Abstract
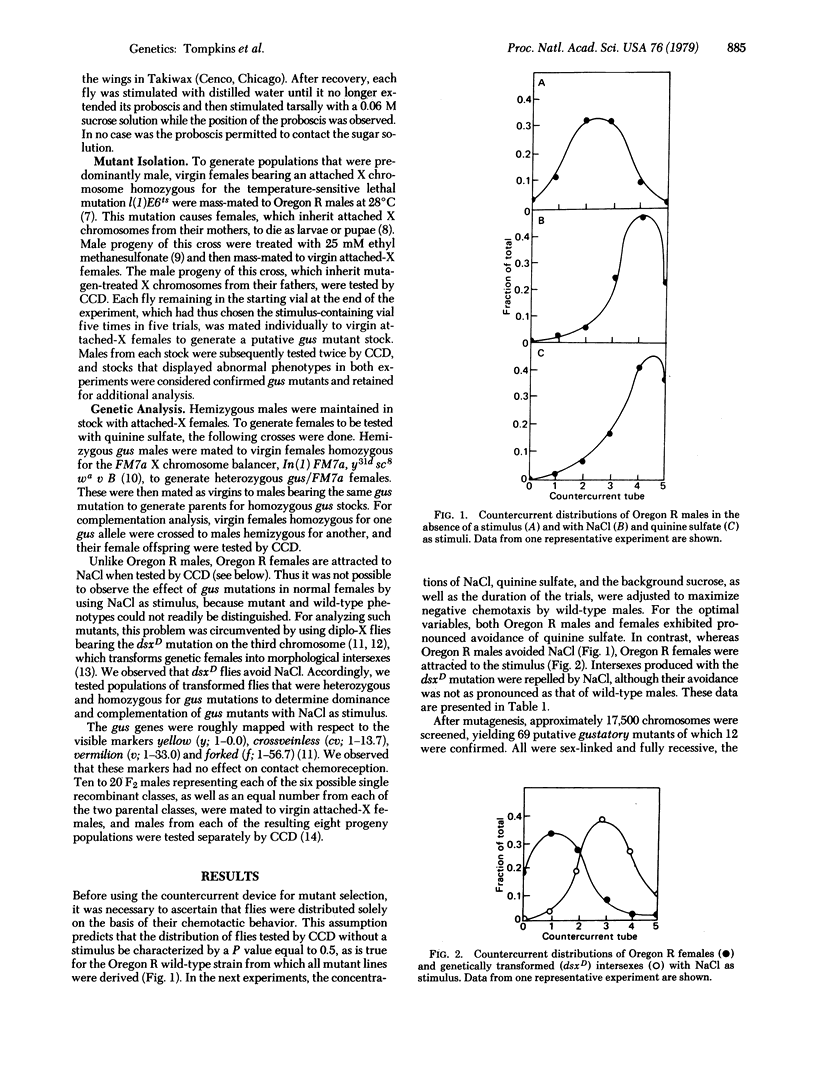
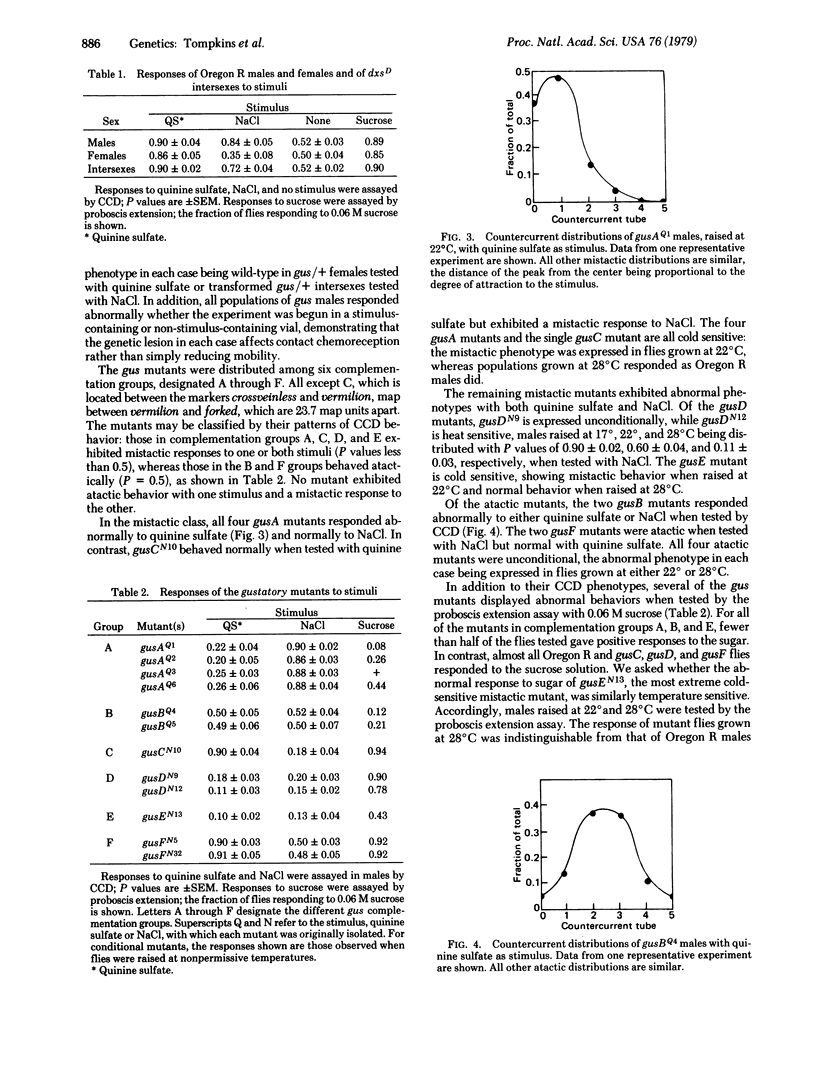
A behavioral countercurrent paradigm has been developed for assaying the chemotactic responses of wild-type and mutant Drosophila melanogaster adults. Oregon R males avoid both quinine sulfate and NaCl, whereas Oregon R females reject the quinine salt but are attracted to NaCl when tested in this paradigm. Wild-type behavior is sufficiently reproducible to allow identification of mutants affecting chemotaxis, and 12 such mutants, in six complementation groups, have now been isolated. Three of the mutants respond abnormally to NaCl, two in one complementation group with atactic behavior (no chemotaxis) and the other, in a separate group, with a mistactic response (attraction to the stimulus). Four mutants in another group respond mistactically to quinine sulfate. Of the remaining mutants, two in one group behave atactically and three, in two groups, respond mistactically to either chemical stimulus. Several of the mutants also show abnormal behavior in a proboscis extension assay when tested individually with sucrose solutions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzer S. BEHAVIORAL MUTANTS OF Drosophila ISOLATED BY COUNTERCURRENT DISTRIBUTION. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN FUNG S. T., GOWEN J. W. The developmental effect of a sex-limited gene in Drosophila melanogaster. J Exp Zool. 1957 Apr;134(3):515–532. doi: 10.1002/jez.1401340306. [DOI] [PubMed] [Google Scholar]
- DETHIER V. G. The limiting mechanism in tarsal chemoreception. J Gen Physiol. 1951 Sep;35(1):55–65. doi: 10.1085/jgp.35.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. R., MELLON D., Jr Stimulation of a primary taste receptor by salts. J Gen Physiol. 1962 Mar;45:651–661. doi: 10.1085/jgp.45.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk R., Atidia J. Mutation affecting taste perception in Drosophila melanogaster. Nature. 1975 Mar 27;254(5498):325–326. doi: 10.1038/254325a0. [DOI] [PubMed] [Google Scholar]
- Grigliatti T. A., Hall L., Rosenbluth R., Suzuki D. T. Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Mol Gen Genet. 1973 Jan 24;120(2):107–114. doi: 10.1007/BF00267238. [DOI] [PubMed] [Google Scholar]
- Grigliatti T., Suzuki D. T. Temperature-sensitive mutations in Drosophila melanogaster. V. A mutation affecting concentrations of pteridines. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1101–1108. doi: 10.1073/pnas.67.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. F. A Direct Method of Testing Color Vision in Lower Animals. Proc Natl Acad Sci U S A. 1922 Dec;8(12):350–353. doi: 10.1073/pnas.8.12.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y., Benzer S. Genetic dissection of the Drosophila nervous system by means of mosaics. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1156–1163. doi: 10.1073/pnas.67.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka R. J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORITA H., YAMASHITA S. Generator potential of insect chemoreceptor. Science. 1959 Oct 9;130(3380):922–922. doi: 10.1126/science.130.3380.922. [DOI] [PubMed] [Google Scholar]
- Quinn W. G., Harris W. A., Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1974 Mar;71(3):708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki D. T. Temperature-sensitive mutations in Drosophila melanogaster. Science. 1970 Nov 13;170(3959):695–706. doi: 10.1126/science.170.3959.695. [DOI] [PubMed] [Google Scholar]
- WOLBARSHT M. L., DETHIER V. G. Electrical activity in the chemoreceptors of the blowfly. I. Responses to chemical and mechanical stimulation. J Gen Physiol. 1958 Nov 20;42(2):393–412. doi: 10.1085/jgp.42.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]


