bars on a bright background (Extended Data Fig. 5a,b), as are often used in fly behavioral studies1,2. Examining orientation tuning across the population of microglomeruli, we observed a strong preference for vertically oriented bars (Fig. 2h and Extended Data Fig. 4h,i for R2, Extended Data Fig. 4c,k,l for R4d, Fig. 2k and Extended Data Fig. 4g,n,o for pan-neuronal line). RFs tuned to vertical orientations are distributed across the visual field (Fig. 2i,j for R2, Extended Data Fig. 4c,d for R4d, Extended Data Fig. 4f for pan-neuronal line). A significant fraction of neurons also shows direction-selectivity (Fig. 2j and Extended Data Fig. 4j for R2, Extended Data Fig. 4e,m for R4d, Fig. 2l, Extended Data Fig. 4p for pan-neuronal line).
Are response properties of ring neuron dendrites stereotyped? We found strong correlations across flies in RF structure for R4d (Fig. 3a–b, Extended Data Fig. 6,8), and R2 (Fig. 3c, Extended Data Fig. 7,8).
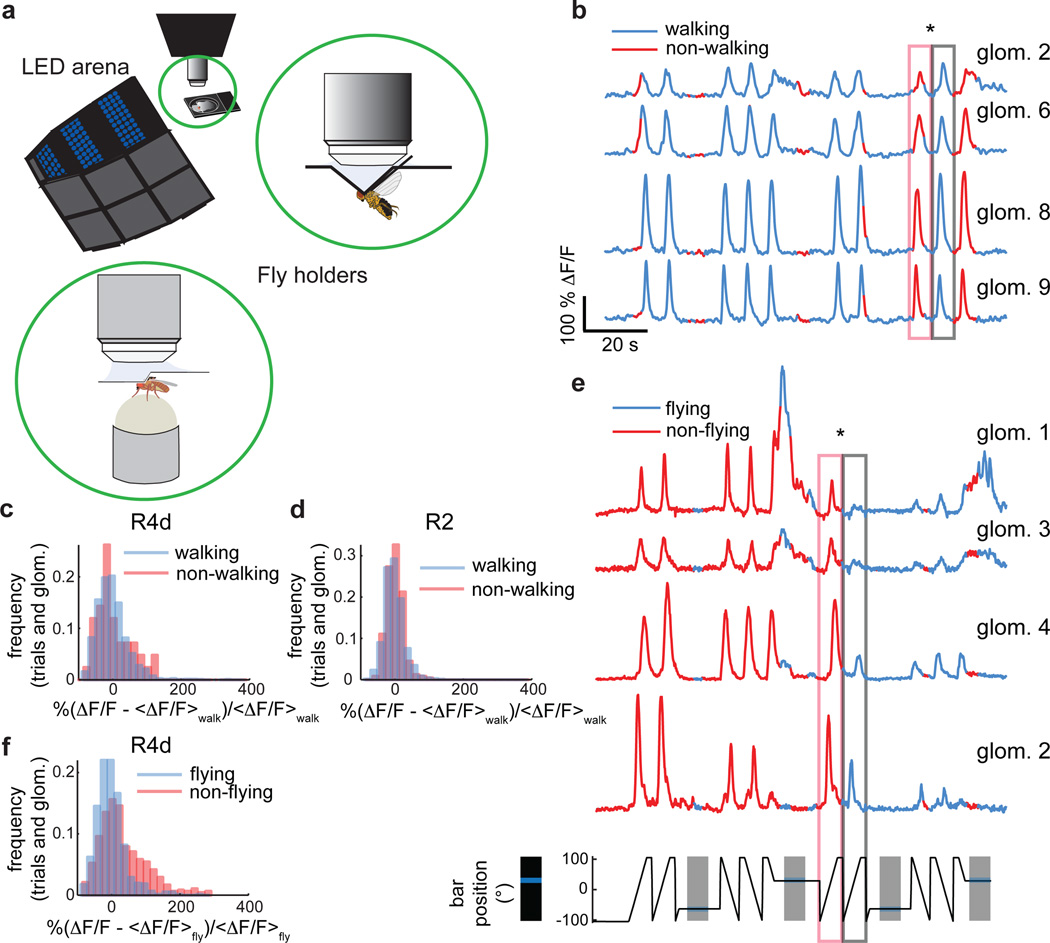
Ring neurons and the EB have often been ascribed a role in complex visuomotor tasks2,3. We examined the possible motor function of ring neurons by assessing potential correlations between neural activity and locomotion in tethered flies walking on an air-supported ball14 or flying15, in darkness or in the presence of a bright stripe moving left or right in front of the fly (Fig. 4a, see also Methods and Supplementary Video 6,7). Although some R3/R4d neurons did show occasional correlations with locomotion when flies walked in the dark, and responses from visually stimulated animals showed occasional modulation during walking, the changes were within the expected variability of visual responses in stationary flies (Fig. 4b–d). Responses were also insensitive to walking direction. Overall, LTr visual responses could be modeled accurately without taking walking state into account (Extended Data Fig. 9a–e and 10). By contrast, responses to visual stimuli were consistently diminished during tethered flight (Fig. 4e,f, Extended Data Fig. 9f–h, Supplementary Video 7), but showed no obvious correlations with flight direction as assessed by differences in wingbeat amplitude envelope. Thus, ring neuron LTr responses were modulated by motor state, but not in a manner consistent with a direct role in motor coordination, and in a markedly different manner than in the optic lobe15,16.
Figure 4. Ring neuron visual responses are not significantly modulated during walking but diminished during flight.
a, Setup for two-photon imaging in behaving flies. Insets: Schematic of fly tethered in flying holder, and positioned on air-supported ball in walking holder. b, Subset of simultaneously recorded R2 neurons during walking. Starred boxes: responses to identical visual stimuli when fly is stationary versus walking. Azimuthal position of visual stimulus shown in e (bottom). c, Distributions of R4d neuron visual responses during walking and non-walking conditions are not significantly different (n = 14 flies, trialswalking = 1722, trialswalking<50% = 42, meanwalking = 0±44.3, meanwalking<50% = 1.1±49.7, p = 0.45). d, Same as c for R2 neurons (n = 8 flies, trialswalking = 2015, trialswalking<50% = 245, meanwalking = 0±28.1, meanwalking<50% = −2.2±24.4, p = 0.37). e, Subset of simultaneously recorded R4d microglomeruli during flight. Starred boxes: diminished responses to identical visual stimuli during flight. f, Distributions of all R4d microglomeruli recorded shows significant shift towards lower responses during flight (n = 13 flies, trialsflying = 759, trialsflying<50% = 481, meanflying = 0±42.2, meanflying<50% = 31.2±72.3, p = 6·10−15). All p-values: two-sample Kolmogorov-Smirnov test.
Behavioral genetics studies in Drosophila have suggested that the central complex is required for a wide range of important sensorimotor functions. However, in the absence of physiological recordings from the region in flies, it has been challenging to constrain its role in the diversity of behaviors that it has been implicated in. We studied the visuomotor responses of ring neurons, which provide input from the LTr to the ellipsoid body of the central complex. Analogous neurons in other insects respond to polarized and unpolarized light17–19, and to mechanosensory stimulation20, one of the sensory modalities that we did not explore but that may partly account for unresponsive neurons in our study. We found that R2 and R4d ring neurons are visually responsive, and these responses are not significantly modulated by walking state. Although visual responses are diminished during flight, they do not vary systematically with wingbeat patterns associated with turns. It is possible that outputs of these ring neurons within the EB rings could be more sensitive to motor actions, but our physiological results in their dendrites during behavior are inconsistent with a major role for these ring neurons in motor coordination in the fly. Further, the high degree of stereotypy that we observe in ring neuron receptive field characteristics across flies suggests that, rather than directly performing motor coordination, these neurons likely provide downstream central complex circuits with similar behaviorally relevant visual feature sets on which to base motor decisions. As a striking example, the strong vertical tuning preference we observe in the LTr may partly underlie the tendency of flies to fixate on vertical edges during both flight and walking1,2,21. Recent work has suggested that vertical stripe fixation during walking largely relies on a hypothesized position system sensitive to local luminance changes that can operate independently of neural circuits involved in optomotor responses to widefield motion21,22. The response properties of R2 and R4D neurons is consistent with a role in such a position system22.
The retinotopic bias, structure of excitatory and inhibitory subfields, orientation tuning and direction selectivity we see are reminiscent of those seen in calcium imaging studies in simple cells in mammalian visual cortex13, providing an interesting example of how evolutionarily distant visual systems with different types of eyes nonetheless use similar feature sets to process visual scenes. From Hubel and Wiesel’s findings several decades ago23 to the present, significant progress has been made on identifying neural representations used at different stages in the mammalian visual system. However, mechanisms underlying simple cell responses are not yet fully understood24. With its array of genetic tools, Drosophila melanogaster may allow us to uncover how spatiotemporal interactions of excitatory and inhibitory inputs might produce similar orientation tuning and direction selectivity in the LTr25,26. There is considerable evidence for spatially tuned visual responses in the lobula complex and anterior optic tubercle in other insects27–30, suggesting that components of ring neuron input response properties may also arise from selective averaging of weaker and more broadly distributed tuning preferences in such areas.
Overall, our findings lay the groundwork for future research into how this genetic model organism’s small brain uses feature and pattern information for visual orientation and navigation.
METHODS
Fly stocks
All experiments were performed with female flies, with ages chosen based on expression levels of relevant fluorescent proteins. At least six animals were used for any single condition tested – specific sample sizes are noted for each set of experiments and were chosen based on the level of variability observed in initial experiments. Flies were randomly picked from their housing vials for all experiments. We used all data that passed a quality threshold based on the observed health of the fly during an experiment and the signal-to-noise ratio of the imaging signal.
Calcium imaging experiments to measure RFs were performed with UAS-GCaMP3; c232-GAL4, UAS-GCaMP3;EB1-GAL4, and pJFRC7-20XUAS-GCaMP5.003 (VK00005)/R57C10-GAL4 flies. We used pGP-JFRC7-20XUAS-IVS-GCaMP6f 15.693(attP40)/R57C10-GAL4 flies for pan-neuronal orientation tuning experiments (2–5 days old) and pGP-JFRC7-20XUAS-IVS-GCaMP6s 15.641 (attP40)/c232-GAL4 for orientation tuning experiments (1–2 weeks old) with dark bars. c232-GAL4 and EB1-GAL4 were gifts from M. Heisenberg and T. Lee, respectively.
To label dendrites and axons in different ring neurons, EB1-GAL4 and c232-GAL4 were each crossed to pJFRC67-3XUAS-IVS-Syt-GFP(attP18)31,32, pJFRC118-10XUAS-DenMark(attP40)32,33, and pJFRC119-10XUAS-IVS-myr::TopHat2(VK00005) (gift of B.D. Pfeiffer, unpublished).
To compare the expression patterns of EB1-GAL4 and c232-GAL4 to the pan-neuronal line R57C10 in the same fly, EB1-GAL4 and c232-GAL4 were each crossed to pJFRC19-13XLexAop2-IVS-myr::GFP (su(Hw)attP8)32, pJFRC21-10XUAS-IVS-mCD8::RFP (attP18)32; Sco/Cyo; R57C10-LexA::p65 (attP2)34.
For stochastic single cell labeling of EB1-GAL4 and c232-GAL4 with three colors a “flip-out”-based approach35 (Nern et al., in preparation) was used. In brief, heat-shock induced expression of FLP recombinase was used to excise FRT-flanked interruption cassettes from UAS reporter constructs carrying HA, V5 and FLAG epitope tags, and stained with epitope-tag specific antibodies. This results in labeling of a subset of the cells in the expression pattern with a stochastic combination of the three labels.
Anatomy: fly dissections, immunohistochemistry and confocal imaging
Confocal stacks were recorded with a 40x or 63x objective on a Zeiss confocal microscope. Dissections and staining were performed as previously described36,37. The primary antibody mixture consisted of 1:1000 sheep anti-GFP (AbD Serotec), 1:1000 rabbit anti-DsRed (Clontech), 1:100 rat anti-HA (for Syt/DenMark/HA staining; Roche), and 10% normal donkey serum (Jackson ImmunoResearch) in PBS-TX.
Fly preparation for RF mapping
RF measurements were performed using a preparation described previously14, but with the behavioral apparatus removed to maximize the fly’s visual field. The fly was briefly anesthetized on ice and transferred to a cold plate held at 4°C. The proboscis of the fly was fixed by either pressing it into the head and fixing it with wax, or by stretching it with a pair of tweezers mounted on a micromanipulator and then fixing it with a mixture of wax and colophony. We additionally removed either the front legs or all legs for RF measurements. The fly was glued to a pin and positioned in the holder using a micromanipulator and fixed in the holder with UV gel14. An opening was cut into the head to obtain optical access to the brain. To stop brain movement due to pulsation of muscle M16, we cut the muscle or the nerves innervating the muscles with dissection needles. The fly holder (including the micromanipulator) was then transferred to the microscope and mounted using magnetic mounts. Flies were dark adapted for 5 to 10 minutes before recordings started.
Fly preparation for imaging during walking
For walking experiments, an air-supported ball was positioned under the fly with a three-axis micromanipulator as described previously14 and the walking velocity of the fly was monitored using a camera system.
Fly preparation for imaging during tethered flight
For flying fly experiments a holder similar to those described previously14,15,38 was used. The holder was made out of two pieces of stainless steel shim (thickness 12.7 µm). The shim is cut using a laser mill and then folded into its pyramidal shape. One piece of the holder is glued onto a liquid chamber similar to the one used for walking behavior using epoxy14. After removing the front legs of the fly to prevent it from grabbing the holder, the fly is glued to a pin and inserted into the holder with a micromanipulator. The second piece of the holder is then inserted to close the pyramidal shape around the fly’s head.
The setup for combined imaging and flight behavior was similar to the one described in15. The fly was illuminated with IR light from below. The wing beat of the fly was recorded using a mirror placed beneath the fly and a camera (Basler 602f, operating at 100 or 150 Hz frame rate)15.
Two-photon imaging
Calcium imaging was performed using a custom-built two photon microscope controlled with ScanImage39. Fluorescence was detected using a photomultiplier tube (H7422PA-40, Hamamatsu). We used an Olympus 40x objective (LUMPlanFl/IR, NA 0.8) and typically adjusted the power to below 20 mW at the back aperture of the objective. We imaged at a frame rate of 6.7 Hz. Focal planes were selected based on the anatomy and visual responses of microglomeruli. We focused on microglomeruli because this approach allowed us to distinguish different neurons in a labeled population (such distinctions are difficult to make in the EB, where axons of different neurons arborize in close proximity).
Visual stimulation
LED arena
Visual stimuli were presented using a curved visual display40 that was covered with a color filter to prevent cross-talk between fluorescence detection and visual stimulation as described14. Additionally, to avoid reflections of stimuli from the curved surface of the display, we covered the display with a diffuser (tracing paper). Under such low-contrast conditions, we found that the signal-to-noise ratio (SNR) of calcium responses when stimulated by dark-on-bright-background stimuli (e.g., dark bar on bright surround) was low. This motivated our preference for bright-on-dark-background stimuli, which produced higher SNR calcium responses (comparison in Extended Data Fig. 5).
For RF measurements the display spanned 270° in azimuth and 120° in elevation. The top left and top right corners of the display (three square panels in each corner, each with a size of 30° by 30°) could not be seen by the fly (since they were occluded by the fly holder) and were excluded from the display. For behavioral experiments, a similar but smaller display was used, spanning 210° in azimuth and 90° in elevation.
Flashing dots for RF mapping
Excitatory RFs for c232-GAL4 and EB1-GAL4 flies were measured with stationary bright square dots (7.5° by 7.5°) appearing randomly in the visual field of the fly for 1s followed by a dark period of 1s. The measurements were repeated until the entire visual field covered by the display was stimulated once. The display was sampled with a spatial resolution of the stimulus (7.5°). Measurements were performed in 8 blocks of 140s each, presenting a total of 468 stimuli covering the entire display.
Horizontally moving bars for RF mapping
We used bright bars (15° in elevation, 7.5° in azimuth) that moved at a speed of 30 °/s left and right (in azimuthal direction) in the fly’s field of view.
Two repetitions with stimulation in one direction were followed by two repetitions of movement in the opposite direction. To map responses over the entire visual field spanned by the display the stimulus was shifted in steps of 15° in elevation from the top most part of the visual display towards the bottom part and the left and right moving stimulation was repeated in each row.
White noise
For white-noise stimulation37,41 we subdivided the display into squares of 11.25° by 11.25°. Each stimulus frame consisted of 20 randomly selected bright squares while the remaining squares remained dark (in a few trials for one fly we used 30 squares). The stimulus appeared for one second followed by a dark period of one second. We presented 60 frames of random stimuli in a trial of 140 s. For c232-GAL4 flies we used an average of 33 +/− 20 trials in 7 flies (59, 61, 29, 21, 21, 14, and 26 trials, respectively). For EB1-GAL4 flies we used an average of 41 +/− 13 trials in 6 flies, (38, 25, 39, 63, 35, and 47 trials, respectively). The color scale (Fig. 2, Extended Data Fig. 8) was adjusted to give equal weight to excitatory and inhibitory subfields (mean set to 0).
To validate white noise based responses we stimulated the fly with bright bars of 56.25 degrees by 18.75 degrees oriented either vertically, horizontally, or rotated by 45 or −45 degrees with respect to the vertical direction. To stimulate the receptive fields of all microglomeruli in an unbiased way, the bar stimuli were presented at a random position on the display and the display was sampled with a resolution of 11.25° in both elevation and azimuth.
Visual stimulation for measuring orientation tuning curves
For orientation tuning curve measurement we used a stimulus that extended across the entire display, from 120° in azimuth for vertical orientation (90°) to 270° azimuth for horizontal orientation. The width of the bar was 15° and it was moved at a speed of 75°/s. The angle was changed incrementally in steps of 11.25° starting with either horizontally or vertically oriented bars for one direction of movement, and then repeated in the opposite direction of movement. To obtain tuning curves with dark stimuli we inverted the contrast of bright and dark, and removed the diffuser.
Visual stimulation during walking and flying experiments
For walking and flying experiments we used a vertical bright bar spanning 90° in elevation and 15° in azimuth. The bar moved horizontally at a velocity of 15 °/s. The bar stayed stationary for 10 s after moving in one direction for 17 s and then resumed moving in the opposite direction for 17 s.
Data analysis
All data analysis was performed in MATLAB (MathWorks, Inc., Natick, MA). All errors and error bars shown are standard deviation (s.d.). All p-values shown are based on t-tests, unless otherwise noted.
Frame alignment and movement correction
Data recorded using two-photon imaging were aligned in the XY plane on a frame-by-frame basis. Data were thresholded to distinguish arborizations in the lateral triangle from background. All above-threshold pixels were set to the same value. Frames were aligned by cross-correlating each thresholded frame to a single frame at the beginning of the measurement. Multiple trials were aligned by cross-correlation of trial-averaged frames thresholded as above.
Calculation of fluorescence changes
The baseline for calculating ΔF/F was selected by averaging over the 10% of frames with lowest intensity in each trial or by using the baseline at the beginning of the experiment. Due to the low baseline intensity of GCaMP6, background fluorescence was not subtracted in measurements with GCaMP6. Calcium traces recorded from behaving flies were smoothed with a third order Savitzky-Golay filter over 7 frames for comparisons with behavioral data.
ROI selection
For RF measurements in stationary flies, ROIs corresponding to microglomeruli were selected manually in videos of ΔF/F. Overlapping parts of ROIs were excluded from further analysis.
For experiments in behaving flies, ROIs were selected using visually supervised k-means clustering (Extended Data Fig. 10a–d). We used a subset of three trials (2820 frames) for clustering-based selection of ROIs. We then used correlation-based k-means clustering between the calcium traces in all thresholded pixels. The number of clusters was selected based on an estimate of the number of microglomeruli. If not all microglomeruli could be separated into different clusters, the number of clusters was increased in a second run. We then set a threshold to remove clusters that were smaller than a certain number of pixels (60). We additionally removed clusters that had an average cross-correlation value lower than a threshold (0.2). We further split anatomically disconnected regions of the same cluster and again removed those parts that were smaller than a size threshold (30 pixels). In a final check, the remaining ROIs were overlaid with the frames of the calcium video that showed the largest response in this ROI, and ROIs that did not correspond to microglomeruli were removed manually.
RF mapping
RFs were smoothed using a Gaussian filter (4 pixels with a standard deviation of 1 pixel by default; 5 pixels with a standard deviation of 4 pixels for white noise measurements).
For RF measurements (e.g., in Fig. 1), we combined responses to left- and right-moving stimuli (each averaged over two trials). The onset of the calcium response was correlated with the bar’s movement into the RF. Due to the size of the RF, a bar moving from the right side of the fly towards the left side entered the RF from the right side and induced a calcium onset starting at the right side of the (excitatory part of the) RF. Similarly, a bar entering the receptive field from the left side induced a calcium onset starting at the left side of the RF. Due to the faster on-response than off-response of GCaMP the onset of the RF was better defined than the offset. To find the center of the excitatory RF measured with moving bars, we defined the RF center as the weighted centroid of the average of responses to thresholded left- and right-moving stimuli (Extended Data Fig. 1 b). This was equivalent to delimiting the RF by its calcium onset response. This procedure made the location of computed RF centers invariant to the kinetics of the calcium indicator.
We used the following parameters to characterize the excitatory parts of RFs measured with single stationary dots (measured at 50% of the maximum ΔF/F response of each RF) (see Extended Data Figure 7c): area, major axis (of an ellipse that has the same normalized second central moments as the region as determined with the MATLAB ‘regionprops’ function), minor axis, eccentricity, orientation (the angle between the horizontal x axis and the major axis of the ellipse, 0 corresponding to horizontal orientation), retinotopic correlation (the correlation coefficient between the center of the RF, determined as the weighted centroid of the RF area, and the center of the corresponding ROI).
RF display
To display microglomerular RFs, we colored LED arena positions in proportion to the ΔF/F response elicited by the stimulus presented in that position. For moving stimuli, the calcium response in each pixel was determined as the average over all response frames that were recorded while the stimulus was at that position. Calcium responses were interpolated to account for mismatches between frame rates and movement of stimuli. For stimulation with stationary stimuli, the ΔF/F values shown in RF plots correspond to the maximum ΔF/F during the stimulation period. To prevent cross talk between sequentially presented stimulus frames due to the slow decay of the calcium response (which extended beyond stimulus presentation) we only considered the calcium increase and not the calcium decay in assigning ΔF/F values to stimulus frames.
Retinotopy
To assess retinotopy, we calculated the correlation coefficient between microglomerulus centers and centers of their RFs. This was compared to the correlation coefficient obtained after randomly shuffling correspondences.
Additionally, we performed principal component analysis (PCA) on the (x,y) values of RF centers. The first PC gave us the direction of maximum variation of RF centers, which we consider to be the primary retinotopic axis (in the fly’s visual field) for the RF population.
Orientation tuning
To measure orientation tuning (Fig. 2), single 15°-wide bars spanning the entire visual display were presented, and their orientation changed in steps of 11.25°. Responses from multiple trials were then averaged, and tuning curves were fit using the sum of two circular Gaussians42,43.
where, a1 and a2 are amplitudes, θ1 and θ2 are the maximum angles and k1 and k2 are width parameters.
The preferred orientation for each microglomerulus was the maximum of its fitted tuning curve.
Orientation selectivity
The orientation selectivity index44, OSI, was computed as the difference between the response in the preferred direction, ΔF/Fmax, and the direction orthogonal to it (preferred direction +/− 90°), ΔF/Fortho, normalized by the sum of the responses in the two directions:
Direction selectivity
The direction selectivity index42, DI, was calculated as the difference between responses in the preferred direction, ΔF/Fmax, and anti-preferred direction (preferred direction+180°), ΔF/Fopposite, normalized by their sum:
The sign of the DI was defined as positive for front-to-back movement and negative for back-to-front movement.
White-noise-based RFs and response predictions
RFs were reconstructed by thresholding calcium traces at 30% ΔF/F and averaging over all frames that induced a response larger than this threshold, weighted by peak calcium response. Only the rising slope of the calcium response was considered. The displayed RFs are the weighted averages of stimulus frames and the mean value (background) is subtracted. Max is the maximum of the weighted average after subtracting the mean value, and min is the minimum of this average. The mean value is set to 0.
To predict responses to oriented bars (Fig. 2), we multiplied the mean-subtracted white-noise-based RF with stimulus values in each pixel and summed over all pixels. We convolved the result with a calcium response function. The amplitude and time constants of the calcium response function were fitted using responses to bars of one orientation and then used in the prediction of responses to the remaining three orientations.
The calcium response function, crf, used in predicting responses to oriented stimuli and in the analysis of calcium responses recorded during behavior (see below) is given by
where t0 is the onset time, and ton and toff, are the rise and decay times, of the calcium indicator.
Walking behavior analysis
Ball movement was recorded with a sampling rate of 4 kHz and velocities were calculated with a time base of 250 ms. Velocities were then averaged over all velocity values in each two-photon imaging frame. Average velocities were calculated by detecting epochs in which the fly was moving and then averaged over this period. Due to strong walking activity in behaving animals, there were few recordings that allowed us to compare microglomerular responses in walking and stationary conditions (Extended Data Fig. 10e–n).
Flight behavior analysis
The wing angle was detected by first manually identifying the wing hinge in both wings. The wing was then detected by first subtracting the background recorded while the fly was not flying, smoothing with a mean filter, and setting a threshold for detecting the wings in two ROIs surrounding the wings. The wing angle was then defined as the angle between the wing hinge and the tip of the wing. Flight was typically intermittent (Extended Data Fig. 10o,p).
Model for fitting responses of visual microglomeruli during behavior
For describing the calcium responses of visual microglomeruli during walking behavior we fitted the responses with a model consisting of a single excitatory and inhibitory Gaussian function. Model fitting was only performed for microglomeruli that responded with a single peak during one passage of the stimulus on the display, not for bilateral receptive fields. The initial position of each Gaussian, the (common) standard deviation and the amplitude were used as fit parameters. Since the inhibitory responses could not be directly observed in our calcium signals, we set all negative values to zero.
where, x0 and x1 are the location of the excitatory and inhibitory receptive fields, respectively, and σx is the width of the receptive field. The calcium signal s (ΔF/F) was then modeled as the convolution of the receptive field with the calcium response function, crf, and a constant offset, bg,
Since the response in flying flies depended on the state of the fly we used a model for the receptive field that additionally included the left and right wing angles as parameters
where, behavior = a · lw + b · rw + c depends linearly, with the parameters a, b and c, on the left wing angle, lw, and the right wing angle, rw.
Supplementary Material
a, Same as Extended Data Fig. 6a, measured in R2 neurons in 8 flies. b, Cross-correlation coefficient between fly 1 and all other RFs in each column for R2 neurons shown in a (mean = 0.75 ± 0.12). c, Table showing characteristics of excitatory receptive fields for R4d and R2 ring neurons measured with single dot stimulation calculated at half maximum ΔF/F. The retinotopic correlation coefficients are significantly different from the control distributions, with p = 0.0283 for R4d (n = 8 flies, 159 RFs), and p = 0.0069 for R2 (n = 12 flies, 219 RFs).
a, RFs measured using white-noise stimulation in 6 flies aligned according to similarity. We used an average of 41 ± 13 trials (6 flies, number of trials: 38, 25, 39, 63, 35, and 47). Each trial consisted of presentation of 60 stimulus frames (see Methods). b, RFs measured using white-noise stimulation in 7 flies aligned by similarity (subset of this data is shown in Fig. 3). We used an average of 33 ± 20 trials (7 flies, number of trials: 59, 61, 29, 21, 21, 14, and 26).
a, Microglomeruli selected by visually assisted k-means clustering in R2 neurons, a subset of which are shown in Fig. 4b. b, Average over n = 24 trials of responses of R2 microglomeruli to a bright bar (15°×90°) moving at 30 °/s shown during a period when the fly is walking more than 50 % of the time (blue) and less than 50% of the time (red) during rightward moving stimulation (envelopes show s.d.). c, Correlation coefficient (mean = 0.72 ± 0.25) between model (see Methods) and data for R2 microglomeruli recorded in n = 8 flies, and, d, for R4d microglomeruli (n = 14 flies, mean 0.7 ± 0.25) that have unilateral RFs. e, Concatenation of seven epochs during walking behavior with EB1-GAL4, UAS-GCaMP3 flies during stimulation with a left and right moving bar: data (blue) and model (red) fit (see Methods). f, Microglomeruli in R4d selected using visually assisted k-means clustering. g, Visual responses during flying and non-flying behavior during stimulation with a bar moving from left to right (averaged over n = 14 trials for flying, n = 14 trials for non-flying), and during stimulation with a bar moving from right to left (n = 17 for flying, and n = 11 trials for non-flying) for the fly shown in Fig. 4e (envelopes show s.d.). h, Correlation coefficient between model (see Methods) and data for R4d neurons with a linear dependence of the visual response on both wing angles (mean = 0.65 ± −0.27) recorded in n = 14 flies.
a, Cross-correlation matrix between all pixels above threshold (see Methods for details). b, Cross-correlation matrix sorted according to clusters found by k-means clustering. c, Resulting regions of interest. d, Overlay of resulting regions of interest and selected frames of calcium video. Regions of interest that do not correspond to microglomeruli (based on comparison with the calcium video) are removed. e, Forward, f, side, and, g, rotational velocities for all c232-GAL4, UAS-GCaMP3 flies recorded during walking behavior. h, Percentage of walking for all EB1-GAL4, UAS-GCaMP3 flies recorded. i, Forward, j, sidewise, k, and rotational velocities, and l, percentage of walking for all EB1-GAL4, UAS-GCaMP3 flies recorded during walking behavior. m, Number of 130s epochs recorded for each c232-GAL4, UAS-GCaMP3 walking fly. n, Number of 130s epochs recorded for each EB1-GAL4, UAS-GCaMP3 walking fly. o, Percentage of time flying during recording for all c232-GAL4, UAS-GCaMP3 flies recorded during flight behavior. p, Number of 130s epochs recorded for each c232-GAL4, UAS-GCaMP3 flying fly.
a, Selected two-photon imaging frames showing calcium response in the LTr with pan-neuronal expression of GCaMP5. Highlighted in red are the microglomeruli that were selected for RF reconstruction. Scale bar = 5 µm. b, Schematic of visual stimulation used, RFs (two-trial averages) measured with left-and right-moving visual stimuli and their intersection labeled at FWHM intensity (white ellipse) with the weighted centroid (white star) of the distribution. c, Center of each RF plotted against the center of its corresponding microglomerulus. d, Correlation coefficient of data in d (r = 0.39, p = 0) and correlation coefficient of same data after randomly permuting the microglomeruli (n = 3000 permutations, mean = (4.6 ± 0.076) · 10−4). e, Principal component axes of RF centers (see Methods).
a, A frame of a confocal stack of the LTr labeled with antibody staining against green fluorescent protein (GFP) using a pan-neuronal driver line, R57C10-LexA and antibody staining against red fluorescent protein (RFP) of EB1-GAL4. b, A section of a confocal stack of the LTr labeled with GFP using a pan-neuronal driver line, R57C10-LexA and RFP labeling of c232-GAL4. c, Multicolor FLP-out labeling of c232-GAL4 (see Methods). d, Antibody staining of c232-GAL4 expressing the dendritic marker, DenMark (red) and an axonal label, synaptotagmin-tagged GFP (green). Entire cell is outlined with membrane-tagged HA (blue) (see Methods). Top panel is merge of all colors. e, Same as d for EB1-GAL4.
a, Overlay of all regions of interest of R4d microglomeruli recorded in a fly during visual stimulation over an average of all frames in a calcium video (940 frames), and selected frames of the calcium video showing responses of individual microglomeruli. Scale bar = 5 µm. b, Top: An example of the single dot stimulus appearing at the specified azimuth and elevation for 1s followed by a dark period of 1s (colored dot indicates both the stimulus and the subsequent off period). Bottom: ΔF/F for microglomerulus 3 in a responding to the stimulus. c, Excitatory RFs for all microglomeruli shown in a. d, Overlay of all regions of interest selected among R2 microglomeruli recorded during visual stimulation over an average of all frames (n = 940) in a calcium video, and selected frames of the calcium video showing responses of individual microglomeruli. Scale bar = 5 µm. e, Top: Azimuth and elevation of the single bright dot presented for 1s followed by a 1s interval without stimulus. Bottom: ΔF/F for microglomerulus 1 in d responding to the visual stimulus. f, Excitatory RFs for all microglomeruli shown in d. g, Overlapping RFs of R4d neurons. Ellipses have the same normalized second central moments as the RFs shown in c thresholded at 30% ΔF/F. Intensity scale indicates how many RFs overlap in a given region. h, Same as g for R2 neurons shown in f. i, Average RFs measured across all flies with single dot stimulation for R4d neurons (green, n = 8 flies, 159 RFs), and j, for R2 neurons (purple, n = 12 flies, 219 RFs), and k, for both (overlay). Recordings from the right lateral triangle were flipped to the contralateral side for averaging. Note: Although a large fraction of R2 and R4d ring neurons we imaged were visually responsive, there were ring neurons that did not respond in our experiments. Such neurons may have RFs in areas of the fly’s visual field that we did not sample (for example, very lateral areas), be tuned to visual dimensions that we did not explore (such as color or polarization or complex shapes), or be selectively responsive to other sensory modalities (such as mechanosensation or thermosensation).
a, Correlation coefficients between predicted and measured responses for R2 neurons (histogram over all trials and RFs) (n = 6 flies). b, Same as a, for R4d neurons (n = 6 flies). c, Microglomeruli of R4d neurons colored according to orientation preference and orientation selectivity (six-trial averages). d, Azimuth and elevation of the center of the excitatory RFs of the neurons shown in c (two-trial averages, measured using moving bars as described in Extended Data Fig. 1 and Methods). e, Direction selectivity for neurons in c (six-trial averages). f, Microglomeruli shown in Fig. 2k,l labeled according to the azimuth and elevation of their excitatory RF center (measured using moving stimuli as in Extended Data Fig. 1 and, g, their orientation selectivity (four-trial averages). h–p, Distributions of preferred orientation, orientation selectivity and direction index for R2 (n = 10 flies), R4d (n = 12) and pan-neuronal (n = 11 flies) microglomeruli.
a, Orientation tuning curves (GCaMP6s) of three R4d microglomeruli measured using (top row) bright bars on a dark background (as in Fig. 2f) and dark bars on a bright background (exact contrast inversion of bright-bar stimulus). As suggested by excitatory-inhibitory structure of RFs (Fig. 2c, Extended Data Fig. 8), tuning curves measured with the two stimuli are qualitatively similar, but bright bars produce stronger responses (with our low-contrast, filtered LED array display, see Methods). b, Fitted tuning curves measured using dark and bright bars are highly correlated (mean = 0.648 ± 0.26, n = 7 flies).
a, Excitatory RFs measured using single dot stimulation in one fly (fly1), and RFs recorded in n=12 other flies aligned by similarity. b, Cross-correlation coefficient between fly 1 and all other RFs in each column (mean = 0.74 ± 0.2).
ACKNOWLEDGMENTS
We thank A. Nern for sharing flies and generous technical advice, B. Pfeiffer and G. Rubin for providing pJFRC64, pJFRC118, and pJFRC119 flies, and R. Harris for building fly lines, sharing preliminary results, and providing critical feedback in the use of fly reagents. We thank K. Hibbard, D. Hall, J. Kao and the Janelia Fly Core for fly crosses and support, E. Chiappe for initial anatomy experiments, M. Reiser and J. Truman for sharing equipment, J. Liu for technical support, V. Iyer for ScanImage support, T. Adelman for helpful suggestions, and L. Looger and the Janelia GENIE team for GCaMP5 and GCaMP6. We are grateful to A. Leonardo, M. Reiser, E. Chiappe, J. Freeman, A. Karpova, S. Huston and members of Vivek’s lab for useful discussions and comments on the manuscript. This work was supported by the Howard Hughes Medical Institute.
Footnotes
AUTHOR CONTRIBUTIONS
Both authors designed the study and wrote the manuscript. J.S. carried out the experiments and data analysis.
The authors declare no competing financial interests.
REFERENCES
- 1.Bausenwein B, Muller NR, Heisenberg M. Behavior-dependent activity labeling in the central complex of Drosophila during controlled visual stimulation. J Comp Neurol. 1994;340:255–268. doi: 10.1002/cne.903400210. [DOI] [PubMed] [Google Scholar]
- 2.Neuser K, Triphan T, Mronz M, Poeck B, Strauss R. Analysis of a spatial orientation memory in Drosophila. Nature. 2008;453:1244–1247. doi: 10.1038/nature07003. [DOI] [PubMed] [Google Scholar]
- 3.Ofstad TA, Zuker CS, Reiser MB. Visual place learning in Drosophila melanogaster. Nature. 2011;474:204–207. doi: 10.1038/nature10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu G, et al. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439:551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- 5.Pan YF, et al. Differential roles of the fan-shaped body and the ellipsoid body in Drosophila visual pattern memory. Learn Memory. 2009;16:289–295. doi: 10.1101/lm.1331809. [DOI] [PubMed] [Google Scholar]
- 6.Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- 7.Young JM, Armstrong JD. Structure of the adult central complex in Drosophila: Organization of distinct neuronal subsets. Journal of Comparative Neurology. 2010;518:1500–1524. doi: 10.1002/cne.22284. [DOI] [PubMed] [Google Scholar]
- 8.Hanesch U, Fischbach KF, Heisenberg M. Neuronal architecture of the central complex in Drosophila melanogaster. Cell Tissue Res. 1989;257:343–366. [Google Scholar]
- 9.Renn SCP, et al. Genetic analysis of the Drosophila ellipsoid body neuropil: Organization and development of the central complex. Journal of Neurobiology. 1999;41:189–207. [PubMed] [Google Scholar]
- 10.Trager U, Wagner R, Bausenwein B, Homberg U. A novel type of microglomerular synaptic complex in the polarization vision pathway of the locust brain. Journal of Comparative Neurology. 2008;506:288–300. doi: 10.1002/cne.21512. [DOI] [PubMed] [Google Scholar]
- 11.Jenett A, et al. A GAL4-Driver Line Resource for Drosophila Neurobiology. Cell reports. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Zugates CT, Liang IH, Lee CHJ, Lee TM. Drosophila Dscam is required for divergent segregation of sister branches and suppresses ectopic bifurcation of axons. Neuron. 2002;33:559–571. doi: 10.1016/s0896-6273(02)00570-6. [DOI] [PubMed] [Google Scholar]
- 13.Bonin V, Histed MH, Yurgenson S, Reid RC. Local diversity and fine-scale organization of receptive fields in mouse visual cortex. J Neurosci. 2011;31:18506–18521. doi: 10.1523/JNEUROSCI.2974-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seelig JD, et al. Two-photon calcium imaging from head-fixed Drosophila during optomotor walking behavior. Nat Methods. 2010 doi: 10.1038/nmeth.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maimon G, Straw AD, Dickinson MH. Active flight increases the gain of visual motion processing in Drosophila. Nat Neurosci. 2010;13:393–399. doi: 10.1038/nn.2492. [DOI] [PubMed] [Google Scholar]
- 16.Chiappe ME, Seelig JD, Reiser MB, Jayaraman V. Walking modulates speed sensitivity in Drosophila motion vision. Curr Biol. 2010;20:1470–1475. doi: 10.1016/j.cub.2010.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinze S, Reppert SM. Sun compass integration of skylight cues in migratory monarch butterflies. Neuron. 2011;69:345–358. doi: 10.1016/j.neuron.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Heinze S, Homberg U. Maplike representation of celestial E-vector orientations in the brain of an insect. Science. 2007;315:995–997. doi: 10.1126/science.1135531. [DOI] [PubMed] [Google Scholar]
- 19.Rosner R, Homberg U. Widespread sensitivity to looming stimuli and small moving objects in the central complex of an insect brain. J Neurosci. 2013;33:8122–8133. doi: 10.1523/JNEUROSCI.5390-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo P, Ritzmann RE. Neural activity in the central complex of the cockroach brain is linked to turning behaviors. J Exp Biol. 2013;216:992–1002. doi: 10.1242/jeb.080473. [DOI] [PubMed] [Google Scholar]
- 21.Bahl A, Ammer G, Schilling T, Borst A. Object tracking in motion-blind flies. Nat Neurosci. 2013;16:730–738. doi: 10.1038/nn.3386. [DOI] [PubMed] [Google Scholar]
- 22.Poggio T, Reichard W. Theory of pattern induced flight orientation of fly Musca domestica. Kybernetik. 1973;12:185–203. doi: 10.1007/BF00270572. [DOI] [PubMed] [Google Scholar]
- 23.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol-London. 1962;160 doi: 10.1113/jphysiol.1962.sp006837. 106-&. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priebe NJ, Ferster D. Mechanisms of neuronal computation in mammalian visual cortex. Neuron. 2012;75:194–208. doi: 10.1016/j.neuron.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freifeld L, Clark DA, Schnitzer MJ, Horowitz MA, Clandinin TR. GABAergic lateral interactions tune the early stages of visual processing in Drosophila. Neuron. 2013;78:1075–1089. doi: 10.1016/j.neuron.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egelhaaf M, Borst A. A look into the cockpit of the fly: visual orientation, algorithms, and identified neurons. J Neurosci. 1993;13:4563–4574. doi: 10.1523/JNEUROSCI.13-11-04563.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeiffer K, Kinoshita M, Homberg U. Polarization-sensitive and light-sensitive neurons in two parallel pathways passing through the anterior optic tubercle in the locust brain. Journal of Neurophysiology. 2005;94:3903–3915. doi: 10.1152/jn.00276.2005. [DOI] [PubMed] [Google Scholar]
- 28.Mu LY, Ito K, Bacon JP, Strausfeld NJ. Optic glomeruli and their inputs in Drosophila share an organizational ground pattern with the antennal lobes. Journal of Neuroscience. 2012;32:6061–6071. doi: 10.1523/JNEUROSCI.0221-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collett T. Visual neurones for tracking moving targets. Nature. 1971;232:127–130. doi: 10.1038/232127a0. [DOI] [PubMed] [Google Scholar]
- 30.O’Carroll D. Feature-detecting neurons in dragonflies. Nature. 1993;362:541–543. [Google Scholar]
ADDITONAL REFERENCES
- 31.Zhang YQ, Rodesch CK, Broadie K. Living synaptic vesicle marker: synaptotagmin-GFP. Genesis. 2002;34:142–145. doi: 10.1002/gene.10144. [DOI] [PubMed] [Google Scholar]
- 32.Pfeiffer BD, et al. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicolai LJJ, et al. Genetically encoded dendritic marker sheds light on neuronal connectivity in Drosophila. Proc Natl Acad Sci U S A. 2010;107:20553–20558. doi: 10.1073/pnas.1010198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeiffer BD, Truman JW, Rubin GM. Using translational enhancers to increase transgene expression in Drosophila. Proc Natl Acad Sci U S A. 2012;109:6626–6631. doi: 10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- 36.Peng H, et al. BrainAligner: 3D registration atlases of Drosophila brains. Nat Methods. 2011;8:493–500. doi: 10.1038/nmeth.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber F, Machens CK, Borst A. Spatiotemporal response properties of optic-flow processing neurons. Neuron. 2010;67:629–642. doi: 10.1016/j.neuron.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Suver MP, Mamiya A, Dickinson MH. Octopamine neurons mediate flight-induced modulation of visual processing in Drosophila. Curr Biol. 2012 doi: 10.1016/j.cub.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 39.Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed Eng Online. 2003;2:13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiser MB, Dickinson MH. A modular display system for insect behavioral neuroscience. J Neurosci Methods. 2008;167:127–139. doi: 10.1016/j.jneumeth.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Chichilnisky EJ. A simple white noise analysis of neuronal light responses. Network. 2001;12:199–213. [PubMed] [Google Scholar]
- 42.Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swindale NV. Orientation tuning curves: empirical description and estimation of parameters. Biological Cybernetics. 1998;78:45–56. doi: 10.1007/s004220050411. [DOI] [PubMed] [Google Scholar]
- 44.Swindale NV, Grinvald A, Shmuel A. The spatial pattern of response magnitude and selectivity for orientation and direction in cat visual cortex. Cerebral Cortex. 2003;13:225–238. doi: 10.1093/cercor/13.3.225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a, Same as Extended Data Fig. 6a, measured in R2 neurons in 8 flies. b, Cross-correlation coefficient between fly 1 and all other RFs in each column for R2 neurons shown in a (mean = 0.75 ± 0.12). c, Table showing characteristics of excitatory receptive fields for R4d and R2 ring neurons measured with single dot stimulation calculated at half maximum ΔF/F. The retinotopic correlation coefficients are significantly different from the control distributions, with p = 0.0283 for R4d (n = 8 flies, 159 RFs), and p = 0.0069 for R2 (n = 12 flies, 219 RFs).
a, RFs measured using white-noise stimulation in 6 flies aligned according to similarity. We used an average of 41 ± 13 trials (6 flies, number of trials: 38, 25, 39, 63, 35, and 47). Each trial consisted of presentation of 60 stimulus frames (see Methods). b, RFs measured using white-noise stimulation in 7 flies aligned by similarity (subset of this data is shown in Fig. 3). We used an average of 33 ± 20 trials (7 flies, number of trials: 59, 61, 29, 21, 21, 14, and 26).
a, Microglomeruli selected by visually assisted k-means clustering in R2 neurons, a subset of which are shown in Fig. 4b. b, Average over n = 24 trials of responses of R2 microglomeruli to a bright bar (15°×90°) moving at 30 °/s shown during a period when the fly is walking more than 50 % of the time (blue) and less than 50% of the time (red) during rightward moving stimulation (envelopes show s.d.). c, Correlation coefficient (mean = 0.72 ± 0.25) between model (see Methods) and data for R2 microglomeruli recorded in n = 8 flies, and, d, for R4d microglomeruli (n = 14 flies, mean 0.7 ± 0.25) that have unilateral RFs. e, Concatenation of seven epochs during walking behavior with EB1-GAL4, UAS-GCaMP3 flies during stimulation with a left and right moving bar: data (blue) and model (red) fit (see Methods). f, Microglomeruli in R4d selected using visually assisted k-means clustering. g, Visual responses during flying and non-flying behavior during stimulation with a bar moving from left to right (averaged over n = 14 trials for flying, n = 14 trials for non-flying), and during stimulation with a bar moving from right to left (n = 17 for flying, and n = 11 trials for non-flying) for the fly shown in Fig. 4e (envelopes show s.d.). h, Correlation coefficient between model (see Methods) and data for R4d neurons with a linear dependence of the visual response on both wing angles (mean = 0.65 ± −0.27) recorded in n = 14 flies.
a, Cross-correlation matrix between all pixels above threshold (see Methods for details). b, Cross-correlation matrix sorted according to clusters found by k-means clustering. c, Resulting regions of interest. d, Overlay of resulting regions of interest and selected frames of calcium video. Regions of interest that do not correspond to microglomeruli (based on comparison with the calcium video) are removed. e, Forward, f, side, and, g, rotational velocities for all c232-GAL4, UAS-GCaMP3 flies recorded during walking behavior. h, Percentage of walking for all EB1-GAL4, UAS-GCaMP3 flies recorded. i, Forward, j, sidewise, k, and rotational velocities, and l, percentage of walking for all EB1-GAL4, UAS-GCaMP3 flies recorded during walking behavior. m, Number of 130s epochs recorded for each c232-GAL4, UAS-GCaMP3 walking fly. n, Number of 130s epochs recorded for each EB1-GAL4, UAS-GCaMP3 walking fly. o, Percentage of time flying during recording for all c232-GAL4, UAS-GCaMP3 flies recorded during flight behavior. p, Number of 130s epochs recorded for each c232-GAL4, UAS-GCaMP3 flying fly.
a, Selected two-photon imaging frames showing calcium response in the LTr with pan-neuronal expression of GCaMP5. Highlighted in red are the microglomeruli that were selected for RF reconstruction. Scale bar = 5 µm. b, Schematic of visual stimulation used, RFs (two-trial averages) measured with left-and right-moving visual stimuli and their intersection labeled at FWHM intensity (white ellipse) with the weighted centroid (white star) of the distribution. c, Center of each RF plotted against the center of its corresponding microglomerulus. d, Correlation coefficient of data in d (r = 0.39, p = 0) and correlation coefficient of same data after randomly permuting the microglomeruli (n = 3000 permutations, mean = (4.6 ± 0.076) · 10−4). e, Principal component axes of RF centers (see Methods).
a, A frame of a confocal stack of the LTr labeled with antibody staining against green fluorescent protein (GFP) using a pan-neuronal driver line, R57C10-LexA and antibody staining against red fluorescent protein (RFP) of EB1-GAL4. b, A section of a confocal stack of the LTr labeled with GFP using a pan-neuronal driver line, R57C10-LexA and RFP labeling of c232-GAL4. c, Multicolor FLP-out labeling of c232-GAL4 (see Methods). d, Antibody staining of c232-GAL4 expressing the dendritic marker, DenMark (red) and an axonal label, synaptotagmin-tagged GFP (green). Entire cell is outlined with membrane-tagged HA (blue) (see Methods). Top panel is merge of all colors. e, Same as d for EB1-GAL4.
a, Overlay of all regions of interest of R4d microglomeruli recorded in a fly during visual stimulation over an average of all frames in a calcium video (940 frames), and selected frames of the calcium video showing responses of individual microglomeruli. Scale bar = 5 µm. b, Top: An example of the single dot stimulus appearing at the specified azimuth and elevation for 1s followed by a dark period of 1s (colored dot indicates both the stimulus and the subsequent off period). Bottom: ΔF/F for microglomerulus 3 in a responding to the stimulus. c, Excitatory RFs for all microglomeruli shown in a. d, Overlay of all regions of interest selected among R2 microglomeruli recorded during visual stimulation over an average of all frames (n = 940) in a calcium video, and selected frames of the calcium video showing responses of individual microglomeruli. Scale bar = 5 µm. e, Top: Azimuth and elevation of the single bright dot presented for 1s followed by a 1s interval without stimulus. Bottom: ΔF/F for microglomerulus 1 in d responding to the visual stimulus. f, Excitatory RFs for all microglomeruli shown in d. g, Overlapping RFs of R4d neurons. Ellipses have the same normalized second central moments as the RFs shown in c thresholded at 30% ΔF/F. Intensity scale indicates how many RFs overlap in a given region. h, Same as g for R2 neurons shown in f. i, Average RFs measured across all flies with single dot stimulation for R4d neurons (green, n = 8 flies, 159 RFs), and j, for R2 neurons (purple, n = 12 flies, 219 RFs), and k, for both (overlay). Recordings from the right lateral triangle were flipped to the contralateral side for averaging. Note: Although a large fraction of R2 and R4d ring neurons we imaged were visually responsive, there were ring neurons that did not respond in our experiments. Such neurons may have RFs in areas of the fly’s visual field that we did not sample (for example, very lateral areas), be tuned to visual dimensions that we did not explore (such as color or polarization or complex shapes), or be selectively responsive to other sensory modalities (such as mechanosensation or thermosensation).
a, Correlation coefficients between predicted and measured responses for R2 neurons (histogram over all trials and RFs) (n = 6 flies). b, Same as a, for R4d neurons (n = 6 flies). c, Microglomeruli of R4d neurons colored according to orientation preference and orientation selectivity (six-trial averages). d, Azimuth and elevation of the center of the excitatory RFs of the neurons shown in c (two-trial averages, measured using moving bars as described in Extended Data Fig. 1 and Methods). e, Direction selectivity for neurons in c (six-trial averages). f, Microglomeruli shown in Fig. 2k,l labeled according to the azimuth and elevation of their excitatory RF center (measured using moving stimuli as in Extended Data Fig. 1 and, g, their orientation selectivity (four-trial averages). h–p, Distributions of preferred orientation, orientation selectivity and direction index for R2 (n = 10 flies), R4d (n = 12) and pan-neuronal (n = 11 flies) microglomeruli.
a, Orientation tuning curves (GCaMP6s) of three R4d microglomeruli measured using (top row) bright bars on a dark background (as in Fig. 2f) and dark bars on a bright background (exact contrast inversion of bright-bar stimulus). As suggested by excitatory-inhibitory structure of RFs (Fig. 2c, Extended Data Fig. 8), tuning curves measured with the two stimuli are qualitatively similar, but bright bars produce stronger responses (with our low-contrast, filtered LED array display, see Methods). b, Fitted tuning curves measured using dark and bright bars are highly correlated (mean = 0.648 ± 0.26, n = 7 flies).
a, Excitatory RFs measured using single dot stimulation in one fly (fly1), and RFs recorded in n=12 other flies aligned by similarity. b, Cross-correlation coefficient between fly 1 and all other RFs in each column (mean = 0.74 ± 0.2).