Abstract
We have previously reported excellent outcomes with liver transplantation for selected patients with early-stage perihilar cholangiocarcinoma (CCA) following neoadjuvant chemoradiotherapy. Our aim was to identify predictors of dropout before transplantation and predictors of cancer recurrence after transplantation.
We reviewed all patients with unresectable perihilar CCA treated with neoadjuvant chemoradiation in anticipation for transplantation between 1993 and 2010. Predictors were identified by uni- and multivariable Cox regression analysis of clinical variables.
In total 199 patients were enrolled, of whom 62 dropped out and 131 underwent transplantation at our institution, with 6 undergoing transplantation elsewhere. Predictors of dropout were CA 19–9 ≥ 500 U/ml (HR 2.3; P=.04), mass ≥ 3 cm (HR 2.2; P=.01), malignant brushing or biopsy (HR 3.6; P=.001) and MELD score ≥ 20 (HR 3.5; P=.02). An online risk calculator was developed for clinical purposes. Post-transplant recurrence-free 5-year survival was 68%. Predictors of recurrence were elevated CA 19–9 (HR 1.8; P=.01), portal vein encasement (HR 3.3; P=.007) and residual tumor on explant (HR 9.8; P<.001). PSC, age, history of cholecystectomy and waiting time were not independent predictors.
Conclusion
Outcome following neoadjuvant chemoradiation and liver transplantation for perihilar CCA is excellent. Risk of dropout is related to patient and tumor characteristics and the risk calculator can be used to guide patient counseling prior to enrolment. Recurrence risk is mostly associated with presence of residual cancer on explant. PSC patients do not have an independent survival advantage over de-novo patients, but present with more favorable tumor characteristics.
Keywords: Bile duct cancer, liver transplantation, neoadjuvant therapy, outcomes
Perihilar cholangiocarcinoma (CCA) constitutes approximately two thirds of all cholangiocarcinomas and is the second most common liver cancer with an annual incidence of approximately 1.2 in 100,000 (1). Known risk factors in the western world include Primary Sclerosing Cholangitis (PSC), with a life time risk of 5–15%, and much less frequently, choledochal cysts, Caroli Syndrome, and cirrhosis of any cause (2).
Clinical challenges of CCA are twofold. First, the diagnosis is difficult to establish. The location is difficult to access and the tumor is highly desmoplastic with a tropism for bile, leading to initial growth along the bile duct rather then into the parenchyma, making it hard to visualize by cross-sectional imaging. The desmoplastic nature of the tumor also results in paucicellular cytologic specimens, rendering a confident diagnosis of malignancy challenging. Intraluminal brushing or biopsy of a dominant stricture on endoscopic retrograde cholangiopancreaticography (ERCP), polysomy on Fluorescence In Situ Hybridization (FISH), elevated tumor marker carbohydrate antigen 19–9 (CA 19–9) and visualization of a mass on imaging, preferably in combination, lead to a sensitivity of only 45–50%, though a specificity of 96–100% (3).
Second, curative therapeutic options are limited. Resection is the standard of care and can provide an opportunity for long-term survival, though many patients present with unresectable disease. After resection, 5-year survival rates generally range from 20–30% (with improvement up to 40–50% with R0 margins) with regional metastasis limiting long-term survival (4–8). While liver transplantation alone was a dismal failure for patients with perihilar CCA (9, 10), when used in combination with neoadjuvant chemoradiotherapy it has evolved to a highly promising alternative for unresectable cases. Encouraged by results from our small series in which 22% 5-year survival was achieved chemoradiation alone (11) and that of the University of Nebraska using neoadjuvant high dose brachytherapy and chemotherapy prior to transplantation (12), in 1993 we developed a protocol combining neoadjuvant EBRT, brachytherapy, chemotherapy, and liver transplantation. Subsequent reports have shown excellent results, with 5-year recurrence-free survival rates of approximately 70% (13–16).
Although liver transplantation appears beneficial in those who complete the protocol, there is a group of patients who do not make it to transplantation (i.e drop out) due to cancer progression or complications of the treatment. However, as of yet there is no literature on predictive markers of dropout, which limits our ability to counsel patients about their individual chances of successfully completing the protocol. Furthermore, after transplantation, recurrence of cholangiocarcinoma has been reported to occur in 17% (15). In our earlier experience with 65 transplanted patients, we found increased age, elevated CA 19-9, prior cholecystectomy, mass on imaging, and residual tumor in explant with perineural invasion to be associated with post-transplant recurrence. However, small sample size precluded multivariate analyses and hence identification of statistically independent predictors. Due to the shortage of liver allografts as well as the potential morbidity of the neoadjuvant therapy and transplantation, we feel it is essential to identify who will most benefit. Thus, the aim of the present study was to identify independent predictors of dropout before transplantation, as well as predictors of cancer recurrence after transplantation.
METHODS
Study design, patient population and transplant protocol
Since January 1993, all patients with unresectable perihilar cholangiocarcinoma presenting to our institution were considered for a treatment protocol consisting of neoadjuvant chemoradiation followed by liver transplantation as previously described (17–19). All patients were evaluated by an experienced hepatobiliary surgeon and determined unresectable in the setting of bilobar involvement of major perihilar structures or underlying PSC, the latter due to the presence of underlying parenchymal disease and/or possible multi-focal disease. All patients treated in accord with this protocol between 1/1993 and 10/2010 were prospectively followed through 12/2010.
Diagnosis was established in the presence of 1) positive or strongly suspicious intraluminal brush or biopsy or, 2) a radiographic malignant appearing stricture plus either CA 19-9 > 100 U/ml in the absence of acute bacterial cholangitis, polysomy on FISH (available since 2003), or well-defined mass on cross-sectional imaging. Patients were excluded if they had any evidence of extrahepatic disease or regional lymph node involvement (as determined by endoscopic ultrasound with fine needle aspiration in all patients since 2002), a previous malignancy (excluding skin or cervical cancer) 5 years prior, prior abdominal radiotherapy, uncontrolled infection, previous attempt at surgical resection with violation of the tumor plane, or any medical condition precluding transplantation. Of note, vascular encasement and stricture/mass extension along the duct were not contra-indications, although a mass with a clear radial diameter of greater than 3 cm was generally excluded.
Patients received neoadjuvant therapy according to our previously published protocol (17–19). External beam radiotherapy (EBRT) was administered to a total dose of 4500 cGy in 30 fractions of 150 cGy twice daily for three weeks. The radiosensitizing chemotherapy regimen has changed over time from daily bolus Fluorouracil (5-FU) at 500 mg/m2 for the first three days to continuous infusion of 5-FU given for the duration of EBRT. After a two week break, a transluminal radiation boost is delivered through transcatheter Iridium-192 seeds placed under fluoroscopy guidance. The delivery hereof has changed from giving 2000 cGy over 24 hours using low dose-rate brachytherapy, to most recently high-dose brachytherapy of either 1200–1600 cGy in 2–4 fractions. Whenever brachytherapy was technically not possible, an extra boost of EBRT (1000–1500 cGy) was given. Patients then begin chemotherapy consisting of oral capecitabine at 2000 mg/m2 in two divided doses for 2 out of every 3 weeks until transplantation. An open staging laparotomy (since 2004, a hand-assisted laparoscopy) with routine biopsy of hepatic artery and peri-choledochal lymph nodes plus any suspicious lesion was performed. Timing of staging has varied with differences in allocation system over time, though since 2007, staging has been performed as time for transplantation nears. Only those with negative staging operation remain eligible.
Transplantation techniques have been described extensively (13). Both deceased and living donor transplants were performed. The distal bile duct margin was sent for frozen section and if tumor was found, additional pancreaticoduodenectomy was performed. All patients received standard immunosuppression (currently short-term mycophenolate mofetil and prednisone taper with tacrolimus monotherapy). Post-transplant follow-up was performed at our institution and included CA 19-9, and abdominal cross-sectional imaging. This study was approved by the Mayo Clinic Rochester Institutional Review Board.
Data collection
Data were prospectively collected since initiation of this protocol. A mass was defined as a well-defined lesion on either Magnetic Resonance Imaging (MRI) or Computerized Tomography (CT). Perihilar thickening or enhancement was not considered a mass. Mass size was defined as the diameter measured radially or longitudinally, whichever was largest. The MELD value represents the calculated MELD (20).
Outcome definition and statistical analyses
Dropout is defined as positive staging, tumor metastasis, death or withdrawal due to intolerable side effects at any time before transplantation. Recurrence is defined as radiographic or pathologically confirmed evidence of cholangiocarcinoma post-transplantation. Continuous variables were expressed as median (range) of their natural or logarithmic scale, and categorical variables were expressed as N (% of total).
Statistical analyses included uni- and multivariable Cox regression analyses of baseline predictors for time to dropout or recurrence (Pin=.05 and Pout=.10). For the prediction of dropout, T0 was set at time of presentation to our transplant clinic. A dropout risk score was developed as the sum of the coefficients of the independent variables in the final multivariate model. The predicted dropout hazard over time for each score was defined as S(t)=S0(t) ^ EXP(risk score) with S0(t) representing patients without risk factors. For the prediction of recurrence, two models were created: one with T0 at presentation (baseline predictors) and one with T0 at transplant (transplant predictors). Survival was calculated using Kaplan Meier analysis and compared by log-rank testing. Comparison between sub-groups was based on Chi-square (categorical) or Mann-Whitney-U testing (continuous variables). Statistical significance was set at P<.05. All analyses were performed in SPSS (version 16.0, Chicago, IL, USA).
RESULTS
Total population
A total of 199 patients were enrolled. Median age was 51 years (range 19–70), 144 (72%) patients were male and 127 (64%) had underlying PSC. Of PSC patients, 36 (28%) presented with CCA as their first manifestation. Table 1 summarizes the diagnostic criteria. There were 103 (52%) patients with definite pathological confirmation. Of those 96 who met other criteria but did not have an initial tissue diagnosis, over half (55%) either dropped out before transplant from disease metastasis (N=22), had evidence of residual tumor on explant (N=30) and/ or developed recurrent cancer (N=14). Median follow-up was 2.6 years (0.11–17.8). Overall (intent-to-treat) survival is presented in Figure 1a.
Table 1.
Diagnostic criteria for all eligible patients with perihilar cholangiocarcinoma who were enrolled and underwent neoadjuvant therapy in anticipation for transplantation (N=199) in association with their underlying etiology and outcomes. All results are expressed in absolute numbers (N).
| Description of diagnostic criteria | N | PSC (N=127) versus De novo (N=72) | Drop-out (N=62) | Tumor on explants (N=61) | Recurrence (N=26) | |
|---|---|---|---|---|---|---|
| Definite evidence of malignancy on intraluminal brushing or biopsy (i.e. positive for adenocarcinoma) | 103 | PSC | 71 | 26 | 18 | 5 |
| De novo | 32 | 14 | 13 | 7 | ||
| Stricture + combination of 2 or more of CA 19-9>100, mass, suspicious brushing or biopsy and/or polysomy | 69 | PSC | 41 | 7 | 11 | 4 |
| De novo | 28 | 10 | 12 | 4 | ||
| Stricture + CA 19-9>100 | 6 | PSC | 3 | 1 | 1 | 1 |
| De novo | 3 | 0 | 2 | 1 | ||
| Stricture + mass | 5 | PSC | 0 | 0 | 0 | 0 |
| De novo | 5 | 2 | 2 | 2 | ||
| Stricture + polysomy | 5 | PSC | 5 | 0 | 0 | 0 |
| Stricture + suspicious brushing | 1 | PSC | 1 | 1 | 0 | 0 |
| Stricture + weight loss + obstructive jaundice | 5 | PSC | 1 | 0 | 0 | 0 |
| De novo | 4 | 1 | 0 | 1 | ||
| Diffuse strictures + CA 19-9>100 + mass | 1 | PSC | 1 | 0 | 1 | 1 |
| Diffuse strictures + suspicious brushing + polysomy | 1 | PSC | 1 | 0 | 1 | 0 |
| Intraductal polypoid lesion on ERCP + polysomy | 1 | PSC | 1 | 0 | 0 | 0 |
| Diffuse strictures + suspicious brushing + trisomy (repeatedly) | 1 | PSC | 1 | 0 | 0 | 0 |
| Diffuse strictures + polysomy | 1 | PSC | 1 | 0 | 0 | 0 |
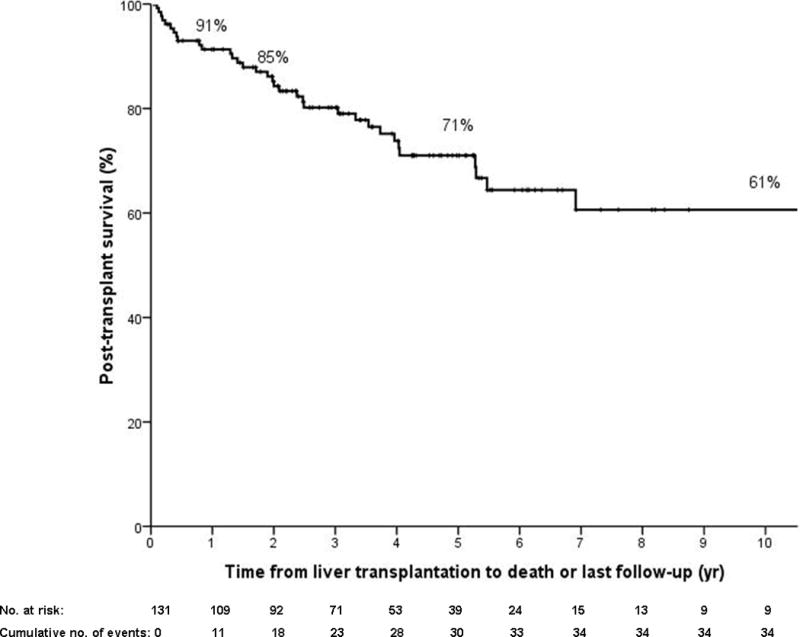
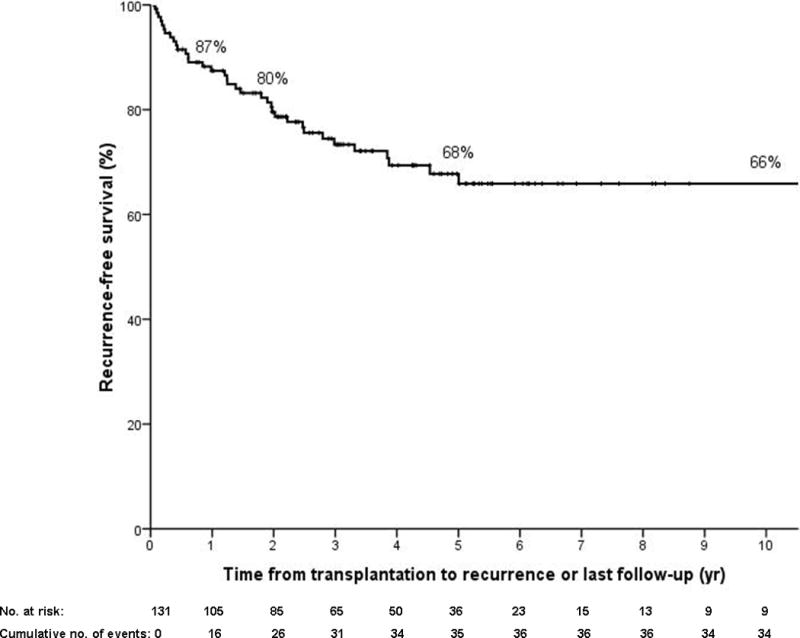
Figure 1.
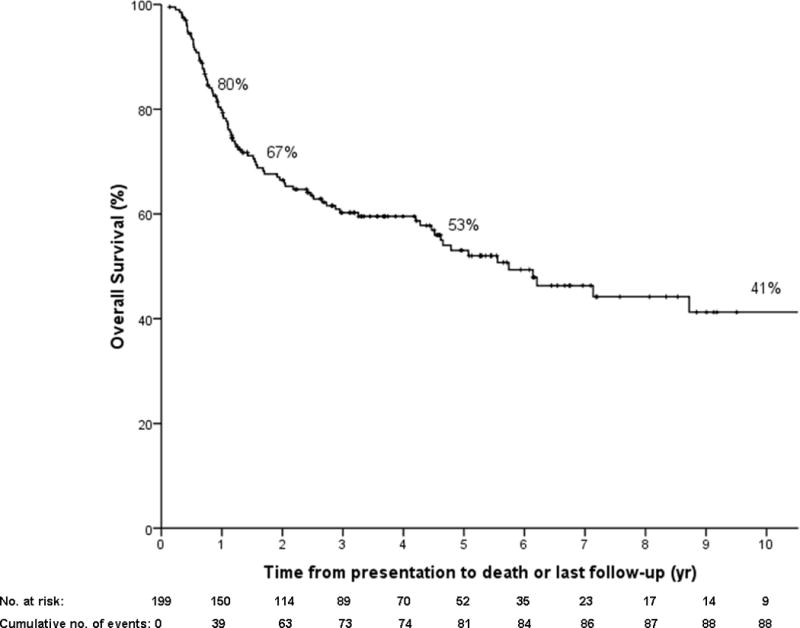
a) Overall Kaplan Meier survival curve in all patients enrolled in our protocol (N=199) from presentation to death (N=90). Patients are censored at last follow-up if alive regardless of treatment received (intent to treat).
b) Kaplan Meier survival post-transplantation in all patients (N=131; N=36 deaths). Patients are censored at last follow-up if alive.
c) Recurrence-free Kaplan Meier survival in all patients (N=131) from transplantation to cholangiocarcinoma recurrence or death (N=38 recurrence or death). Patients are censored at last follow-up if alive and without recurrence.
Dropout pre-transplantation
As summarized in Figure 2, a total of 62 patients (31%) dropped-out from the protocol after a median of 4.7 months (1.1–17.1). Fifty five patients had progression of their cancer, of whom 19 were diagnosed clinically and 36 at the time of operative staging with metastatic disease found in regional lymph nodes (N=14), intrahepatic (N=6) and extrahepatic lesions (N=16). Of these, 45 (82%) died from cancer progression, and the remaining ten left our institution immediately after dropout and were lost to follow-up. One patient was no longer a transplant candidate due to progressive clinical deterioration, and she died from liver failure. Five patients died from cardiovascular arrest (N=2), sepsis (N=1), liver failure (N=1) or GI bleeding (N=1) without evidence for cancer progression. Total mortality in the dropped-out patients was thus 83% (N=52) and death occurred after a median of 3.6 months from detection of metastasis (range 0–22.1 months with one patient surviving up to 96.7 months after detection of metastatic disease on staging). There were no differences (including waiting time) between patients (N=6) who dropped out after initial negative staging as compared to those who did so before staging (N=20), or those who underwent transplant (N=131; data not shown).
Figure 2.
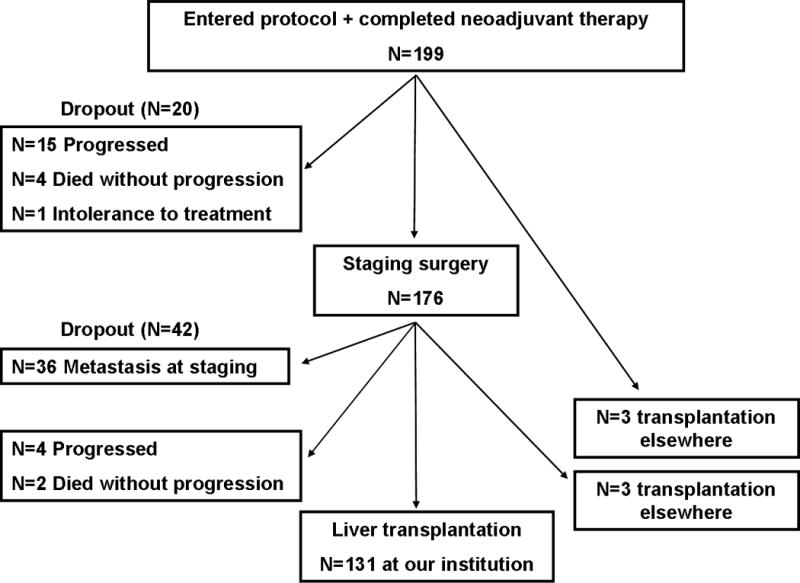
Flow of patients from start of neoadjuvant treatment to transplantation from January 1993 to October 2010. A total of 62 patients dropped-out and 137 either were transplanted at our institution (N=131) or elsewhere (N=6).
The results of the uni- and multivariable analyses of waiting list dropout are presented in Table 2. Presentation with painless jaundice or weight loss, visible tumor mass on cross-sectional imaging (especially with largest diameter ≥ 3 cm), positive or suspicious intraluminal brushing or biopsy, elevated CA 19–9, vascular encasement and higher MELD score were all significant predictors of dropout in the univariate analysis. Interestingly, underlying PSC, age, history of cholecystectomy or use of percutaneous biliary drainage was not predictive. The final multivariable model identified the following independent predictors: mass size ≥ 3 cm, positive or suspicious intraluminal brushing or biopsy, elevated CA 19–9 and higher MELD score. Based on the coefficients in the final model, a dropout risk score was formulated as follows: 0.78 × (size ≥ 3) + 1.29 × (positive/ suspicious cyto-pathology) + 0.44 × (CA 19–9 100–500 U/ml) + 0.82 × (CA 19–9 ≥ 500 U/ml) + 0.30 × (meld 10–20) + 1.24 × (meld ≥ 20). All predictors are scored as 1 if true and 0 if not true. Table 3 illustrates the translation of this score into dropout risk over time. For clinical use, an online calculator is now available at no cost: (website in progress).
Table 2.
Univariate and multivariate Cox models of baseline characteristics for patients who dropped out from the protocol (N=62) versus those who remained eligible for transplant (defined as ‘success’, N=137). Categorical variables are expressed in N (%) and continuous variables as median (range).
| UNIVARIATE | MULTIVARIATE | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Baseline characteristic | Drop-out N=62 | Success N=137 | Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value |
| Age | 52.2 (27–70) | 50.6 (19–67) | 1.01 | 0.99–1.04 | .38 | – | – | – |
| PSC | 35 (56) | 92 (67) | 0.74 | 0.43–1.17 | .17 | – | – | – |
| Inflammatory bowel disease | 31 (50) | 76 (55) | 0.86 | 0.52–1.41 | .54 | – | – | – |
| PTC drain vs. ERCP stent | 10 (16) 33 (53) |
20 (15) 64 (48) |
1.02 | 0.50–2.07 | .96 | – | – | – |
| Cholecystectomy | 10 (16) | 33 (24) | 0.74 | 0.38–1.47 | .39 | – | – | – |
| Onset of symptoms (days) | 81 (31–707) | 103 (22–866) | 1.00 | 1.00–1.00 | .67 | – | – | – |
| Painless jaundice | 47 (76) | 76 (55) | 2.07 | 1.16–3.71 | .01 | – | – | – |
| Weight loss | 42 (68) | 76 (55) | 1.69 | 0.99–2.89 | .05 | – | – | – |
| Opioids use for abdominal pain | 4 (6) | 6 (4) | 1.64 | 0.60–4.54 | .34 | – | – | – |
| Mass seen on imaging | 43 (69) | 68 (50) | 2.24 | 1.30–3.85 | .004 | – | – | – |
| Mass largest diameter (cm) | 3.0 (0.9–5.0) | 2.5 (0.6–4.5) | 1.61 | 1.18–2.20 | .003 | – | – | – |
| Mass ≥ 3 cm | 19 (31) | 16 (11) | 3.07 | 1.78–5.31 | <.001 | 2.17 | 1.17–4.03 | .01 |
| Positive / suspicious brushing or biopsy | 49 (79) | 91 (66) | 2.28 | 1.15–4.50 | .02 | 3.63 | 1.67–7.89 | .001 |
| Polysomy on FISH | 15 (24) | 40 (29) | 1.26 | 0.59–2.70 | .56 | – | – | – |
| CA 19-9 (log)* | 2.34 (0–4.12) | 1.77 (0–4.46) | 1.68 | 1.27–2.25 | <.001 | – | – | – |
| CA 19-9 (U/ml) | ||||||||
| <100 (ref) | 24 (39) | 83 (61) | 0.87–3.18 | .002 | 0.78–3.08 | .04 | ||
| 100–499 | 15 (24) | 34 (25) | 1.67 | 1.60–5.06 | 1.55 | 1.22–4.26 | ||
| ≥500 | 23 (53) | 18 (13) | 2.85 | 1.02–1.10 | 2.28 | |||
| MELD score | 12 (6–26) | 10 (6–35) | 1.06 | .007 | – | – | – | |
| MELD score | ||||||||
| <10 | 22 (35) | 73 (53) | 1.10–3.35 | .02 | 0.73–2.48 | .02 | ||
| 10–20 | 30 (48) | 55 (41) | 1.93 | 1.24–5.88 | 1.35 | 1.46–8.27 | ||
| ≥20 | 9 (15) | 9 (7) | 2.70 | 3.47 | ||||
| Vascular encasement | 31 (50) | 44 (32) | 1.82 | 1.10–2.99 | .02 | – | – | – |
| Atrophy / hypertrophy complex | 20 (32) | 42 (31) | 0.95 | 0.56–1.63 | .86 | – | – | – |
Given the wide distribution of tumor marker CA 19-9, a logarithmic conversion is used for the continuous variable. Each log changes signifies a change of 101 (e.g. from 10 to 100, or 100 to 1000). Median CA 19-9 values were 221 (1-13200) and 51.3 U/ml (0-28750) for the drop-out and success group, respectively.
Table 3.
Expected drop-out risk at 6, 12, and 18 months based on the most common combinations of predictors in our dataset (i.e. ≥ 4 patients per score). Drop-out Risk Score = 0.7 × (size ≥ 3) + 1.29 × (positive/ suspicious cyto-pathology) + 0.44 × (CA 19-9 100–500 U/ml) + 0.82 × (CA 19-9 ≥ 500 U/ml) + 0.30 × (meld 10–20) + 1.24 × (meld ≥ 20). All predictors are scored as 1 if true and 0 is not true.
| N | Drop-out Risk Score | Mass size (cm) on cross-sectional imaging | Brushing or biopsy result | CA 19-9 at presentation | Calculated MELD | Drop-out risk at 6 months (%) | Drop-out risk at 12 months (%) | Drop-out risk at 18 months (%) |
|---|---|---|---|---|---|---|---|---|
| 11 | 0 | < 3 | Negative | <100 | <10 | 5 | 8 | 13 |
| 8 | 0.299 | < 3 | Negative | <100 | 10–20 | 7 | 10 | 16 |
| 5 | 0.440 | < 3 | Negative | 100–499 | <10 | 8 | 12 | 19 |
| 8 | 0.739 | < 3 | Negative | 100–499 | 10–20 | 11 | 16 | 24 |
| 45 | 1.890 | < 3 | Positive / Suspicious | <100 | <10 | 18 | 25 | 38 |
| 27 | 1.588 | < 3 | Positive / Suspicious | <100 | 10–20 | 23 | 33 | 48 |
| 12 | 1.729 | < 3 | Positive / Suspicious | 100–499 | <10 | 26 | 37 | 53 |
| 8 | 2.028 | < 3 | Negative | ≥ 500 | ≥ 20 | 33 | 46 | 64 |
| 4 | 2.066 | < 3 | Positive / Suspicious | 100–499 | 10–20 | 34 | 47 | 65 |
| 10 | 2.112 | < 3 | Positive / Suspicious | ≥ 500 | <10 | 36 | 49 | 67 |
| 5 | 2.364 | ≥ 3 | Positive / Suspicious | <100 | 10–20 | 43 | 58 | 76 |
| 10 | 2.411 | < 3 | Positive / Suspicious | ≥ 500 | 10–20 | 45 | 59 | 77 |
| 5 | 2.532 | < 3 | Positive / Suspicious | <100 | ≥ 20 | 49 | 64 | 81 |
| 7 | 2.804 | ≥ 3 | Positive / Suspicious | 100–499 | 10–20 | 58 | 74 | 89 |
| 4 | 3.187 | ≥ 3 | Positive / Suspicious | ≥ 500 | 10–20 | 72 | 86 | 96 |
Transplantation
A total of 131 patients (66%) underwent liver transplantation at our institution after a median wait time of 6.9 months (range 1.97–34.3) with a deceased donor in 88 (67%), a living donor in 42 (32%) and a familial amyloidosis donor in 1 patient. Median calculated MELD was 11 (6–40), and median CA 19–9 was 55 U/ml (0–2370). On explant, residual tumor was found in 61 patients (47%) of whom 24 had perineural and three regional lymphovascular invasion. Histological grade was G1 (well differentiated; N=1), G2 (moderately differentiated; N=22), G3 (poorly differentiated; N=29) and G4 (undifferentiated; N=4). In five patients, grading could not be performed due to radiation effects (i.e. Gx).
Eleven patients required re-transplantation (8%) after a median of 1.2 months (0.03–163.6) for primary non-function (N=3), hepatic artery thrombosis (N=6), ischemic cholangiopathy (N=1) and recurrent PSC (N=1). In total, 36 patients died (27%), due to cancer recurrence (N=24), multiorgan failure (N=3), invasive intracranial aspergillosis (N=1), graft-versus-host disease (N=1), massive pulmonary embolism (N=1), sepsis (N=1), subdural hematoma (N=1), pulmonary infection (N=1), massive intra-operative intra-abdominal hemorrhage (N=1) and PTLD (N=1). One patient died in an outside institution with unknown cause with no evidence of recurrence at 6.7 months of follow-up. Post-transplant 1, 2 and 5 year survival was 91% (95% CI 86–96), 85% (95% CI 79–91) and 71% (95% CI 62–80%), respectively (Figure 1b).
Recurrence post-transplantation
A total of 26 patients (20%) developed cancer recurrence at a median of 23 months (1–128) after transplantation. There were 17 with distant and 9 with locoregional metastases. Median survival post-recurrence was 6.9 months (0–40). All but two patients (alive at 1 and 10 months at end of follow-up) died post-recurrence. Recurrence-free 1, 2 and 5 year survival was 87% (95% CI 82–93), 80% (95% CI 72–87) and 68% (95% CI 59–77), respectively (Figure 1c).
Table 4 summarizes predictors of recurrence. Of variables available at enrolment, age, mass, CA 19-9 and vascular encasement (particularly encasement of the portal vein) were significantly associated with risk of recurrence. Also, patients with PSC-related CCA had a lower risk of recurrence than those with de-novo CCA. In the multivariable model, however, only elevated CA 19-9 at presentation and complete portal vein encasement remained independent baseline predictors of recurrence post-transplant.
Table 4.
Univariate and multivariate Cox models of characteristics at presentation and at time of transplantation for transplanted patients who developed recurrence (N=26) versus those who remained free of recurrence (N=105) at our institution. Categorical variables are expressed in N (%) and continuous variables as median (range).
| UNIVARIATE | MULTIVARIATE | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Recurrence N=26 | Free of recurrence (N=105) | Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value | |
| Baseline Characteristics | ||||||||
|
| ||||||||
| Age | 53 (36–67) | 50 (22–66) | 1.06 | 1.01–1.10 | .01 | – | – | – |
| PSC | 11 (42) | 76 (72) | 0.35 | 0.16–0.77 | .009 | – | – | – |
| PTC drain vs. ERCP stent | 8 (31) 14 (54) |
12 (11) 47 (45) |
0.61 | 0.26–1.45 | .26 | – | – | – |
| Cholecystectomy | 9 (35) | 24 (23) | 1.49 | 0.66–3.34 | .34 | – | – | – |
| Onset of symptoms (d) | 104 (51–274) | 111 (22–866) | 1.00 | 0.99–1.00 | .41 | – | – | – |
| Mass seen on imaging | 18 (69) | 48 (46) | 3.66 | 1.56–8.59 | .003 | – | – | – |
| Mass largest diameter (cm) | 2.5 (2.0–4.0) | 2.4 (0.6–4.5) | 1.79 | 0.89–3.59 | .10 | – | – | – |
| Mass ≥ 3 cm | 6 (23) | 10 (9) | 3.97 | 1.53–10.35 | .005 | – | – | – |
| Positive / suspicious brushing or biopsy | 17 (65) | 71 (68) | 1.07 | 0.47–2.41 | .87 | – | – | – |
| CA 19-9 (log)* | 2.05 (0.48–4.46) | 1.70 (0–4.42) | 1.85 | 1.24–2.78 | .003 | 1.79 | 1.14–2.80 | .011 |
| CA 19-9 (U/ml) | ||||||||
| <100 (ref) | 13 (50) | 65 (62) | 1.57 | 0.59–4.17 | .12 | – | – | – |
| 100–499 | 6 (23) | 27 (26) | 2.61 | 1.04–6.54 | ||||
| ≥500 | 7 (27) | 11 (10) | ||||||
| MELD score | 10 (6–25) | 10 (6–35) | 1.00 | 0.93–1.07 | .96 | – | – | – |
| Vascular encasement | 12 (46) | 30 (29) | 2.25 | 1.03–4.88 | .04 | – | – | – |
| Complete portal vein encasement | 8 (31) | 11 (11) | 4.19 | 1.78–9.87 | .001 | 3.30 | 1.38–7.86 | .007 |
|
| ||||||||
| Transplant characteristics | ||||||||
|
| ||||||||
| Time on waitlist until OLT (d) | 200 (65–423) | 211 (60–1044) | 1.00 | 1.00–1.00 | .19 | – | – | – |
| Waitlist time ≥1 year | 2 (8) | 29 (28) | 0.25 | 0.06–1.08 | .06 | – | – | – |
| Living donor vs. | 7 (27) | 36 (34) | 0.97 | 0.41–2.33 | .95 | – | – | – |
| Deceased donor (ref) | 19 (73) | 69 (66) | ||||||
| Extended criteria donor# vs. Standard (ref) | 3 (12) 23 (88) |
22 (21) 83 (79) |
0.79 | 0.23–2.63 | .70 | – | – | – |
| Pancreaticoduodenectomy | 4 (15) | 8 (31) | 1.85 | 0.64–5.37 | .26 | – | – | – |
| Cell Saver derived autologous blood transfusion | 11 (42) | 39 (37) | 1.64 | 0.75–3.60 | .22 | – | – | – |
| CA 19-9 (log)* | 2.15 (0.95–3.37) | 1.73 (0.48–3.23) | 2.33 | 1.14–4.75 | .02 | – | – | – |
| MELD | 10 (6–23) | 12 (6–41) | 0.98 | 0.92–1.04 | .51 | – | – | – |
| Residual tumor tissue on explant | 23 (88) | 38 (36) | 9.83 | 2.95–32.76 | <.001 | 9.83 | 2.95–32.76 | <.001 |
| Perineural invasion | 11 (42) | 13 (12) | 4.05 | 1.86–8.83 | <.001 | |||
Given the wide distribution of tumor marker CA 19-9, a logarithmic conversion is used. Each log changes signifies a change of 101 (e.g. from 10 to 100, or 100 to 1000). At presentation, actual median CA 19-9 values were 112 (0-28750) and 45.8 U/ml (0-26200) for the recurrence and no-recurrence group, respectively. At transplant, median CA 19-9 values were 132 (0-2370) and 47.3 U/ml (0-1701) for the recurrence and no-recurrence group, respectively.
ECD criteria included age >65 (N=14), Death after Cardiac Death (N=6), >20% macrosteatosis (N=3) and high risk donor (N=2).
Of variables available at the time of transplant, CA 19-9, residual tumor on the explant, and perineural invasion were significantly associated with increased recurrence risk in univariate analyses. Interestingly, waitlist time was not significantly associated with recurrence, even after stratification for MELD era or living vs. deceased donors (data not shown). In the multi-variable model, the most significant predictor of recurrence was residual tumor on explant with an HR of 9.83 (P<.001).
It is important to note, however, that 38 out of 61 patients with residual cells (62%) did not develop recurrence. We found no significant differences in clinical characteristics (including waiting time and follow-up time) between patients who did or did not develop recurrence, indicating there may be yet unknown factors such as inherent tumor biology, which may determine responsiveness to treatment and recurrence.
Subgroup analyses
Two subgroup analyses were performed to examine the robustness of our results. First, we compared patients with PSC-related CCA to those with de-novo CCA to explain why univariately, PSC patients appear to do better but in multivariate models this effect disappears. We found that this is likely due to patients with PSC having a more favorable profile with younger age (median 48 versus 55; P<.001), less mass formation (47% versus 72%; P=.001), less vascular encasement (26% versus 58%; P<.001), lower CA 19-9 levels (median 51 versus 150 U/ml; P=.001), less painless jaundice (51% versus 81%; P<.001) and less weight loss (53% versus 71%; P=.02). In a sensitivity analysis (repeating the univariate Cox analyses of dropout for each subset separately) PSC patients had the same significant predictors as the overall population (data not shown). For de-novo patients the same held true, though the differences were non-significant given smaller number of patients and lower statistical power, for all variables except mass size ≥ 3 cm (22% in dropout vs. 27% in success group; HR 1.07; P=.89) and MELD score (median 10.6 vs. 11.0, respectively; HR 0.99; P=.79).
Second, although the majority of patients (N=183; 92%) eventually had definite proof of CCA by either pre-transplant intraluminal brushing or biopsy, radiological evidence of a well-defined tumor mass, tumor tissue on explant or CCA recurrence, 16 patients (8%) did not despite having constitutional symptoms, a malignant stricture, CA 19-9 > 100 U/ml and/or positive FISH. A sensitivity analysis showed that removal of these patients did not alter survival (Figure 3) or the results of the uni- and multivariable models (data not shown).
Figure 3.
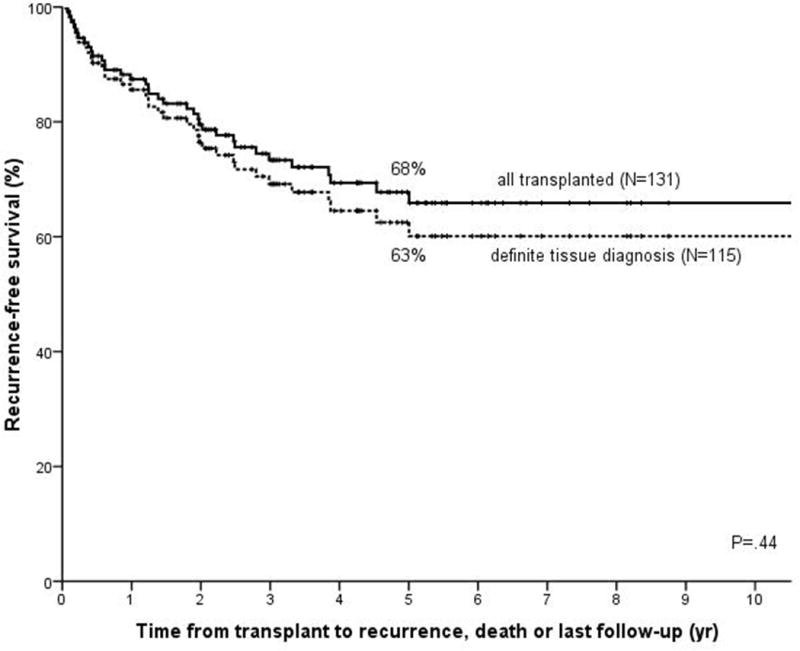
Kaplan Meier recurrence-free survival curve when patients with no mass on diagnostic imaging, negative brushing or biopsy, no residual tumor, and no tumor recurrence (N=16) are excluded (dotted line; N=115; N=38 recurrence or death) as compared to the total population (solid line, same as Figure 1c). Comparison based on log-rank testing.
DISCUSSION
Since 1993, we have offered a protocol combining the known benefits of neoadjuvant chemoradiotherapy with liver transplantation in an attempt to develop an effective therapy for patients with unresectable perihilar cholangiocarcinoma. Outcomes utilizing this multimodality protocol are superior to liver transplantation alone. Indeed, our current post-transplant survival of 91%, 85% and 71% at 1, 2, and 5 years is similar to that achieved for other standard indications for OLT such as hepatocellular carcinoma or HCV. Despite the aggressive pre-transplant protocol however, 26 patients (20%) developed disease recurrence. Additionally, there were 62 patients (31%) who had disease progression or other issues, which precluded transplantation. Given the marked allograft shortage as well as the considerable toxicity of the treatment, this study contributes to the field by identifying those factors important in predicting risk for dropout, as well as recurrence. We found that a mass of 3 cm or greater, CA 19-9 levels above 500 U/ml, positive or suspicious results on intraluminal brushing or biopsy, and MELD above 20 predicted an increased risk of dropout pre-transplantation. Factors, which predicted recurrent CCA post-transplantation included elevated CA 19-9, portal vein encasement and more importantly, presence of residual tumor on explant pathology.
The predictors of dropout are biologically plausible. A mass size ≥ 3 cm was associated with a 2-fold increased risk. Growth may represent a greater opportunity to metastasize or more aggressive tumor biology, particularly since mass formation is not a typical early feature of CCA (21). It is important to note, however, that these measurements are subject to interpretation due to surrounding inflammation, which obscures its borders. In addition, we believe extension along the bile ducts does not portend the same prognosis as radial growth into the parenchyma. An elevated CA 19-9 at enrolment, especially if over 500 U/ml, was also predictive of dropout. Although CA 19-9 can be elevated in numerous biliary conditions such as obstruction or cholangitis, in our study it was only recorded after resolution of these conditions. This finding could therefore represent overall tumor burden or biology and should heighten clinical awareness. Presence of positive or suspicious cytology or pathology also likely represents a tumor that is more advanced and thus more amenable to localization on endoscopy. Endoscopic biopsies and brushing are performed under fluoroscopic guidance and hence require obvious changes in duct caliber. The yield of diagnostic sampling is therefore generally unsatisfying, as was also apparent in our patients. Importantly, however, 55% of those with an initial negative result developed overt tumor progression, disease recurrence or definite tissue diagnosis at explant. The use of direct visualization and biopsy via cholangiopancreatoscopy (aka Spyscope) may eventually improve the diagnostic sensitivity of endoscopic biopsy though thus far, data are limited (22). Finally, a higher MELD score was associated with a 3.5 fold increased risk of dropout, which was mostly driven by elevations in bilirubin and INR. A higher MELD may thus represent greater tumor burden. However, it may also reflect more advanced underlying liver disease, which may make patients more prone to complications of therapy. Indeed, chemoradiation regimens are often interrupted and doses reduced in patients with hyperbilirubinemia. It is therefore imperative to alleviate cholestasis by biliary dilatation and/or stenting when needed.
The abovementioned factors have all been incorporated into an online risk calculator, which predicts waitlist dropout risk over time (website in progress). This tool should supplement, but not replace, clinical judgment and expertise by providing data regarding risk and benefit assessment when counseling patients at start of the protocol. Although we would have desired to perform an external validation using an independent patient population before widespread implementation of the score, in realty it is impossible to find a large enough cohort given that very few centers have a similar protocol.
For the prediction of recurrence in the post-transplant setting, CA 19-9 at enrolment again was significant as was encasement of the portal vein. We are uncertain of the basis for the latter but suspect that it may also reflect tumor burden. The fact that vascular invasion was not predictive of recurrence confirms that this radiographic finding is due to tumor extension along the vein rather than invasion, unlike in hepatocellular carcinoma. Of all transplant related variables, having residual tumor cells was the strongest predictor of recurrence, with a near 10 fold increased risk. Interestingly, however, the 62% with residual tumor who did not develop recurrence were not different from those who did, based on known parameters. Unfortunately, we do not know if there are inherent biologic principles of the tumor which make it less radiosensitive or more prone to early dissemination, or simply that some tumors cannot be completely eradicated by the chemo-radiotherapy. Further research in this field is desperately needed. Although finding residual cancer in the explant is an anxiety-provoking event, there is currently no proven benefit of adjuvant therapy.
The predictors of recurrence we found in our current study are not all in line with our earlier experience (15). For example, we did not find waiting time, age, history of cholecystectomy and PSC to be significant. Part of the discrepancy lies in the substantial difference in sample size; in our earlier study, 65 patients were in size; in our earlier study, 65 patients were included with only 11 events, versus 131 and 26 now. At the same token, results were only based on univariate analyses. In the present study, waiting time was not associated with risk of recurrence, even after correction for MELD era or living vs. deceased donation. Although outliers existed, the overall spread in waiting time for those who made it to transplant was likely too homogenous to demonstrate a difference. Furthermore, the actual key factor may be the response to neoadjuvant therapy, rather than time. Unfortunately, the lack of accessible tissue limits our ability to determine this serially, though certainly future advances in complex molecular analysis of these tumors may allow this question to be addressed.
It was surprising that neither age nor PSC were significant in the multivariable analyses. Comparing patients with PSC-related CCA and de-novo CCA taught us that PSC patients usually presented with more favorable tumor characteristics, potentially because they are monitored more closely or undergo a more rapid evaluation. Notably, those who do present with more advanced disease do as poorly as de-novo CCA patients. In our sensitivity analyses, we found the same factors to be predictive of dropout in the PSC group as in the total group. On the other hand, in de-novo patients, mass size and MELD score did not seem to discriminate between those who dropped out versus those who proceeded to transplant. Though statistical power is low due to smaller numbers of patients, it may be that MELD score is less informative in patients with de-novo CCA due to lack of parenchymal disease, resulting in lower scores with less variability. Lack of parenchymal disease in de-novo patients may also lead to easier mass detection. This is suggested by the observation that 72% of these patients had a visible mass (compared to 47% in PSC) and a median size of 2.6 cm. Further research in this field is needed. At this point, however, mass size or higher MELD in patients with de-novo CCA appear less predictive than in PSC.
Our results continue to demonstrate excellent outcomes following neoadjuvant therapy and liver transplantation for early-stage perihilar CCA, similar to earlier studies (14, 15, 18, 19). Indeed, our 71% 5-year post-transplant survival is similar to the 68% for all deceased liver transplants based on the latest Organ Procurement Transplantation Network data. We believe this is achieved by a combination of rigorous selection criteria and the effectiveness of neoadjuvant radiation and chemotherapy to eliminate or at least contain the tumor while awaiting transplantation. In a recent series by Hong et al. (23), outcomes of patients transplanted with more advanced disease, including both intrahepatic (N=26) and perihilar CCA (N=14), were described. Based on variables that predicted higher recurrence risk (multifocality, perineural and lymphovascular invasion, infiltrative growth, PSC, perihilar CCA, and absence of neoadjuvant therapy.), patients were classified into 3 risk groups. While the 5-year survival in the low risk group (78%) mimics ours, it is important to note that only 2 out of the 11 low risk patients had perihilar CCA. The 5-year recurrence-free survival for the intermediate and high risk groups, which had the majority of the perihilar cancers, however, was very poor. While the authors postulate that this would improve with increased use of neoadjuvant therapy, this has not yet been demonstrated. Additionally, the proposed use of direct tumor biopsies pre- and post-neoadjuvant treatment to obtain prognostic information is worrisome as this has been associated with a higher risk for tumor dissemination (24). Although any attempt to optimize treatment options for patients with this devastating disease is highly encouraged, as of yet there are no curative options for those with advanced disease.
Our study has limitations. First, although this is the largest series to date, overall numbers from a statistical standpoint are still small, especially for the analysis of recurrence risk. We therefore limited the list of potential predictors to only the most clinically relevant. Along the same line, the dropout risk score is based only on our study population and is of yet not externally validated. However, at the current time no suitable external cohort is available in the US. Second, even though most patients are followed at our institution, we could not assess final outcome for ten patients who left our clinic after dropout, who typically returned to their local centers for palliative care. Lastly, despite all our efforts to obtain a definite tissue diagnosis in patients whose clinical picture is highly compatible with CCA, we were unable to obtain definite evidence in 16 patients. However, we showed that excluding these patients did not significantly alter our results.
In conclusion, patients who undergo neoadjuvant chemoradiation followed by liver transplantation have excellent 5-year recurrence-free survival. Elevated CA 19-9, mass ≥ 3 cm, malignant brushing or biopsy and higher MELD score were all associated with increased risk of dropout before transplantation. The currently available online risk calculator can be used to supplement clinical judgment when counseling patients prior to enrolment. Elevated CA 19-9, encasement of the portal vein and, most importantly, residual tumor cells were associated with increased risk for recurrence after transplantation. Ideally, novel therapies will become available in the future which could be offered to those patients identified as higher risk of disease progression prior to transplantation or recurrence after transplantation.
Acknowledgments
Grant Support
Sarwa Darwish Murad is a recipient of the 2010/2011 AASLD/LIFER Clinical and Translational Research Fellowship in Liver Diseases Award
Abbreviations (alphabetically)
- CCA
Cholangiocarcinoma
- CA
19-9 Carbohydrate antigen 19-9
- CT
Computed Tomography
- EBRT
External beam radiotherapy
- ERCP
Endoscopic Retrograde Cholangio-Pancreaticography
- FISH
Fluorescence In-Situ Hybridization
- 5-FU
5-Fluorouracil
- MELD
Model for End-stage Liver Disease
- MRI
Magnetic Resonance Imaging
- OLT
Orthotopic Liver Transplantation
- PET
Positron Emission Tomography
- PSC
Primary Sclerosing Cholangitis
- PTC
Percutaneous Transhepatic Cholangiography
Footnotes
Disclosures
Sarwa Darwish Murad nothing to disclose, no conflict of interest
W. Ray Kim nothing to disclose, no conflict of interest
Gregory J. Gores nothing to disclose, no conflict of interest
Charles B. Rosen nothing to disclose, no conflict of interest
Terry Therneau nothing to disclose, no conflict of interest
James A. Martenson nothing to disclose, no conflict of interest
Steven R. Alberts nothing to disclose, no conflict of interest
Julie K. Heimbach nothing to disclose, no conflict of interest
Author Contributions
Sarwa Darwish Murad study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analyses; obtained funding
W. Ray Kim study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; study supervision
Gregory J. Gores study concept and design; critical revision of the manuscript for important intellectual content; study supervision
Charles B. Rosen study concept and design; critical revision of the manuscript for important intellectual content
Terry Therneau analysis and interpretation of data; critical revision of the manuscript for important intellectual content
James A. Martenson analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Steven R. Alberts analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Julie K. Heimbach study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; study supervision; obtained funding
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl 6):VI1–9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–1117. doi: 10.1002/hep.22441. [DOI] [PubMed] [Google Scholar]
- 4.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, Youssef BM, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. discussion 517–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi A, Miwa S, Nakata T, Miyagawa S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg. 2010;97:56–64. doi: 10.1002/bjs.6788. [DOI] [PubMed] [Google Scholar]
- 7.Rea DJ, Munoz-Juarez M, Farnell MB, Donohue JH, Que FG, Crownhart B, Larson D, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004;139:514–523. doi: 10.1001/archsurg.139.5.514. discussion 523–515. [DOI] [PubMed] [Google Scholar]
- 8.Washburn WK, Lewis WD, Jenkins RL. Aggressive surgical resection for cholangiocarcinoma. Arch Surg. 1995;130:270–276. doi: 10.1001/archsurg.1995.01430030040006. [DOI] [PubMed] [Google Scholar]
- 9.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633–1637. doi: 10.1097/00007890-200004270-00019. [DOI] [PubMed] [Google Scholar]
- 10.Shimoda M, Farmer DG, Colquhoun SD, Rosove M, Ghobrial RM, Yersiz H, Chen P, et al. Liver transplantation for cholangiocellular carcinoma: analysis of a single-center experience and review of the literature. Liver Transpl. 2001;7:1023–1033. doi: 10.1053/jlts.2001.29419. [DOI] [PubMed] [Google Scholar]
- 11.Foo ML, Gunderson LL, Bender CE, Buskirk SJ. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:929–935. doi: 10.1016/s0360-3016(97)00299-x. [DOI] [PubMed] [Google Scholar]
- 12.Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw B, Jr, McCashland T, Sorrell M, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774–779. doi: 10.1034/j.1600-6143.2002.20812.x. [DOI] [PubMed] [Google Scholar]
- 13.Rea DJ, Rosen CB, Nagorney DM, Heimbach JK, Gores GJ. Transplantation for cholangiocarcinoma: when and for whom? Surg Oncol Clin N Am. 2009;18:325–337. ix. doi: 10.1016/j.soc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 14.De Vreede I, Steers JL, Burch PA, Rosen CB, Gunderson LL, Haddock MG, Burgart L, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6:309–316. doi: 10.1053/lv.2000.6143. [DOI] [PubMed] [Google Scholar]
- 15.Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Pedersen R, Kremers W, Nyberg SL, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703–1707. doi: 10.1097/01.tp.0000253551.43583.d1. [DOI] [PubMed] [Google Scholar]
- 16.Heimbach JK, Gores GJ, Nagorney DM, Rosen CB. Liver transplantation for perihilar cholangiocarcinoma after aggressive neoadjuvant therapy: a new paradigm for liver and biliary malignancies? Surgery. 2006;140:331–334. doi: 10.1016/j.surg.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Heimbach JK. Successful liver transplantation for hilar cholangiocarcinoma. Curr Opin Gastroenterol. 2008;24:384–388. doi: 10.1097/MOG.0b013e3282f706ce. [DOI] [PubMed] [Google Scholar]
- 18.Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Nyberg SL, Ishitani MB, Rosen CB. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004;24:201–207. doi: 10.1055/s-2004-828896. [DOI] [PubMed] [Google Scholar]
- 19.Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, Gores GJ, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–458. doi: 10.1097/01.sla.0000179678.13285.fa. discussion 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 21.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–969. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- 22.Draganov PV, Lin T, Chauhan S, Wagh MS, Hou W, Forsmark CE. Prospective evaluation of the clinical utility of ERCP-guided cholangiopancreatoscopy with a new direct visualization system. Gastrointest Endosc. 2011;73:971–979. doi: 10.1016/j.gie.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Hong JC, Petrowsky H, Kaldas FM, Farmer DG, Durazo FA, Finn RS, Saab S, et al. Predictive index for tumor recurrence after liver transplantation for locally advanced intrahepatic and hilar cholangiocarcinoma. J Am Coll Surg. 2011;212:514–520. doi: 10.1016/j.jamcollsurg.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13:356–360. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


