Abstract
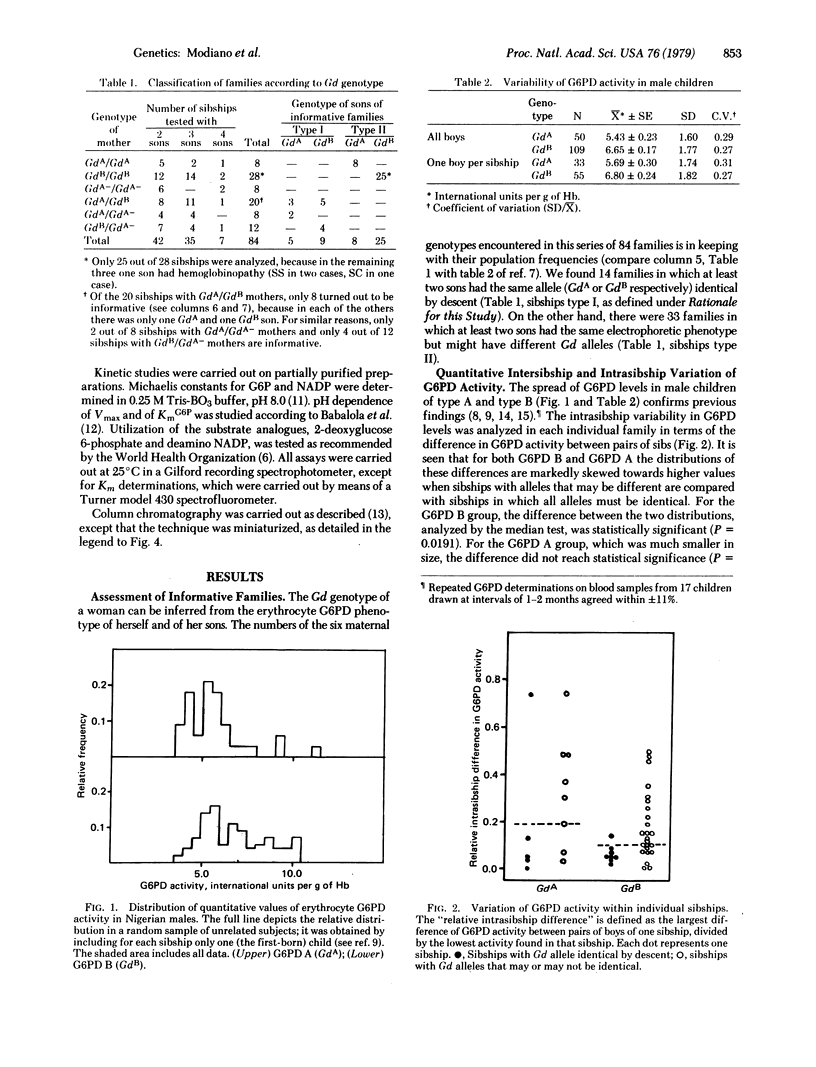
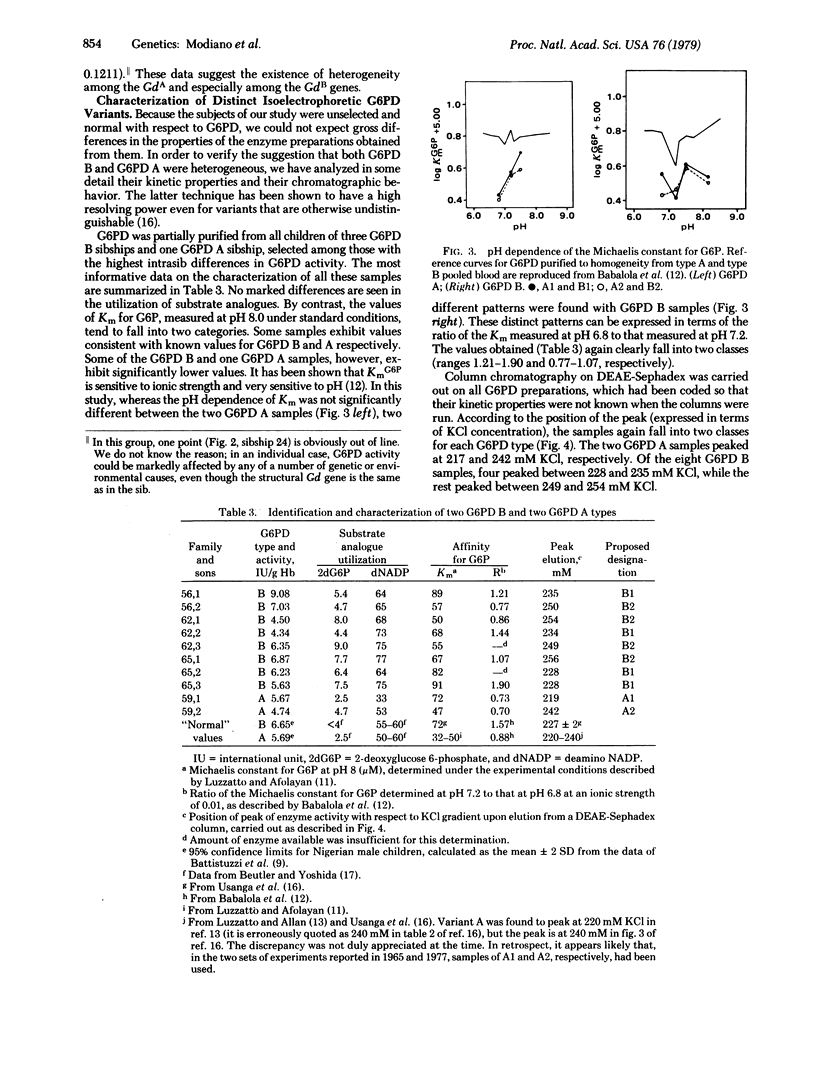
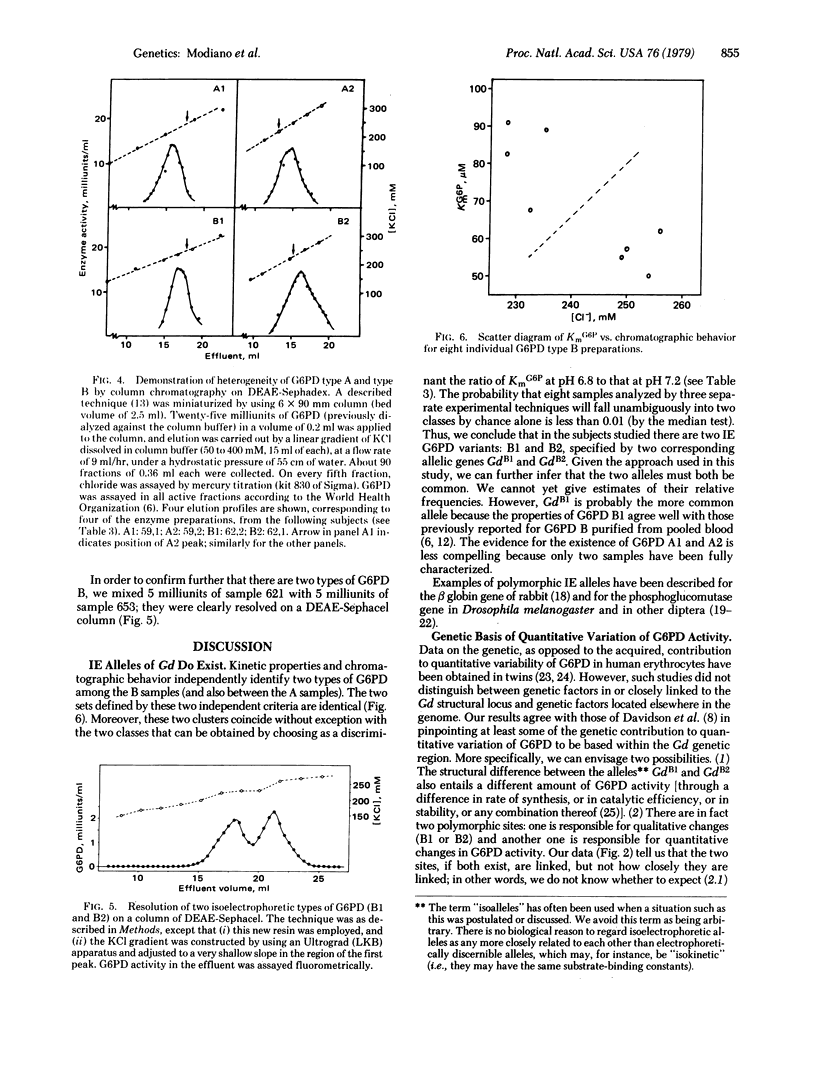
Quantitative determination of glucose-6-phosphate dehydrogenase (G6PD; D-glucose-6-phosphate: NADP+ 1-oxidoreductase, EC 1.1.1.49) activity was carried out in 214 male Nigerian children of 84 mothers with known Gd genotype. The relative intrasibship difference in G6PD activity (normalized to the lowest value within the sibship) was below 0.18 in all cases but one when the children were known to have the same Gd+ allele (identical by descent); whereas it was higher than 0.18 in 18 out of 33 sibships in which children might have had either of the two maternal (electrophoretically identical) Gd+ alleles. G6PD from 10 (8 G6PD B and 2 G6PD A) children belonging to four of the sibships possessing high quantitative variation in G6PD activity was partially purified and extensively characterized. The 8 G6PD type B samples fell unambiguously into two classes on the basis of Km values for glucose 6-phosphate (determined at variuos pH values), and KCl gradient elution from DEAE-Sephadex columns. The two types of G6PD B were resolved from an artificial mixture on a DEAE-Sephacel column. The two G6PD type A samples were also different from each other by the same criteria. We conclude that "normal" G6PD is genetically heterogeneous and that the structural Gd alleles concerned are all polymorphic in the Nigerian population. In this instance, a human enzyme polymorphism, not associated with enzyme deficiency, is revealed by an approach other than electrophoresis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babalola A. O., Beetlestone J. G., Luzzatto L. Genetic variants of human erythrocyte glucose-6-phosphate dehydrogenase. Kinetic and thermodynamic parameters of variants A, B, and A- in relation to quaternary structure. J Biol Chem. 1976 May 25;251(10):2993–3002. [PubMed] [Google Scholar]
- Battistuzzi G., Esan G. J., Fasuan F. A., Modiano G., Luzzatto L. Comparison of GdA and GdB activities in Nigerians. A study of the variation of the G6PD activity. Am J Hum Genet. 1977 Jan;29(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Battistuzzi G., Esan G. J., Fasuan F. A., Modiano G., Luzzatto L. Response to Nance letter. Am J Hum Genet. 1977 Sep;29(5):543–544. [PMC free article] [PubMed] [Google Scholar]
- Beiguelman B., Colli-Inglez G., Bonder-Itskan S., Saldanha P. H. Glucose-6-phosphate dehydrogenase activity among Caucasoid twins. Hum Hered. 1970;20(5):535–539. doi: 10.1159/000152356. [DOI] [PubMed] [Google Scholar]
- Beutler E., Yoshida A. Human glucose-6-phosphate dehydrogenase variants: a supplementary tabulation. Ann Hum Genet. 1973 Oct;37(2):151–155. doi: 10.1111/j.1469-1809.1973.tb01823.x. [DOI] [PubMed] [Google Scholar]
- Bienzle U., Ayeni O., Lucas A. O., Luzzatto L. Glucose-6-phosphate dehydrogenase and malaria. Greater resistance of females heterozygous for enzyme deficiency and of males with non-deficient variant. Lancet. 1972 Jan 15;1(7742):107–110. doi: 10.1016/s0140-6736(72)90676-9. [DOI] [PubMed] [Google Scholar]
- Brewer G. J., Gall J. C., Honeyman M., Gershowitz H., Shreffler D. C., Dern R. J., Hames C. Inheritance of quantitative expression of erythrocyte glucose-6-phosphate dehydrogenase activity in the negro--a twin study. Biochem Genet. 1967 Jun;1(1):41–53. doi: 10.1007/BF00487735. [DOI] [PubMed] [Google Scholar]
- DAVIDSON R. G., CHILDS B., SINISCALCO M. GENETIC VARIATIONS IN THE QUANTITATIVE CONTROL OF ERYTHROCYTE GLUCOSE-6-PHOSPHATE DEHYDROGENASE ACTIVITY. Ann Hum Genet. 1964 Sep;28:61–70. doi: 10.1111/j.1469-1809.1964.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Garrick M. D., Bricker J., Garrick L. M. An electrophoretically silent polymorphism for the beta chains of rabbit hemoglobin and associated polyribosome patterns. Genetics. 1974 Jan;76(1):99–108. doi: 10.1093/genetics/76.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livincstone F. B. Malaria and human polymorphisms. Annu Rev Genet. 1971;5:33–64. doi: 10.1146/annurev.ge.05.120171.000341. [DOI] [PubMed] [Google Scholar]
- Luzzatto L., Afolayan A. Enzymic properties of different types of human erythrocyte glucose-6-phosphate dehydrogenase, with characterization of two new genetic variants. J Clin Invest. 1968 Aug;47(8):1833–1842. doi: 10.1172/JCI105873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L., Allan N. C. Different properties of glucose 6-phosphate dehydrogenase from human erythrocytes with normal and abnormal enzyme levels. Biochem Biophys Res Commun. 1965 Dec 21;21(6):547–554. doi: 10.1016/0006-291x(65)90520-6. [DOI] [PubMed] [Google Scholar]
- Luzzatto L., Allan N. C. Relationship between the genes for glucose-6-phosphate dehydrogenase and for haemoglobin in a Nigerian population. Nature. 1968 Sep 7;219(5158):1041–1042. doi: 10.1038/2191041a0. [DOI] [PubMed] [Google Scholar]
- Luzzatto L. Genetic factors in malaria. Bull World Health Organ. 1974;50(3-4):195–202. [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L. Genetic heterogeneity and pathophysiology of G6PD deficiency. Br J Haematol. 1974 Oct;28(2):151–155. doi: 10.1111/j.1365-2141.1974.tb06649.x. [DOI] [PubMed] [Google Scholar]
- Luzzatto L., Usanga F. A., Reddy S. Glucose-6-phosphate dehydrogenase deficient red cells: resistance to infection by malarial parasites. Science. 1969 May 16;164(3881):839–842. doi: 10.1126/science.164.3881.839. [DOI] [PubMed] [Google Scholar]
- Nance W. E. Quantitative studies of glucose-6-phosphate dehydrogenase. Am J Hum Genet. 1977 Sep;29(5):537–544. [PMC free article] [PubMed] [Google Scholar]
- Rattazzi M. C. Isolation and purification of human erythrocyte glucose-6-phosphate dehydrogenase from small amounts of blood. Biochim Biophys Acta. 1969 May;181(1):1–11. doi: 10.1016/0005-2795(69)90221-9. [DOI] [PubMed] [Google Scholar]
- Singh R. S., Lewontin R. C., Felton A. A. Genetic heterogeneity within electrophoretic "alleles" of xanthine dehydrogenase in Drosophila pseudoobscura. Genetics. 1976 Nov;84(3):609–629. doi: 10.1093/genetics/84.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippa G., Catamo A., Lombardozzi A., Cicchetti R. A simple approach for discovering common nonelectrophoretic enzyme variability: a heat denaturation study in Drosophila melanogaster. Biochem Genet. 1978 Apr;16(3-4):299–305. doi: 10.1007/BF00484086. [DOI] [PubMed] [Google Scholar]
- Usanga E. A., Bienzle U., Cancedda R., Fasuan F. A., Ajayi O., Luzzatto L. Genetic variants of human erythrocyte glucose 6-phosphate dehydrogenase: new variants in West Africa characterized by column chromatography. Ann Hum Genet. 1977 Jan;40(3):279–286. doi: 10.1111/j.1469-1809.1977.tb00192.x. [DOI] [PubMed] [Google Scholar]


